Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization
Abstract
:1. Introduction
2. Results and Discussion
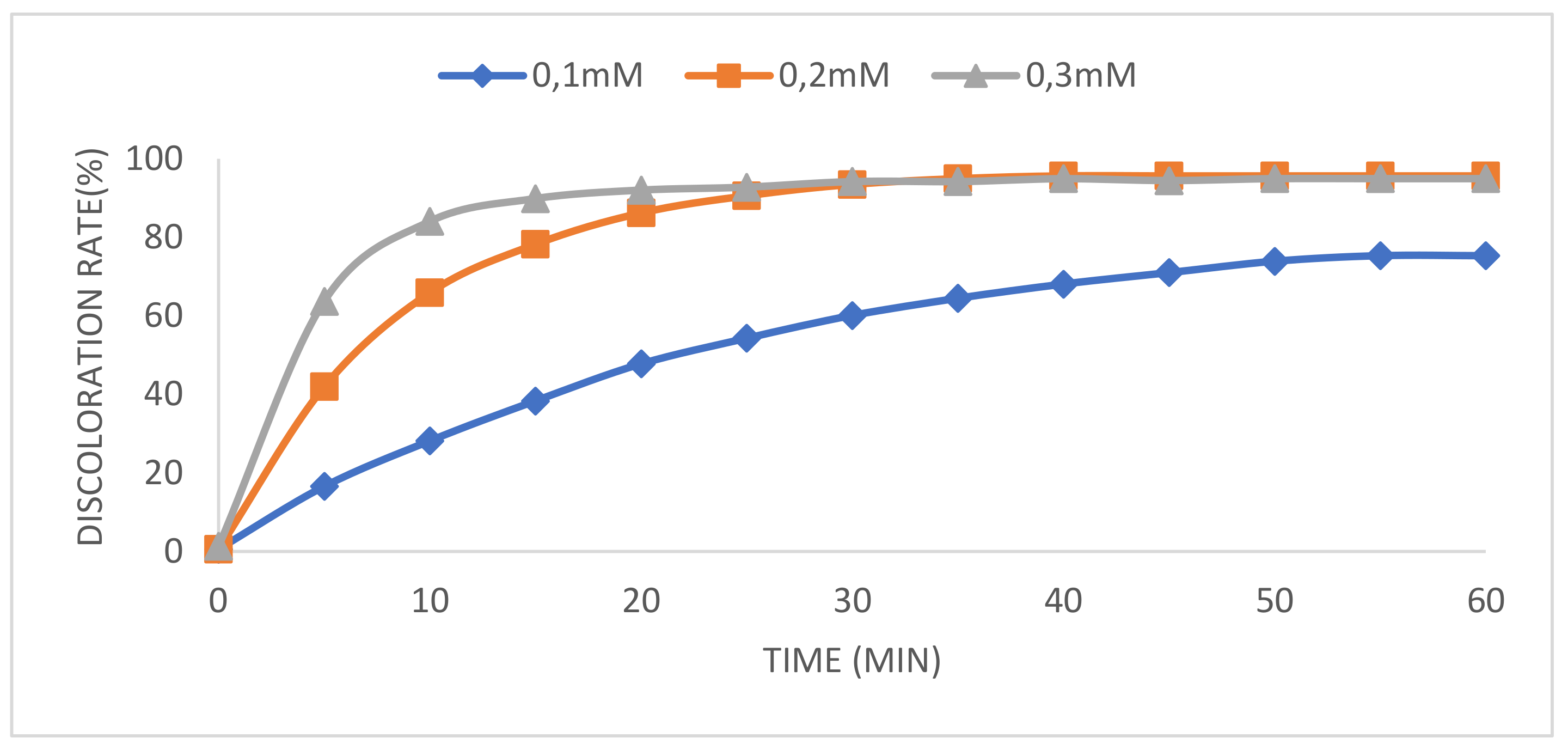
2.1. Effect of Catalyst Concentration
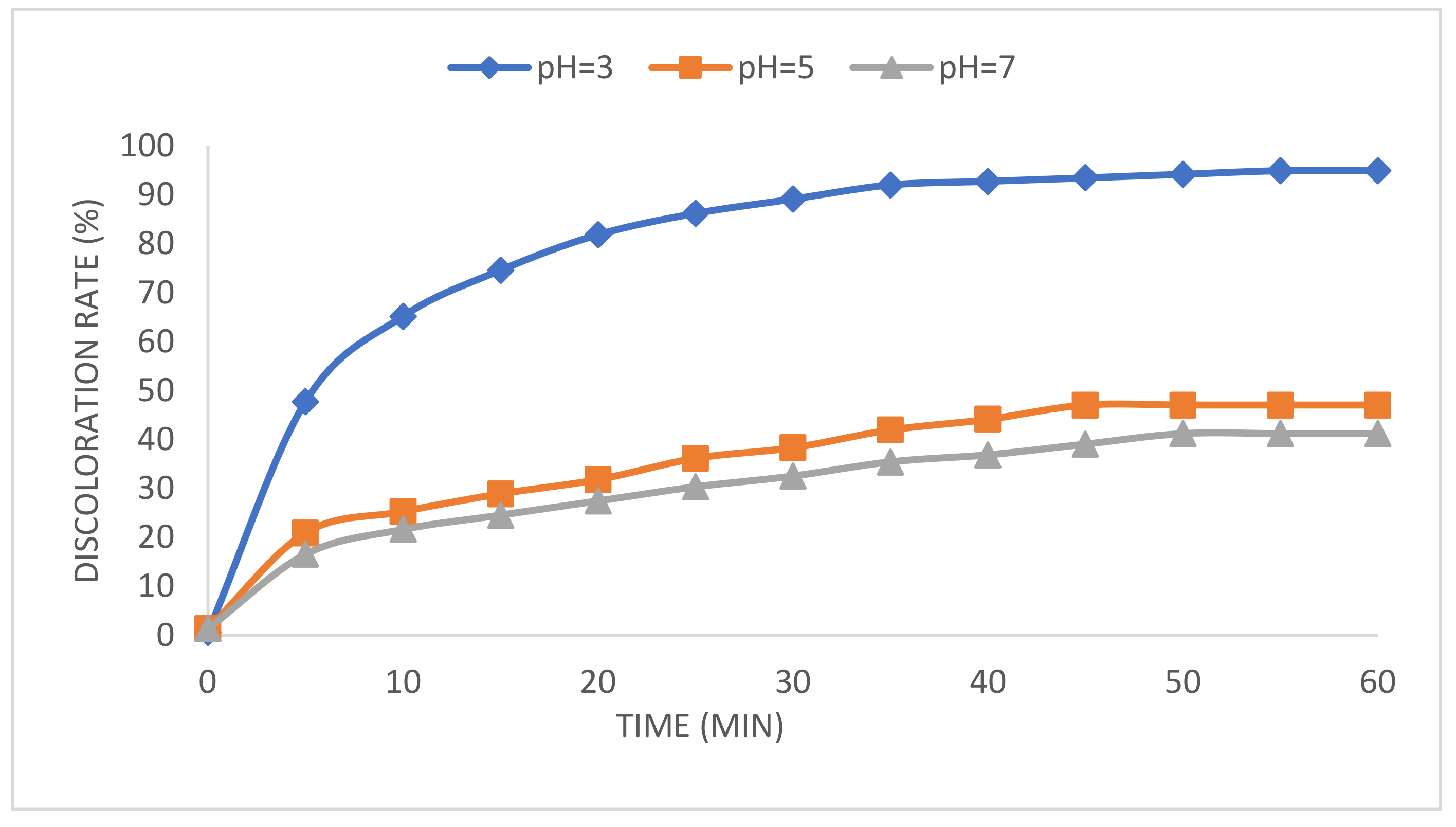
2.2. Effect of Solution pH
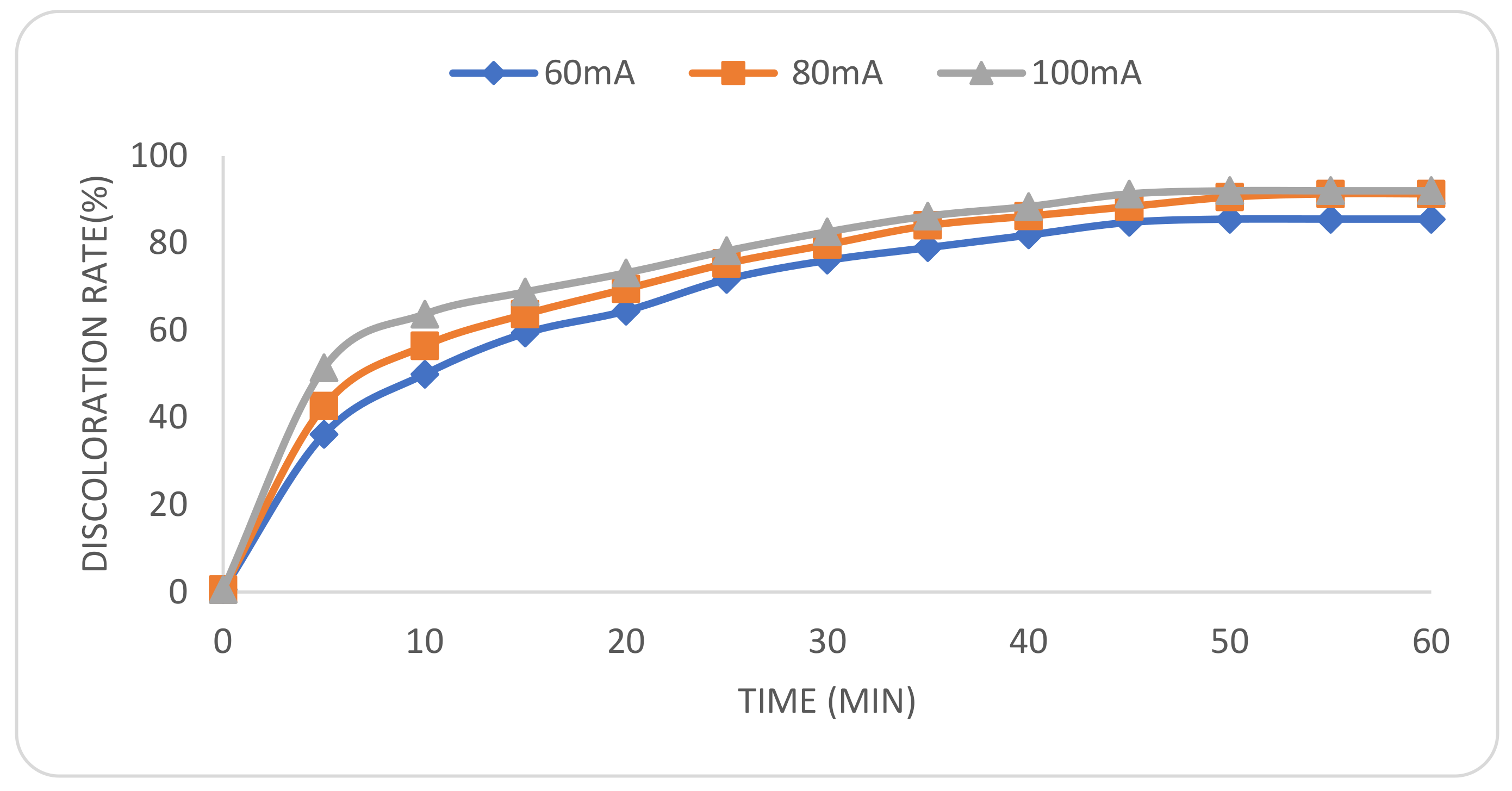
2.3. Effect of Applied Current
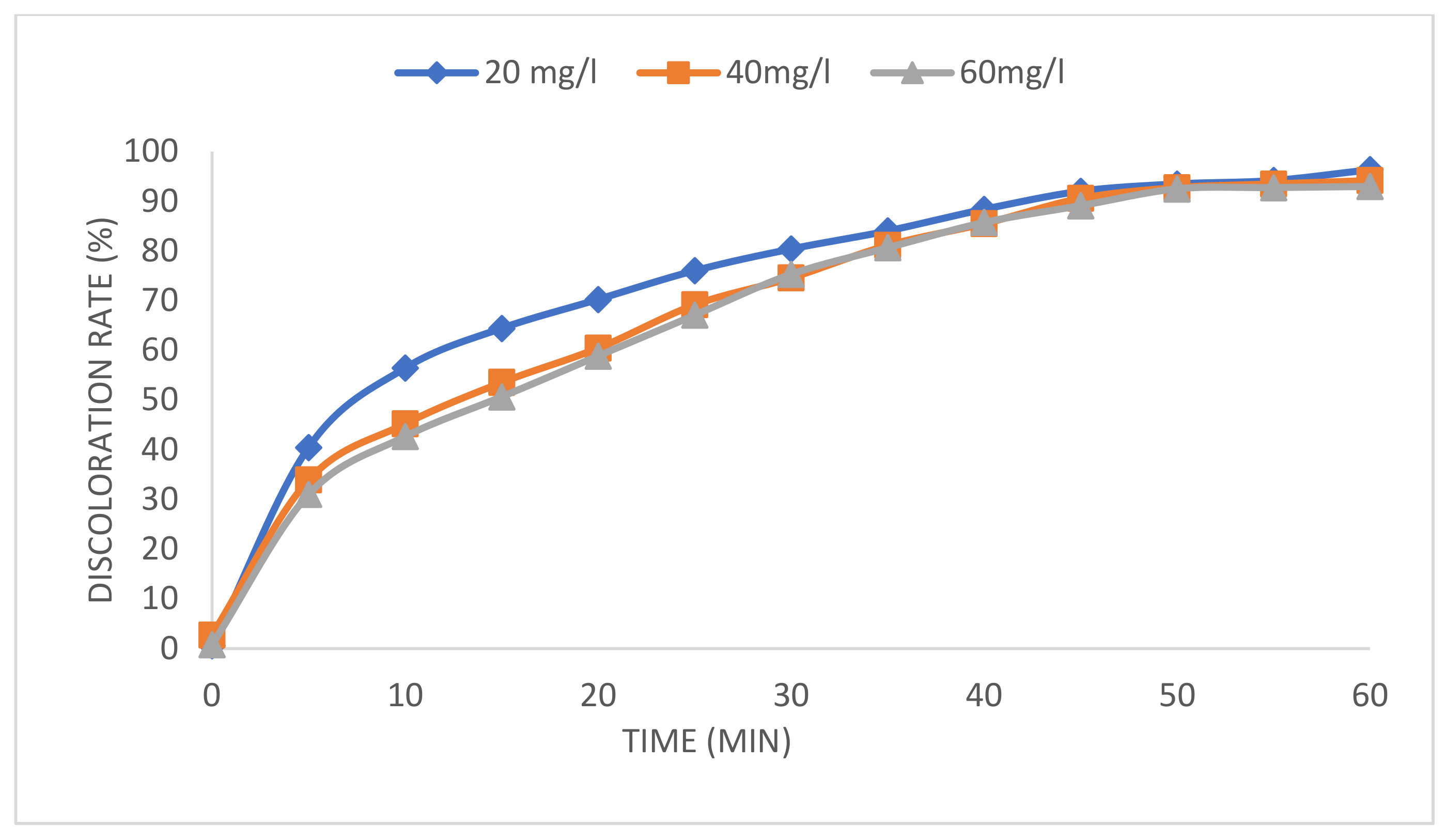
2.4. Effect of Initial Dye Concentrations
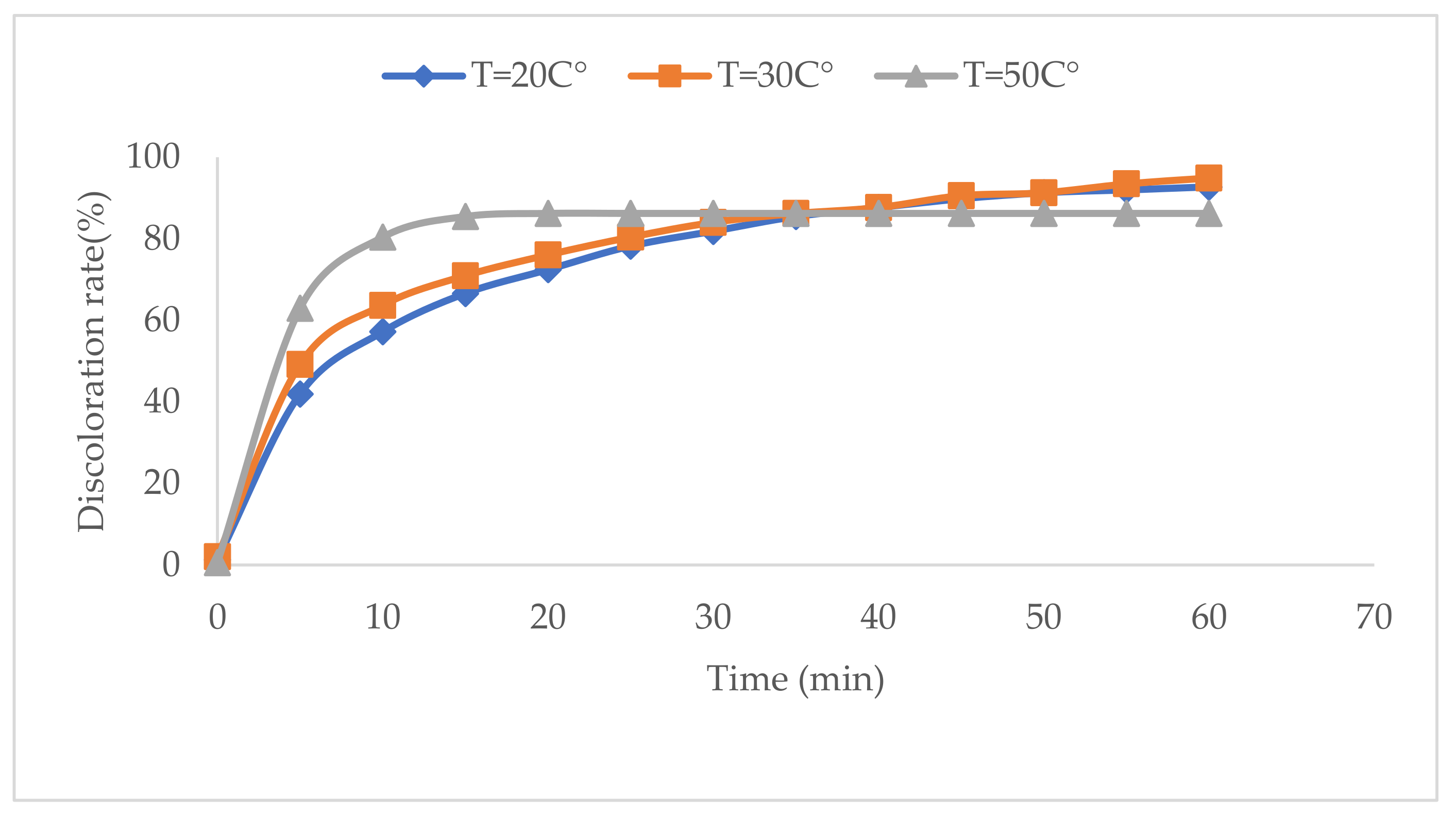
2.5. Effect of Temperature
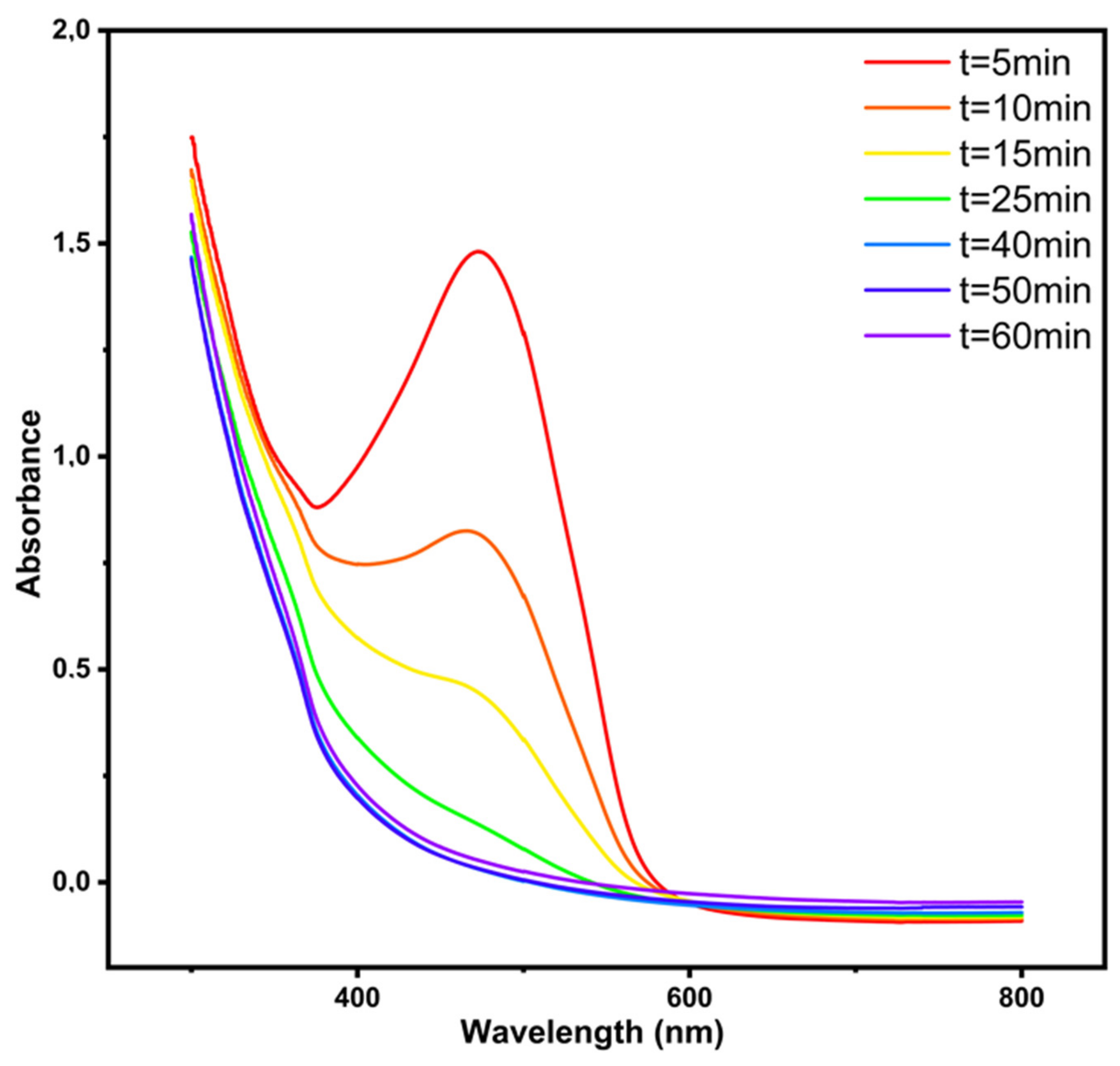
2.6. Evolution of the Methyl Orange Solution’s UV-Visible Spectra
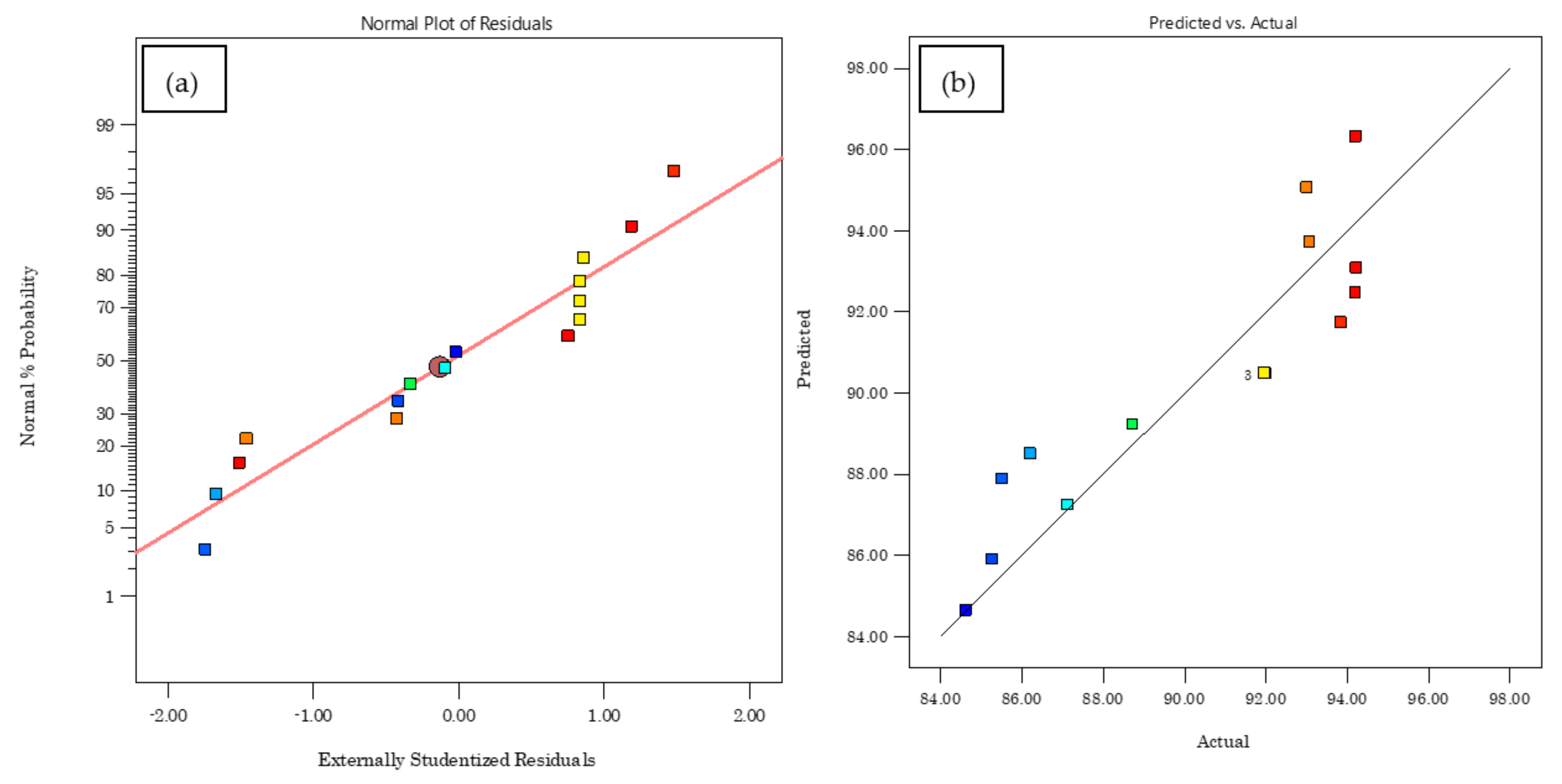
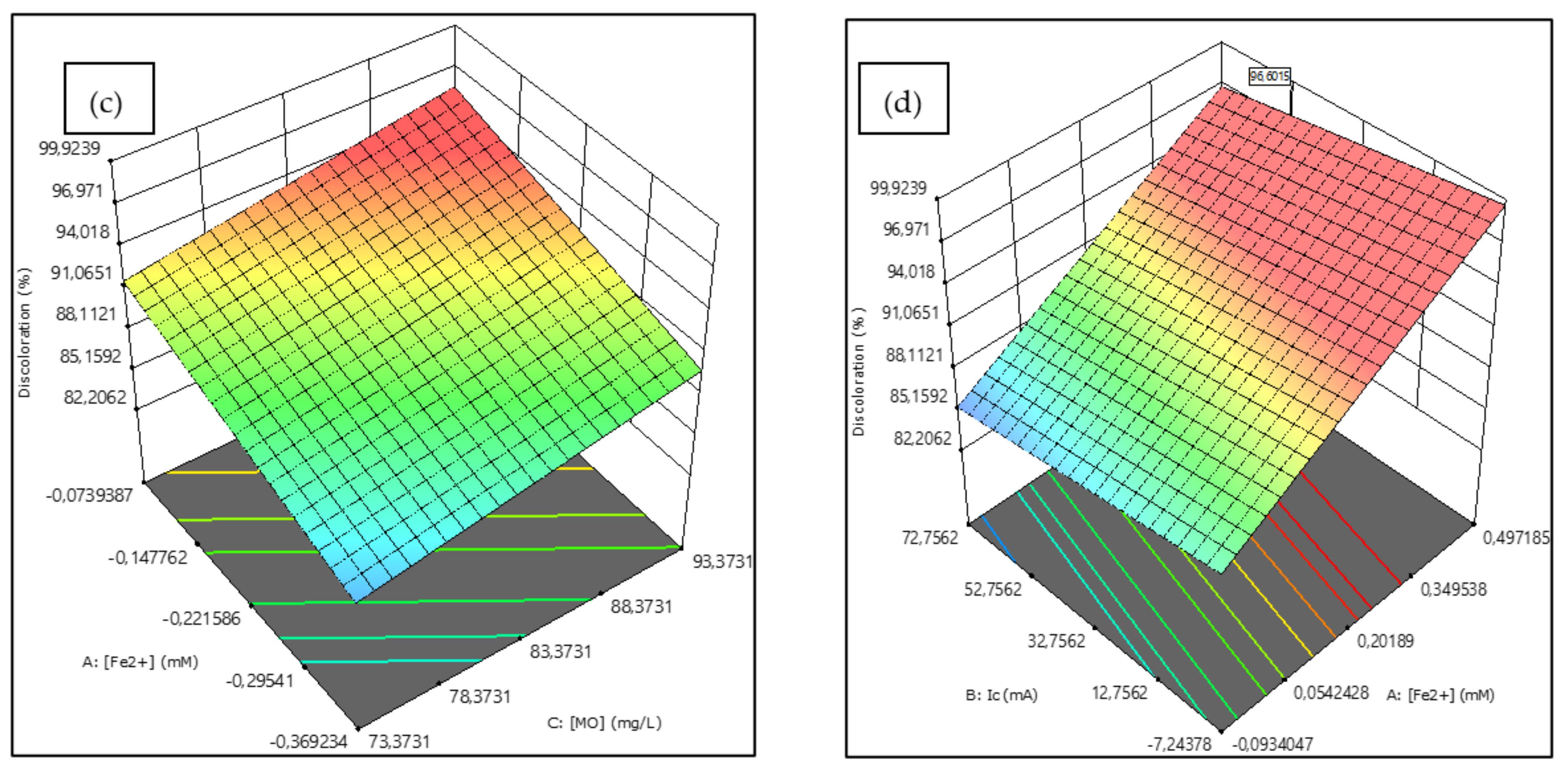
2.7. BBD Plan Optimization and Statistical Modelling
2.8. Numerical Optimization Using the Desirability Function
3. Materials and Methods
3.1. Chemical Product
3.2. Electrochemical Equipment and Procedures
3.3. Experimental Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic Technologies for Environmental Applications. J. Photochem. Photobiol. A Chem. 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Benmessaoud, S.; Anissi, J.; Kara, M.; Assouguem, A.; AL-Huqail, A.A.; Germoush, M.O.; Ullah, R.; Ercisli, S.; Bahhou, J. Isolation and Characterization of Three New Crude Oil Degrading Yeast Strains, Candida Parapsilosis SK1, Rhodotorula Mucilaginosa SK2 and SK3. Sustainability 2022, 14, 3465. [Google Scholar] [CrossRef]
- Sala, M.; Gutiérrez-Bouzán, M.C. Electrochemical Treatment of Industrial Wastewater and Effluent Reuse at Laboratory and Semi-Industrial Scale. J. Clean. Prod. 2014, 65, 458–464. [Google Scholar] [CrossRef]
- Lou, W.; Kane, A.; Wolbert, D.; Rtimi, S.; Assadi, A.A. Study of a Photocatalytic Process for Removal of Antibiotics from Wastewater in a Falling Film Photoreactor: Scavenger Study and Process Intensification Feasibility. Chem. Eng. Process. Process Intensif. 2017, 122, 213–221. [Google Scholar] [CrossRef]
- Ganzenko, O.; Oturan, N.; Sirés, I.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Fast and Complete Removal of the 5-Fluorouracil Drug from Water by Electro-Fenton Oxidation. Environ. Chem. Lett. 2018, 16, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Isarain-Chávez, E.; Rodríguez, R.M.; Cabot, P.L.; Centellas, F.; Arias, C.; Garrido, J.A.; Brillas, E. Degradation of Pharmaceutical Beta-Blockers by Electrochemical Advanced Oxidation Processes Using a Flow Plant with a Solar Compound Parabolic Collector. Water Res. 2011, 45, 4119–4130. [Google Scholar] [CrossRef]
- Sirés, I.; Oturan, N.; Oturan, M.A. Electrochemical Degradation of β-Blockers. Studies on Single and Multicomponent Synthetic Aqueous Solutions. Water Res. 2010, 44, 3109–3120. [Google Scholar] [CrossRef]
- Ganzenko, O.; Trellu, C.; Oturan, N.; Huguenot, D.; Péchaud, Y.; van Hullebusch, E.D.; Oturan, M.A. Electro-Fenton Treatment of a Complex Pharmaceutical Mixture: Mineralization Efficiency and Biodegradability Enhancement. Chemosphere 2020, 253, 126659. [Google Scholar] [CrossRef]
- Kamagate, M.; Assadi, A.A.; Kone, T.; Giraudet, S.; Coulibaly, L.; Hanna, K. Use of Laterite as a Sustainable Catalyst for Removal of Fluoroquinolone Antibiotics from Contaminated Water. Chemosphere 2018, 195, 847–853. [Google Scholar] [CrossRef]
- Lei, H.; Li, H.; Li, Z.; Li, Z.; Chen, K.; Zhang, X.; Wang, H. Electro-Fenton Degradation of Cationic Red X-GRL Using an Activated Carbon Fiber Cathode. Process Saf. Environ. Prot. 2010, 88, 431–438. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Almeida, L.C.; Bocchi, N.; Brillas, E. Solar Photoelectro-Fenton Degradation of the Herbicide 4-Chloro-2-Methylphenoxyacetic Acid Optimized by Response Surface Methodology. J. Hazard. Mater. 2011, 194, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.A.; Anderson, N.G. Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications. Org. Process Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Spessato, L.; Duarte, V.A.; Viero, P.; Zanella, H.; Fonseca, J.M.; Arroyo, P.A.; Almeida, V.C. Optimization of Sibipiruna Activated Carbon Preparation by Simplex-Centroid Mixture Design for Simultaneous Adsorption of Rhodamine B and Metformin. J. Hazard. Mater. 2021, 411, 125166. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Gençten, M. Investigation of Acid Red 88 Oxidation in Water by Means of Electro-Fenton Method for Water Purification. Chemosphere 2016, 146, 245–252. [Google Scholar] [CrossRef]
- Özcan, A.; Oturan, M.A.; Oturan, N.; Şahin, Y. Removal of Acid Orange 7 from Water by Electrochemically Generated Fenton’s Reagent. J. Hazard. Mater. 2009, 163, 1213–1220. [Google Scholar] [CrossRef]
- Al-Qaradawi, S.; Salman, S.R. Photocatalytic Degradation of Methyl Orange as a Model Compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Akyol, A.; Yatmaz, H.C.; Bayramoglu, M. Photocatalytic Decolorization of Remazol Red RR in Aqueous ZnO Suspensions. Appl. Catal. B Environ. 2004, 54, 19–24. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Elyemni, M.; Lahkimi, A.; Lhassani, A.; Chaouch, M.; Taleb, M. Mesoporous Carbon from Optimized Date Stone Hydrochar by Catalytic Hydrothermal Carbonization Using Response Surface Methodology: Application to Dyes Adsorption. Int. J. Chem. Eng. 2021, 2021, 5555406. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Sablas, M.M.; Hung, C.M.; Chen, C.W.; Garcia-Segura, S.; Dong, C. Di Modeling and Optimization of Imidacloprid Degradation by Catalytic Percarbonate Oxidation Using Artificial Neural Network and Box-Behnken Experimental Design. Chemosphere 2020, 251, 126254. [Google Scholar] [CrossRef]
- Jawad, A.H.; Sahu, U.K.; Jani, N.A.; ALOthman, Z.A.; Wilson, L.D. Magnetic Crosslinked Chitosan-Tripolyphosphate/MgO/Fe3O4 Nanocomposite for Reactive Blue 19 Dye Removal: Optimization Using Desirability Function Approach. Surf. Interfaces 2022, 28, 101698. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. Science of the Total Environment A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhou, Z. Technology Electro-Fenton Process for Water and Wastewater Treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of Azo Dyes Using Low Iron Concentration of Fenton and Fenton-like System. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Cerisola, G. Electro-Fenton Degradation of Synthetic Dyes. Water Res. 2009, 43, 339–344. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Journal of Environmental Chemical Engineering A Comparative Study of Electrocoagulation, Electrochemical Fenton, Electro-Fenton and Peroxi-Coagulation for Decolorization of Real Textile Wastewater Electrical Energy Consumption and Biodegradability Imp. Biochem. Pharmacol. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Hodaifa, G.; Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Martinez-Ferez, A. Optimization of Continuous Reactor at Pilot Scale for Olive-Oil Mill Wastewater Treatment by Fenton-like Process. Chem. Eng. J. 2013, 220, 117–124. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Ezenwa, C.P. Removal of Endocrine Disrupting Chemicals in Wastewater Treatment by Fenton-like Oxidation. Water Air Soil Pollut. 2011, 217, 213–220. [Google Scholar] [CrossRef]
- Wang, S. A Comparative Study of Fenton and Fenton-like Reaction Kinetics in Decolourisation of Wastewater. Dyes Pigment. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Kuleyin, A.; Gök, A.; Akbal, F. Treatment of Textile Industry Wastewater by Electro-Fenton Process Using Graphite Electrodes in Batch and Continuous Mode. J. Environ. Chem. Eng. 2021, 9, 104782. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.J.; Frades, J.; Buxeda, P. Oxidation of P-Hydroxybenzoic Acid by Fenton’s Reagent. Water Res. 2001, 35, 387–396. [Google Scholar] [CrossRef]
- Pajootan, E.; Arami, M.; Rahimdokht, M. Discoloration of Wastewater in a Continuous Electro-Fenton Process Using Modified Graphite Electrode with Multi-Walled Carbon Nanotubes/Surfactant. Sep. Purif. Technol. 2014, 130, 34–44. [Google Scholar] [CrossRef]
- Ruiz, E.J.; Arias, C.; Brillas, E.; Hernández-ramírez, A.; Peralta-hernández, J.M. Chemosphere Mineralization of Acid Yellow 36 Azo Dye by Electro-Fenton and Solar Photoelectro-Fenton Processes with a Boron-Doped Diamond Anode. Chemosphere 2011, 82, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kubo, D.; Kawase, Y. Hydroxyl Radical Generation in Electro-Fenton Process with in Situ Electro-Chemical Production of Fenton Reagents by Gas-Diffusion-Electrode Cathode and Sacrificial Iron Anode. J. Clean. Prod. 2018, 203, 685–695. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, N.; Olvera-Vargas, H.; Brillas, E.; Gadri, A.; Ammar, S.; Oturan, M.A. Pyrite as a Sustainable Catalyst in Electro-Fenton Process for Improving Oxidation of Sulfamethazine. Kinetics, Mechanism and Toxicity Assessment. Water Res. 2016, 94, 52–61. [Google Scholar] [CrossRef]
- Samet, Y.; Agengui, L.; Abdelhédi, R. Electrochemical Degradation of Chlorpyrifos Pesticide in Aqueous Solutions by Anodic Oxidation at Boron-Doped Diamond Electrodes. Chem. Eng. J. 2010, 161, 167–172. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Liu, M.; Ma, L.; Yang, H. A Novel Vertical-Flow Electro-Fenton Reactor for Organic Wastewater Treatment. Chem. Eng. J. 2016, 298, 55–67. [Google Scholar] [CrossRef]
- Moreira, F.C.; Soler, J.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes for Sanitary Landfill Leachate Remediation: Evaluation of Operational Variables. Appl. Catal. B Environ. 2016, 182, 161–171. [Google Scholar] [CrossRef]
- Hamza, M.; Abdelhedi, R.; Brillas, E.; Sirés, I. Comparative Electrochemical Degradation of the Triphenylmethane Dye Methyl Violet with Boron-Doped Diamond and Pt Anodes. J. Electroanal. Chem. 2009, 627, 41–50. [Google Scholar] [CrossRef]
- Sun, J.H.; Sun, S.P.; Wang, G.L.; Qiao, L.P. Degradation of Azo Dye Amido Black 10B in Aqueous Solution by Fenton Oxidation Process. Dyes Pigment. 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Wang, C.T.; Hu, J.L.; Chou, W.L.; Kuo, Y.M. Removal of Color from Real Dyeing Wastewater by Electro-Fenton Technology Using a Three-Dimensional Graphite Cathode. J. Hazard. Mater. 2008, 152, 601–606. [Google Scholar] [CrossRef]
- Wang, C.T.; Chou, W.L.; Chung, M.H.; Kuo, Y.M. COD Removal from Real Dyeing Wastewater by Electro-Fenton Technology Using an Activated Carbon Fiber Cathode. Desalination 2010, 253, 129–134. [Google Scholar] [CrossRef]
- Zyoud, A.; Zu’bi, A.; Helal, M.H.S.; Park, D.H.; Campet, G.; Hilal, H.S. Optimizing Photo-Mineralization of Aqueous Methyl Orange by Nano-ZnO Catalyst under Simulated Natural Conditions. J. Environ. Health Sci. Eng. 2015, 13, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Zee, F.P.; Villaverde, S. Combined Anaerobic-Aerobic Treatment of Azo Dyes—A Short Review of Bioreactor Studies. Water Res. 2005, 39, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M. Experimental Design and Response Surface Methodology in Energy Applications: A Tutorial Review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Amiri, F.; Mousavi, S.M.; Yaghmaei, S. Enhancement of Bioleaching of a Spent Ni/Mo Hydroprocessing Catalyst by Penicillium Simplicissimum. Sep. Purif. Technol. 2011, 80, 566–576. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Comparison of Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Modelling for Supercritical Fluid Extraction of Phytochemicals from Terminalia Chebula Pulp and Optimization Using RSM Coupled with Desirability Function (DF) and Genetic Algorithm (GA) and ANN with GA. Ind. Crops Prod. 2021, 170, 113769. [Google Scholar] [CrossRef]
- Sharifpour, E.; Khafri, H.Z.; Ghaedi, M.; Asfaram, A.; Jannesar, R. Isotherms and Kinetic Study of Ultrasound-Assisted Adsorption of Malachite Green and Pb2+ Ions from Aqueous Samples by Copper Sulfide Nanorods Loaded on Activated Carbon: Experimental Design Optimization. Ultrason. Sonochem. 2018, 40, 373–382. [Google Scholar] [CrossRef]

| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 155.57 | 3 | 51.86 | 16.31 | 0.0002 | significant |
| A-[Fe2+] | 29.74 | 1 | 29.74 | 9.35 | 0.0099 | |
| B-Ic | 3.61 | 1 | 3.61 | 1.14 | 0.3075 | |
| C-[MO] | 122.22 | 1 | 122.22 | 38.44 | <0.0001 | |
| Residual | 38.16 | 12 | 3.18 | |||
| Lack of Fit | 38.16 | 9 | 4.24 | 13,726.38 | <0.0001 | significant |
| Optimal Condition: X1 = 0.232 mM, X2 = 80 mA, X3 = 60 mg/L with D = 1 | |||
|---|---|---|---|
| Predicted | Experimental | Error | |
| Discoloration rate (%) | 95 | 94.9 | 0.1 (0.1%) |
| Factor | Name | Units | Minimum | Maximum | Coded Low | Coded High |
|---|---|---|---|---|---|---|
| X1 | [Fe2+] | mM | 0.10 | 0.30 | −1 ↔ 0.10 | +1 ↔ 0.30 |
| X2 | Ic | mA | 60.00 | 100.00 | −1 ↔ 60.00 | +1 ↔ 100.00 |
| X3 | [MO] | mg/L | 20.00 | 60.00 | −1 ↔ 20.00 | +1 ↔ 60.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, A.; Ouadrhiri, F.E.; Kara, M.; El Manssouri, I.; Assouguem, A.; Almutairi, M.H.; Bayram, R.; Mohamed, H.R.H.; Peluso, I.; Eloutassi, N.; et al. Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization. Catalysts 2022, 12, 665. https://doi.org/10.3390/catal12060665
Adachi A, Ouadrhiri FE, Kara M, El Manssouri I, Assouguem A, Almutairi MH, Bayram R, Mohamed HRH, Peluso I, Eloutassi N, et al. Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization. Catalysts. 2022; 12(6):665. https://doi.org/10.3390/catal12060665
Chicago/Turabian StyleAdachi, Abderrazzak, Faiçal El Ouadrhiri, Mohammed Kara, Ibtissam El Manssouri, Amine Assouguem, Mikhlid H. Almutairi, Roula Bayram, Hanan R. H. Mohamed, Ilaria Peluso, Noureddine Eloutassi, and et al. 2022. "Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization" Catalysts 12, no. 6: 665. https://doi.org/10.3390/catal12060665
APA StyleAdachi, A., Ouadrhiri, F. E., Kara, M., El Manssouri, I., Assouguem, A., Almutairi, M. H., Bayram, R., Mohamed, H. R. H., Peluso, I., Eloutassi, N., & Lahkimi, A. (2022). Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization. Catalysts, 12(6), 665. https://doi.org/10.3390/catal12060665










