A HCl-Mediated, Metal- and Oxidant-Free Photocatalytic Strategy for C3 Arylation of Quinoxalin(on)es with Arylhydrazine
Abstract
:1. Introduction
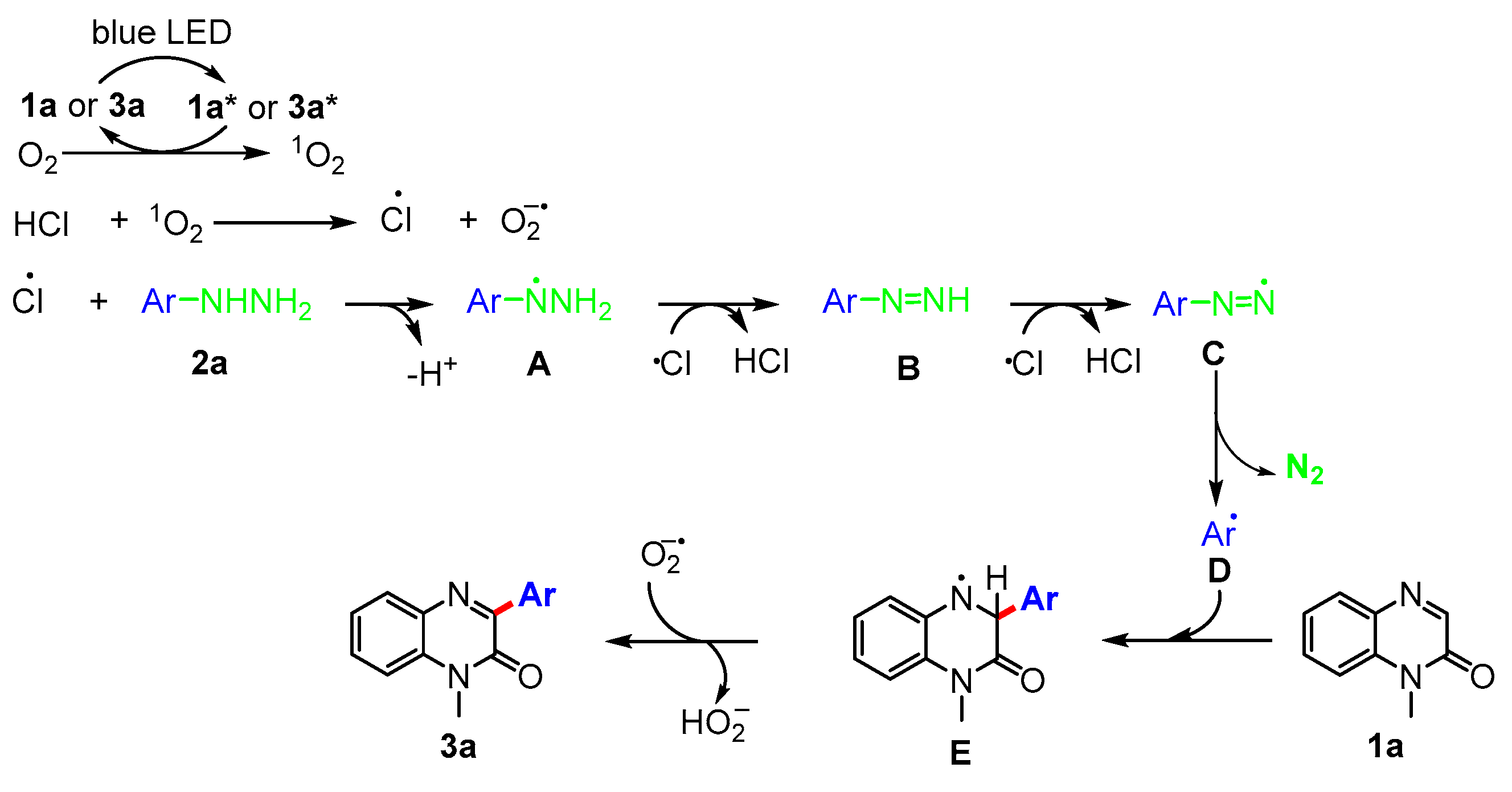
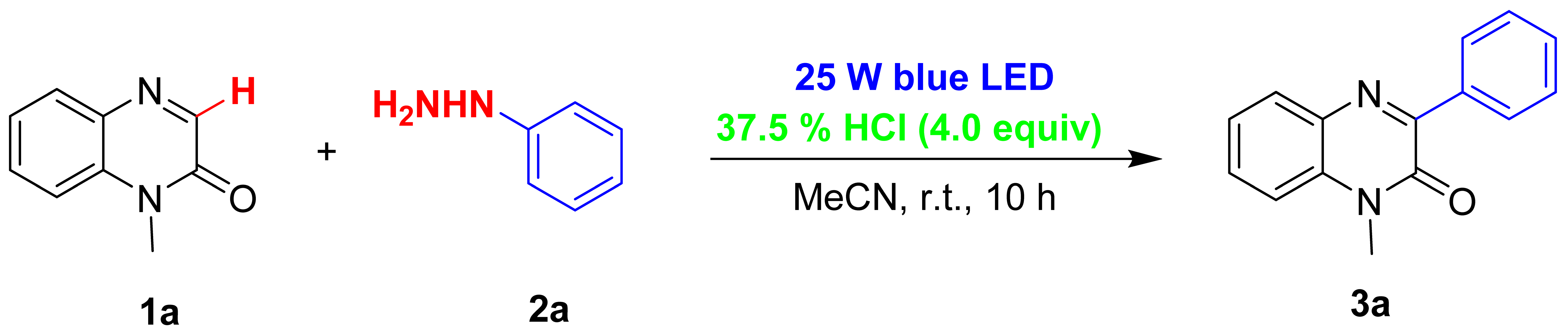
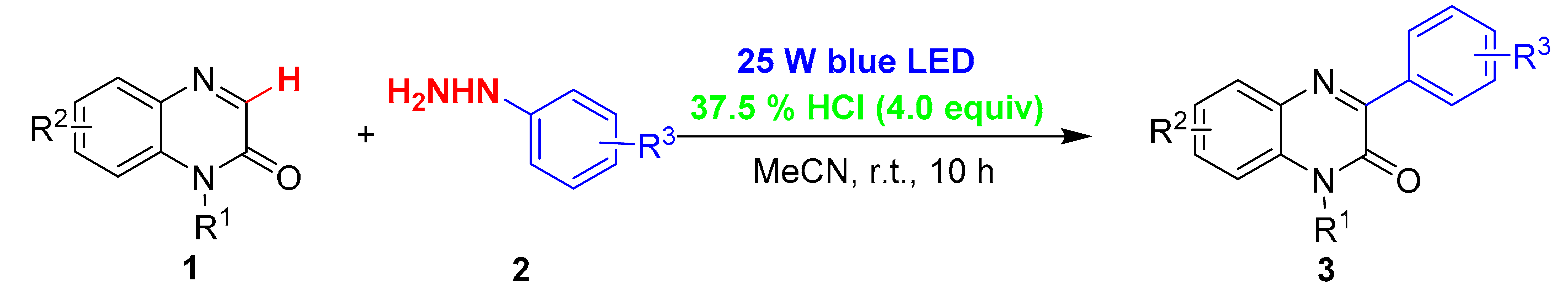
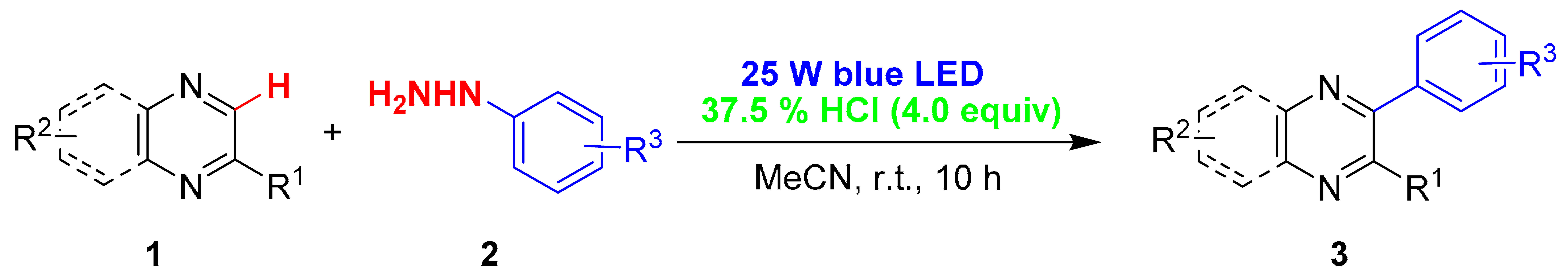
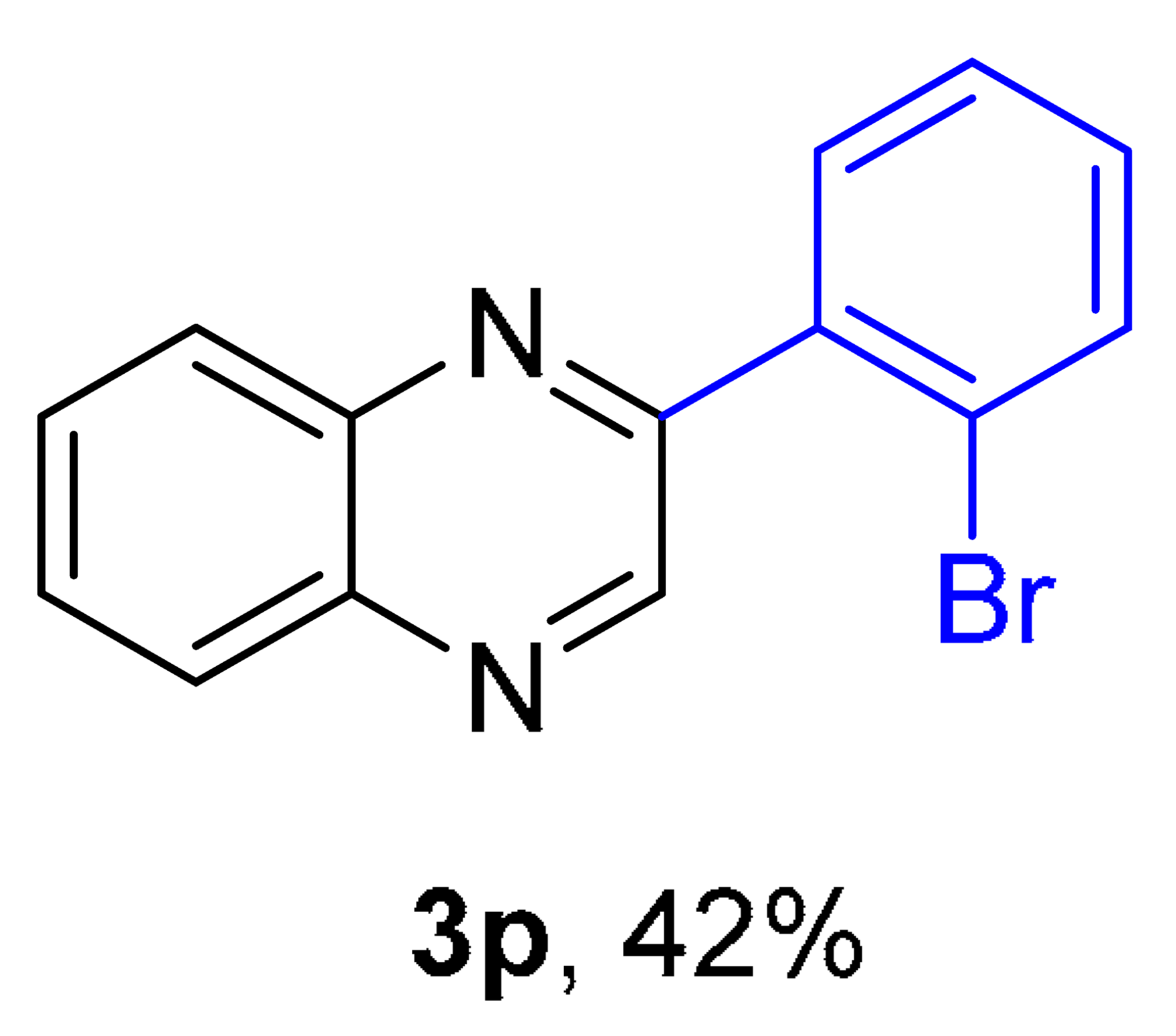
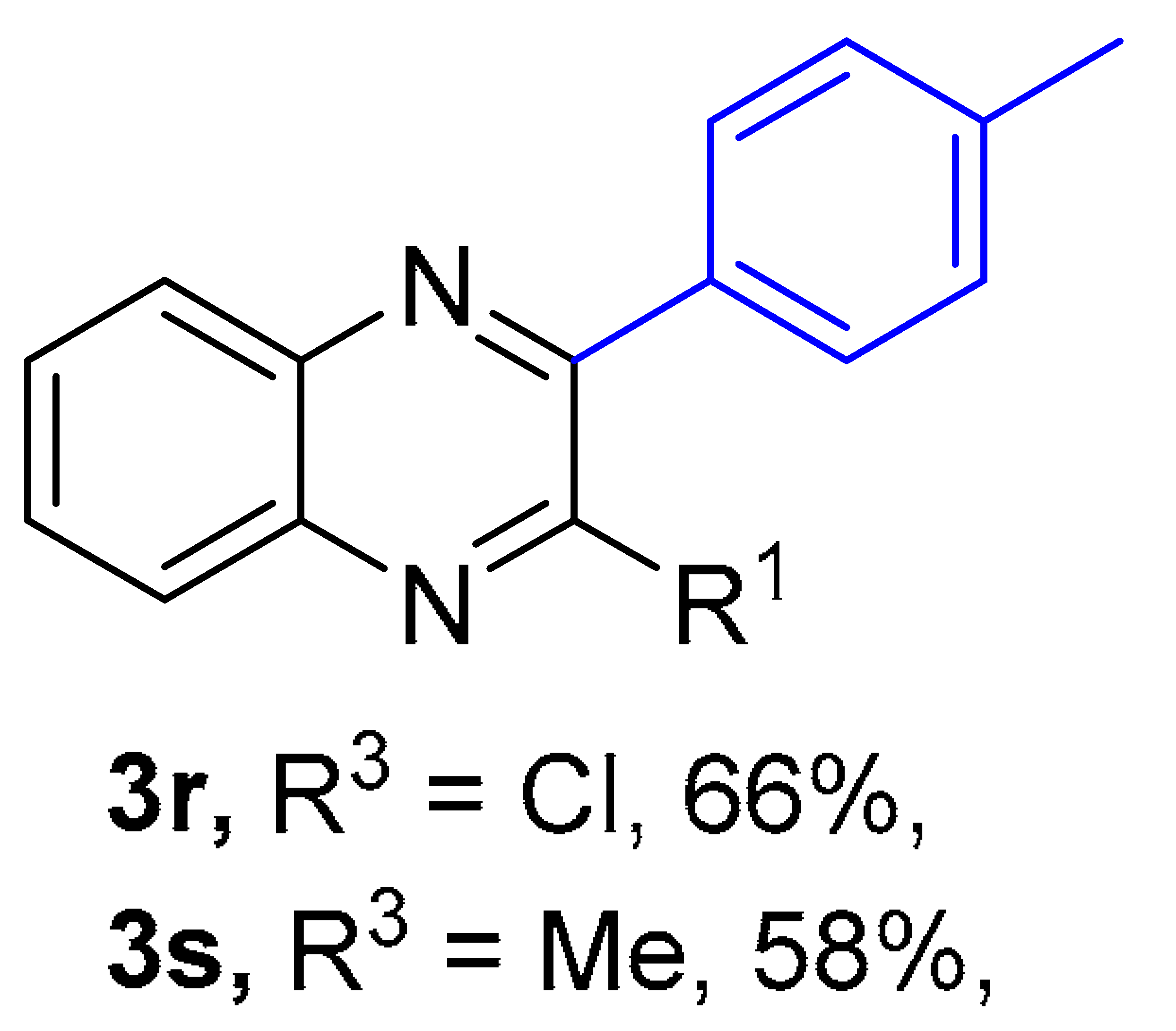
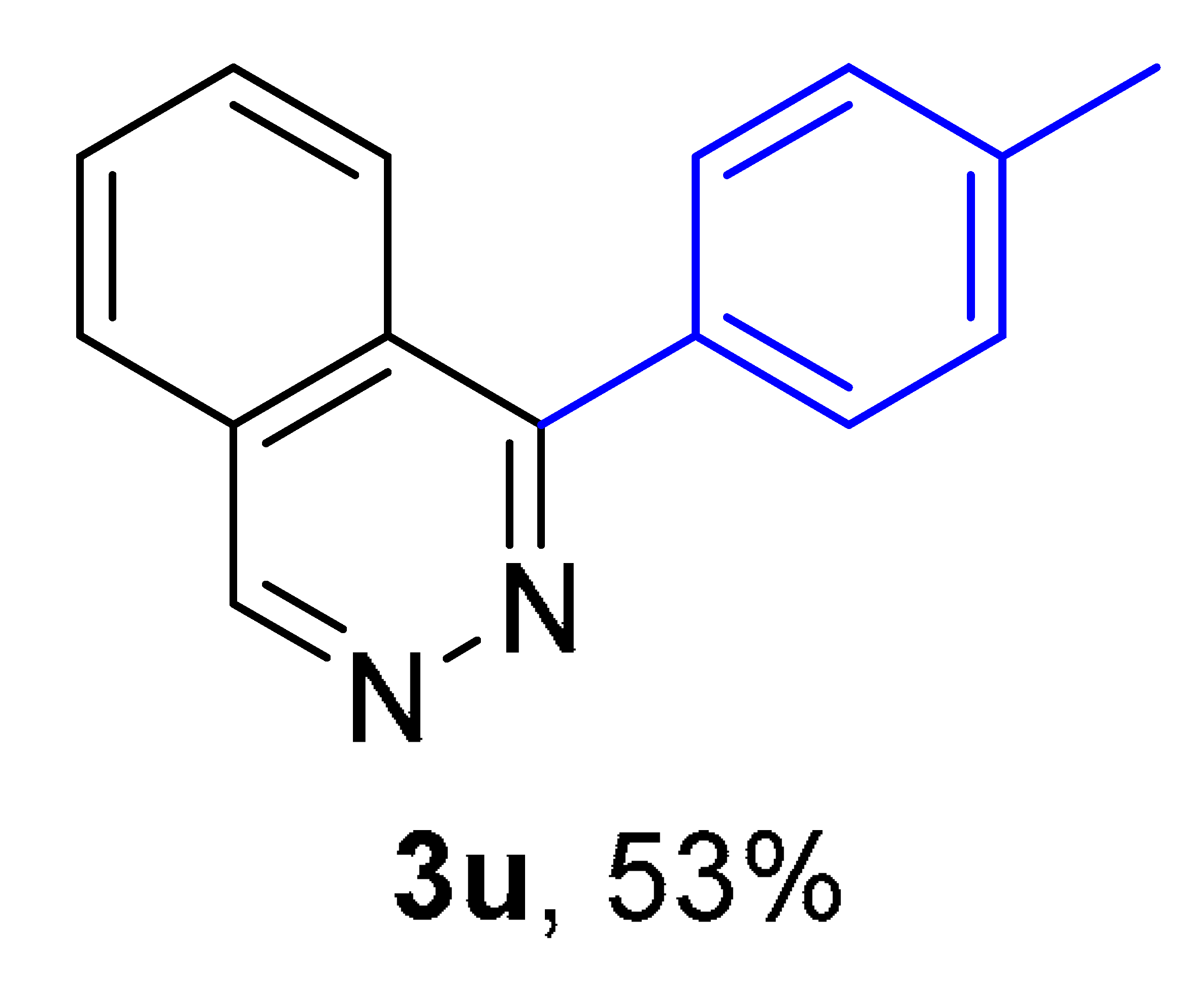
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- TenBrink, R.E.; Im, W.B.; Sethy, V.H.; Tang, A.H.; Carter, D.B. Antagonist, Partial Agonist, and Full Agonist Imidazo[1,5-a]quinoxaline Amides and Carbamates Acting through the GABAA/Benzodiazepine Receptor. J. Med. Chem. 1994, 37, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Abouzid, K.A.M.; Hussein, M.H.M. Synthesis of Certain Substituted Quinoxalines as Antimicrobial Agents (Part II). Arch. Pharmacal. Res. 2003, 26, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Weïwer, M.; Spoonamore, J.; Wei, J.; Guichard, B.; Ross, N.T.; Masson, K.; Silkworth, W.; Dandapani, S.; Palmer, M.; Scherer, C.A.; et al. A Potent and Selective Quinoxalinone-Based STK33 Inhibitor Does Not Show Synthetic Lethality in KRAS-Dependent Cells. ACS Med. Chem. Lett. 2012, 3, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bao, W. An Efficient Domino Synthesis of Quinoxalin-2(1H)-ones via an SNAr/Coupling/Demesylation Reaction Catalyzed by Copper(I) as Key Step. Adv. Synth. Catal. 2010, 352, 955–960. [Google Scholar] [CrossRef]
- Wen, J.; Wei, W.; Xue, S.; Yang, D.; Lou, Y.; Gao, C.; Wang, H. Metal-Free Oxidative Spirocyclization of Alkynes with Sulfonylhydrazides Leading to 3-Sulfonated Azaspiro[4,5]trienones. J. Org. Chem. 2015, 80, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Y.; Qu, J.; Peng, S.; Liu, K.-J.; Wang, Z.; Ding, M.-H.; Wang, Y.; Cao, Z.; He, W.-M. Selectfluor-mediated Regioselective Nucleophilic Functionalization of N-heterocycles under Metal- and Base-free Conditions. Green Chem. 2018, 20, 760–764. [Google Scholar] [CrossRef]
- Quinn, J.; Guo, C.; Ko, L.; Sun, B.; He, Y.; Li, Y. Pyrazino[2,3-g]quinoxaline-2,7-dione Based π-conjugated Polymers with Affinity Towards Acids and Semiconductor Performance in Organic Thin Film Transistors. RSC Adv. 2016, 6, 22043–22501. [Google Scholar] [CrossRef]
- Jeon, S.O.; Lee, J.Y. Comparison of Symmetric and Asymmetric Bipolar type high Triplet Energy host Materials for Deep Blue Phosphorescent Organic Light-emitting Diodes. J. Mater. Chem. 2012, 22, 7239–7244. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Habib, N.S.; Kassem, M.A. Synthesis of Some New Quinoxalines and 1,2,4-Triazolo[4,3-a]-quinoxalines for Evaluation of in vitro Antitumor and Antimicrobial Activities. Arch. Pharm. Chem. Life Sci. 2006, 339, 564–571. [Google Scholar] [CrossRef]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Rodrigues, F.A.R.; Bomfim, I.D.S.; Cavalcanti, B.C.; Pessoa, C.D.Ó.; Wardell, J.L.; Wardell, S.M.S.V.; Pinheiro, A.C.; Kaiser, C.R.; Nogueira, T.C.M.; Low, J.N.; et al. Design, Synthesis and Biological Evaluation of (E)-2-(2-arylhydrazinyl)quinoxalines, a Promising and Potent New Class of Anticancer Agents. Bioorg. Med. Chem. Lett. 2014, 24, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Newahie, A.M.S.E.; Ismail, N.S.M.; Ella, D.A.A.E.; Abouzid, K.A.M. Quinoxaline-Based Scaffolds Targeting Tyrosine Kinases and Their Potential Anticancer Activity. Arch. Pharm. Chem. Life Sci. 2016, 349, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Kánai, K.; Arányi, P.; Böcskei, Z.; Ferenczy, G.; Harmat, V.; Simon, K.; Bátori, S.; Náray-Szabó, G.; Hermecz, I. Prolyl Oligopeptidase Inhibition by N-Acyl-pro-pyrrolidine-type Molecules. J. Med. Chem. 2008, 51, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; et al. Design and Synthesis of Potent and Multifunctional Aldose Reductase Inhibitors Based on Quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [CrossRef]
- Viswanadham, K.K.D.R.; Reddy, M.P.; Sathyanarayana, P.; Ravi, O.; Kant, R.; Bathula, S.R. Iodine-mediated oxidative annulation for one-pot synthesis of pyrazines and quinoxalines using a multipathway coupled domino strategy. Chem. Commun. 2014, 50, 13517–13520. [Google Scholar] [CrossRef]
- Hazarika, D.; Phukan, P. Metal Free Synthesis of Quinoxalines from Alkynes via a Cascade Process using TsNBr2. Tetrahedron 2017, 73, 1374–1379. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Lv, G.; Lai, R.; Lv, S.; Li, J.; Hai, L.; Wu, Y. Iridium-Catalyzed Carbenoid Insertion of Sulfoxonium Ylides for Synthesis of Quinoxalines and β-Keto Thioethers in Water. Eur. J. Org. Chem. 2020, 2020, 4635–4638. [Google Scholar] [CrossRef]
- Gulevskaya, A.V.; Burov, O.N.; Pozharskii, A.F.; Kletskii, M.E.; Korbukova, I.N. Oxidative Alkylamination of Azinones as a Direct route to Aminoazinones: Study of some Condensed Diazinones. Tetrahedron 2008, 64, 696–707. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Wang, L.; Cui, X. Copper-catalysed Oxidative Amination of Quinoxalin-2(1H)-ones with Aliphatic Amines. Org. Biomol. Chem. 2016, 14, 8428–8432. [Google Scholar] [CrossRef]
- Gupta, A.; Deshmukh, M.S.; Jain, N. Iodine-Catalyzed C–N Bond Formation: Synthesis of 3-Aminoquinoxalinones under Ambient Conditions. J. Org. Chem. 2017, 82, 4784–4792. [Google Scholar] [CrossRef]
- Wei, W.; Wang, L.; Bao, P.; Shao, Y.; Yue, H.; Yang, D.; Yang, X.; Zhao, X.; Wang, H. Metal-Free C(sp2)–H/N–H Cross-Dehydrogenative Coupling of Quinoxalinones with Aliphatic Amines under Visible-Light Photoredox Catalysis. Org. Lett. 2018, 20, 7125–7130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Fu, J.; Yin, J.; Dong, Z.; Xiao, Y.; Mao, P.; Qu, L. Transition-metal-free Direct C-3 Alkylation of Quinoxalin-2(1H)-ones with Ethers. Org. Chem. Front. 2018, 5, 2820–2828. [Google Scholar] [CrossRef]
- Sun, A.C.; McClain, E.J.J.; Beatty, W.; Stephenson, C.R.J. Visible Light-Mediated Decarboxylative Alkylation of Pharmaceutically Relevant Heterocycles. Org. Lett. 2018, 20, 3487–3490. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.X.; Su, Y.P.; Wang, K.-H.; Zhang, R.; Feng, Y.W.; Cao, L.D.; Huang, D.F.; Hu, Y. Visible-light induced Decarboxylative Alkylation of Quinoxalin-2(1H)-ones at the C3-position. Org. Biomol. Chem. 2019, 17, 6654–6661. [Google Scholar] [CrossRef]
- Xu, J.; He, L.; Liang, C.F.; Yue, X.G.; Ouyang, Y.N.; Zhang, P.F. Multicomponent Bifunctionalization of Methyl Ketones Enabled by Heterogeneous Catalysis and Solar Photocatalysis in Water. ACS Sustain. Chem. Eng. 2021, 9, 13663–13671. [Google Scholar] [CrossRef]
- Niu, K.K.; Hao, Y.K.; Song, L.Y.; Liu, Y.X.; Wang, Q.M. Electro-oxidative C–H Alkylation of Quinoxalin-2(1H)-ones with Organoboron Compounds. Green Chem. 2021, 23, 302–306. [Google Scholar] [CrossRef]
- Xie, L.Y.; Chen, Y.L.; Qin, L.; Wen, Y.; Xie, J.W.; Tan, J.X.; Huang, Y.; Cao, Z.; He, W.M. Visible-light-promoted Direct C–H/S–H Cross-coupling of Quinoxalin-2(1H)-ones with Thiols Leading to 3-sulfenylated Quinoxalin-2(1H)-ones in air. Org. Chem. Front. 2019, 6, 3950–3955. [Google Scholar] [CrossRef]
- Xie, L.Y.; Liu, Y.S.; Ding, H.R.; Gong, S.F.; Tan, J.X.; He, J.Y.; Cao, Z.; He, W.M. C(sp2)–H/O–H Cross-dehydrogenative Coupling of Quinoxalin-2(1H)-ones with Alcohols under Visible-light Photoredox Catalysis. Chin. J. Catal. 2020, 41, 1168–1173. [Google Scholar] [CrossRef]
- Xu, X.; Xia, C.C.; Li, X.; Sun, J.; Hao, L.Q. Visible-light-induced aerobic C3–H Fluoroalkoxylation of Quinoxalin-2(1H)-ones with Fluoroalkyl Alcohols. RSC Adv. 2020, 10, 2016–2026. [Google Scholar] [CrossRef] [Green Version]
- Carrër, A.; Brion, J.D.; Alami, M.; Messaoudi, S. Assisted Tandem Palladium(II)/Palladium(0)-Catalyzed C- and N-Arylations of Quinoxalin-2(1 H)-ones in Water. Adv. Synth. Catal. 2014, 356, 3821–3830. [Google Scholar] [CrossRef]
- Paul, S.; Khanal, H.D.; Clinton, C.D.; Kim, S.H.; Lee., Y.R. Pd(TFA)2-catalyzed Direct Arylation of Quinoxalinones with Arenes. Org. Chem. Front. 2019, 6, 231–235. [Google Scholar] [CrossRef]
- Paul, S.; Ha, J.H.; Park, G.E.; Lee, Y.R. Transition Metal-Free Iodosobenzene-Promoted Direct Oxidative 3-Arylation of Quinoxalin-2(H)-ones with Arylhydrazines. Adv. Synth. Catal. 2017, 359, 1515–1521. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, R.H. Transition-Metal-Free Direct C–H Arylation of Quinoxalin-2(1H)-ones with Diaryliodonium Salts at Room Temperature. Org. Lett. 2017, 19, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.W.; Liu, S.N.; Qu, L.B. Transition Metal-Free Direct C-3 Arylation of Quinoxalin-2(1H)-ones with Arylamines under Mild Conditions. Adv. Synth. Catal. 2017, 359, 4197–4207. [Google Scholar] [CrossRef]
- Song, S.J.; Shi, X.J.; Zhu, Y.S.; Ren, Q.L.; Zhou, P.; Zhou, J.D.; Li, J.J. Electrochemical Oxidative C–H Arylation of Quinoxalin(on)es with Arylhydrazine Hydrochlorides under Mild Conditions. J. Org. Chem. 2022, 87, 4764–4776. [Google Scholar] [CrossRef]
- Liu, K.-J.; Wang, Z.; Lu, L.-H.; Chen, J.-Y.; Zeng, F.; Lin, Y.-W.; Cao, Z.; Yu, X.Y.; He, W.-M. Synergistic Cooperative Effect of CF3SO2Na and Bis(2-butoxyethyl)ether Towards Selective Oxygenation of Sulfides with Molecular Oxygen under Visible-light Irradiation. Green Chem. 2021, 23, 496–500. [Google Scholar] [CrossRef]
- Zhu, X.J.; Liu, Y.; Liu, C.; Yang, H.; Fu, J.H. Light and Oxygen-enabled Sodium Trifluoromethanesulfinate-mediated Selective Oxidation of C–H Bonds. Green Chem. 2020, 22, 4357–4363. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E.J. Controlled Fluoroalkylation Reactions by Visible-Light Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 2284–2294. [Google Scholar] [CrossRef]
- Wang, L.L.; Bao, P.L.; Liu, W.W.; Liu, S.T.; Hu, C.S.; Yue, H.L.; Yang, D.S.; Wei, W. Direct C-H 3-Arylation of Quinoxalin-2(H)-ones with Aryl Diazonium Salts under Visible-Light Irradiation. Chin. J. Org. Chem. 2018, 38, 3189–3196. [Google Scholar] [CrossRef]
- Tian, M.; Liu, S.; Bu, X.; Yu, J.P.; Yang, X. Covalent Organic Frameworks: A Sustainable Photocatalyst toward Visible-Light-Accelerated C3 Arylation and Alkylation of Quinoxalin-2(1H)-ones. Chem. Eur. J. 2020, 26, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, H.; Zhao, J.; Ni, Z.; Zhang, P.F.; Shi, B.F.; Li, W.M. Photocatalyst-, Metal- and Additive-free, Direct C–H Arylation of Quinoxalin-2(1H)-ones with Aryl Acyl Peroxides Induced by Visible Light. Org. Chem. Front. 2020, 7, 4031–4042. [Google Scholar] [CrossRef]
- Wang, J.Y.; Sun, B.; Zhang, L.; Xu, T.W.; Xie, Y.Y.; Jin, C. Visible-Light-Induced Trifluoromethylation of Quinoxalin-2(1H)-Ones under Photocatalyst-Free Conditions. Asian J. Org. Chem. 2019, 8, 1942–1946. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Photo-/electrocatalytic Functionalization of Quinoxalin-2(1H)-ones. Chin. J. Catal. 2021, 42, 1921–1943. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Bai, Y.-S.; Xu, X.-Q.; Peng, X.; Tang, H.-S.; Huang, Y.; Lin, Y.-W.; Cao, Z.; He, W.-M. Visible-light-induced Decarboxylative Acylation of Quinoxalin-2(1H)-ones with α-oxo Carboxylic Acids under Metal-, Strong Oxidant- and External Photocatalyst-free Conditions. Green Chem. 2020, 22, 1720–1725. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Mo, Z.-Y.; Wang, H.-S.; Wen, X.-A.; Tang, H.-T.; Pan, Y.-M. Electrochemically Enabled Chemoselective Sulfonylation and Hydrazination of Indoles. Green Chem. 2019, 21, 3807–3811. [Google Scholar] [CrossRef]
- Niu, K.K.; Shi, X.D.; Ding, L.; Liu, Y.X.; Song, H.J.; Wang, Q.M. HCl-Catalyzed Aerobic Oxidation of Alkylarenes to Carbonyls. ChemSusChem 2022, 15, e202102326. [Google Scholar] [CrossRef]

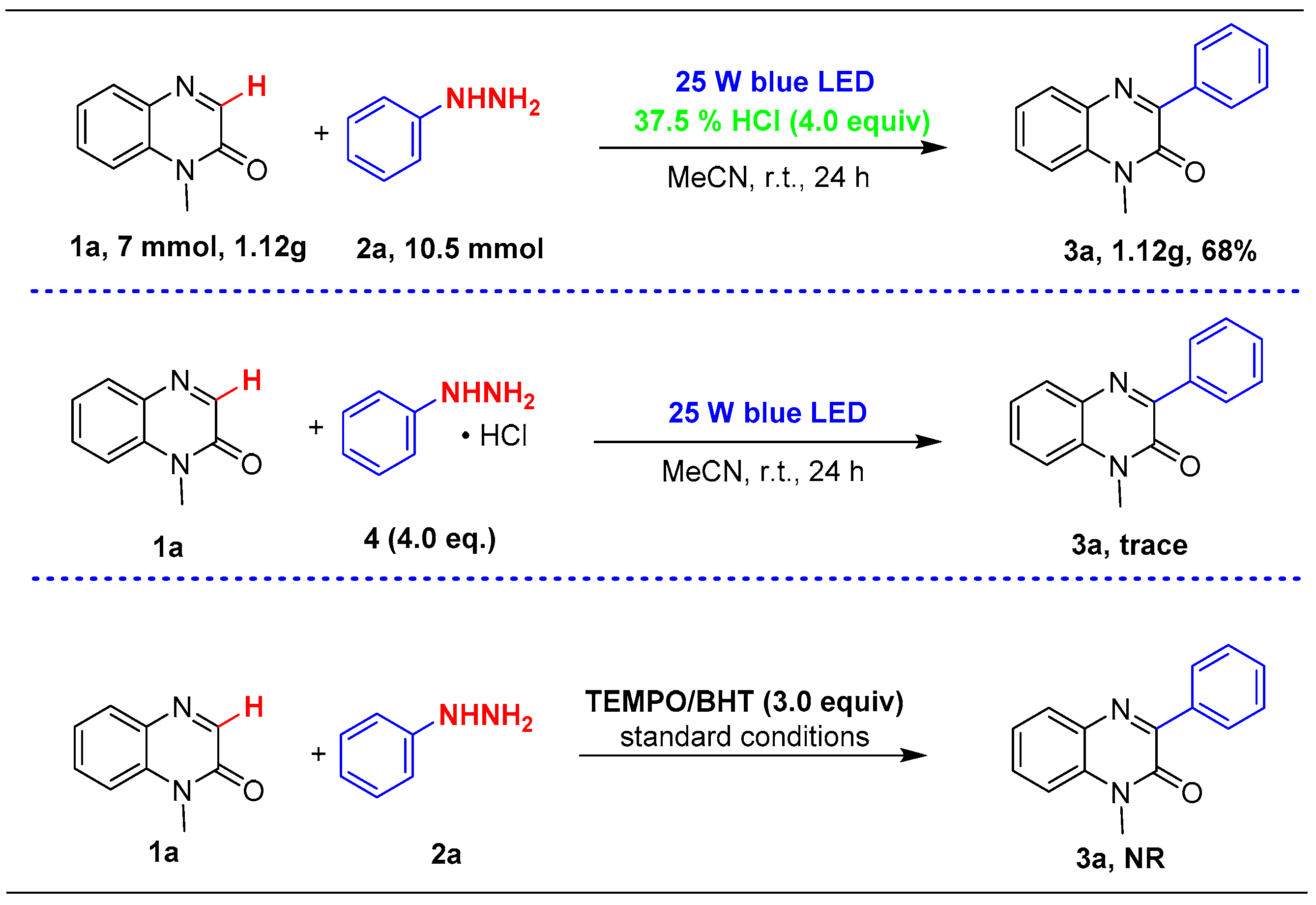
| Entry | Variation from Given Conditions | Yields (%) |
|---|---|---|
| 1 | none | 80 |
| 2 | green LED instead of blue LED | trace |
| 3 | white LED instead of blue LED | 43 |
| 4 | without light | 0 |
| 5 | without HCl | 0 |
| 6 | 3.0 equivs of HCl used | 67 |
| 7 | 5.0 equivs of HCl used | 81 |
| 8 | K2CO3 instead of HCl | 45 |
| 9 | EtOH instead of MeCN | 61 |
| 10 | DMSO instead of MeCN | 56 |
| 11 | MeCN/H2O (v/v = 1:1) instead of MeCN | 47 |
| 12 | H2O instead of MeCN | trace |
| 13 | Reaction was performed under O2 | 83 |
| 14 | Reaction was performed under N2 | 0 |
 | ||
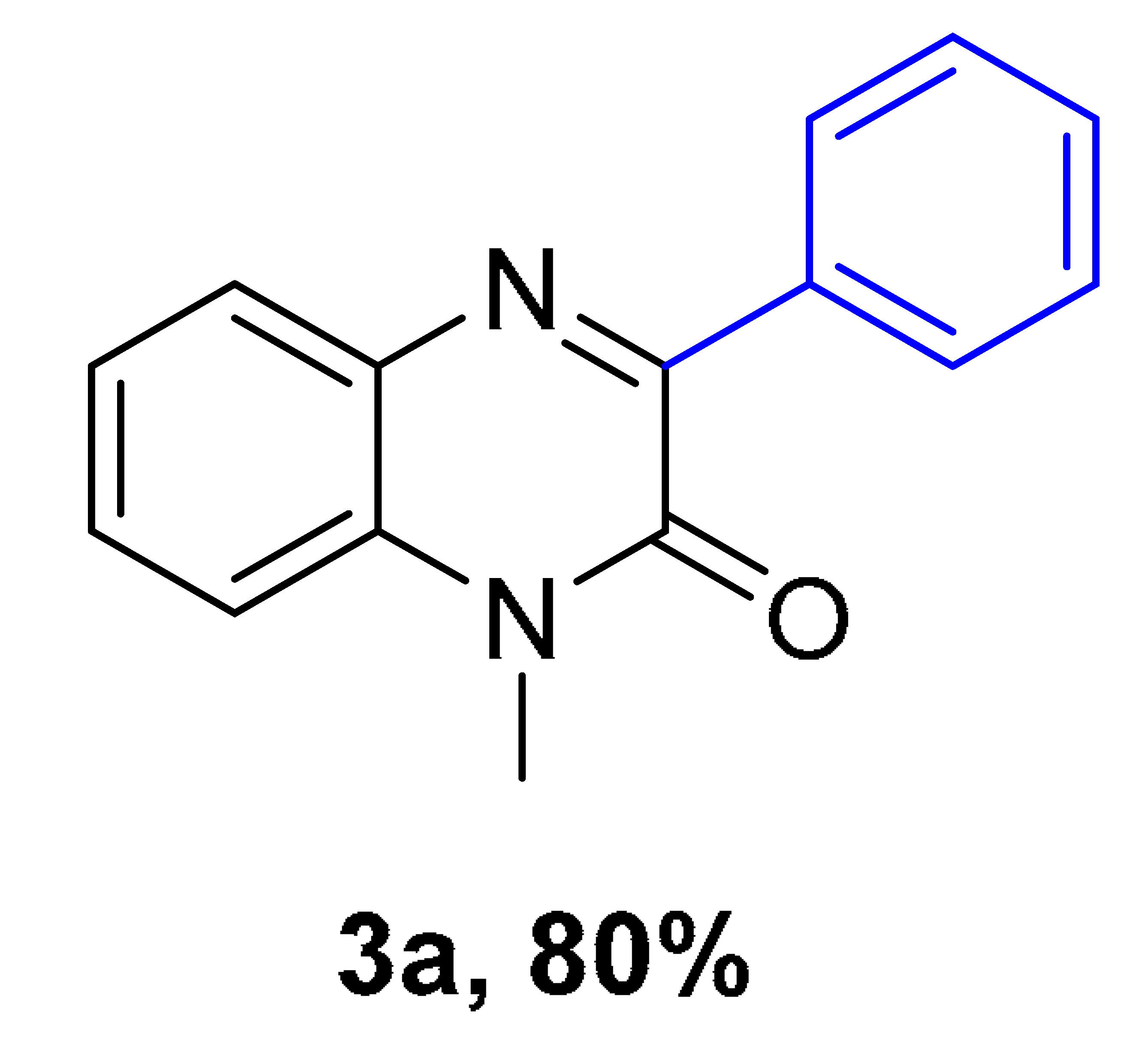 | 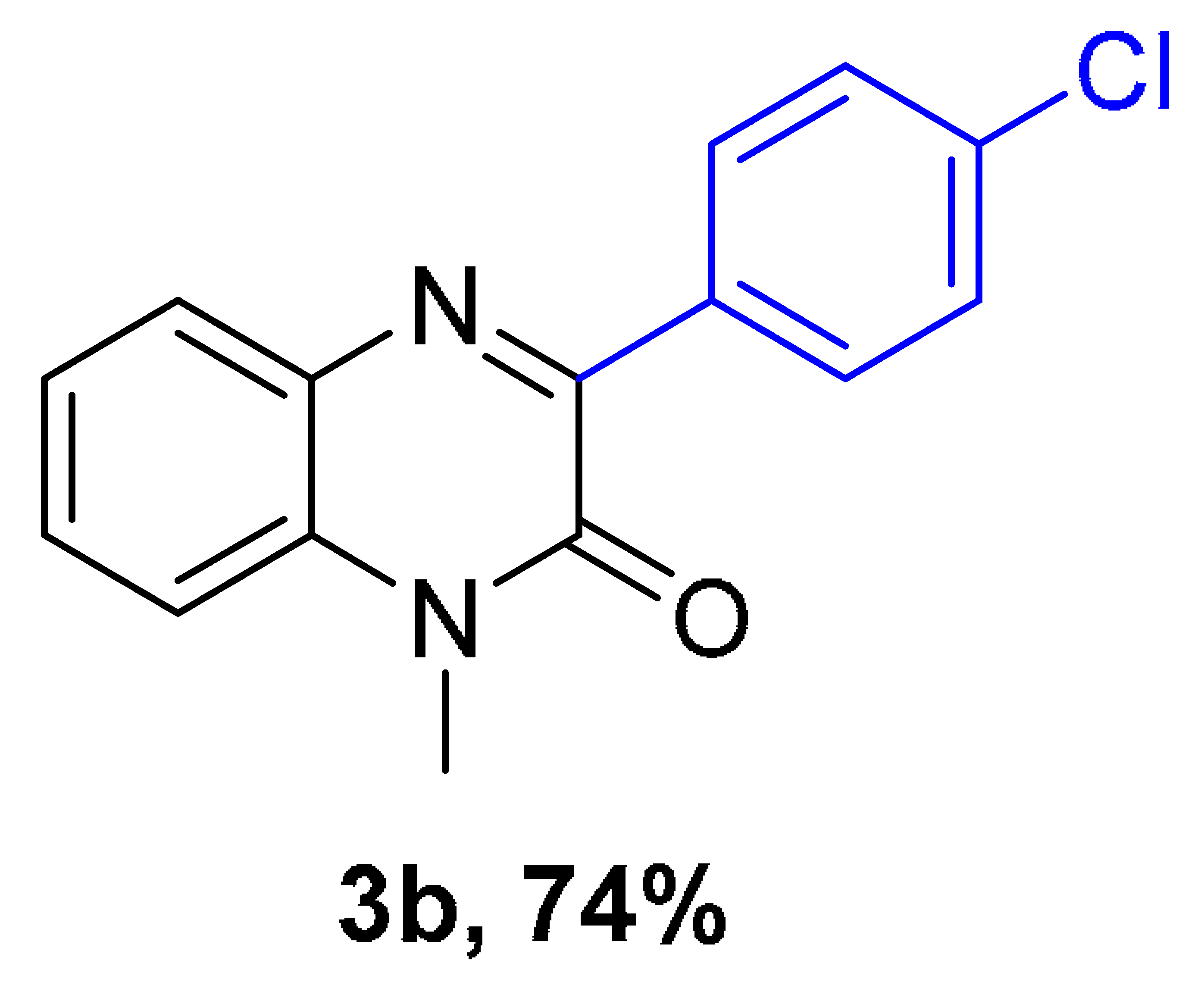 | 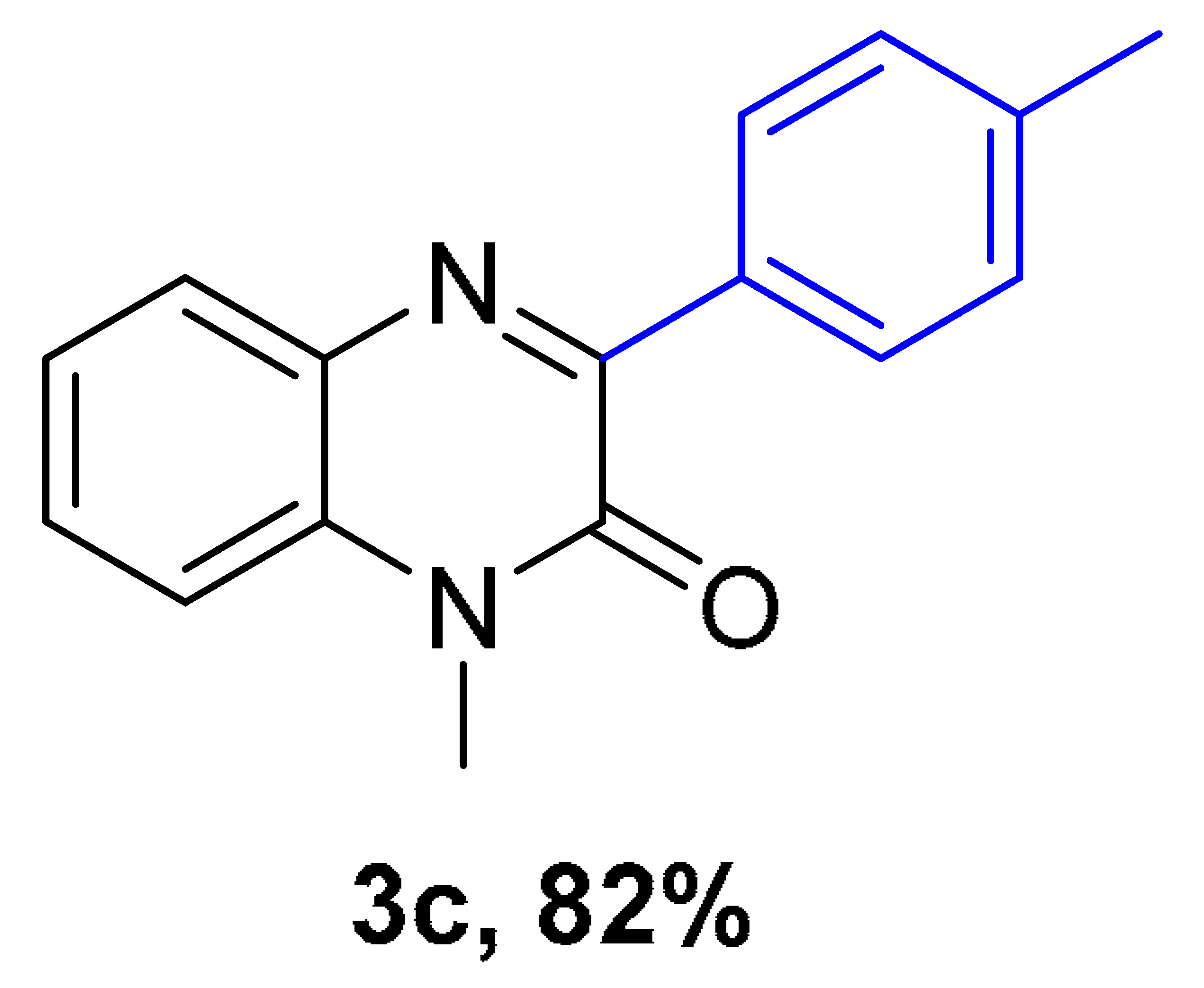 |
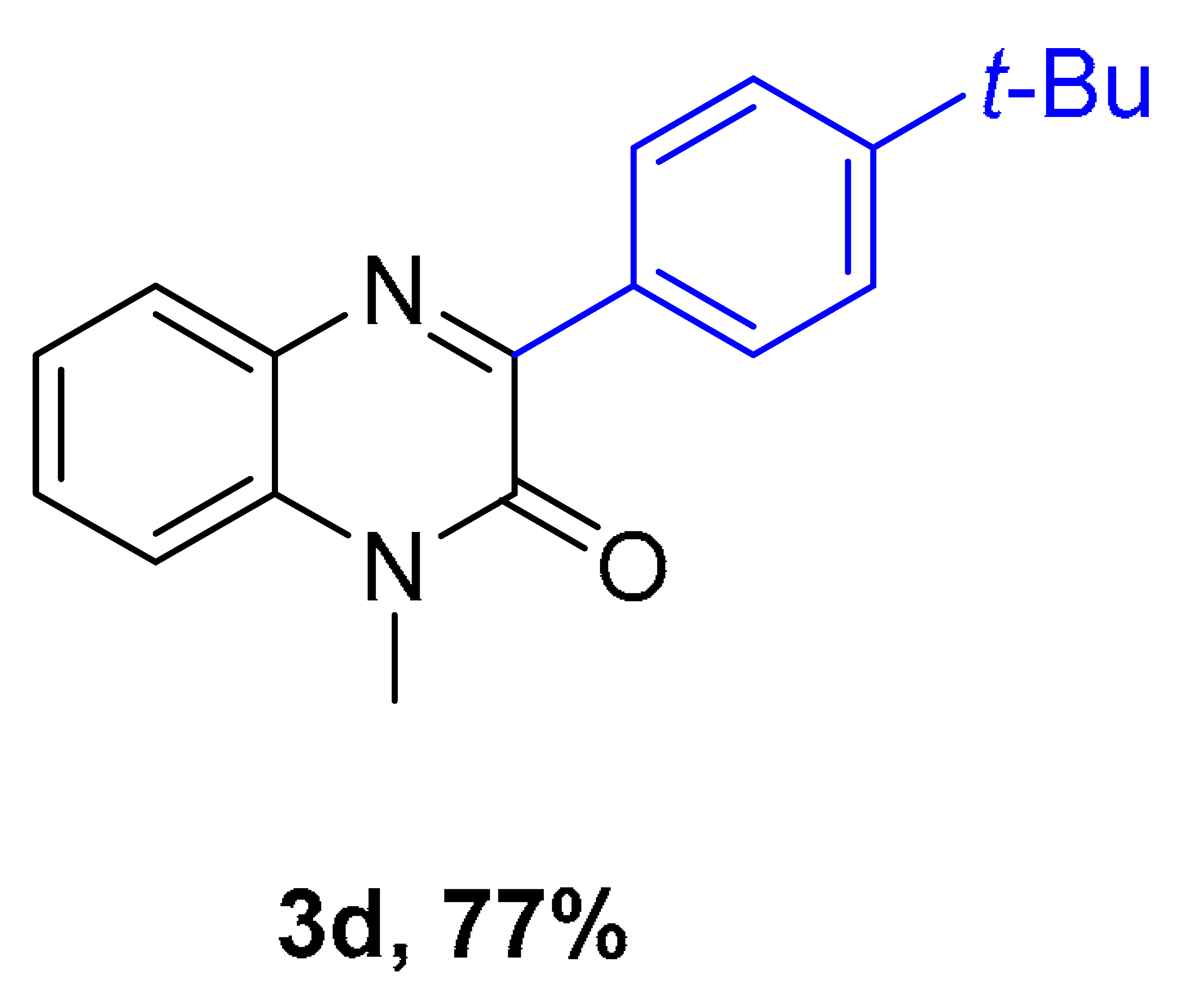 | 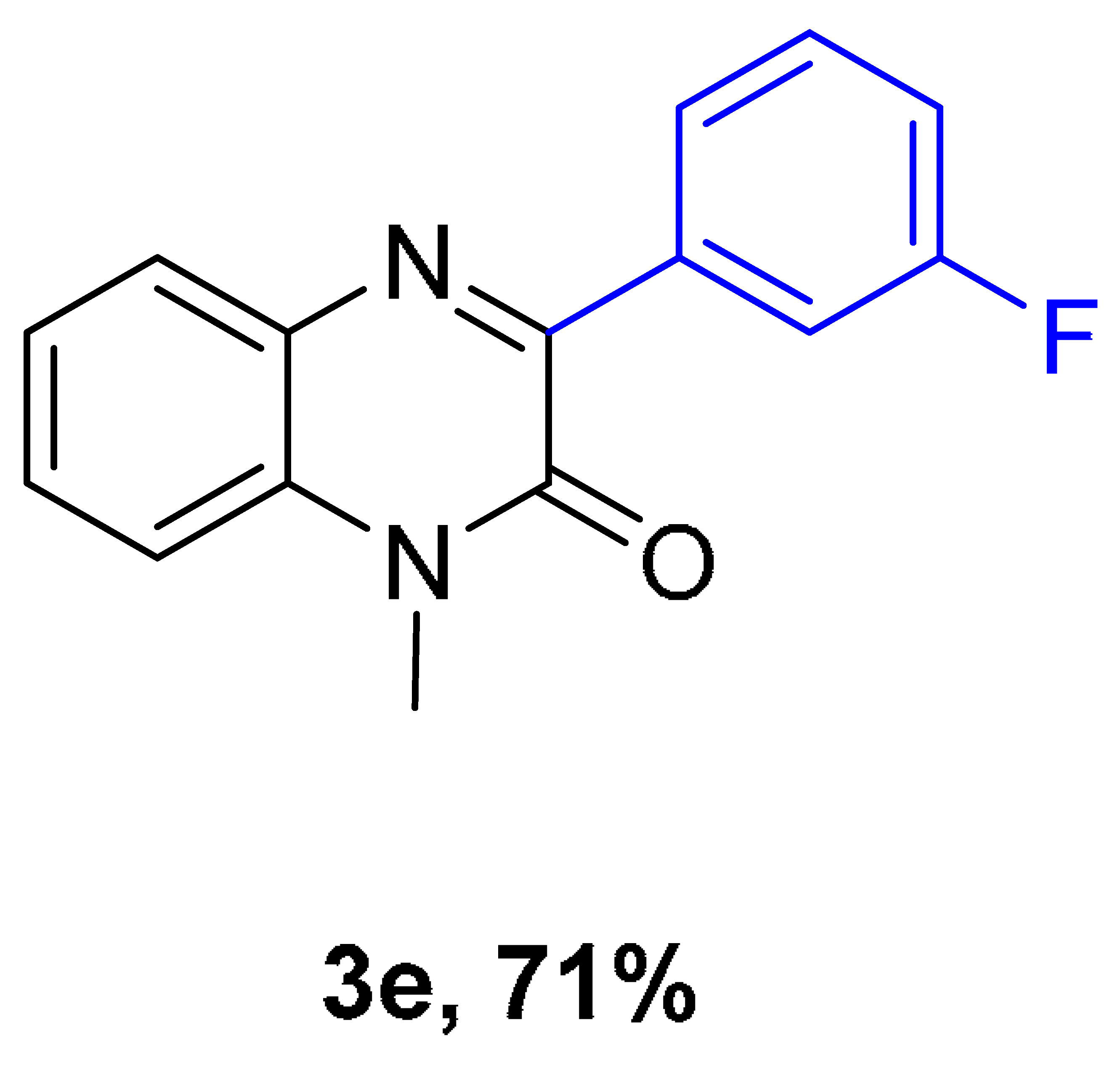 |  |
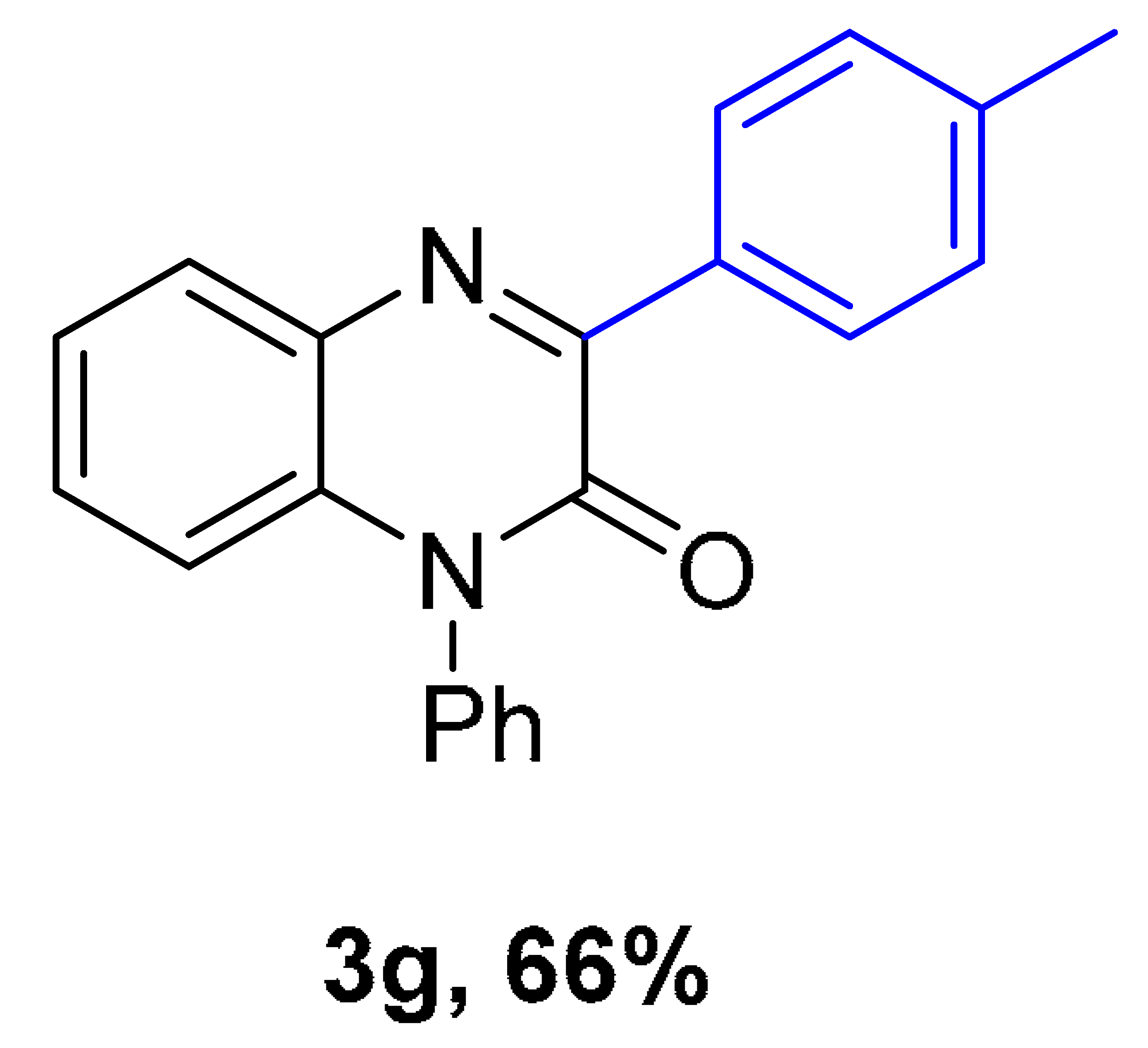 | 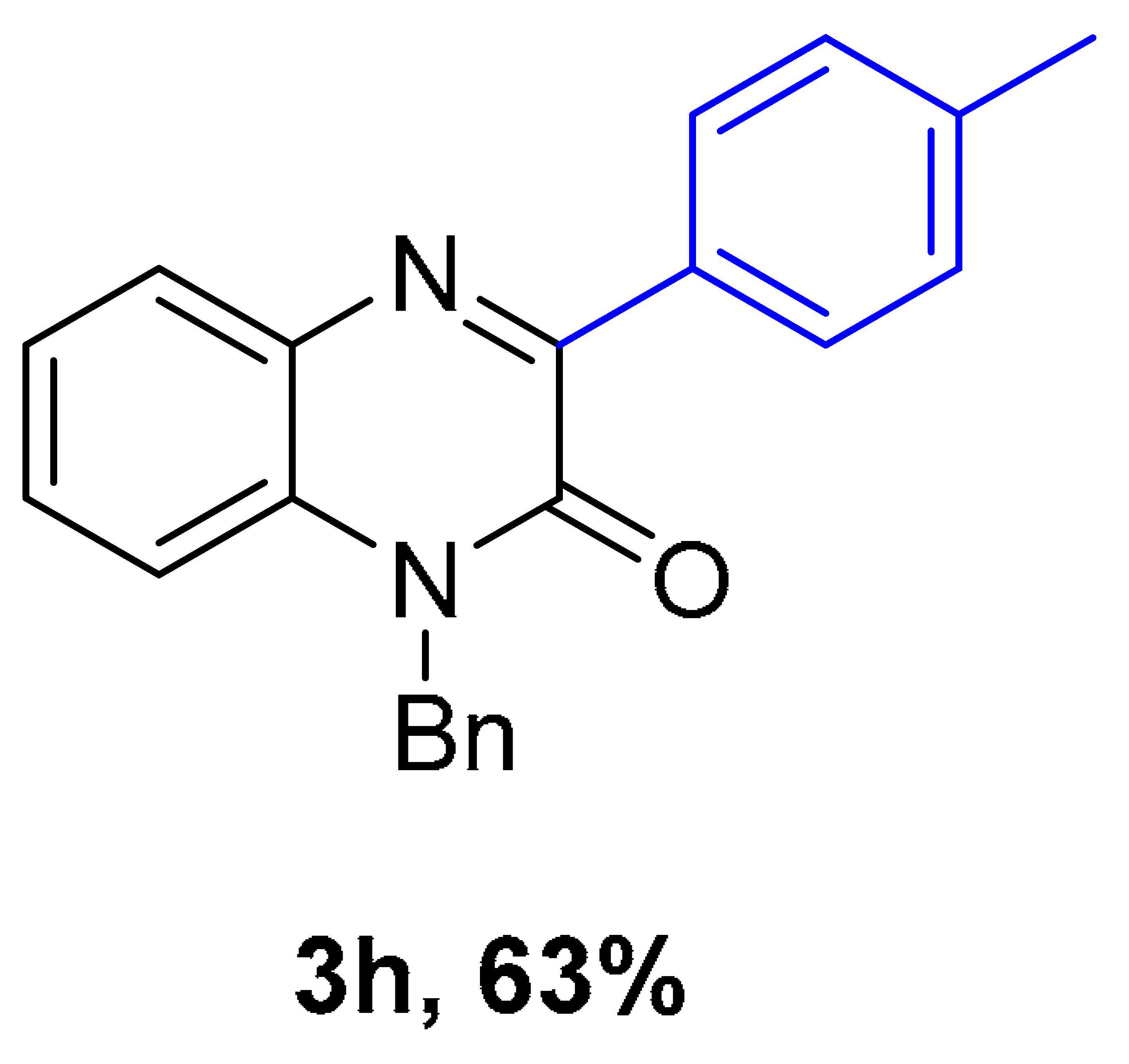 | 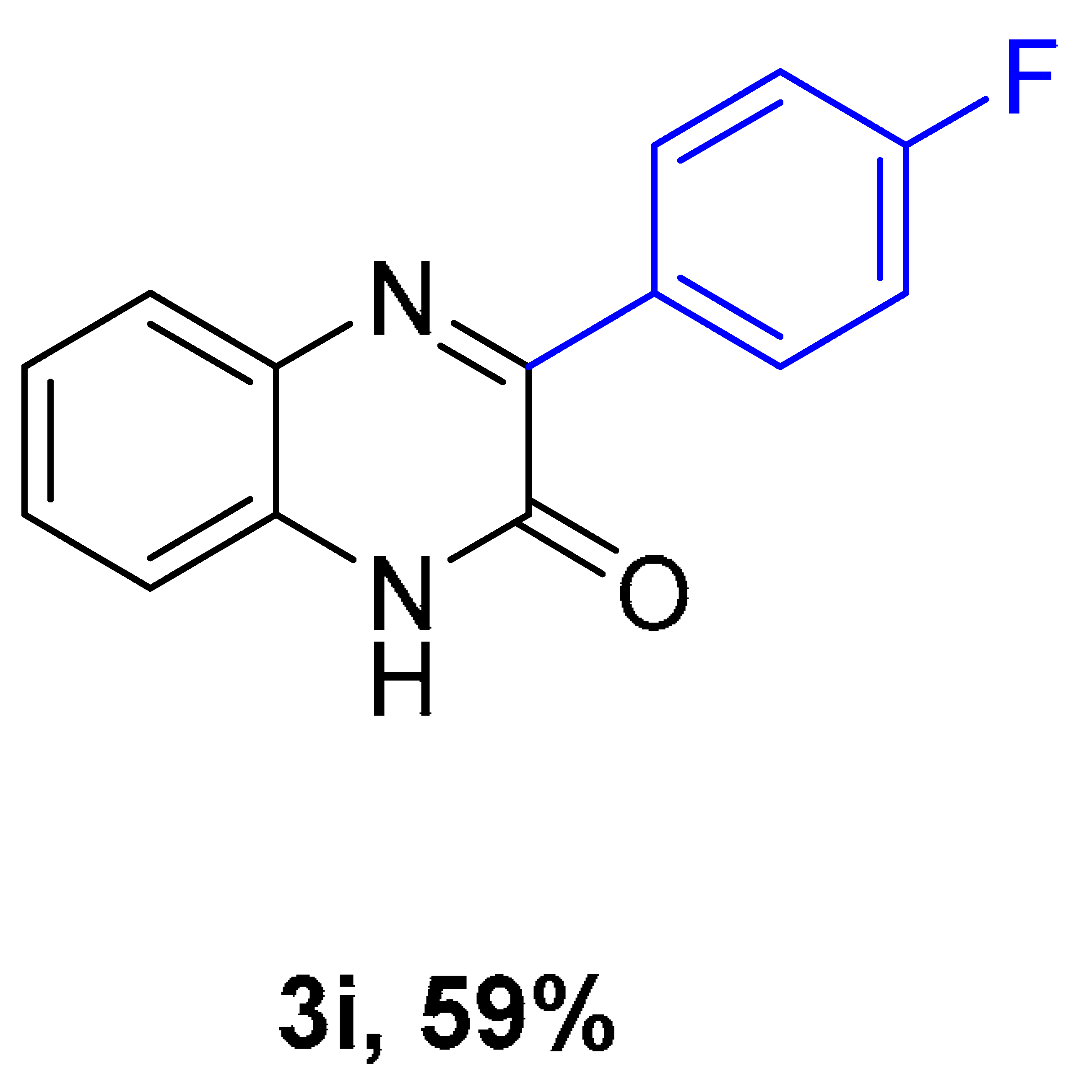 |
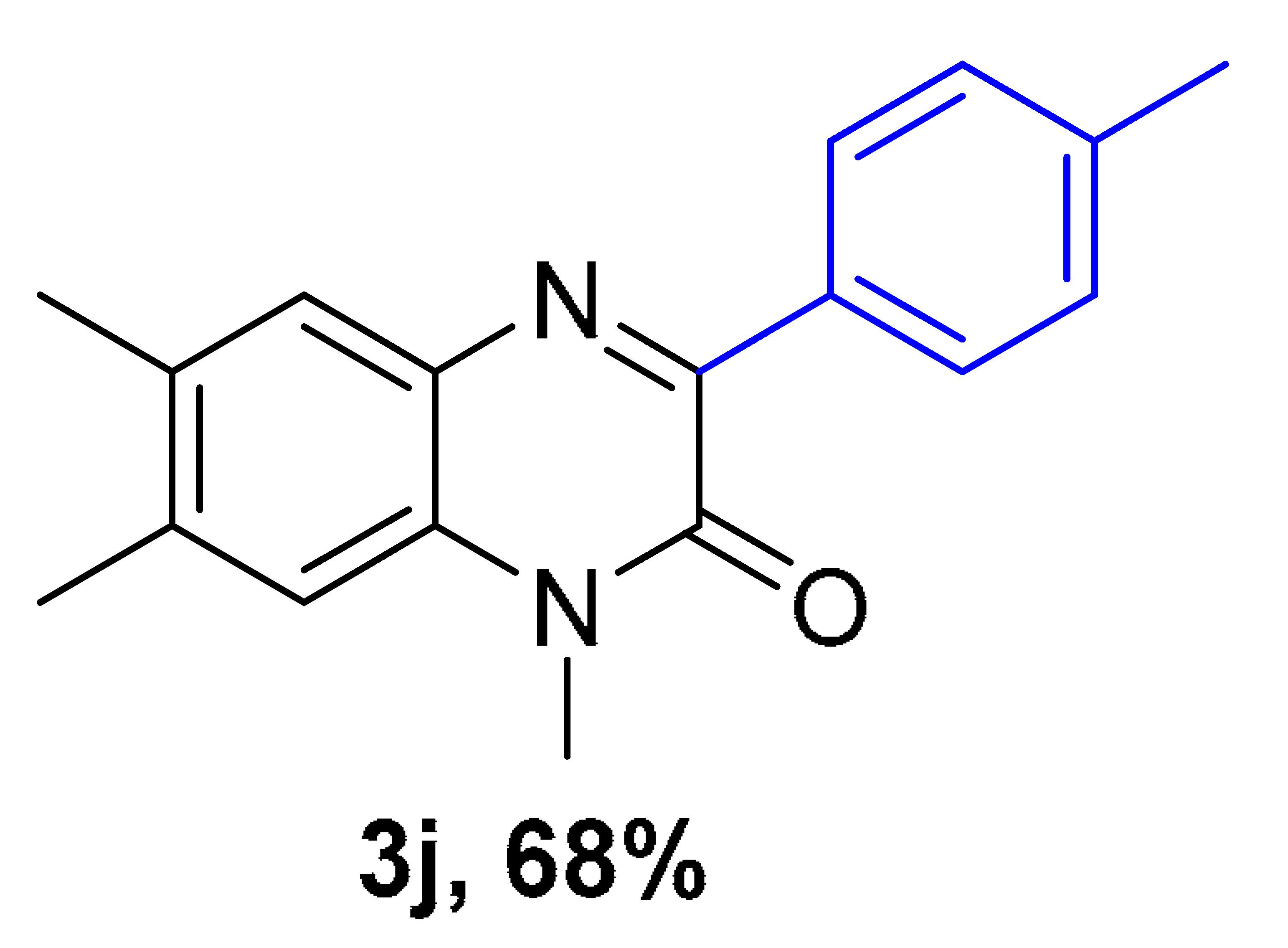 | 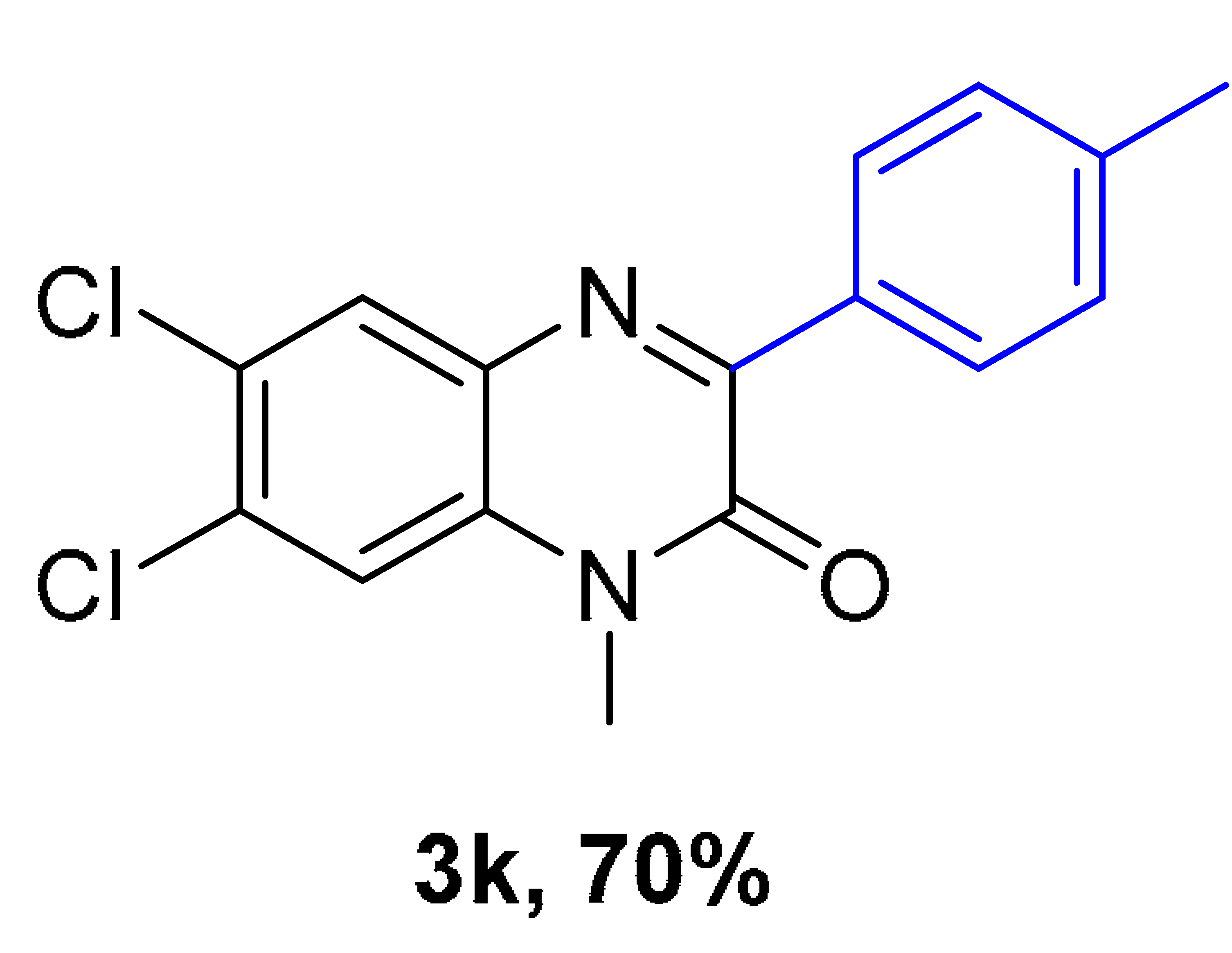 | 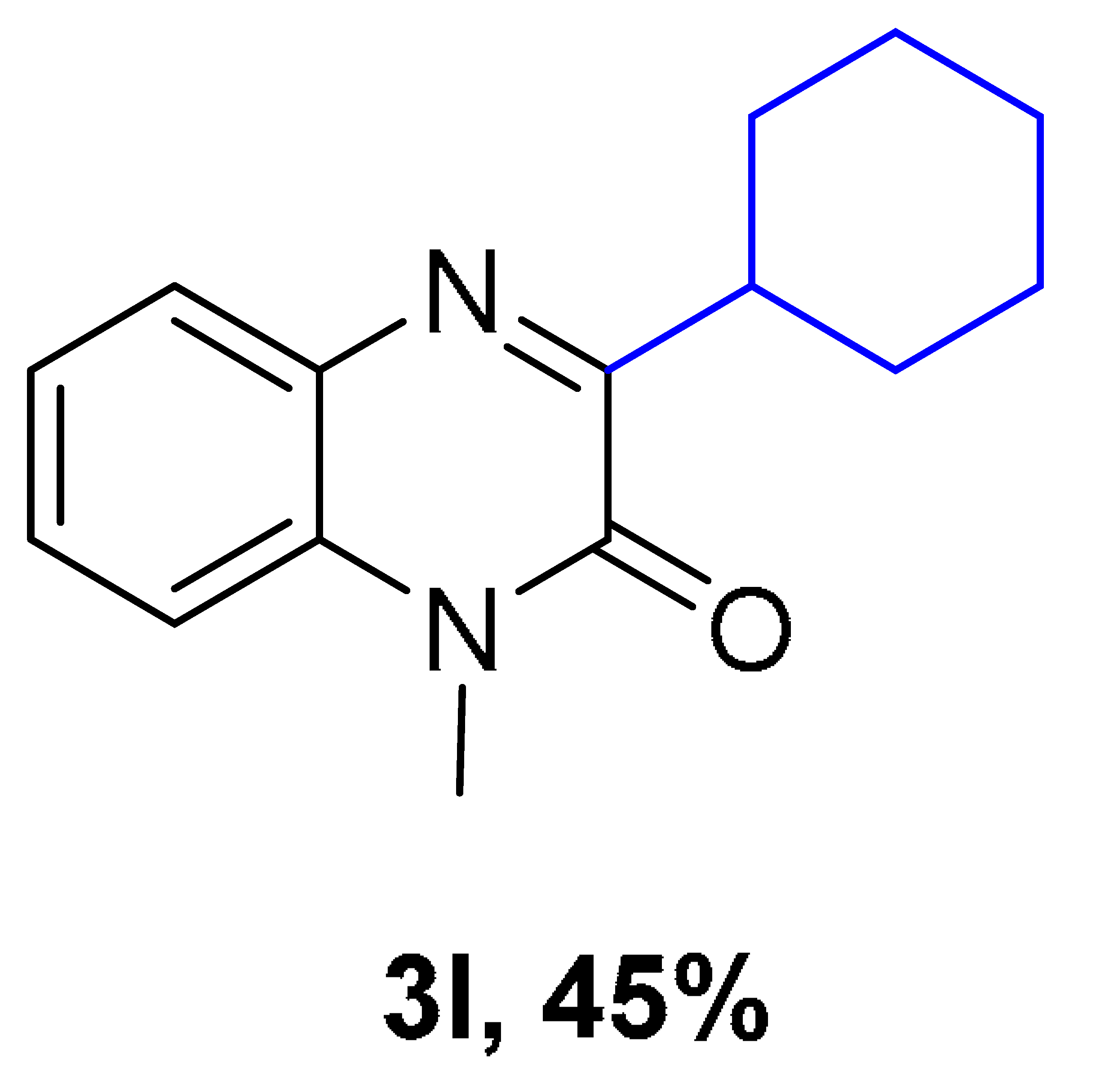 |
 | ||
 |  |  |
 |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xu, W.; Qi, H.; Zhang, P.; Cao, X.-T.; Wang, G. A HCl-Mediated, Metal- and Oxidant-Free Photocatalytic Strategy for C3 Arylation of Quinoxalin(on)es with Arylhydrazine. Catalysts 2022, 12, 633. https://doi.org/10.3390/catal12060633
Wang K, Xu W, Qi H, Zhang P, Cao X-T, Wang G. A HCl-Mediated, Metal- and Oxidant-Free Photocatalytic Strategy for C3 Arylation of Quinoxalin(on)es with Arylhydrazine. Catalysts. 2022; 12(6):633. https://doi.org/10.3390/catal12060633
Chicago/Turabian StyleWang, Kai, Wenjing Xu, Haoran Qi, Pengfei Zhang, Xian-Ting Cao, and Guannan Wang. 2022. "A HCl-Mediated, Metal- and Oxidant-Free Photocatalytic Strategy for C3 Arylation of Quinoxalin(on)es with Arylhydrazine" Catalysts 12, no. 6: 633. https://doi.org/10.3390/catal12060633
APA StyleWang, K., Xu, W., Qi, H., Zhang, P., Cao, X.-T., & Wang, G. (2022). A HCl-Mediated, Metal- and Oxidant-Free Photocatalytic Strategy for C3 Arylation of Quinoxalin(on)es with Arylhydrazine. Catalysts, 12(6), 633. https://doi.org/10.3390/catal12060633






