CeO2-ZrO2 Solid Solution Catalyzed and Moderate Acidic–Basic Sites Dominated Cycloaddition of CO2 with Epoxides: Halogen-Free Synthesis of Cyclic Carbonates
Abstract
:1. Introduction
2. Results
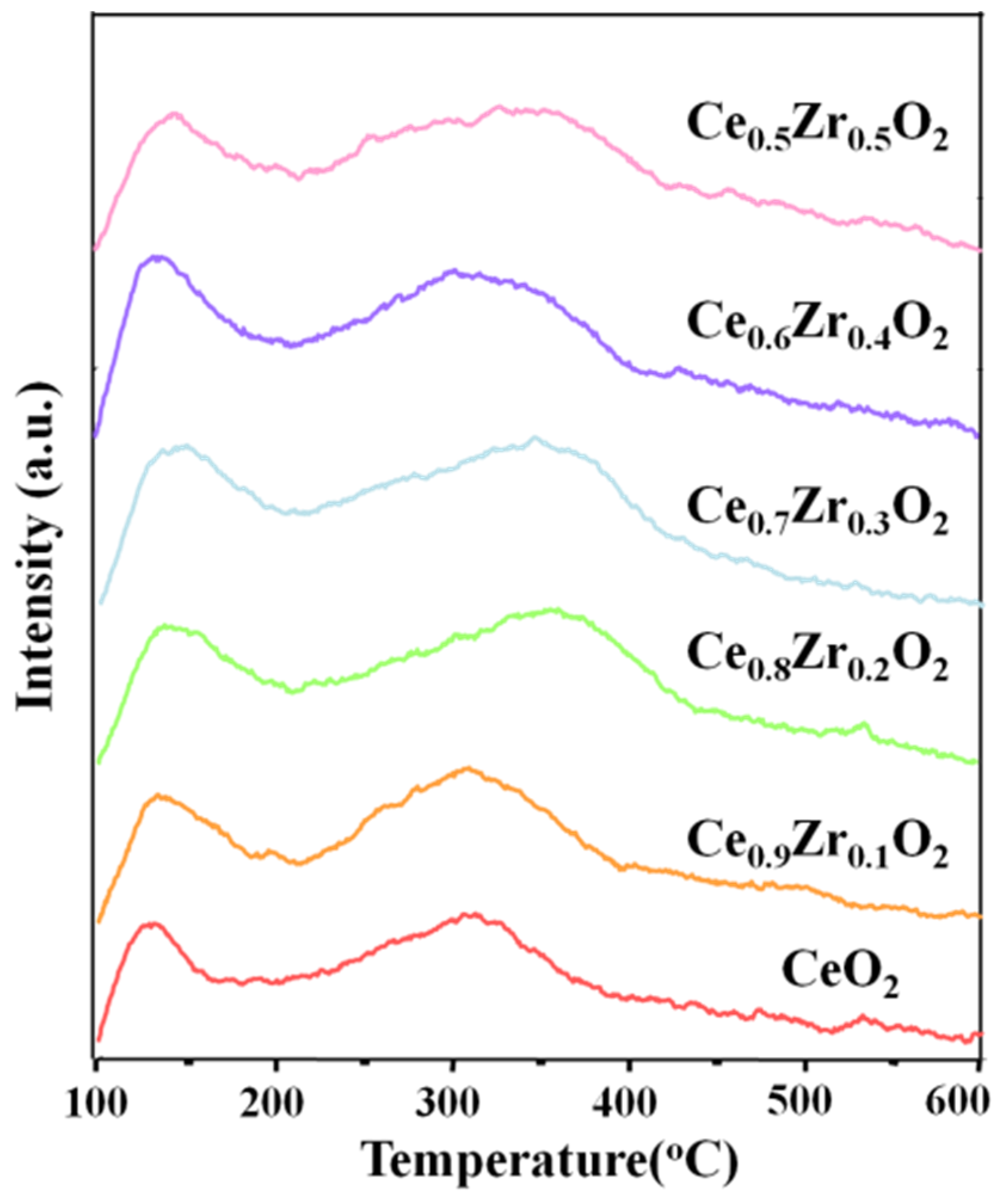
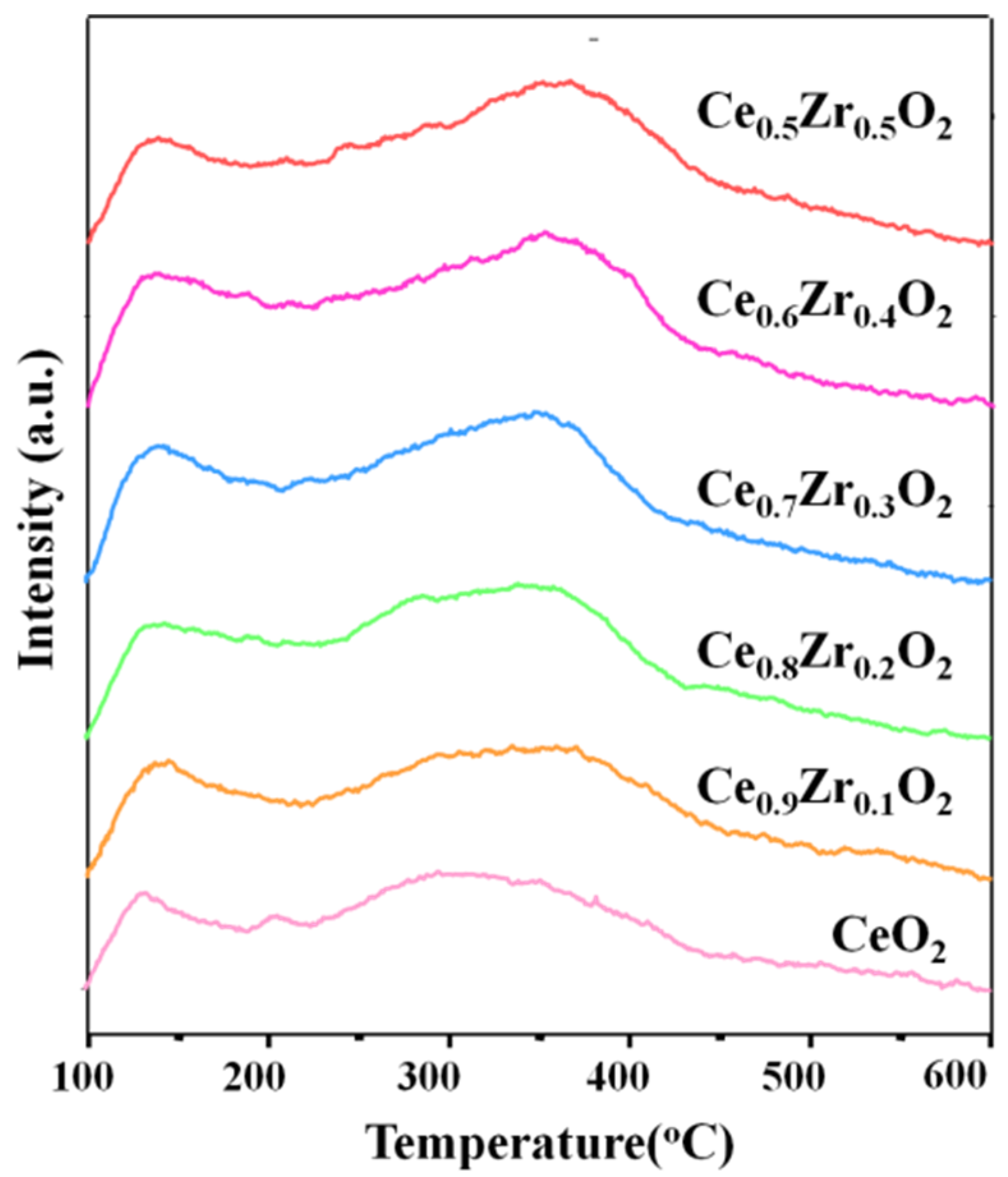
2.1. Characterization of Ce1-xZrxO2 Nanorods
2.2. Catalytic Activity of Ce1-xZrxO2 Nanorods
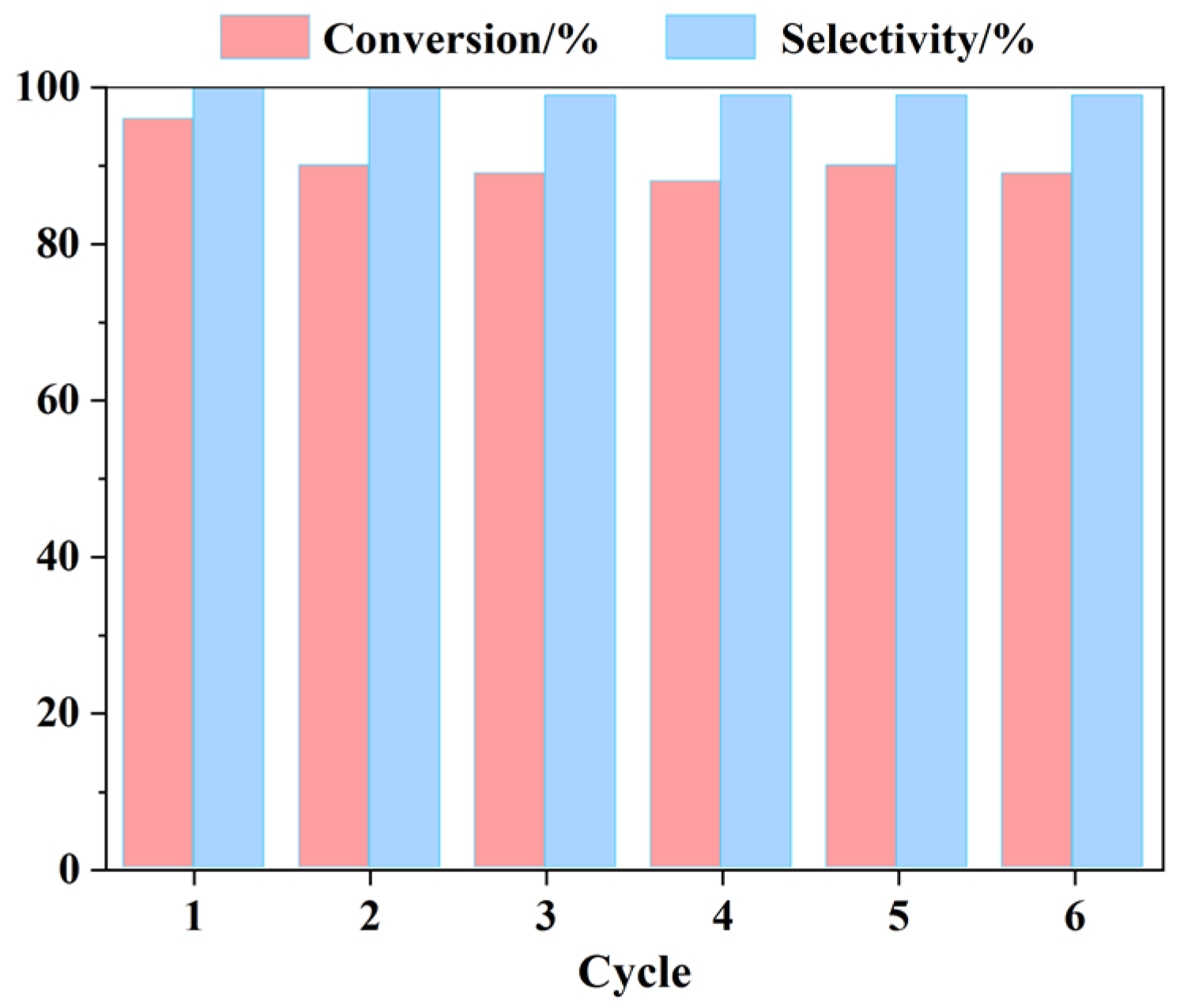
2.3. Reusability of Catalyst
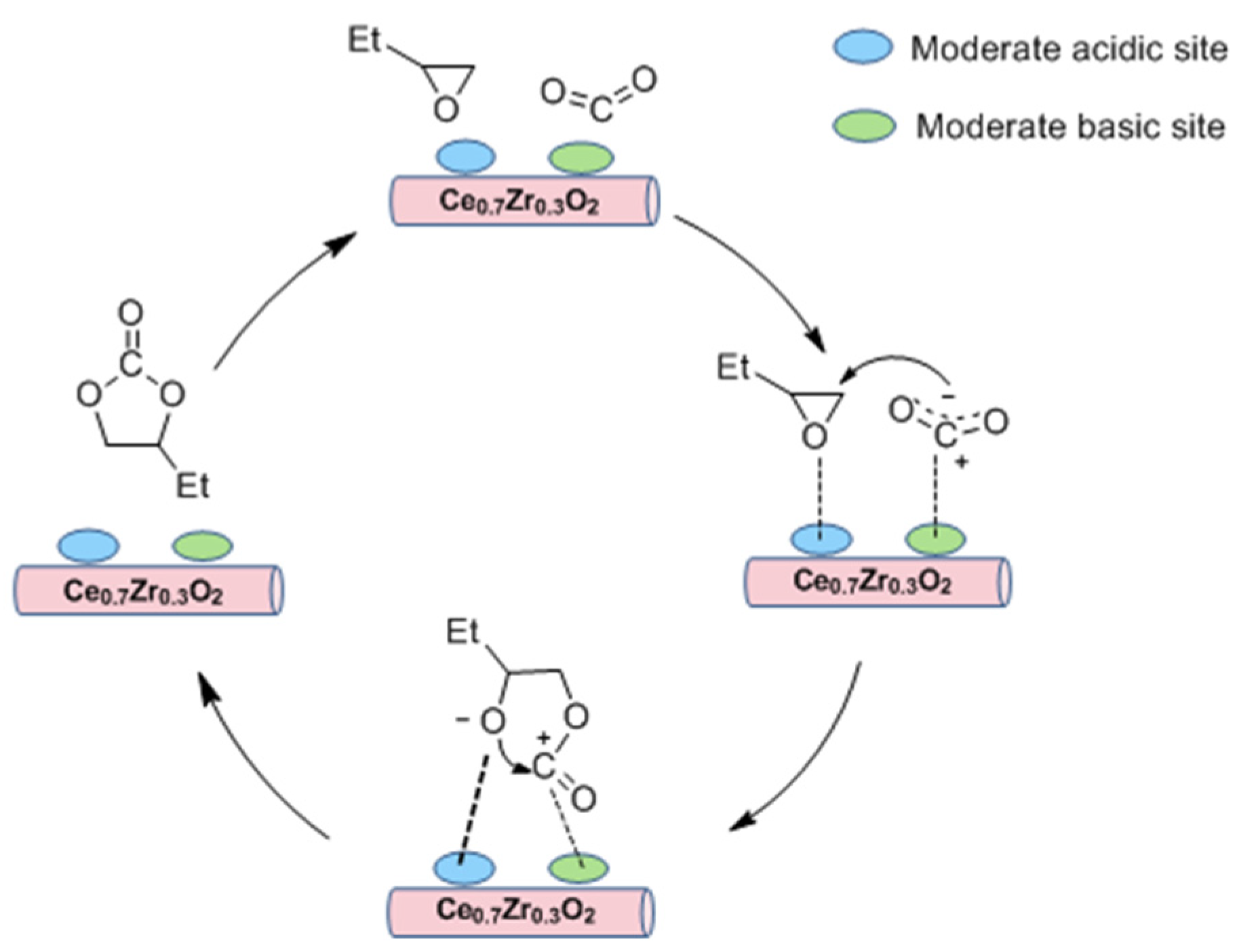
2.4. Plausible Mechanism
3. Experimental Procedures
3.1. Preparation of Zr-Doped CeO2 Nanorods
3.2. General Procedure of Ce1-xZrxO2 Nanorods Catalyzed Cycloaddition of CO2 with Epoxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Zhang, F.; Bulut, S.; Shen, X.; Dong, M.; Wang, Y.; Cheng, X.; Liu, H.; Han, B. Halogen-free fixation of carbon dioxide into cyclic carbonates via bifunctional organocatalysts. Green Chem. 2021, 23, 1147–1153. [Google Scholar] [CrossRef]
- Werner, T.; Büttner, H. Phosphorus-based bifunctional organocatalysts for the addition of carbon dioxide and epoxides. ChemSusChem 2014, 7, 3268–3271. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jupp, A.R.; Ong, M.S.; Burton, K.I.; Chitnis, S.S.; Stephan, D.W. Synthesis of urea derivatives from CO2 and silylamines. Angew. Chem. 2019, 131, 5763–5767. [Google Scholar] [CrossRef]
- Yoshida, M.; Hara, N.; Okuyama, S. Catalytic production of urethanes from amines and alkyl halides in supercritical carbon dioxide. Chem. Commun. 2000, 78, 151–152. [Google Scholar] [CrossRef]
- Grabow, L.; Mavrikakis, M. Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliá-Hernández, F.; Moragas, T.; Cornella, J.; Martin, R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 2017, 545, 84–88. [Google Scholar] [CrossRef]
- Börjesson, M.; Moragas, T.; Gallego, D.; Martin, R. Metal-catalyzed carboxylation of organic (pseudo) halides with CO2. ACS Catal. 2016, 6, 6739–6749. [Google Scholar]
- Ju, T.; Zhou, Y.-Q.; Cao, K.-G.; Fu, Q.; Ye, J.-H.; Sun, G.-Q.; Liu, X.-F.; Chen, L.; Liao, L.-L.; Yu, D.-G. Dicarboxylation of alenes, allenes and (hetero) arenes with CO2 via visible-light photoredox catalysis. Nat. Catal. 2021, 4, 304–311. [Google Scholar] [CrossRef]
- Liao, L.-L.; Wang, Z.-H.; Cao, K.-G.; Sun, G.-Q.; Zhang, W.; Ran, C.-K.; Li, Y.; Chen, L.; Cao, G.-M.; Yu, D.-G. Electrochemical ring-opening dicarboxylation of strained carbon-carbon single bonds with CO2: Facile synthesis of diacids and derivatization into polyesters. J. Am. Chem. Soc. 2022, 144, 2062–2068. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Mohamad Shukri, M.A.A.; Kiatkittipong, W.; Lim, J.W.; Show, P.L.; Lam, M.K.; Assabumrungrat, S. Green pathway in utilizing CO2 via cycloaddition reaction with epoxide—A mini review. Processes 2020, 8, 548. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, J.; Zhang, C.; Wang, Y.; Yuan, D.; Yao, Y. Recyclable single-component rare-earth metal catalysts for cycloaddition of CO2 and epoxides at atmospheric pressure. Inorg. Chem. 2017, 56, 4568–4575. [Google Scholar] [CrossRef]
- Guo, L.P.; Lamb, K.J.; North, M. Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef]
- Qu, J.; Cao, C.Y.; Dou, Z.F.; Liu, H.; Yu, Y.; Li, P.; Song, W.G. Synthesis of cyclic carbonates: Catalysis by an iron-based composite and the role of hydrogen bonding at the solid/liquid interface. ChemSusChem 2012, 5, 652–655. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.-X.; Zhang, W.-Z.; Lu, X.-B. CO2 adducts of phosphorus ylides: Highly active organocatalysts for carbon dioxide transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Jiang, T.; Song, J.; Yang, G.; Han, B. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI. Chem. Commun. 2011, 47, 2131–2133. [Google Scholar] [CrossRef]
- Girard, A.-L.; Simon, N.; Zanatta, M.; Marmitt, S.; Gonçalves, P.; Dupont, J. Insights on recyclable catalytic system composed of task-specific ionic liquids for the chemical fixation of carbon dioxide. Green Chem. 2014, 16, 2815–2825. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, Y.J.; Choi, Y.-S.; Lee, H.; Lee, J.S.; Hong, J.; Jeong, E.-K.; Kim, H.S.; Cheong, M. Zn-containing ionic liquids bearing dialkylphosphate ligands for the coupling reactions of epoxides and CO2. Appl. Catal. B Environ. 2012, 111, 621–627. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Yen, Y.-H.; Chu, Y.-H. Baylis-Hillman reaction in [bdmim][PF6] ionic liquid. Tetrahedron Lett. 2004, 45, 4673–4676. [Google Scholar] [CrossRef]
- Wang, T.-T.; Xie, Y.; Deng, W.-Q. Reaction mechanism of epoxide cycloaddition to CO2 catalyzed by salen-M (M = Co, Al, Zn). J. Phys. Chem. A 2014, 118, 9239–9243. [Google Scholar] [CrossRef]
- Yang, Z.Z.; He, L.N.; Miao, C.X.; Chanfreau, S. Lewis basic ionic liquids-catalyzed conversion of carbon dioxide to cyclic carbonates. Adv. Synth. Catal. 2010, 352, 2233–2240. [Google Scholar] [CrossRef]
- Zou, B.; Hu, C. Halogen-free processes for organic carbonate synthesis from CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 11–16. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Zhang, X.; Zhang, X.; Liu, H.; Han, B. Recent advances in the coupling of CO2 and epoxides into cyclic carbonates under halogen-free condition. Green Chem. Eng. 2020, 1, 82–93. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Tutusaus, O.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Nelson, E.G.; Sevryugina, Y.V. An efficient halogen-free electrolyte for use in rechargeable magnesium batteries. Angew. Chem. 2015, 127, 8011–8015. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M. A heteropolyacid-based ionic liquid immobilized onto fibrous nano-silica as an efficient catalyst for the synthesis of cyclic carbonate from carbon dioxide and epoxides. Green Chem. 2015, 17, 3059–3066. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, W.; Fan, W.; Wang, Y.; Meng, Z.; Zhang, S. Reusable and efficient polymer-supported task-specific ionic liquid catalyst for cycloaddition of epoxide with CO2. Catal. Today 2009, 148, 361–367. [Google Scholar] [CrossRef]
- Cho, S.H.; Dahnum, D.; Cheong, S.-H.; Lee, H.W.; Lee, U.; Ha, J.-M.; Lee, H. Facile one-pot synthesis of ZnBr2 immobilized ion exchange resin for the coupling reaction of CO2 with propylene oxide. J. CO2 Util. 2020, 42, 101324. [Google Scholar] [CrossRef]
- Du, Y.; Cai, F.; Kong, D.-L.; He, L.-N. Organic solvent-free process for the synthesis of propylene carbonate from supercritical carbon dioxide and propylene oxide catalyzed by insoluble ion exchange resins. Green Chem. 2005, 7, 518–523. [Google Scholar] [CrossRef]
- Ji, G.; Yang, Z.; Zhang, H.; Zhao, Y.; Yu, B.; Ma, Z.; Liu, Z. Hierarchically mesoporous o-hydroxyazobenzene polymers: Synthesis and their applications in CO2 capture and conversion. Angew. Chem. 2016, 128, 9837–9841. [Google Scholar] [CrossRef]
- Luo, R.; Liu, X.; Chen, M.; Liu, B.; Fang, Y. Recent advances on imidazolium-functionalized organic cationic polymers for CO2 adsorption and simultaneous conversion into cyclic carbonates. ChemSusChem 2020, 13, 3945–3966. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Z.; Hu, S.; Wu, T.; Jiang, T.; Han, B. MOF-5/n-Bu4NBr: An efficient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2 under mild conditions. Green Chem. 2009, 11, 1031–1036. [Google Scholar] [CrossRef]
- Bai, X.J.; Lu, X.Y.; Ju, R.; Chen, H.; Shao, L.; Zhai, X.; Li, Y.N.; Fan, F.Q.; Fu, Y.; Qi, W. Preparation of MOF film/aerogel composite catalysts via substrate-seeding secondary-growth for the oxygen evolution reaction and CO2 cycloaddition. Angew. Chem. Int. Ed. 2021, 60, 701–705. [Google Scholar] [CrossRef]
- Tharun, J.; Bhin, K.-M.; Roshan, R.; Kim, D.W.; Kathalikkattil, A.C.; Babu, R.; Ahn, H.Y.; Won, Y.S.; Park, D.-W. Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2. Green Chem. 2016, 18, 2479–2487. [Google Scholar] [CrossRef]
- Xiang, W.; Sun, Z.; Wu, Y.; He, L.-N.; Liu, C.-j. Enhanced cycloaddition of CO2 to epichlorohydrin over zeolitic imidazolate frameworks with mixed linkers under solventless and co-catalyst-free condition. Catal. Today 2020, 339, 337–343. [Google Scholar] [CrossRef]
- Yano, T.; Matsui, H.; Koike, T.; Ishiguro, H.; Fujihara, H.; Yoshihara, M.; Maeshima, T. Magnesium oxide-catalysed reaction of carbon dioxide with anepoxide with retention of stereochemistry. Chem. Commun. 1997, 12, 1129–1130. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Fujita, S.-i.; Ikushima, Y.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides, and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity. Appl. Catal. A Gen. 2001, 219, 259–266. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Bhanja, P.; Salam, N.; Bhaumik, A.; Islam, S.M. Magnesium oxide as an efficient catalyst for CO2 fixation and N-formylation reactions under ambient conditions. Mol. Catal. 2018, 450, 46–54. [Google Scholar] [CrossRef]
- Liu, J.; Wang, A.; Jing, H. TiO2-based green heterogeneous catalysts for the cycloaddition of CO2 to epoxides. Chin. J. Catal. 2014, 35, 1669–1675. [Google Scholar] [CrossRef]
- Ramin, M.; van Vegten, N.; Grunwaldt, J.-D.; Baiker, A. Simple preparation routes towards novel Zn-based catalysts for the solventless synthesis of propylene carbonate using dense carbon dioxide. J. Mol. Catal. A Chem. 2006, 258, 165–171. [Google Scholar] [CrossRef]
- Qaroush, A.K.; Alsoubani, F.A.; Ala’a, M.; Nabih, E.; Al-Ramahi, E.; Khanfar, M.F.; Assaf, K.I.; Ala’a, F.E. An efficient atom-economical chemoselective CO2 cycloaddition using lanthanum oxide/tetrabutyl ammonium bromide. Sustain. Energy Fuels 2018, 2, 1342–1349. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Gianfrate, L.; Pastore, C. Nb (V) compounds as epoxides carboxylation catalysts: The role of the solvent. J. Mol. Catal. A Chem. 2003, 204, 245–252. [Google Scholar] [CrossRef]
- Yasuda, H.; He, L.-N.; Takahashi, T.; Sakakura, T. Non-halogen catalysts for propylene carbonate synthesis from CO2 under supercritical conditions. Appl. Catal. A Gen. 2006, 298, 177–180. [Google Scholar] [CrossRef]
- Rasal, K.B.; Yadav, G.D.; Koskinen, R.; Keiski, R. Solventless synthesis of cyclic carbonates by direct utilization of CO2 using nanocrystalline lithium promoted magnesia. Mol. Catal. 2018, 451, 200–208. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg-Al mixed oxides as highly active acid-base catalysts for cycloaddition of carbon dioxide to epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar] [CrossRef]
- Dai, W.-L.; Yin, S.-F.; Guo, R.; Luo, S.-L.; Du, X.; Au, C.-T. Synthesis of propylene carbonate from carbon dioxide and propylene oxide using Zn-Mg-Al composite oxide as high-efficiency catalyst. Catal. Lett. 2010, 136, 35–44. [Google Scholar] [CrossRef]
- Kulal, N.; Vasista, V.; Shanbhag, G.V. Identification and tuning of active sites in selected mixed metal oxide catalysts for cyclic carbonate synthesis from epoxides and CO2. J. CO2 Util. 2019, 33, 434–444. [Google Scholar] [CrossRef]
- Tambe, P.R.; Yadav, G.D. Heterogeneous cycloaddition of styrene oxide with carbon dioxide for synthesis of styrene carbonate using reusable lanthanum–zirconium mixed oxide as catalyst. Clean Technol. Environ. Policy 2018, 20, 345–356. [Google Scholar] [CrossRef]
- Adeleye, A.I.; Patel, D.; Niyogi, D.; Saha, B. Efficient and greener synthesis of propylene carbonate from carbon dioxide and propylene oxide. Ind. Eng. Chem. Res. 2014, 53, 18647–18657. [Google Scholar] [CrossRef]
- Adeleye, A.I.; Kellici, S.; Heil, T.; Morgan, D.; Vickers, M.; Saha, B. Greener synthesis of propylene carbonate using graphene-inorganic nanocomposite catalysts. Catal. Today 2015, 256, 347–357. [Google Scholar] [CrossRef]
- Dai, Q.; Huang, H.; Zhu, Y.; Deng, W.; Bai, S.; Wang, X.; Lu, G. Catalysis oxidation of 1, 2-dichloroethane and ethyl acetate over ceria nanocrystals with well-defined crystal planes. Appl. Catal. B Environ. 2012, 117, 360–368. [Google Scholar] [CrossRef]
- Watanabe, S.; Ma, X.; Song, C. Characterization of structural and surface properties of nanocrystalline TiO2−CeO2 mixed oxides by XRD, XPS, TPR, and TPD. J. Phys. Chem. C 2009, 113, 14249–14257. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, G.; Zou, H.; Cao, Y.; Zhang, Y.; Zhang, R.; Zhang, F.; Xian, M. The base-free and selective oxidative transformation of 1, 3-propanediol into methyl esters by different Au/CeO2 catalysts. Green Chem. 2011, 13, 2690–2695. [Google Scholar] [CrossRef]
- Postole, G.; Chowdhury, B.; Karmakar, B.; Pinki, K.; Banerji, J.; Auroux, A. Knoevenagel condensation reaction over acid–base bifunctional nanocrystalline CexZr1− xO2 solid solutions. J. Catal. 2010, 269, 110–121. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chen, K.-B.; Wang, M.-F.; Weng, S.-F.; Lee, C.-S.; Lin, M.-C. Enhanced catalytic activity of Ce1−xMxO2 (M = Ti, Zr, and Hf) solid solution with controlled morphologies. Chem. Commun. 2010, 46, 3286–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahzadeh-Ghom, S.; Zamani, C.; Andreu, T.; Epifani, M.; Morante, J. Improvement of oxygen storage capacity using mesoporous ceria-zirconia solid solutions. Appl. Catal. B Environ. 2011, 108, 32–38. [Google Scholar] [CrossRef]
- Farneth, W.; Gorte, R. Methods for characterizing zeolite acidity. Chem. Rev. 1995, 95, 615–635. [Google Scholar] [CrossRef]
- Kolobova, E.N.; Pakrieva, E.G.; Carabineiro, S.A.; Bogdanchikova, N.; Kharlanov, A.N.; Kazantsev, S.O.; Hemming, J.; Mäki-Arvela, P.; Pestryakov, A.N.; Murzin, D.Y. Oxidation of a wood extractive betulin to biologically active oxo-derivatives using supported gold catalysts. Green Chem. 2019, 21, 3370–3382. [Google Scholar] [CrossRef] [Green Version]
- Fornasiero, P.; Dimonte, R.; Rao, G.R.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-loaded CeO2-ZrO2 solid-solutions as highly efficient oxygen exchangers: Dependence of the reduction behavior and the oxygen storage capacity on the structural-properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhong, M.; Yang, Q. Hierarchical mesoporous organic polymer with an intercalated metal complex for the efficient synthesis of cyclic carbonates from flue gas. Green Chem. 2016, 18, 6493–6500. [Google Scholar] [CrossRef]
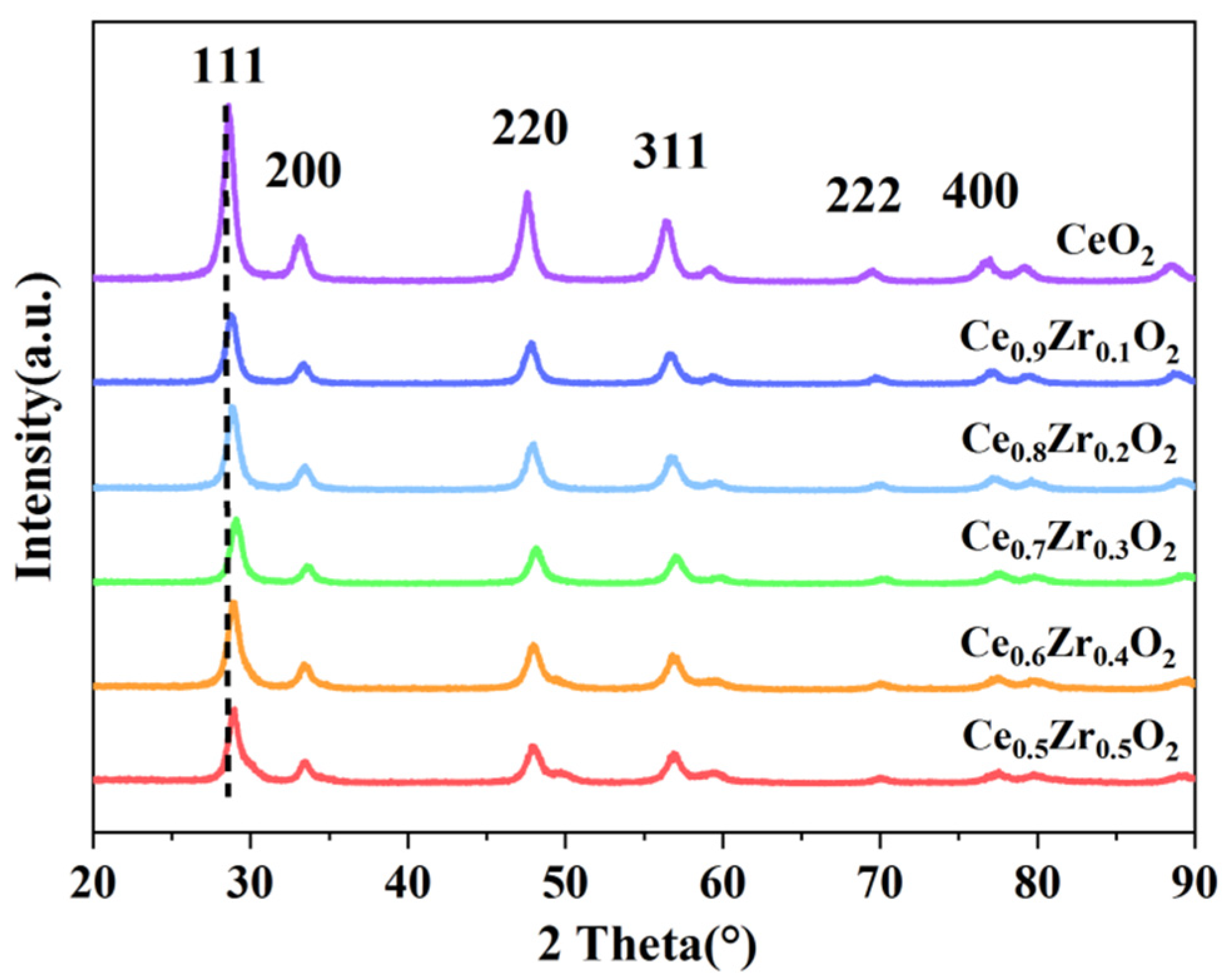
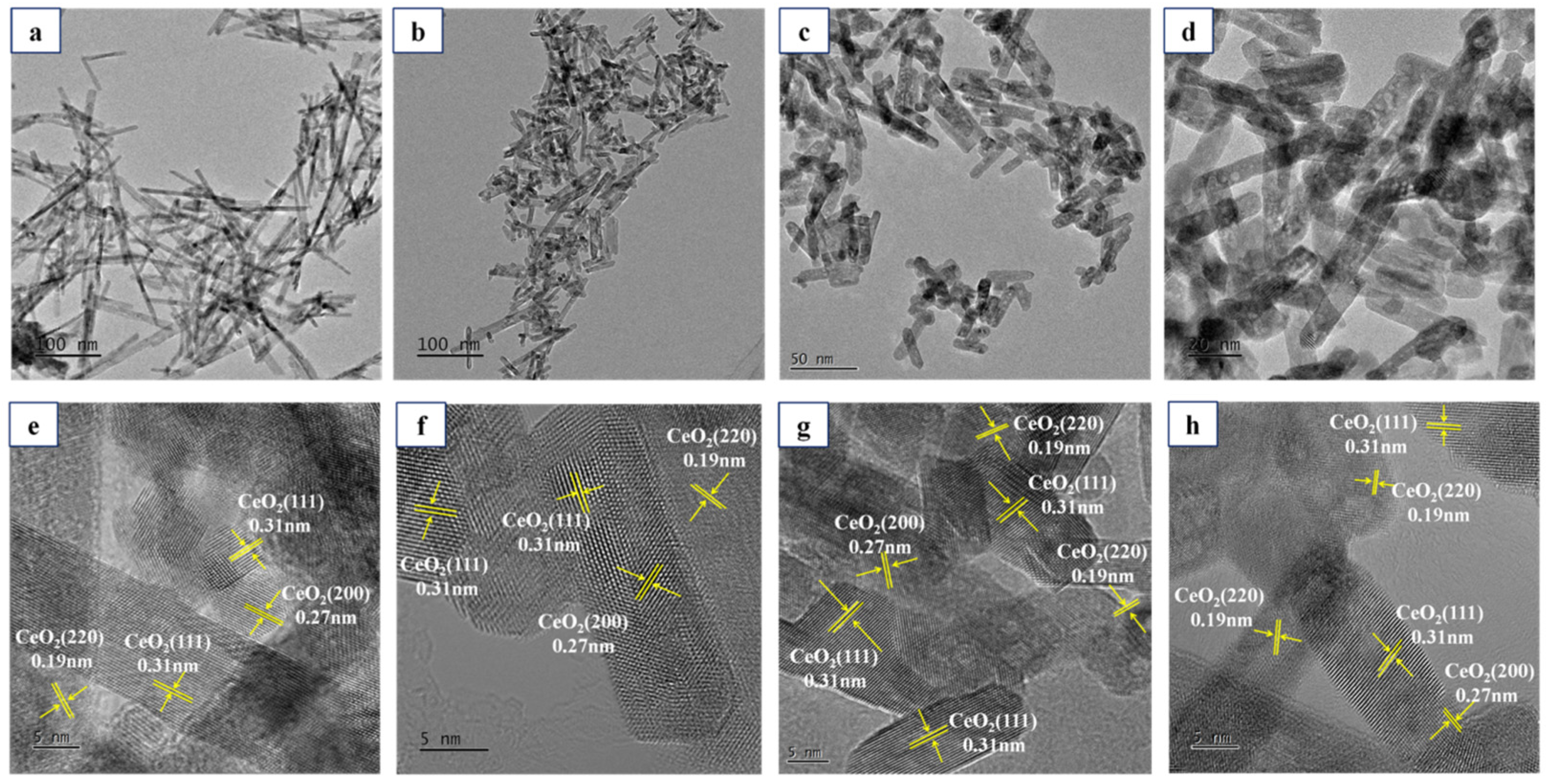
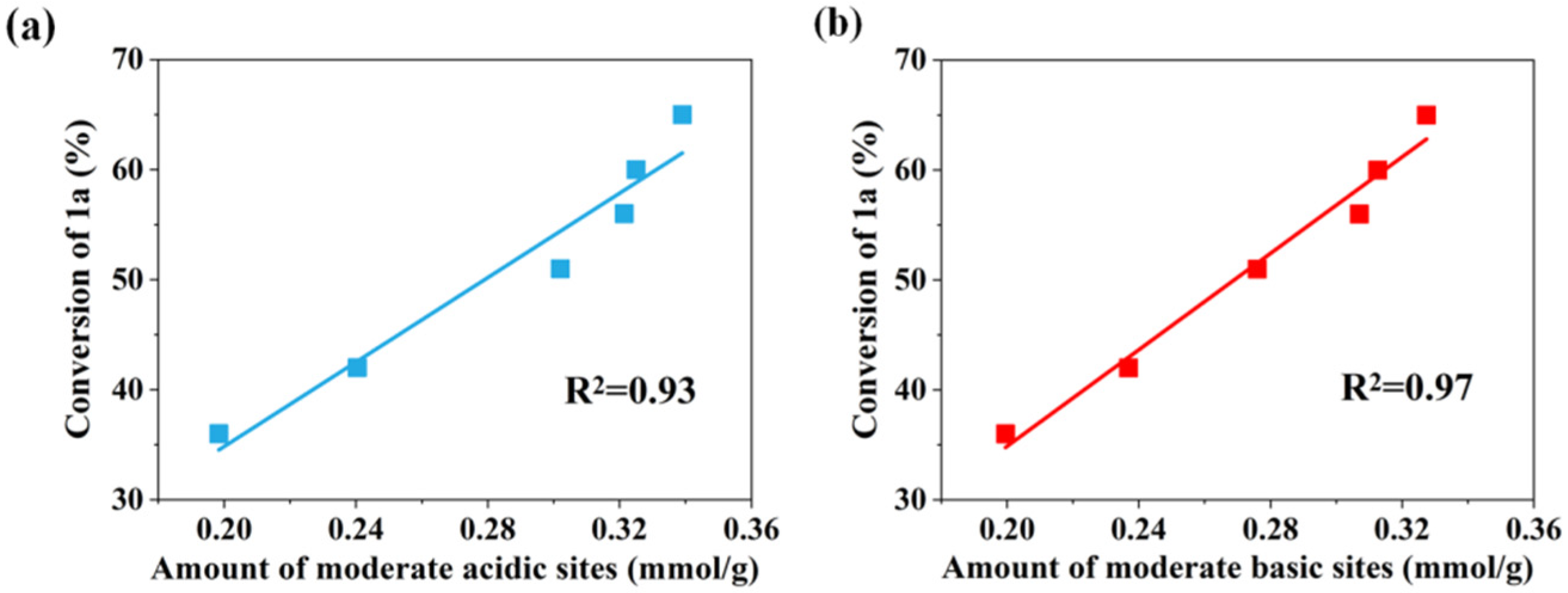
| Catalyst | 2θ (o) a | Interplanar Spacing (Å) a | Lattice Parameter (Å) b | Mol Ratio of Zr/(Ce+Zr) | SBET (m2/g) | VPore (cm3/g) | |
|---|---|---|---|---|---|---|---|
| Bulk Phase c | Surface d | ||||||
| CeO2 | 28.58 | 3.11 | 5.39 | - | - | 77 | 0.59 |
| Ce0.9Zr0.1O2 | 28.70 | 3.10 | 5.38 | 0.09 | 0.11 | 85 | 0.60 |
| Ce0.8Zr0.2O2 | 28.72 | 3.10 | 5.37 | 0.18 | 0.20 | 81 | 0.59 |
| Ce0.7Zr0.3O2 | 28.84 | 3.09 | 5.37 | 0.27 | 0.28 | 76 | 0.58 |
| Ce0.6Zr0.4O2 | 29.07 | 3.07 | 5.36 | 0.35 | 0.39 | 66 | 0.53 |
| Ce0.5Zr0.5O2 | 29.12 | 3.06 | 5.34 | 0.45 | 0.51 | 53 | 0.38 |
| Entry | Catalyst | NH3 Adsorption (mmol/g) a | |||
|---|---|---|---|---|---|
| Weak (100–200 °C) | Moderate (200–400 °C) | Strong (>400 °C) | Total | ||
| 1 | CeO2 | 0.054 | 0.198 | 0.026 | 0.278 |
| 2 | Ce0.9Zr0.1O2 | 0.075 | 0.240 | 0.041 | 0.356 |
| 3 | Ce0.8Zr0.2O2 | 0.082 | 0.302 | 0.012 | 0.398 |
| 4 | Ce0.7Zr0.3O2 | 0.090 | 0.339 | 0.005 | 0.434 |
| 5 | Ce0.6Zr0.4O2 | 0.091 | 0.325 | 0.032 | 0.449 |
| 6 | Ce0.5Zr0.5O2 | 0.068 | 0.321 | 0.021 | 0.411 |
| Entry | Catalyst | CO2 Adsorption (mmol/g) a | |||
|---|---|---|---|---|---|
| Weak (100–200 °C) | Moderate (200–400 °C) | Strong (>400 °C) | Total | ||
| 1 | CeO2 | 0.049 | 0.200 | 0.025 | 0.273 |
| 2 | Ce0.9Zr0.1O2 | 0.066 | 0.237 | 0.027 | 0.330 |
| 3 | Ce0.8Zr0.2O2 | 0.079 | 0.276 | 0.025 | 0.383 |
| 4 | Ce0.7Zr0.3O2 | 0.082 | 0.337 | 0.012 | 0.422 |
| 5 | Ce0.6Zr0.4O2 | 0.099 | 0.313 | 0.011 | 0.423 |
| 6 | Ce0.5Zr0.5O2 | 0.084 | 0.307 | 0.022 | 0.413 |
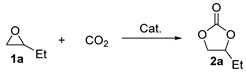 | ||||
| Entry | Catalyst | Conv. b/% | Sel. b/% | Yield b/% |
| 1 | Ce0.9Zr0.1O2 | 73 ± 3 | 99 ± 1 | 72 ± 1 |
| 2 | Ce0.8Zr0.2O2 | 80 ± 1 | 99 ± 1 | 79 ± 2 |
| 3 | Ce0.7Zr0.3O2 | 90 ± 1 | >99 | 90 ± 2 |
| 4 | Ce0.6Zr0.4O2 | 87 ± 1 | 99 ± 1 | 86 ± 2 |
| 5 | Ce0.5Zr0.5O2 | 82 ± 2 | 99 ± 2 | 81 ± 1 |
| 6 | CeO2 | 71 ± 1 | 99 ± 1 | 70 ± 3 |
| 7 | ZrO2 | 62 ± 3 | 98 ± 1 | 61 ± 2 |
| 8 | - | 32 ± 5 | 78 ± 1 | 25 ± 4 |
| 9 c | CeO2 + ZrO2 | 67 ± 1 | 99 ± 2 | 66 ± 3 |
| 10 d | Ce0.7Zr0.3O2 | 62 ± 3 | 99 ± 2 | 61 ± 2 |
| 11 e | Ce0.7Zr0.3O2 | 96 ± 1 | >99 | 96 ± 1 |
| 12 f | Ce0.7Zr0.3O2 | 58 ± 4 | 99 ± 1 | 57 ± 4 |
| 13 g | Ce0.7Zr0.3O2 | 79 ± 2 | >99 | 79 ± 3 |
| 14 h | Ce0.7Zr0.3O2 | 99 ± 2 | 81 ± 2 | 80 ± 3 |
| 15 i | Ce0.7Zr0.3O2 | 84 ± 1 | 97 ± 0 | 81 ± 1 |
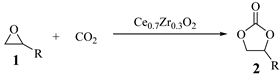 | |||||
| Entry | Epoxide | Product | Conv. b/% | Sel. b/% | Yield b/% |
| 1 | 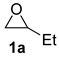 |  | 93 ± 2 | 100 ± 0 | 93 ± 2 |
| 2 | 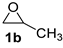 | 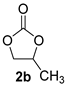 | 96 ± 1 | 100 ± 0 | 96 ± 1 |
| 3 | 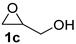 | 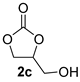 | 91 ± 1 | 99 ± 1 | 90 ± 2 |
| 4 | 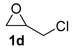 | 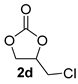 | 91 ± 2 | 99 ± 1 | 90 ± 3 |
| 5 | 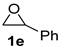 | 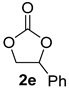 | 90 ± 2 | 97 ± 1 | 87 ± 1 |
| 6 | 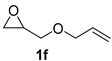 | 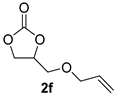 | 86 ± 1 | 98 ± 0 | 84 ± 1 |
| 7 | 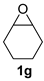 |  | 72 ± 3 | 97 ± 1 | 70 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yue, C.; Wang, H.; Li, J.; Yao, H.; Wang, M.-Y.; Ma, X. CeO2-ZrO2 Solid Solution Catalyzed and Moderate Acidic–Basic Sites Dominated Cycloaddition of CO2 with Epoxides: Halogen-Free Synthesis of Cyclic Carbonates. Catalysts 2022, 12, 632. https://doi.org/10.3390/catal12060632
Gao J, Yue C, Wang H, Li J, Yao H, Wang M-Y, Ma X. CeO2-ZrO2 Solid Solution Catalyzed and Moderate Acidic–Basic Sites Dominated Cycloaddition of CO2 with Epoxides: Halogen-Free Synthesis of Cyclic Carbonates. Catalysts. 2022; 12(6):632. https://doi.org/10.3390/catal12060632
Chicago/Turabian StyleGao, Jie, Chengguang Yue, Hao Wang, Jiaxin Li, He Yao, Mei-Yan Wang, and Xinbin Ma. 2022. "CeO2-ZrO2 Solid Solution Catalyzed and Moderate Acidic–Basic Sites Dominated Cycloaddition of CO2 with Epoxides: Halogen-Free Synthesis of Cyclic Carbonates" Catalysts 12, no. 6: 632. https://doi.org/10.3390/catal12060632
APA StyleGao, J., Yue, C., Wang, H., Li, J., Yao, H., Wang, M.-Y., & Ma, X. (2022). CeO2-ZrO2 Solid Solution Catalyzed and Moderate Acidic–Basic Sites Dominated Cycloaddition of CO2 with Epoxides: Halogen-Free Synthesis of Cyclic Carbonates. Catalysts, 12(6), 632. https://doi.org/10.3390/catal12060632





