Engineering the Activity of Old Yellow Enzyme NemR-PS for Efficient Reduction of (E/Z)-Citral to (S)-Citronellol
Abstract
:1. Introduction
2. Results and Discussion
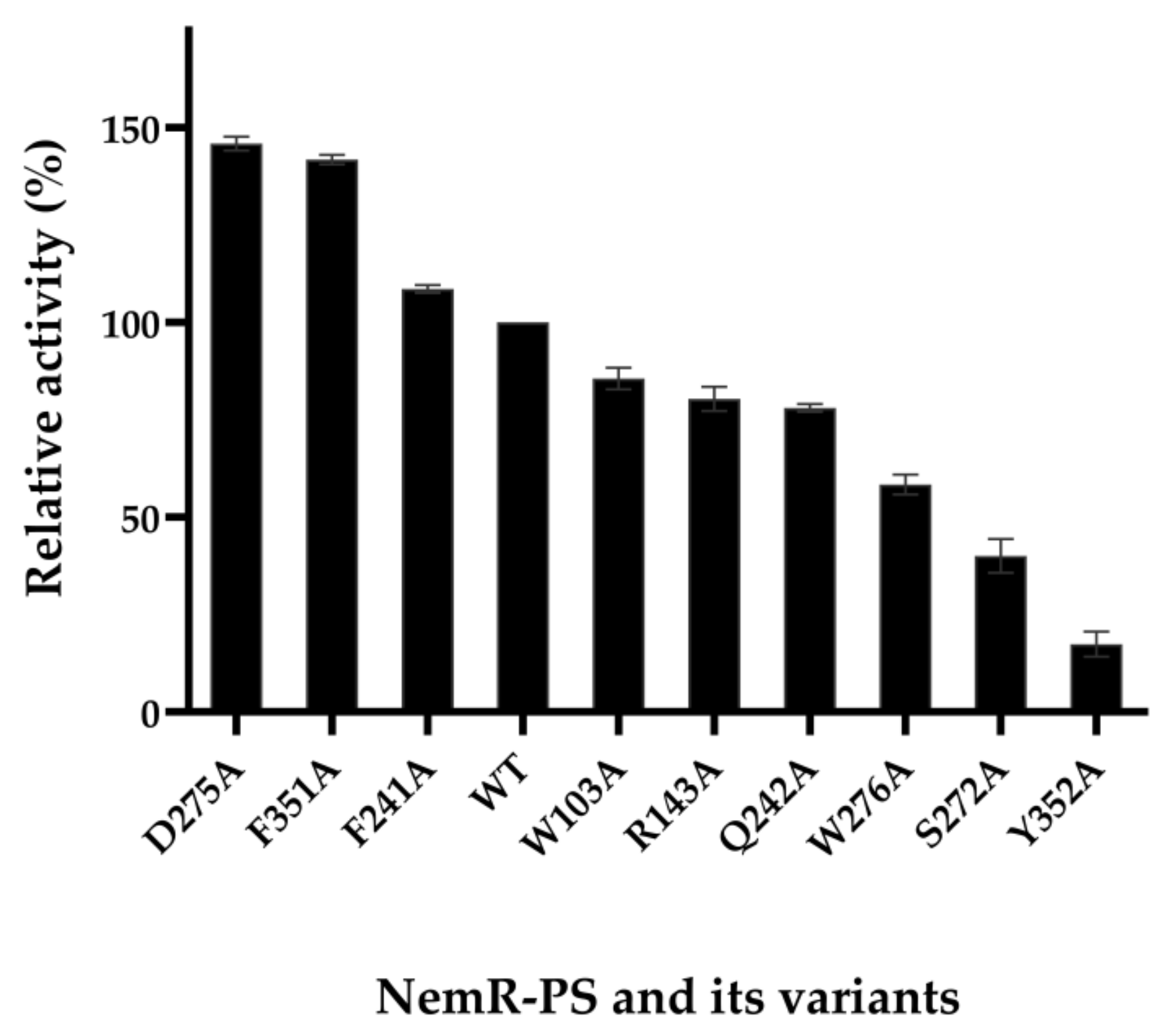
2.1. Homologous Modeling and Alanine Scanning of NemR-PS
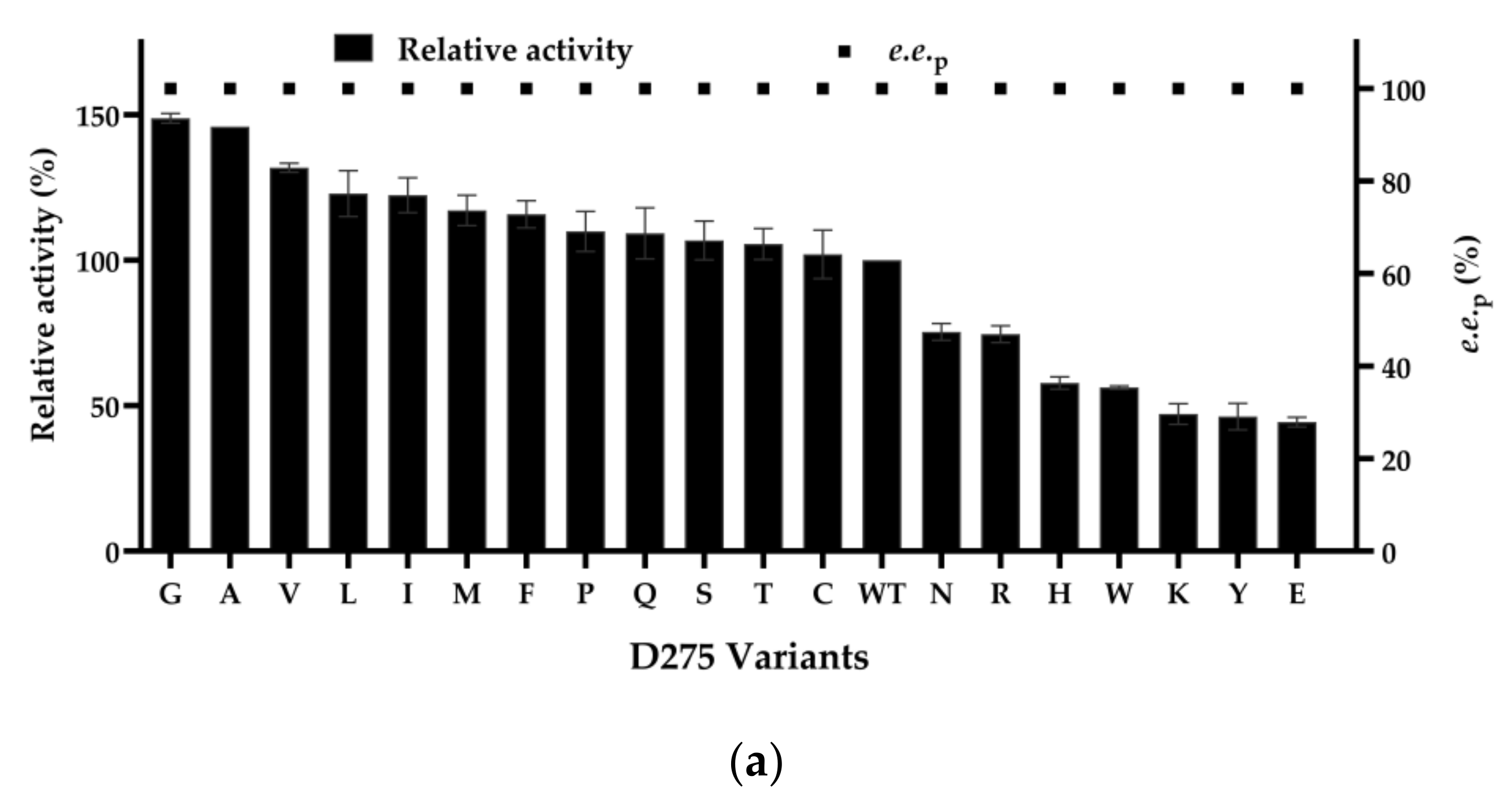
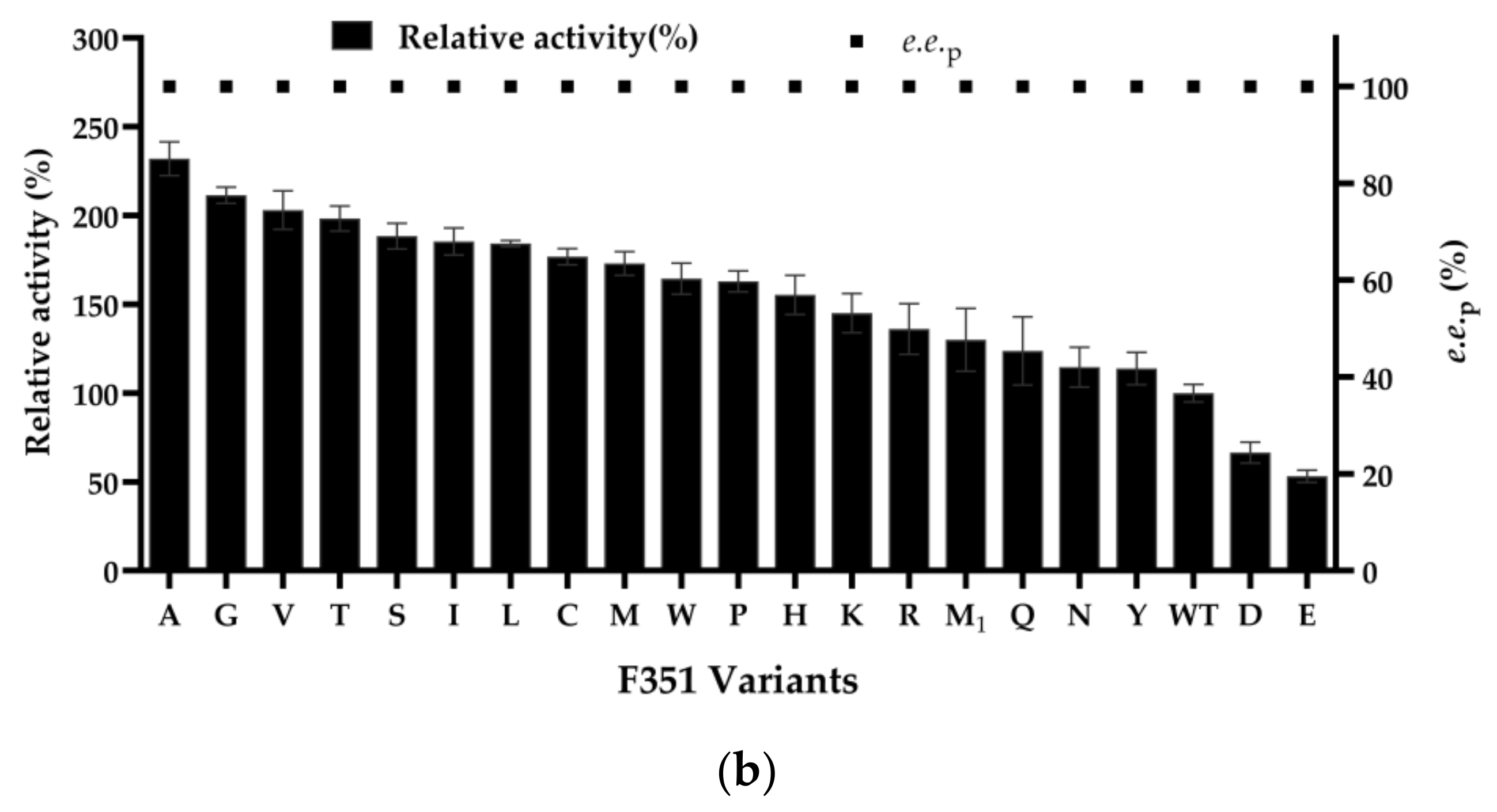
2.2. Site-Saturation Mutagenesis of the Residue D275 and Iterative Saturation Mutagenesis of the Residue F351 in NemR-PS
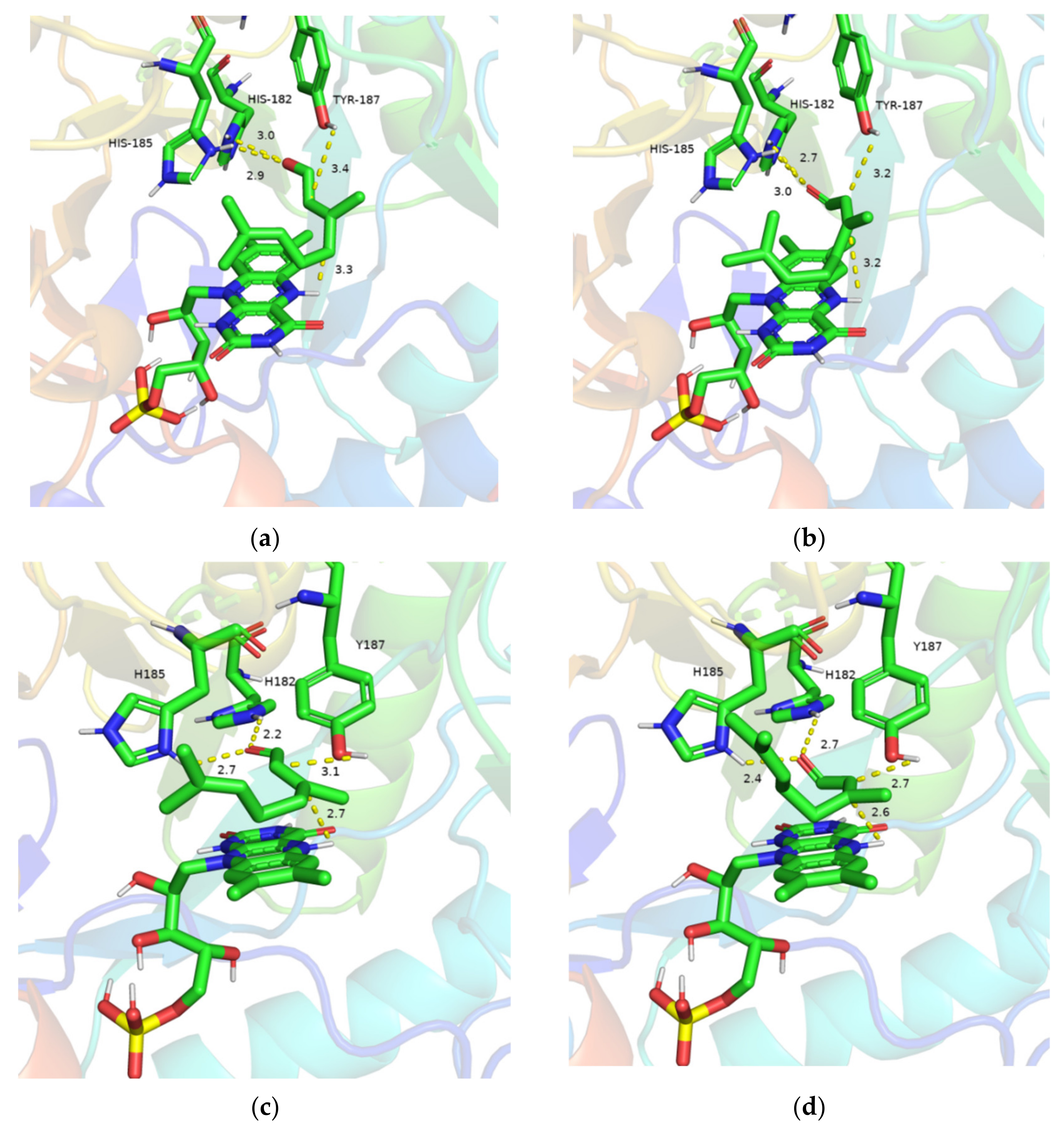
2.3. Kinetic Parameters of the Variant D275/F351A and Molecular Docking Analysis
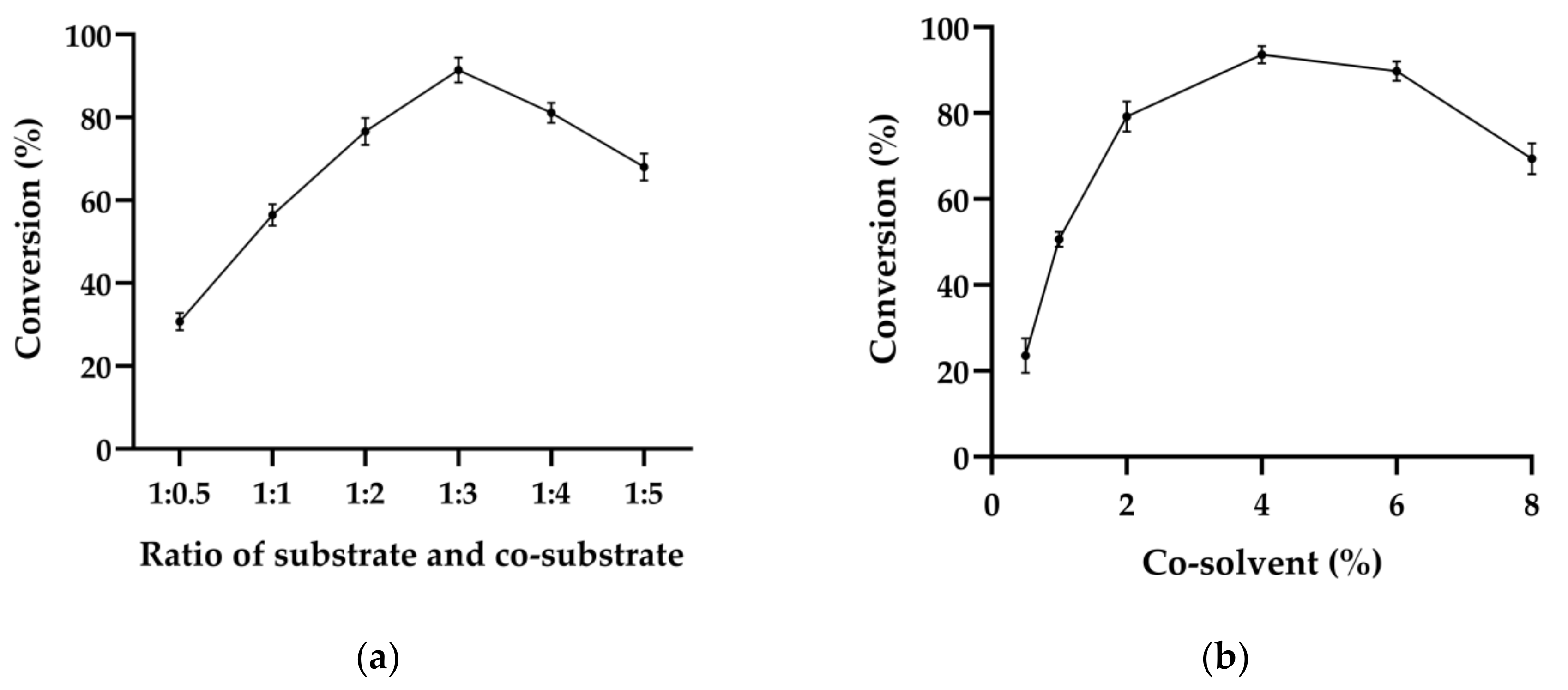
2.4. Key Factors Affecting Whole-Cell Catalyzed Reduction of (E/Z)-Citral to (S)-Citronellol
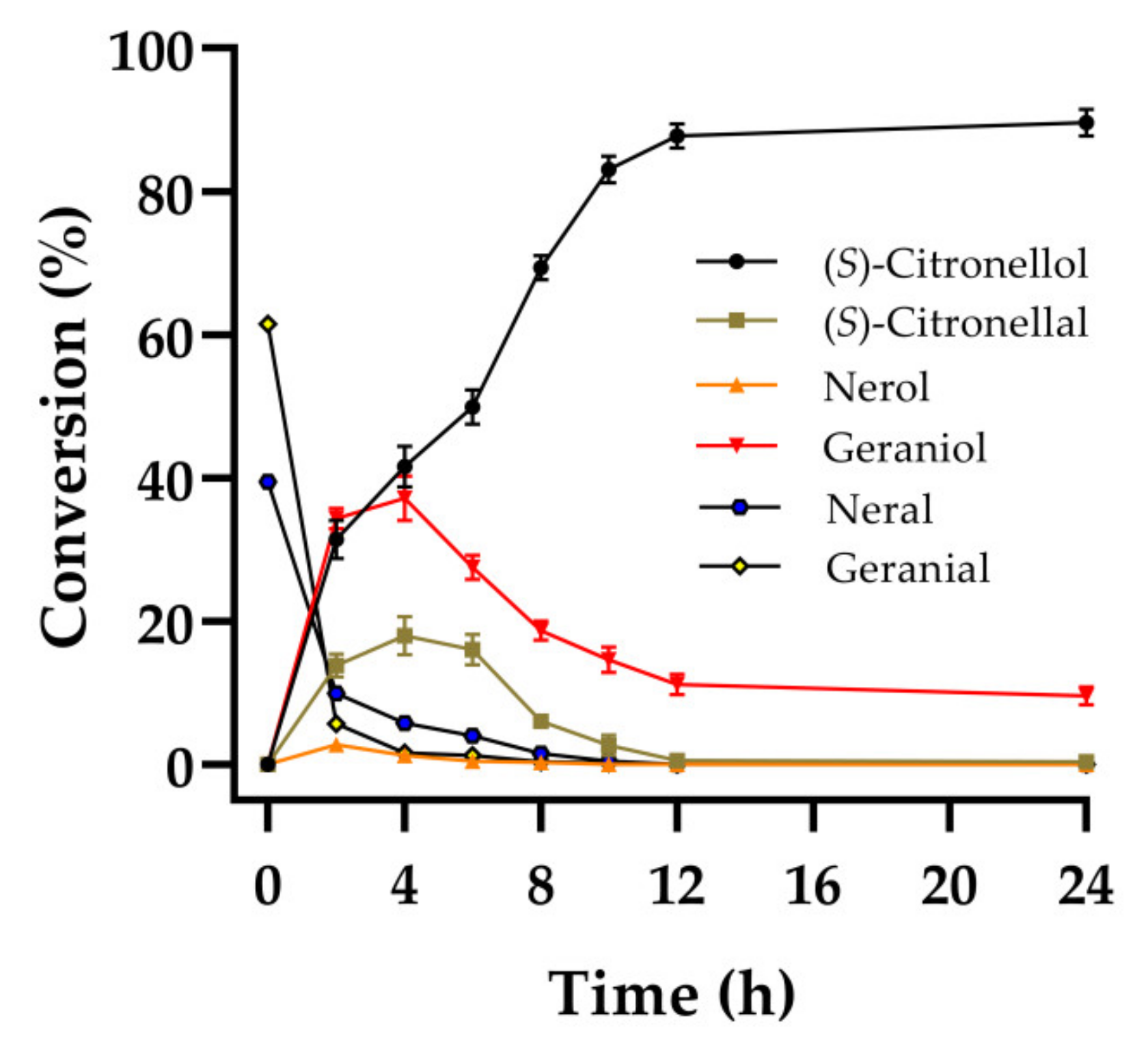
2.5. Synthesis of (S)-Citronellol in the Manner of Substrate Constant Feeding
3. Materials and Methods
3.1. Chemicals, Genes and Organisms
3.2. Expression and Purification of NemR-PS
3.3. Homology Modeling and Molecular Docking
3.4. Site-Directed/Saturation Mutagenesis of NemR-PS
3.5. Activity Assay and Determination of Kinetic Parameters
3.6. Determination of Enantiomeric Excess Value of NemR-PS and Its Variants
3.7. Co-Expression of YsADH, NemR-PS-D275G/F351A and BmGDHM6
3.8. Key Factors on Double Reduction of (E/Z)-Citral to (S)-Citronellol
3.9. Time Courses of the Reduction of 400 mM Citronellol to (S)-Citronellol
3.10. Synthesis of (S)-Citronellol in the Manner of Substrate Constant Feeding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santos, P.L.; Matos, J.; Picot, L.; Almeida, J.; Quintans, J.S.S.; Quintans-Junior, L.J. Citronellol, a monoterpene alcohol with promising pharmacological activities—A systematic review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef]
- Majumder, A.B.; Shah, S.; Gupta, M.N. Enantioselective transacetylation of (R,S)-beta-citronellol by propanol rinsed immobilized Rhizomucor miehei lipase. Chem. Cent. J. 2007, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Matsuda, H.; Utsumi, Y.; Hagiwara, T.; Kanisawa, T. Synthesis and odor of optically active rose oxide. Tetrahedron Lett. 2002, 43, 9077–9080. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Tiainen, L.P.; Lindblad, M.; Demirkan, K.; Kumar, N.; Sjöholm, R.; Ollonqvist, T.; Väyrynen, J.; Salmi, T.; Murzin, D.Y. Liquid-phase hydrogenation of citral for production of citronellol: Catalyst selection. Appl. Catal. A 2003, 241, 271–288. [Google Scholar] [CrossRef]
- Yu, W.; Liu, H.; Liu, M.; Liu, Z. Selective hydrogenation of citronellal to citronellol over polymer-stabilized noble metal colloids. React. Funct. Polym. 2000, 44, 21–29. [Google Scholar] [CrossRef]
- Ribeaucourt, D.; Bissaro, B.; Lambert, F.; Lafond, M.; Berrin, J.G. Biocatalytic oxidation of fatty alcohols into aldehydes for the flavors and fragrances industry. Biotechnol. Adv. 2022, 56, 107787. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Q.; Qiao, J.; Feng, B.; Zhou, X.; Jin, L.; Feng, Y.; Yang, D.; Lu, C.; Ying, X. Cascading old yellow enzyme, alcohol dehydrogenase and glucose dehydrogenase for selective reduction of (E/Z)-citral to (S)-citronellol. Catalysts 2021, 11, 13. [Google Scholar] [CrossRef]
- Hummel, W.; Groger, H. Strategies for regeneration of nicotinamide coenzymes emphasizing self-sufficient closed-loop recycling systems. J. Biotechnol. 2014, 191, 22–31. [Google Scholar] [CrossRef]
- Zheng, L.; Lin, J.; Zhang, B.; Kuang, Y.; Wei, D. Identification of a yeast old yellow enzyme for highly enantioselective reduction of citral isomers to (R)-citronellal. Bioresour. Bioprocess. 2018, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Amato, E.D.; Stewart, J.D. Applications of protein engineering to members of the old yellow enzyme family. Biotechnol. Adv. 2015, 33, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Bougioukou, D.J.; Kille, S.; Taglieber, A.; Reetz, M.T. Directed evolution of an enantioselective enoate-reductase: Testing the utility of iterative saturation mutagenesis. Adv. Synth. Catal. 2009, 351, 3287–3305. [Google Scholar] [CrossRef]
- Zhang, B.; Du, H.; Zheng, Y.; Sun, J.; Shen, Y.; Lin, J.; Wei, D. Design and engineering of whole-cell biocatalyst for efficient synthesis of (R)-citronellal. Microb. Biotechnol. 2022, 15, 1486–1498. [Google Scholar] [CrossRef]
- Daugelavicius, R.; Bakiene, E.; Bamford, D.H. Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob. Agents Chemother. 2000, 44, 2969–2978. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K.; Flickinger, M.C.; Woodley, J.M.; Emanuelsson, E.A.C. Bioprocess intensification: A route to efficient and sustainable biocatalytic transformations for the future. Chem. Eng. Process. 2022, 172, 108793. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Xiong, B.; Wu, T.; Xie, L.; Yu, M.; Wang, Z. Characterization of an allylic/benzyl alcohol dehydrogenase from Yokenella sp. strain WZY002, an organism potentially useful for the synthesis of α,β-unsaturated alcohols from allylic aldehydes and ketones. Appl. Environ. Microbiol. 2014, 80, 2399–2409. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.Z.; Ou, L.; Li, C.X.; Pan, J.; Xu, J.H.; Chen, Q.; Zheng, G.W. Evolution of glucose dehydrogenase for cofactor regeneration in bioredox processes with denaturing agents. Chembiochem 2020, 21, 2680–2688. [Google Scholar] [CrossRef]
- Hulley, M.E.; Toogood, H.S.; Fryszkowska, A.; Mansell, D.; Stephens, G.M.; Gardiner, J.M.; Scrutton, N.S. Focused directed evolution of pentaerythritol tetranitrate reductase by using automated anaerobic kinetic screening of site-saturated Libraries. ChemBioChem 2010, 11, 2433–2447. [Google Scholar] [CrossRef]
- Iorgu, A.I.; Baxter, N.J.; Cliff, M.J.; Waltho, J.P.; Hay, S.; Scrutton, N.S. 1H, 15N and 13C backbone resonance assignments of pentaerythritol tetranitrate reductase from Enterobacter cloacae PB2. Biomol. NMR Assign. 2017, 12, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Morrison, K.L.; Weiss, G.A. Combinatorial alanine-scanning. Curr. Opin. Chem. Biol. 2001, 5, 302–307. [Google Scholar] [CrossRef]
- Fryszkowska, A.; Toogood, H.; Sakuma, M.; Gardiner, J.M.; Stephens, G.M.; Scrutton, N.S. Asymmetric reduction of activated alkenes by pentaerythritol tetranitrate reductase: Specificity and control of stereochemical outcome by reaction optimisation. Adv. Synth. Catal. 2009, 351, 2976–2990. [Google Scholar] [CrossRef]
- Iorgu, A.I.; Hedison, T.M.; Hay, S.; Scrutton, N.S. Selectivity through discriminatory induced fit enables switching of NAD(P)H coenzyme specificity in Old Yellow Enzyme ene-reductases. FEBS J. 2019, 286, 3117–3128. [Google Scholar] [CrossRef] [Green Version]
- Dick, M.; Hartmann, R.; Weiergraber, O.H.; Bisterfeld, C.; Classen, T.; Schwarten, M.; Neudecker, P.; Willboldbd, D.; Pietruszka, J. Mechanism-based inhibition of an aldolase at high concentrations of its natural substrate acetaldehyde: Structural insights and protective strategies. Chem. Sci. 2016, 7, 4492–4502. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Gao, L.; Zhang, L.; Bai, Y.; Chen, L.; Yu, M.; Cheng, F.; Sun, J.; Wang, Z.; Ying, X. Asymmetric reduction of ketopantolactone using a strictly (R)-stereoselective carbonyl reductase through effificient NADPH regeneration and the substrate constant-feeding strategy. Biotechnol. Lett. 2017, 39, 741–1746. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided. Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Laible, M.; Boonrod, K. Homemade site directed mutagenesis of whole plasmids. J. Vis. Exp. 2009, 27, e1135. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ying, X.; Yu, S.; Huang, M.; Wei, R.; Meng, S.; Cheng, F.; Yu, M.; Ying, M.; Zhao, M.; Wang, Z. Engineering the enantioselectivity of yeast old yellow enzyme OYE2y in asymmetric reduction of (E/Z)-citral to (R)-citronellal. Molecules 2019, 24, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| NemR-PS | NemR-PS-D275G/F351A | |
|---|---|---|
| Ki (mM) | 39.79 ± 1.23 | 128.50 ± 1.23 |
| Vmax (U/mg) | 1.98 ± 0.11 | 2.64 ± 0.15 |
| Km (mM) | 0.63 ± 0.13 | 0.35 ± 0.08 |
| Kcat (s−1) | 1.32 ± 0.07 | 1.75 ± 0.10 |
| Kcat/Km (s−1mM−1) | 2.09 ± 0.11 | 5.01 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Li, X.; Jin, L.; Wang, Y.; Tang, Y.; Hua, Y.; Lu, C.; Sun, J.; Zhang, Y.; Ying, X. Engineering the Activity of Old Yellow Enzyme NemR-PS for Efficient Reduction of (E/Z)-Citral to (S)-Citronellol. Catalysts 2022, 12, 631. https://doi.org/10.3390/catal12060631
Feng B, Li X, Jin L, Wang Y, Tang Y, Hua Y, Lu C, Sun J, Zhang Y, Ying X. Engineering the Activity of Old Yellow Enzyme NemR-PS for Efficient Reduction of (E/Z)-Citral to (S)-Citronellol. Catalysts. 2022; 12(6):631. https://doi.org/10.3390/catal12060631
Chicago/Turabian StyleFeng, Binbin, Xia Li, Lijun Jin, Yi Wang, Yi Tang, Yuhao Hua, Chenze Lu, Jie Sun, Yinjun Zhang, and Xiangxian Ying. 2022. "Engineering the Activity of Old Yellow Enzyme NemR-PS for Efficient Reduction of (E/Z)-Citral to (S)-Citronellol" Catalysts 12, no. 6: 631. https://doi.org/10.3390/catal12060631
APA StyleFeng, B., Li, X., Jin, L., Wang, Y., Tang, Y., Hua, Y., Lu, C., Sun, J., Zhang, Y., & Ying, X. (2022). Engineering the Activity of Old Yellow Enzyme NemR-PS for Efficient Reduction of (E/Z)-Citral to (S)-Citronellol. Catalysts, 12(6), 631. https://doi.org/10.3390/catal12060631






