Novel and Green Synthesis of Nitrogen-Doped Carbon Cohered Fe3O4 Nanoparticles with Rich Oxygen Vacancies and Its Application
Abstract
:1. Introduction
2. Results and Discussion
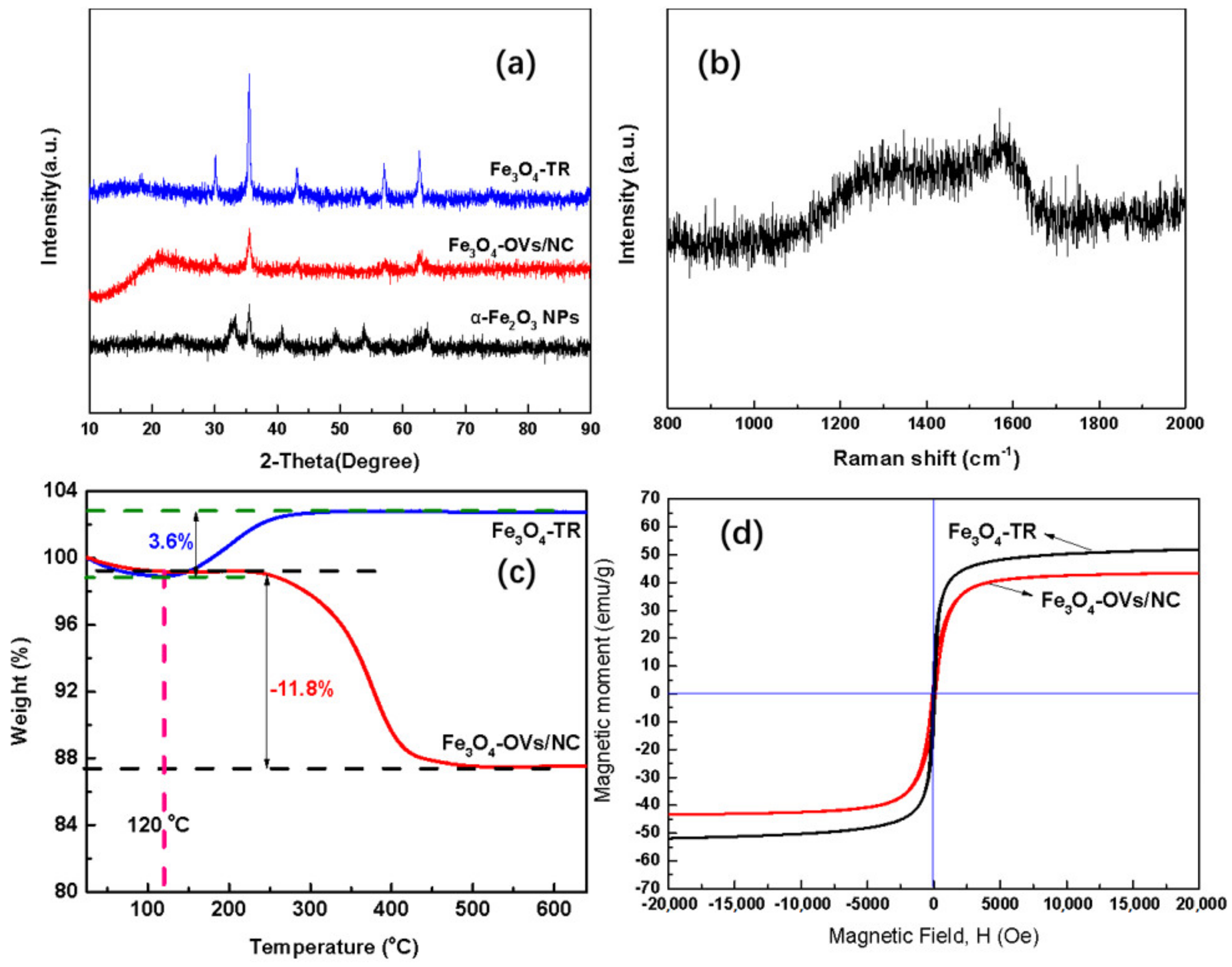
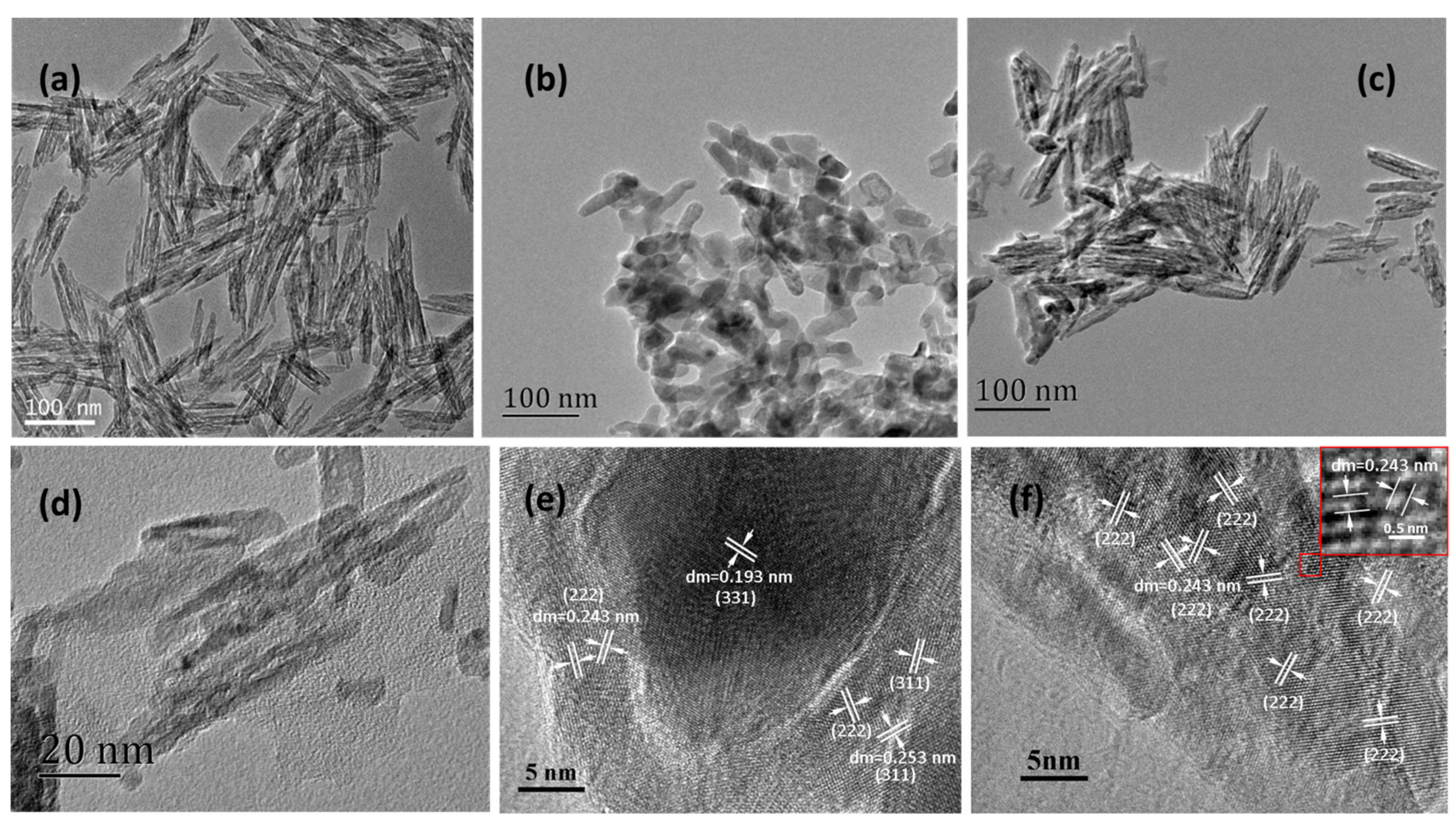
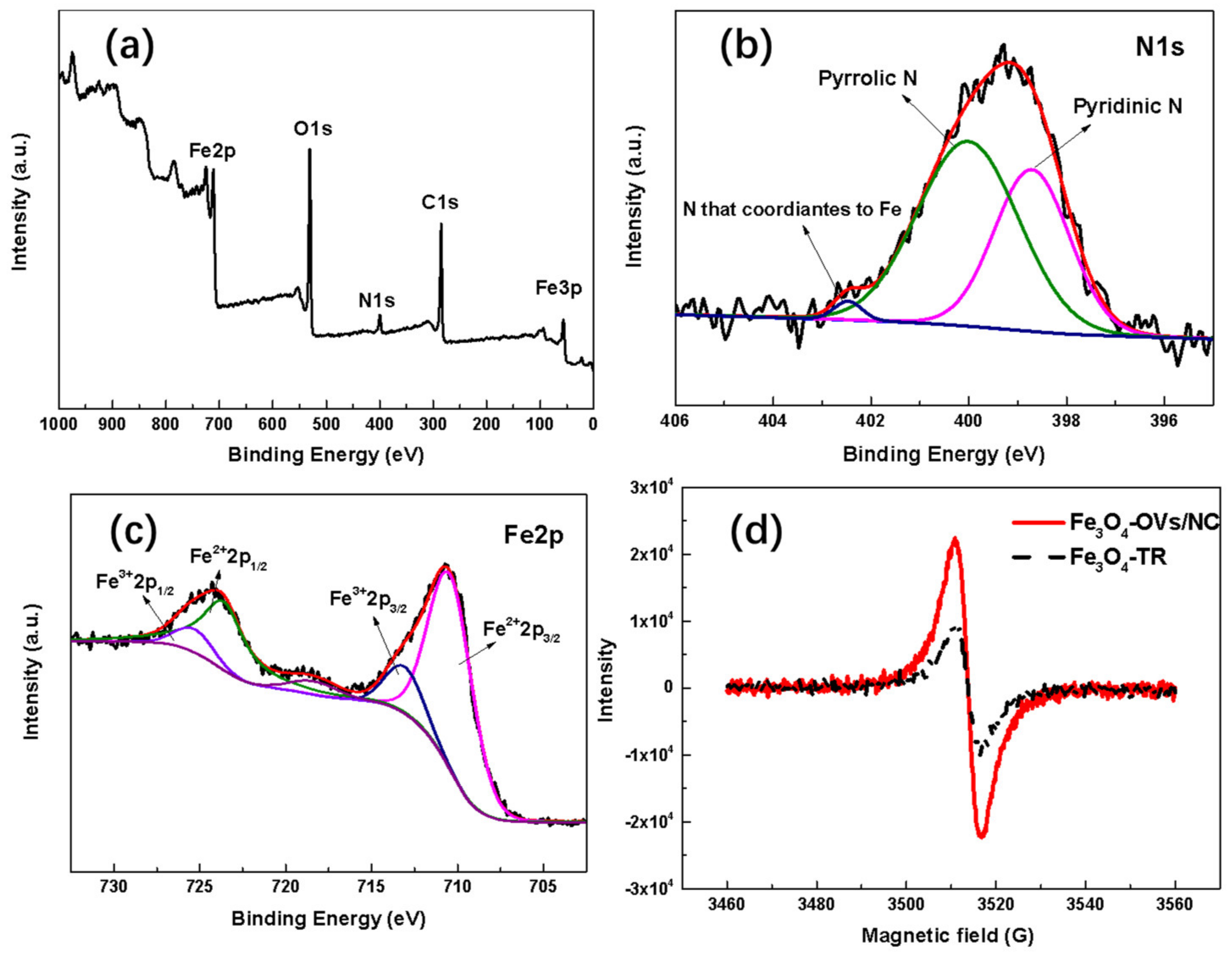
2.1. Microstructural, Morphological and Physio-Chemical Characterization
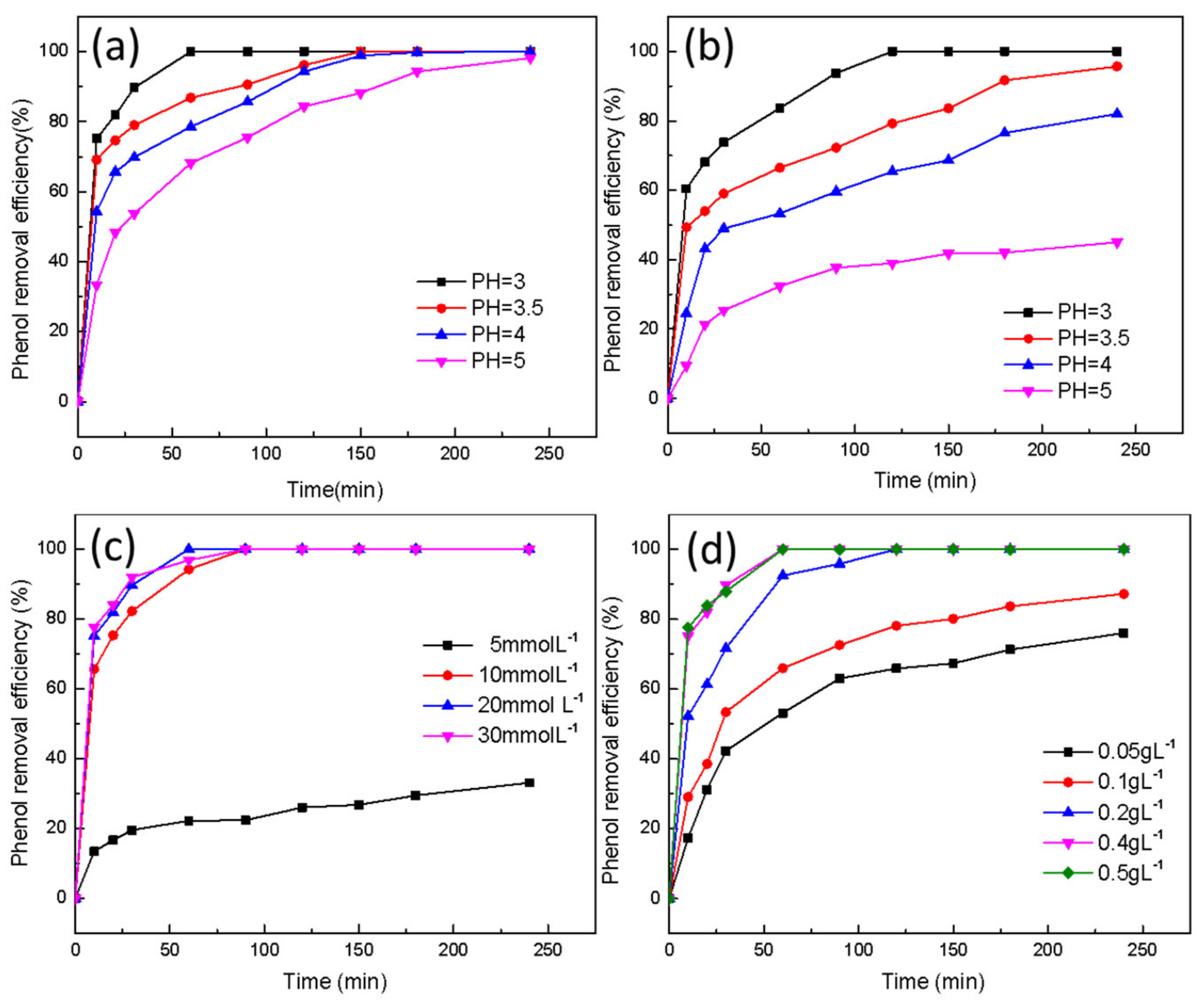
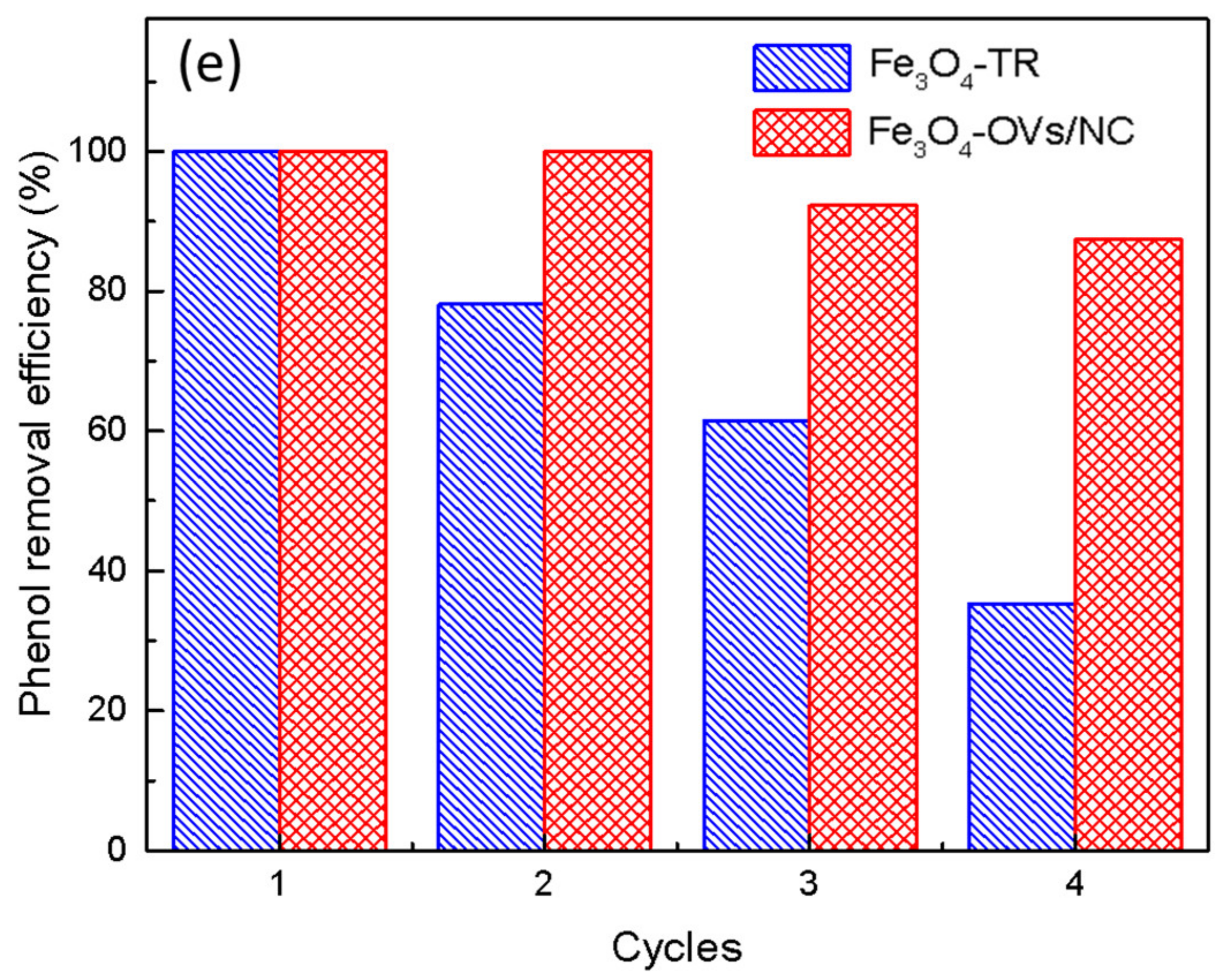
2.2. Catalytic Performance of Fe3O4-TR and Fe3O4-OVs/NC
3. Materials and Methods
3.1. Material Preparation
3.2. Material Characterization
3.3. Catalytic Degradation Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, W.; Guo, L. Iron triad (Fe, Co, Ni) nanomaterials: Structural design, functionalization and their applications. Chem. Soc. Rev. 2015, 44, 6697–6707. [Google Scholar] [CrossRef]
- Wu, S.; Gu, L.; Qin, J.; Zhang, L.; Sun, F.; Liu, Z.; Wang, Y.; Shi, D. Rapid label-free isolation of circulating tumor cells from patients’ peripheral blood using electrically charged Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4193–4203. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ma, C.; Cao, L.; Shao, Z. A self-adhesive graphene nanoscroll/nanosheet paper with confined Fe1-xS/Fe3O4 hetero-nanoparticles for high-performance anode material of flexible Li-ion batteries. Chem. Eng. J. 2019, 370, 536–546. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Xie, Z.; An, C.; Jiang, G.; Wang, Y. 3D graphene-encapsulated nearly monodisperse Fe3O4 nanoparticles as high-performance lithium-ion battery anodes. J. Alloys Compd. 2020, 815, 152337. [Google Scholar] [CrossRef]
- Wang, X.; Pan, F.; Xiang, Z.; Zeng, Q.; Pei, K.; Che, R.; Lu, W. Magnetic vortex core-shell Fe3O4@C nanorings with enhanced microwave absorption performance. Carbon 2020, 157, 130–139. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Veisi, H.; Ebrahimi, Z.; Karmakar, B.; Tamoradi, T. A convenient green protocol for oxidative esterification of arylaldehydes over Pd NPs decorated polyplex encapsulated Fe3O4 microspheres. Int. J. Biol. Macromol. 2022, 200, 132–138. [Google Scholar] [CrossRef]
- Cheng, Q.; Yue, L.; Li, J.; Gao, C.; Ding, Y.; Sun, C.; Xu, M.; Yuan, Z.; Wang, R. Supramolecular tropism driven aggregation of nanoparticles in situ for tumor-specific bioimaging and photothermal therapy. Small 2021, 17, 2101332. [Google Scholar] [CrossRef]
- He, D.; Sun, M.; Cao, D.; He, G.; Chen, H. Rational design of nano-Fe3O4 encapsulated in 3D honeycomb biochar for enhanced lithium storage performance. Nanotechnology 2022, 33, 035401. [Google Scholar] [CrossRef]
- Xue, S.; Wu, G.; Li, M.; Liu, Z.; Deng, Y.; Han, W.; Lv, X.; Wan, S.; Xi, X.; Yang, D.; et al. Generalized assembly of sandwich-like 0D/2D/0D heterostructures with highly exposed surfaces toward superior electrochemical performances. Nano Res. 2022, 15, 255–263. [Google Scholar] [CrossRef]
- Ma, W.; He, P.; Wang, T.; Xu, J.; Liu, X.; Zhuang, Q.; Cui, Z.; Lin, S. Microwave absorption of carbonization temperature-dependent uniform yolk-shell H-Fe3O4@C microspheres. Chem. Eng. J. 2021, 420, 129875. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Ahmadi, A.; Bikhabar, G.; Ramavandi, B. Impact of ZnO and Fe3O4 magnetic nanoscale on the methyl violet 2B removal efficiency of the activated carbon oak wood. Chemosphere 2022, 286, 131632. [Google Scholar] [CrossRef]
- Setvín, M.; Wagner, M.; Schmid, M.; Parkinson, G.S.; Diebold, U. Surface point defects on bulk oxides: Atomically-resolved scanning probe microscopy. Chem. Soc. Rev. 2017, 46, 1772–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, T.; Xie, S.; Yu, M.; Fang, P.; Liang, C.; Lu, X.; Tong, Y. Oxygen vacancies enhancing capacitive properties of MnO2 nanorods for wearable asymmetric supercapacitors. Nano Energy 2014, 8, 255–263. [Google Scholar] [CrossRef]
- Jian, W.; Wang, S.-P.; Zhang, H.-X.; Bai, F.-Q. Disentangling the role of oxygen vacancies on the surface of Fe3O4 and γ-Fe2O3. Inorg. Chem. Front. 2019, 6, 2660–2666. [Google Scholar] [CrossRef]
- Bui, H.T.; Im, S.M.; Kim, K.; Kima, W.; Lee, H. Photocatalytic degradation of phenolic compounds of defect engineered Fe3O4: An alternative approach to solar activation via ligand-to-metal charge transfer. Appl. Surf. Sci. 2020, 509, 144853. [Google Scholar] [CrossRef]
- Deng, Y.; Tian, X.; Shen, G.; Gao, Y.; Lin, C.; Ling, L.; Cheng, F.; Zhang, S.; Liao, S. Coupling hollow Fe3O4 nanoparticles with oxygen vacancy on mesoporous carbon as a high-efficiency ORR electrocatalyst for Zn-air battery. J. Colloid Interface Sci. 2020, 567, 410–418. [Google Scholar] [CrossRef] [PubMed]
- SujayShekar, G.C.; Khaled, A.; Abdo, H.; Ali, A.; Nabil, A.Z.; Lokanath, N.K. Enhanced photo-Fenton activity over a sunlight-driven ignition synthesized α-Fe2O3-Fe3O4/CeO2 heterojunction catalyst enriched with oxygen vacancies. J. Mol. Liq. 2021, 335, 116186. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, W.; Lu, J.; Tian, L.; Yi, S.; Zhang, Y.; Zhou, S.; Niu, B.; Long, D. Oxygen-vacancy-rich Fe3O4/carbon nanosheets enabling high-attenuation and broadband microwave absorption through the integration of interfacial polarization and charge-separation polarization. J. Mater. Chem. A 2022, 10, 8479–8490. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhang, Y.; Zhang, M.; Cheng, M.; Yu, J.; Liu, H.; Ji, M.; Zhu, C.; Xu, J. Fe3O4 encapsulated in porous carbon nanobowls as efficient oxygen reduction reaction catalyst for Zn-air batteries. Chem. Eng. J. 2019, 375, 122058. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Y. Magnetically responsive nanostructures with tunable optical properties. J. Am. Chem. Soc. 2016, 138, 6315–6323. [Google Scholar] [CrossRef]
- Mishra, P.; Patnaik, S.; Parida, K. An overview of recent progress on noble metal modified magnetic Fe3O4 for photocatalytic pollutant degradation and H2 evolution. Catal. Sci. Technol. 2019, 9, 916–941. [Google Scholar] [CrossRef]
- Dong, P.; Liu, W.; Wang, S.; Wang, H.; Wang, Y.; Zhao, C. In suit synthesis of Fe3O4 on carbon fiber paper@polyaniline substrate as novel self-supported electrode for heterogeneous electro-Fenton oxidation. Electrochim. Acta 2019, 308, 54–63. [Google Scholar] [CrossRef]
- Tolba, A.; Alalm, M.G.; Elsamadony, M.; Mostafa, A.; Afify, H.; Dionysiou, D.D. Modeling and optimization of heterogeneous Fenton-like and photo-Fenton processes using reusable Fe3O4-MWCNTs. Process Saf. Environ. 2019, 128, 273–283. [Google Scholar] [CrossRef]
- Jiang, D.; Murugadoss, V.; Wang, Y.; Lin, J.; Ding, T.; Wang, Z.; Shao, Q.; Wang, C.; Liu, H.; Lu, N.; et al. Electromagnetic interference shielding polymers and nanocomposites—A review. Poly. Rev. 2019, 59, 280–337. [Google Scholar] [CrossRef]
- Pujol, A.A.; León, I.; Cárdenas, J.; Sepúlveda-Guzmán, S.; Manríquez, J.; Sirés, I.; Bustos, E. Degradation of phenols by heterogeneous electro-Fenton with a Fe3O4-chitosan composite and a boron-doped diamond anode. Electrochim. Acta 2020, 337, 135784. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Li, S.; Gu, P.; Xue, G. Hydrogel-coated Fe3O4 nanoparticles as an efficient heterogeneous Fenton catalyst for degradation of phenol. J. Mater. Sci. 2019, 54, 10684–10694. [Google Scholar] [CrossRef]
- Silvestre, M.E.; Franzreb, M.; Weidler, P.G.; Shekhah, O.; Wöll, C. Magnetic cores with porous coatings: Growth of metal-organic frameworks on particles using liquid phase epitaxy. Adv. Funct. Mater. 2013, 23, 1210–1213. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, L.; Yan, W.; Li, Z.; Fan, M.; Du, G.; Zhao, W. Controlled preparation of nitrogen-doped hierarchical carbon cryogels derived from Phenolic-Based resin and their CO2 adsorption properties. Energy 2022, 246, 123367. [Google Scholar] [CrossRef]
- Xu, H.; Lv, X.; Wang, H.; Ye, J.; Yuan, J.; Wang, Y.; Zhou, Z.; Sun, S. Impact of pore structure on two-electron oxygen reduction reaction in nitrogen-doped carbon materials: Rotating ring-disk electrode vs. flow cell. ChemSusChem 2022, 15, e202102587. [Google Scholar] [CrossRef]
- Wang, L.; Wei, G.; Dong, X.; Zhao, Y.; Xing, Z.; Hong, H.; Ju, Z. Hollow α-Fe2O3 Nanotubes embedded in graphene aerogel as high-performance anode material for lithium-ion batteries. ChemistrySelect 2019, 4, 11370–11377. [Google Scholar] [CrossRef]
- Jian, Y.; Yu, T.; Jiang, Z.; Yu, Y.; Douthwaite, M.; Liu, J.; Albilali, R.; He, C. In-depth understanding of the morphology effect of α-Fe2O3 on catalytic ethane destruction. ACS Appl. Mater. Interfaces 2019, 11, 11369–11383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Lyu, T.; Xie, W.; Mirjalili, A.; Bradicich, A.; Huitema, R.; Jang, B.W.-L.; Keum, J.K.; More, K.; Liu, C.; et al. Preparation and investigation of Pd doped Cu catalysts forselective hydrogenation of acetylene. Front. Chem. Sci. Eng. 2020, 14, 522–533. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Y.; Liu, L.; Li, W.; Guan, J.; Tong, G. Low-cost carbothermal reduction preparation of monodisperse Fe3O4/C core−shell nanosheets for improved microwave absorption. ACS Appl. Mater. Interfaces 2018, 10, 16511–16520. [Google Scholar] [CrossRef]
- Hong, W.; Hsu, I.; Huang, S.; Lee, C.; Ko, H.; Tsai, P.; Shieh, D.; Huang, C. Assembled growth of 3D Fe3O4@Au nanoparticles for efficient photothermal ablation and SERS detection of microorganisms. J. Mater. Chem. B 2018, 6, 5689–5697. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yu, L.; Chen, Z.; He, H.; Ye, F.; Cheng, G.; Zhang, Q. Microwave-assisted synthesis of Fe3O4 nanocrystals with predominantly exposed facets and their heterogeneous UVA/Fenton catalytic activity. ACS Appl. Mater. Interfaces 2017, 9, 29203–29212. [Google Scholar] [CrossRef]
- Huang, D.; Luo, Y.; Li, S.; Wang, M.; Shen, Y. Hybrid of Fe@Fe3O4 core-shell nanoparticle and iron-nitrogen-doped carbon material as an efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2015, 174, 933–993. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Xu, Y.; Dong, S.; Xiao, J.; Wang, F.; Liu, H.; Xia, B.Y. Hollow nitrogen-doped carbon spheres with Fe3O4 nanoparticles encapsulated as a highly active oxygen-reduction catalyst. ACS Appl. Mater. Interfaces 2017, 9, 10610–10617. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yao, Z.; Zhang, D.; Li, D.; Zhang, Z.; Jiang, Z. Rational synthesis of micronano dendritic ZVI@Fe3O4 modified with carbon quantum dots and oxygen vacancies for accelerating Fenton-like oxidation. Sci. Total Environ. 2019, 671, 1056–1065. [Google Scholar] [CrossRef]
- Yang, W.; Hong, P.; Yang, D.; Yang, Y.; Wu, Z.; Xie, C.; He, J.; Zhang, K.; Kong, L.; Liu, J. Enhanced Fenton-like degradation of sulfadiazine by single atom iron materials fixed on nitrogen-doped porous carbon. J. Colloid Interface Sci. 2021, 597, 56–65. [Google Scholar] [CrossRef]
- Beker, S.A.; Khudur, L.S.; Cole, I.; Ball, A.S. Catalytic degradation of methylene blue using iron and nitrogen-containing carbon dots as Fenton-like catalysts. New J. Chem. 2022, 46, 263–275. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, R.; Rui, N.; Wang, Z.; Wang, J.; Zhou, X.; Liu, C. Co3O4/HZSM-5 catalysts for methane combustion: The effect of preparation methodologies. Catal. Today 2017, 297, 219–227. [Google Scholar] [CrossRef]
- Salamun, N.; Ni, H.X.; Triwahyono, S.; Jalil, A.A.; Karim, A.H. Synthesis and characterization of Fe3O4 nanoparticles by electrodeposition and reduction methods. Mal. J. Fund. Appl. Sci. 2011, 7, 89–92. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zhu, H.; Jang, B.W.-L.; Mirjalili, A.; Yang, C.; Jiang, L.; Tang, S.; Zhang, J.; Qin, J.; Zhang, L. Novel and Green Synthesis of Nitrogen-Doped Carbon Cohered Fe3O4 Nanoparticles with Rich Oxygen Vacancies and Its Application. Catalysts 2022, 12, 621. https://doi.org/10.3390/catal12060621
Cao X, Zhu H, Jang BW-L, Mirjalili A, Yang C, Jiang L, Tang S, Zhang J, Qin J, Zhang L. Novel and Green Synthesis of Nitrogen-Doped Carbon Cohered Fe3O4 Nanoparticles with Rich Oxygen Vacancies and Its Application. Catalysts. 2022; 12(6):621. https://doi.org/10.3390/catal12060621
Chicago/Turabian StyleCao, Xinxiang, Huijie Zhu, Ben W.-L. Jang, Arash Mirjalili, Chunlai Yang, Luoqing Jiang, Siye Tang, Junjie Zhang, Juanjuan Qin, and Long Zhang. 2022. "Novel and Green Synthesis of Nitrogen-Doped Carbon Cohered Fe3O4 Nanoparticles with Rich Oxygen Vacancies and Its Application" Catalysts 12, no. 6: 621. https://doi.org/10.3390/catal12060621
APA StyleCao, X., Zhu, H., Jang, B. W.-L., Mirjalili, A., Yang, C., Jiang, L., Tang, S., Zhang, J., Qin, J., & Zhang, L. (2022). Novel and Green Synthesis of Nitrogen-Doped Carbon Cohered Fe3O4 Nanoparticles with Rich Oxygen Vacancies and Its Application. Catalysts, 12(6), 621. https://doi.org/10.3390/catal12060621




