In Situ Growth and UV Photocatalytic Effect of ZnO Nanostructures on a Zn Plate Immersed in Methylene Blue
Abstract
:1. Introduction
2. Results and Discussion
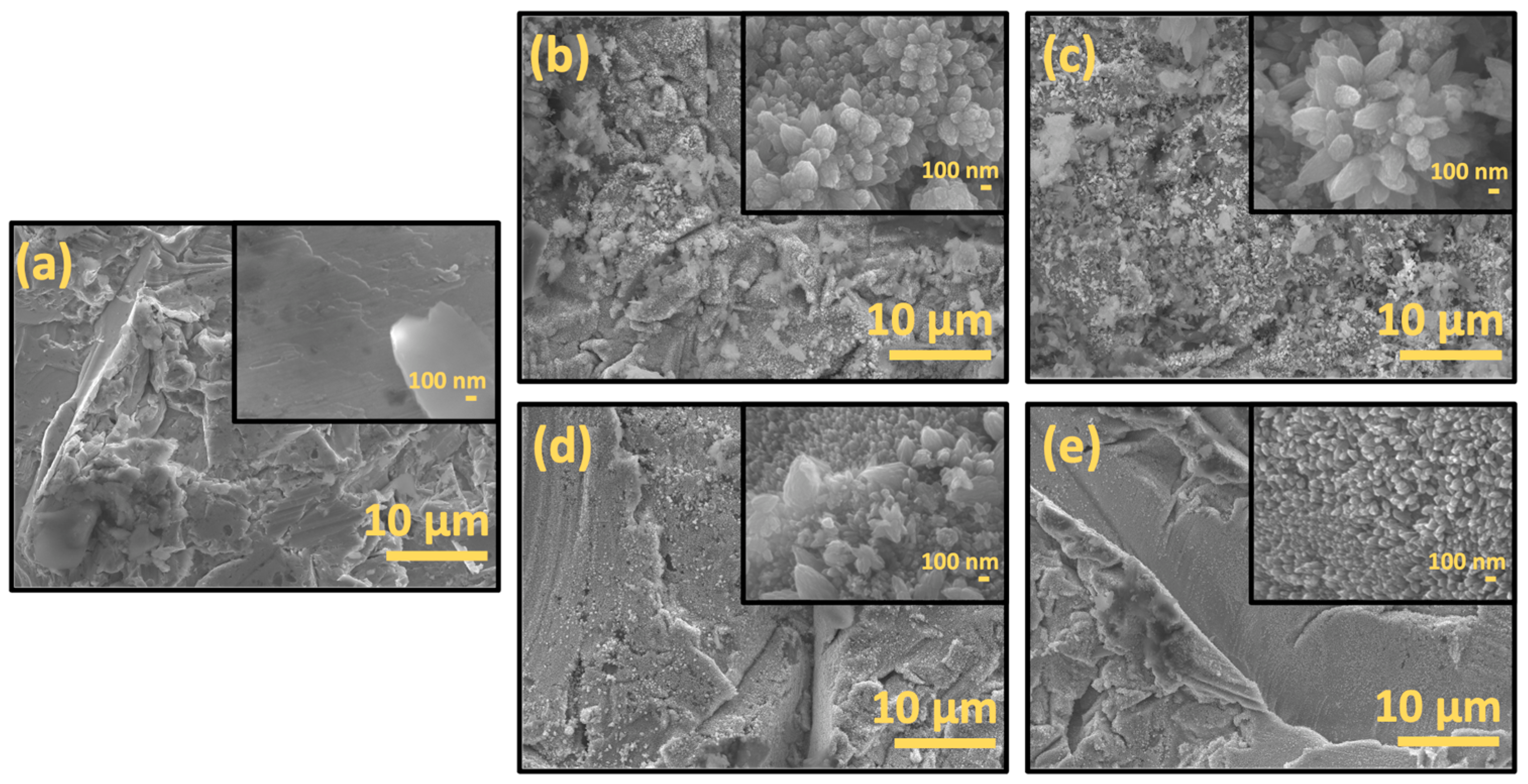
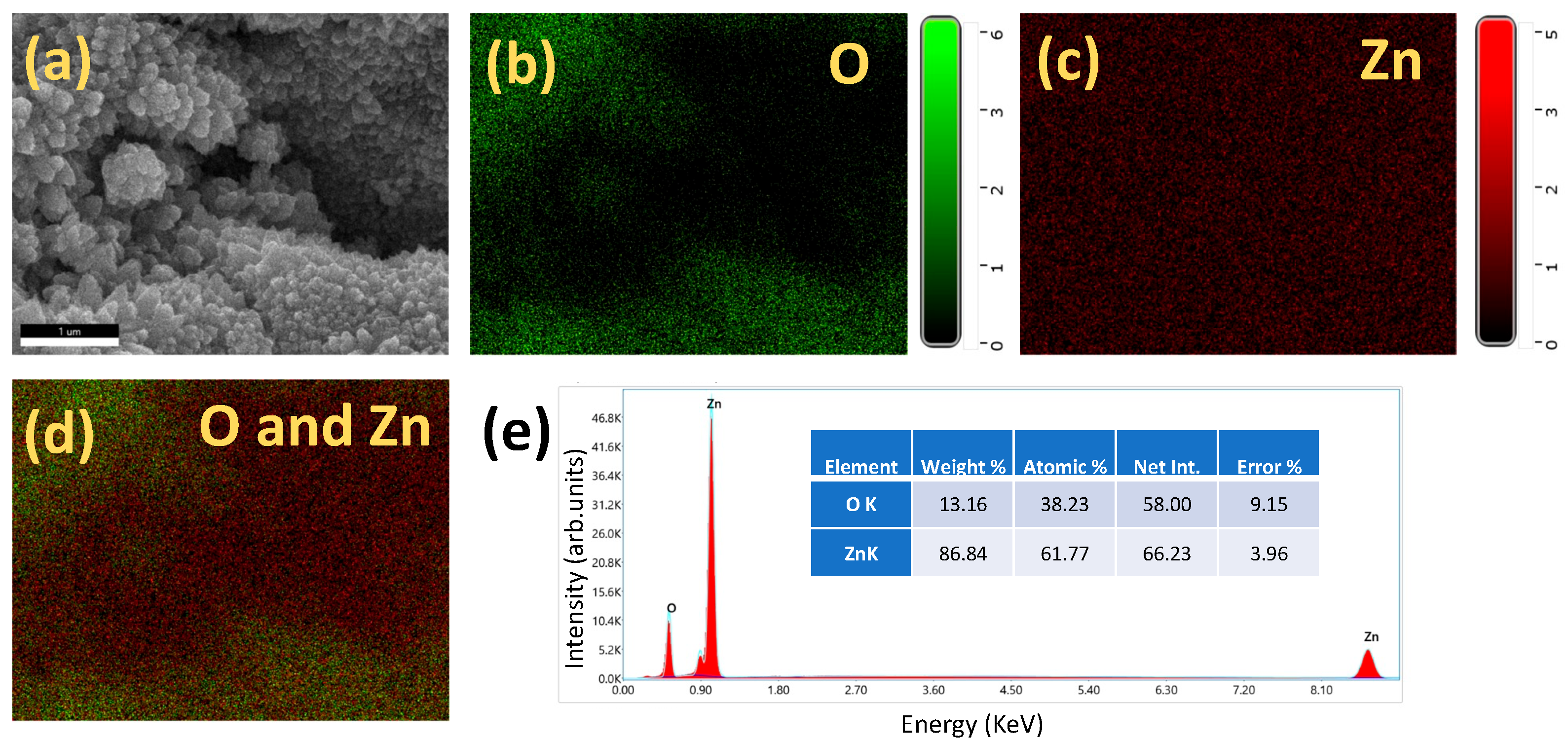
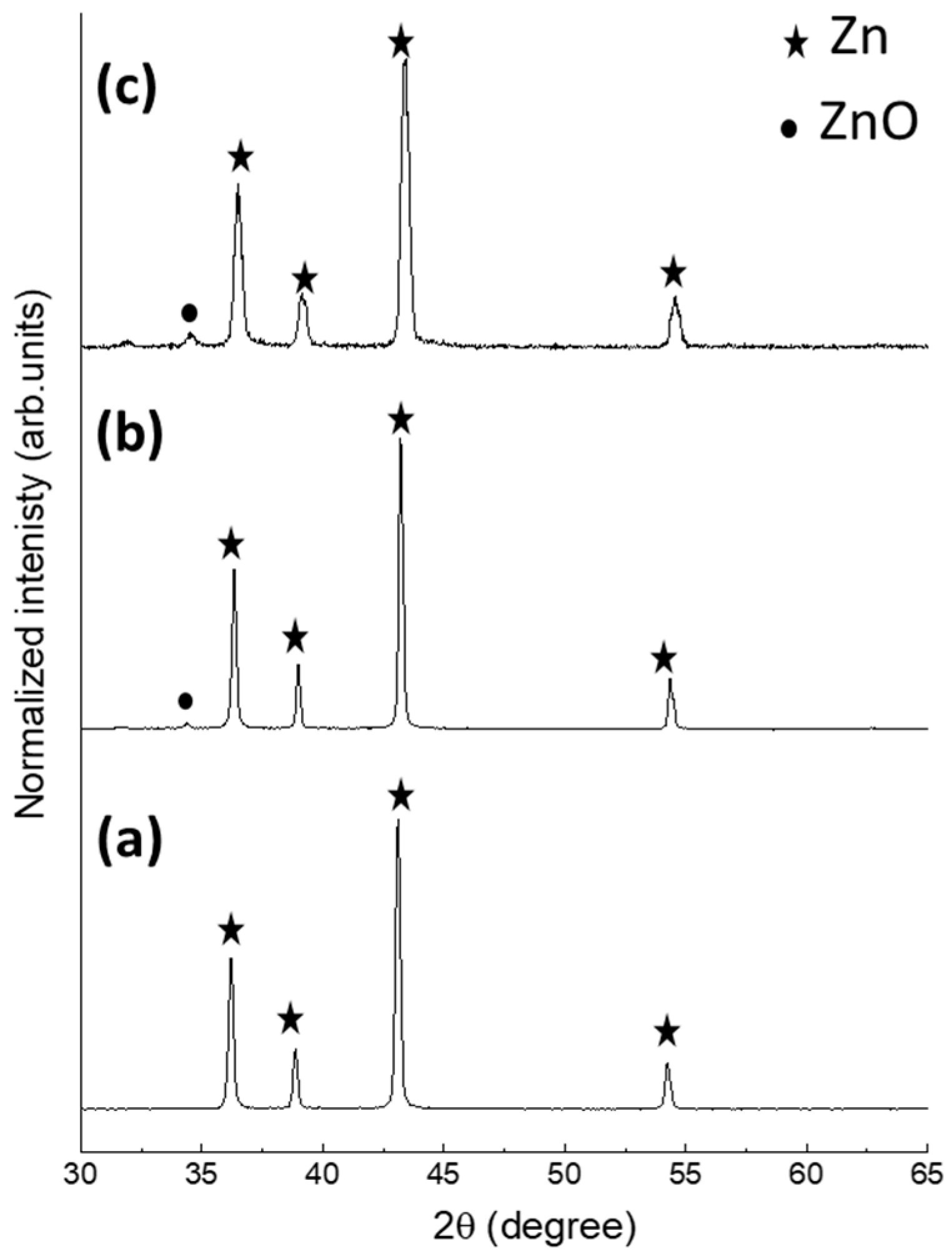
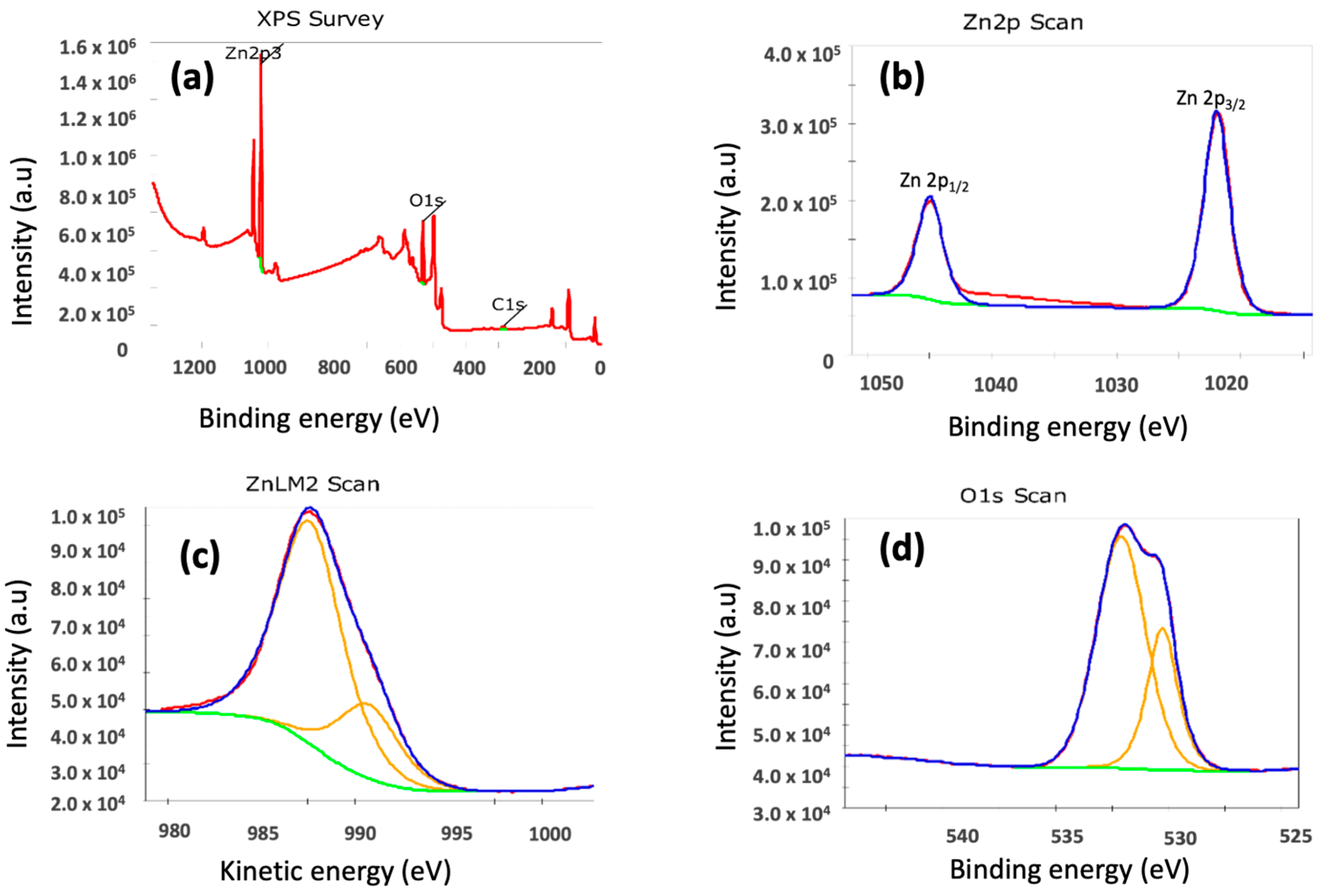
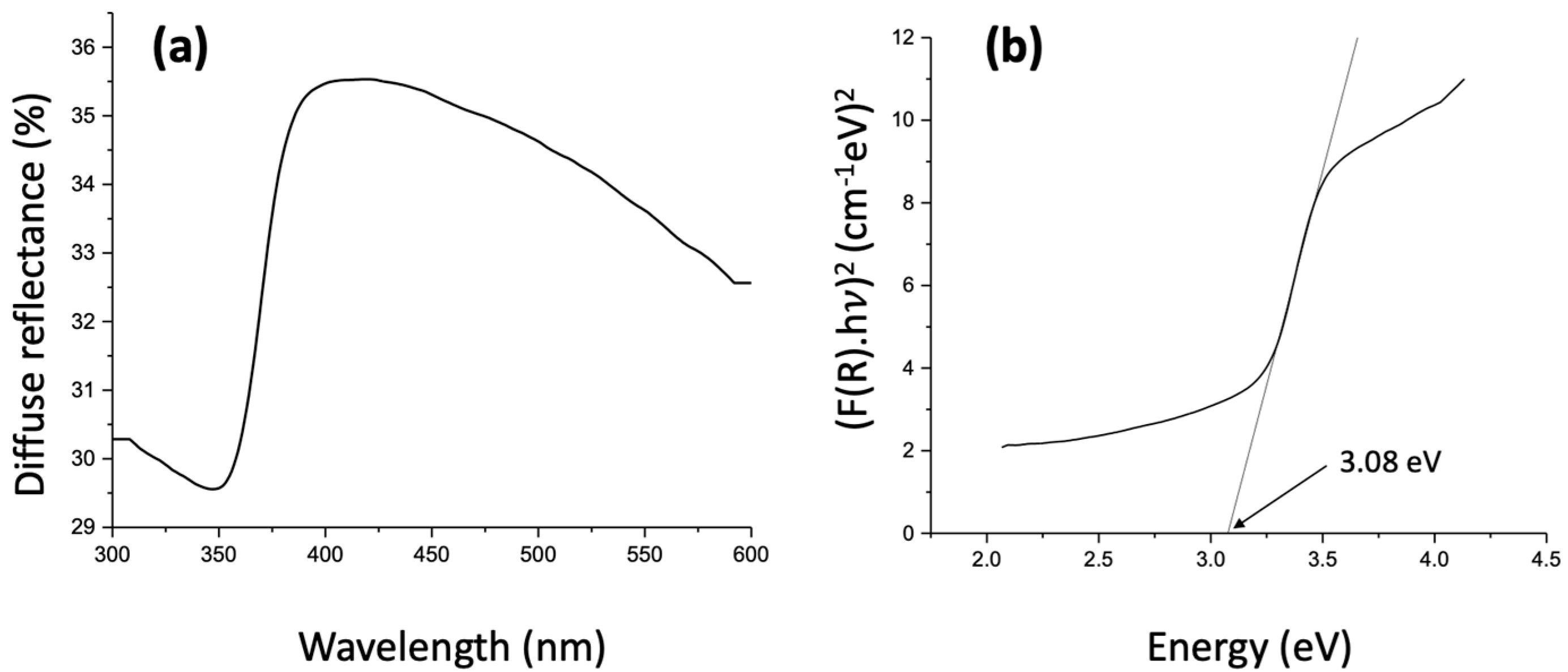
2.1. Materials Charecterization
2.2. Nanostructure Growth Mechanism
2.3. Photocatalytic Degradation of Methylene Blue
| ZnO Synthesis | Organic Dye Solution (Type, Volume, Concentration) | Light Source (Wavelength and Power) | Best Photocatalytic Performance or Rate Constant | Ref. | |||
|---|---|---|---|---|---|---|---|
| Method | Nanostructures immobilized on | Materials used | Reaction parameters | ||||
| Hydrothermal | Si and glass | Zinc acetate, zinc nitrate, hexamethylenetetramine | 60–150 °C, 13 h (approx.) | Methylene Blue, 12 mL, 10 mM | Fluorescent lamp, 375 nm, 18 Watts | 45% in 6 h, 0.0021 min−1 | [50] |
| Hydrothermal | PDMS/PET | Zinc acetate, zinc nitrate, hexamethylenetetramine | 60–90 °C, 10 h (approx.) | Methylene Blue, 40 mL, 10 mM | Fluorescent lamp, 375 nm, 18 Watts | 50% degradation in 6 h, 0.0018 min−1 | [51] |
| Thermal oxidation | Zn | Nil | 500 °C, 12 h | Methylene Blue, 35 × 10−6 M | UV lamp, 312 nm, 15 Watts | 57% degradation in 320 min, 0.1542 h−1 | [37] |
| Spray pyrolysis | FTO coated glass | Zinc acetate, methanol | 425 °C | Methylene Blue, volume not reported, 1 mM | UV light, wavelength and power not reported | 98.1% degradation in 120 min, rate constant not reported | [52] |
| Anodization | Zn | Potassium bicarbonate, orthophosphoric acid, hydrochloric acid, oxalic acid, and sodium hydroxide | 10–40 V DC for anodization, pre and post treatment annealing at 300 °C. | Phenol, 50 mL, 5 ppm | UV lamp, 254 nm, 6 Watts | 82% degradation in 4 h, 0.44 h−1 | [17] |
| Anodization and O2 plasma annealing | Zn | Potassium bicarbonate | 1 V DC, pretreatment annealing at 300 °C and post treatment thermal and O2. plasma annealing (200–350 °C) | Phenol, 50–80 mL, 5–10 ppm | UV lamp, 254 nm, 6 Watts | 70% degradation in 4 h, 0.31 h−1 | [35] |
| Hydrothermal | Zn | Zinc nitrate, hexamethylenetetramine, methylamine, potassium hydroxide | 120–180 °C, 2–12 h | Rhodamine B, 10 mL, 1 × 10−5 M | UV lamp, wavelength and power not reported | 80% degradation of rhodamine B in 3 h, rate constant not reported | [15] |
3. Materials and Methods
3.1. ZnO Nanostructure Synthesis
3.2. Materials Characterization
3.3. In Situ Photocatalytic Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, W.; Wang, W.; Zhang, X.; Liu, Y.; Liu, J. The Perspective and Challenge of Nanomaterials in Oil and Gas Wastewater Treatment. Molecules 2021, 26, 3945. [Google Scholar] [CrossRef]
- Batra, V.; Kaur, I.; Pathania, D.; Sonu; Chaudhary, V. Efficient dye degradation strategies using green synthesized ZnO-based nanoplatforms: A review. Appl. Surf. Sci. Adv. 2022, 11, 100314. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal–metal oxide core–shell nanoparticles for environmental remediation and their recycling process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Marinho, B.A.; de Souza, S.; de Souza, A.A.U.; Hotza, D. Electrospun TiO2 nanofibers for water and wastewater treatment: A review. J. Mater. Sci. 2021, 56, 5428–5448. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Sanakousar, F.M.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Prakash, K. Recent progress on visible-light-driven metal and non-metal doped ZnO nanostructures for photocatalytic degradation of organic pollutants. Mater. Sci. Semicond. Process. 2022, 140, 106390. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Optimization of process parameters using D-optimal design for synthesis of ZnO nanoparticles via sol–gel technique. J. Ind. Eng. Chem. 2013, 19, 99–105. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Li, X.; Dong, W.; Tang, W.; Chen, B.; Li, C.; Li, X.; Zhang, T.; Xu, W. Nanostructured porous ZnO film with enhanced photocatalytic activity. Thin Solid Films 2011, 519, 5673–5678. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, Z.H.; Chen, Z.H.; Shafiq, I.; Zapien, J.A.; Bello, I.; Zhang, W.J.; Lee, S.T. Synthesis, Characterization, and Photocatalytic Application of Different ZnO Nanostructures in Array Configurations. Cryst. Growth Des. 2009, 9, 3222–3227. [Google Scholar] [CrossRef]
- Ramirez-Canon, A.; Medina-Llamas, M.; Vezzoli, M.; Mattia, D. Multiscale design of ZnO nanostructured photocatalysts. Phys. Chem. Chem. Phys. 2018, 20, 6648–6656. [Google Scholar] [CrossRef] [Green Version]
- Anand, V.; Srivastava, V.C. Zinc oxide nanoparticles synthesis by electrochemical method: Optimization of parameters for maximization of productivity and characterization. J. Alloys Compd. 2015, 636, 288–292. [Google Scholar] [CrossRef]
- Kumar, V.R.; Wariar, P.R.S.; Prasad, V.S.; Koshy, J. A novel approach for the synthesis of nanocrystalline zinc oxide powders by room temperature co-precipitation method. Mater. Lett. 2011, 65, 2059–2061. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles-An eco-friendly approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Khatami, M.; Iravani, S. Green and Eco-Friendly Synthesis of Nanophotocatalysts: An Overview. Comments Inorg. Chem. 2021, 41, 133–187. [Google Scholar] [CrossRef]
- Saadi, N.S.; Hassan, L.B.; Karabacak, T. Metal oxide nanostructures by a simple hot water treatment. Sci. Rep. 2017, 7, 7158. [Google Scholar] [CrossRef] [Green Version]
- Hariharalakshmanan, R.K.; Saadi, N.S.; Ergul-Yilmaz, B.; Al-Mayalee, K.H.; Karabacak, T. Zinc Oxide Nanostructures Synthesized by a Simple Hot Water Treatment Method for Photocatalytic Degradation of Organic Pollutants in Water. MRS Adv. 2020, 5, 2457–2465. [Google Scholar] [CrossRef]
- Jeem, M.; bin Julaihi, M.R.M.; Ishioka, J.; Yatsu, S.; Okamoto, K.; Shibayama, T.; Iwasaki, T.; Kato, T.; Watanabe, S. A pathway of nanocrystallite fabrication by photo-assisted growth in pure water. Sci. Rep. 2015, 5, 11429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jeem, M.; Okamoto, K.; Watanabe, S. Photochemistry and the role of light during the submerged photosynthesis of zinc oxide nanorods. Sci. Rep. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, L.B.; Saadi, N.S.; Karabacak, T. Hierarchically rough superhydrophobic copper sheets fabricated by a sandblasting and hot water treatment process. Int. J. Adv. Manuf. Technol. 2017, 93, 1107–1114. [Google Scholar] [CrossRef]
- Le, A.T.; Le, T.D.H.; Cheong, K.-Y.; Pung, S.-Y. Immobilization of zinc oxide-based photocatalysts for organic pollutant degradation: A review. J. Environ. Chem. Eng. 2022, 10, 108505. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Hosseinzadeh, A.; Zhou, J.L.; Huang, Z. Progress in the application of surface engineering methods in immobilizing TiO2 and ZnO coatings for environmental photocatalysis. Catal. Rev. 2021, 1–52. [Google Scholar] [CrossRef]
- Zinc X-ray Photoelectron Spectra, Zinc Electron Configuration, and Other Elemental Information. Available online: https://www.thermofisher.com/us/en/home/materials-science/learning-center/periodic-table/transition-metal/zinc.html (accessed on 28 October 2022).
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic degradation of methylene blue dye by porous zinc oxide nanofibers prepared via electrospinning: When defects become merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Al-Mayalee, K.H.; Saadi, N.; Badradeen, E.; Watanabe, F.; Karabacak, T. Optical and Photoconductive Response of CuO Nanostructures Grown by a Simple Hot-Water Treatment Method. J. Phys. Chem. C 2018, 122, 23312–23320. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, P.; Das, D. Morphological variations of ZnO nanostructures and its influence on the photovoltaic performance when used as photoanodes in dye sensitized solar cells. Sol. Energy Mater. Sol. Cells 2022, 243, 111811. [Google Scholar] [CrossRef]
- Wahyuono, R.A.; Hermann-Westendorf, F.; Dellith, A.; Schmidt, C.; Dellith, J.; Plentz, J.; Schulz, M.; Presselt, M.; Seyring, M.; Rettenmeyer, M.; et al. Effect of annealing on the sub-bandgap, defects and trapping states of ZnO nanostructures. Chem. Phys. 2017, 483–484, 112–121. [Google Scholar] [CrossRef]
- Taylor, C.M.; Ramirez-Canon, A.; Wenk, J.; Mattia, D. Enhancing the photo-corrosion resistance of ZnO nanowire photocatalysts. J. Hazard. Mater. 2019, 378, 120799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, Y.; Wang, C.; Xu, H.; Fang, J.; Yang, Y. Seeded growth of ZnO nanowires in dye-containing solution: The submerged plant analogy and its application in photodegradation of dye pollutants. CrystEngComm 2020, 22, 4154–4161. [Google Scholar] [CrossRef]
- Florica, C.; Costas, A.; Preda, N.; Beregoi, M.; Kuncser, A.; Apostol, N.; Popa, C.; Socol, G.; Diculescu, V.; Enculescu, I. Core-shell nanowire arrays based on ZnO and CuxO for water stable photocatalysts. Sci. Rep. 2019, 9, 17268. [Google Scholar] [CrossRef] [Green Version]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Hu, Y.-L.; Pelligra, C.; Chen, C.-H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Z.-Y.; Kuang, Q.; Xie, Z.-X.; Huang, R.-B.; Zheng, L.-S. Shape-controlled fabrication of porous ZnO architectures and their photocatalytic properties. J. Solid State Chem. 2009, 182, 115–121. [Google Scholar] [CrossRef]
- Boppella, R.; Anjaneyulu, K.; Basak, P.; Manorama, S.V. Facile Synthesis of Face Oriented ZnO Crystals: Tunable Polar Facets and Shape Induced Enhanced Photocatalytic Performance. J. Phys. Chem. C 2013, 117, 4597–4605. [Google Scholar] [CrossRef]
- Jang, E.S.; Won, J.-H.; Hwang, S.-J.; Choy, J.-H. Fine Tuning of the Face Orientation of ZnO Crystals to Optimize Their Photocatalytic Activity. Adv. Mater. 2006, 18, 3309–3312. [Google Scholar] [CrossRef]
- Song, D.M.; Li, J.C. First principles study of band gap of Cu doped ZnO single-wall nanotube modulated by impurity concentration and concentration gradient. Comput. Mater. Sci. 2012, 65, 175–181. [Google Scholar] [CrossRef]
- Yayapao, O.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Sonochemical synthesis, photocatalysis and photonic properties of 3% Ce-doped ZnO nanoneedles. Ceram. Int. 2013, 39, S563–S568. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, L.-C.; Xie, Y.; Liu, G.; Ma, X.; Cheng, H.-M. Crystallinity-dependent substitutional nitrogen doping in ZnO and its improved visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 400, 18–23. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Gupta, J.; Barick, K.C.; Bahadur, D. Defect mediated photocatalytic activity in shape-controlled ZnO nanostructures. J. Alloys Compd. 2011, 509, 6725–6730. [Google Scholar] [CrossRef]
- Zhang, Y.; Mandal, R.; Ratchford, D.C.; Anthony, R.; Yeom, J. Si Nanocrystals/ZnO Nanowires Hybrid Structures as Immobilized Photocatalysts for Photodegradation. Nanomaterials 2020, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, X.; Yeom, J. A Floatable Piezo-Photocatalytic Platform Based on Semi-Embedded ZnO Nanowire Array for High-Performance Water Decontamination. Nano-Micro Lett. 2019, 11, 11. [Google Scholar] [CrossRef]
- Suryavanshi, R.D.; Mohite, S.V.; Bagade, A.A.; Shaikh, S.K.; Thorat, J.B.; Rajpure, K.Y. Nanocrystalline immobilised ZnO photocatalyst for degradation of benzoic acid and methyl blue dye. Mater. Res. Bull. 2018, 101, 324–333. [Google Scholar] [CrossRef]
- Zhou, Q.; Wen, J.Z.; Zhao, P.; Anderson, W.A. Synthesis of Vertically-Aligned Zinc Oxide Nanowires and Their Application as a Photocatalyst. Nanomaterials 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hariharalakshmanan, R.K.; Watanabe, F.; Karabacak, T. In Situ Growth and UV Photocatalytic Effect of ZnO Nanostructures on a Zn Plate Immersed in Methylene Blue. Catalysts 2022, 12, 1657. https://doi.org/10.3390/catal12121657
Hariharalakshmanan RK, Watanabe F, Karabacak T. In Situ Growth and UV Photocatalytic Effect of ZnO Nanostructures on a Zn Plate Immersed in Methylene Blue. Catalysts. 2022; 12(12):1657. https://doi.org/10.3390/catal12121657
Chicago/Turabian StyleHariharalakshmanan, Ranjitha K., Fumiya Watanabe, and Tansel Karabacak. 2022. "In Situ Growth and UV Photocatalytic Effect of ZnO Nanostructures on a Zn Plate Immersed in Methylene Blue" Catalysts 12, no. 12: 1657. https://doi.org/10.3390/catal12121657






