Synthesis and Application of Domestic Glassy Carbon TiO2 Nanocomposite for Electrocatalytic Triclosan Detection
Abstract
1. Introduction
2. Results and Discussion
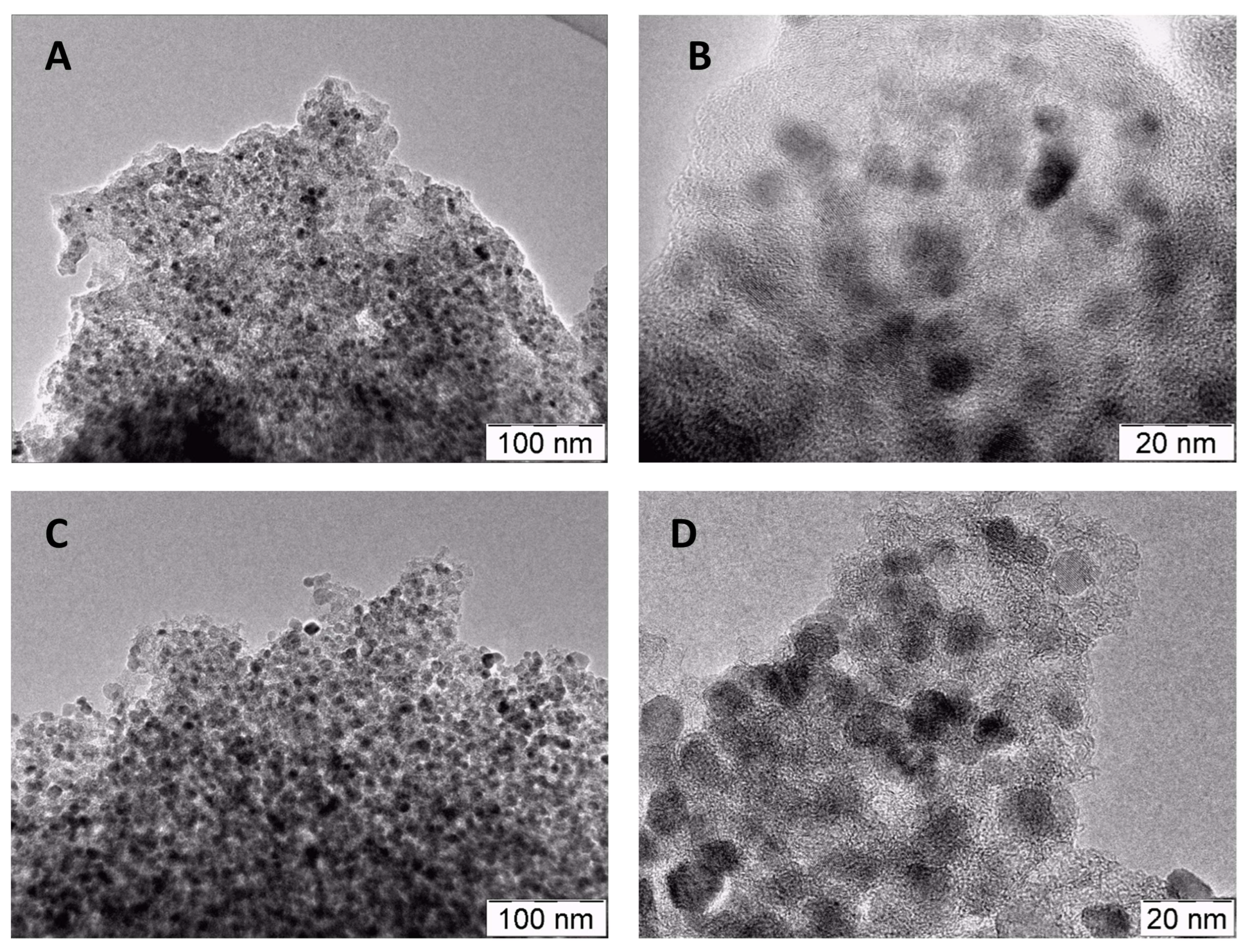
2.1. Morphological Characterization of the Materials
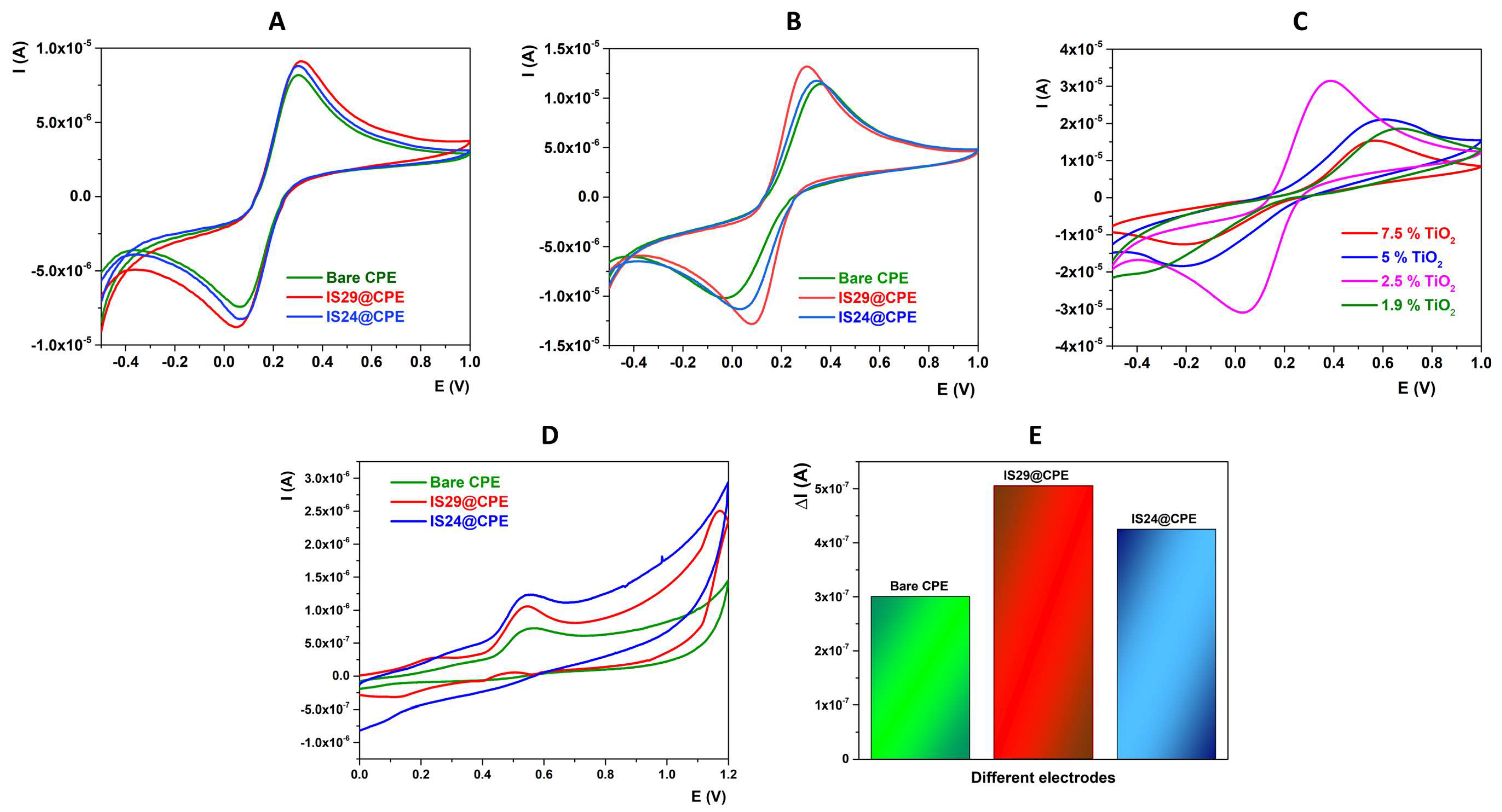
2.2. Electrochemical Characterization of the Electrodes
2.3. Optimization of Experimental Parameters
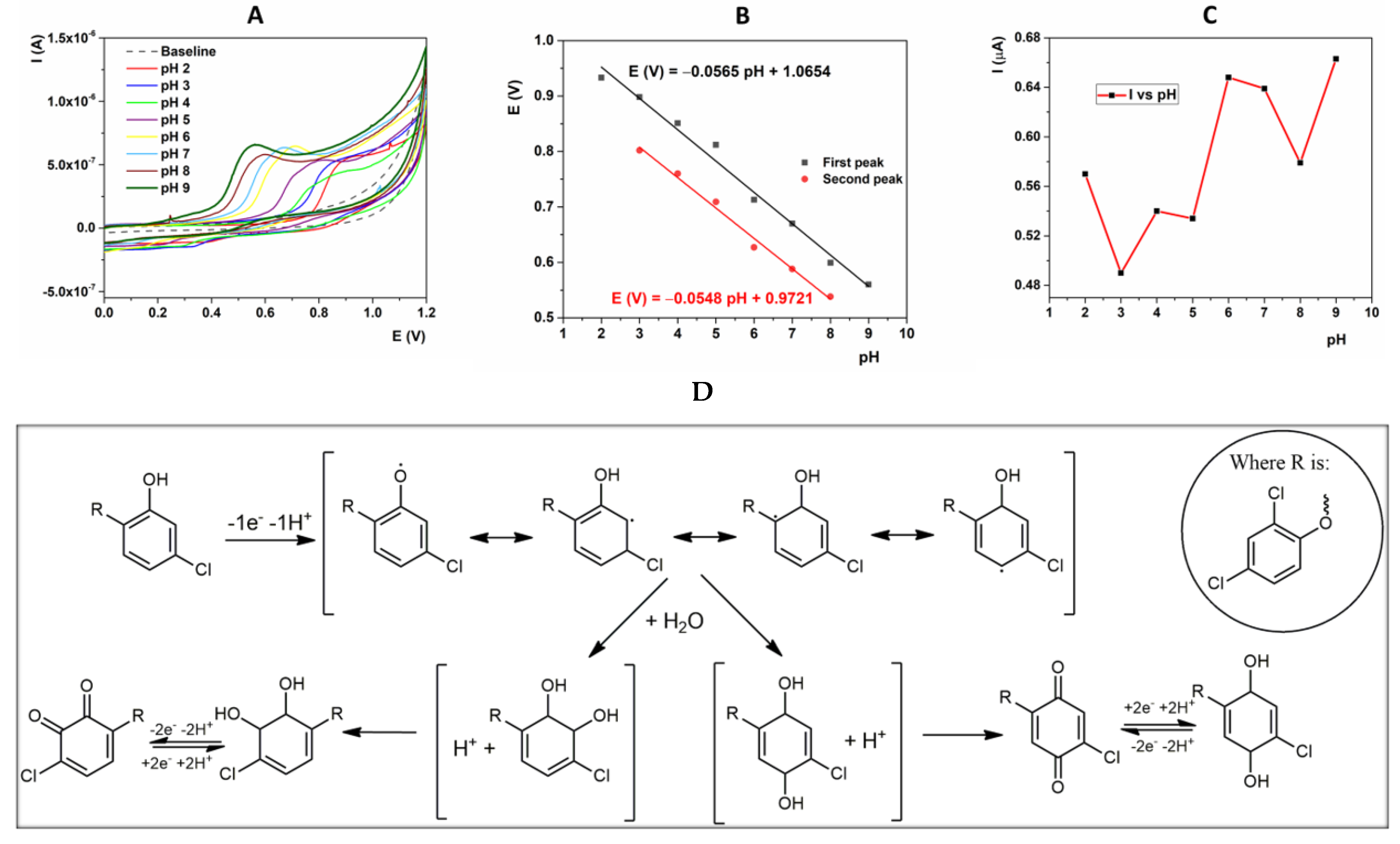
2.3.1. Effect of the pH of the Supporting Electrolyte
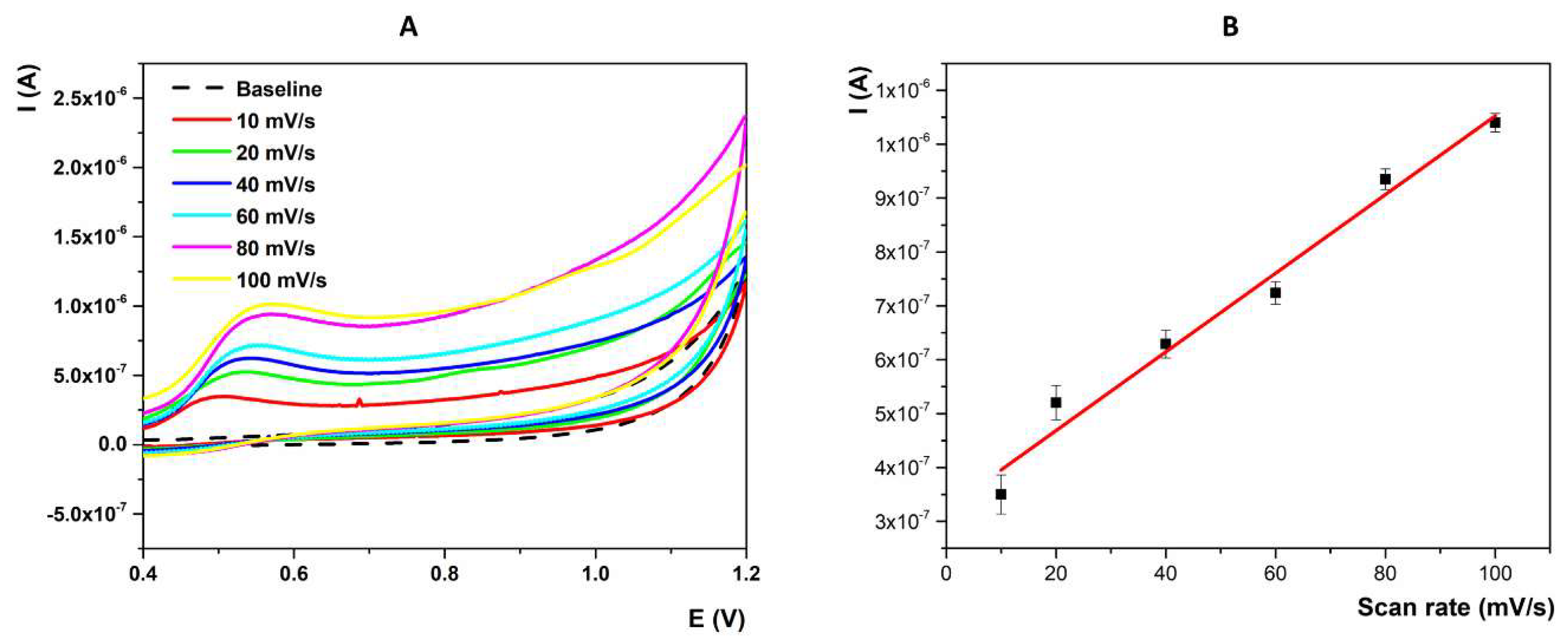
2.3.2. Nature of the Electrochemical Reaction at the Interface Electrode/Testing Solution
2.4. Analytical Parameters of the Developed Method
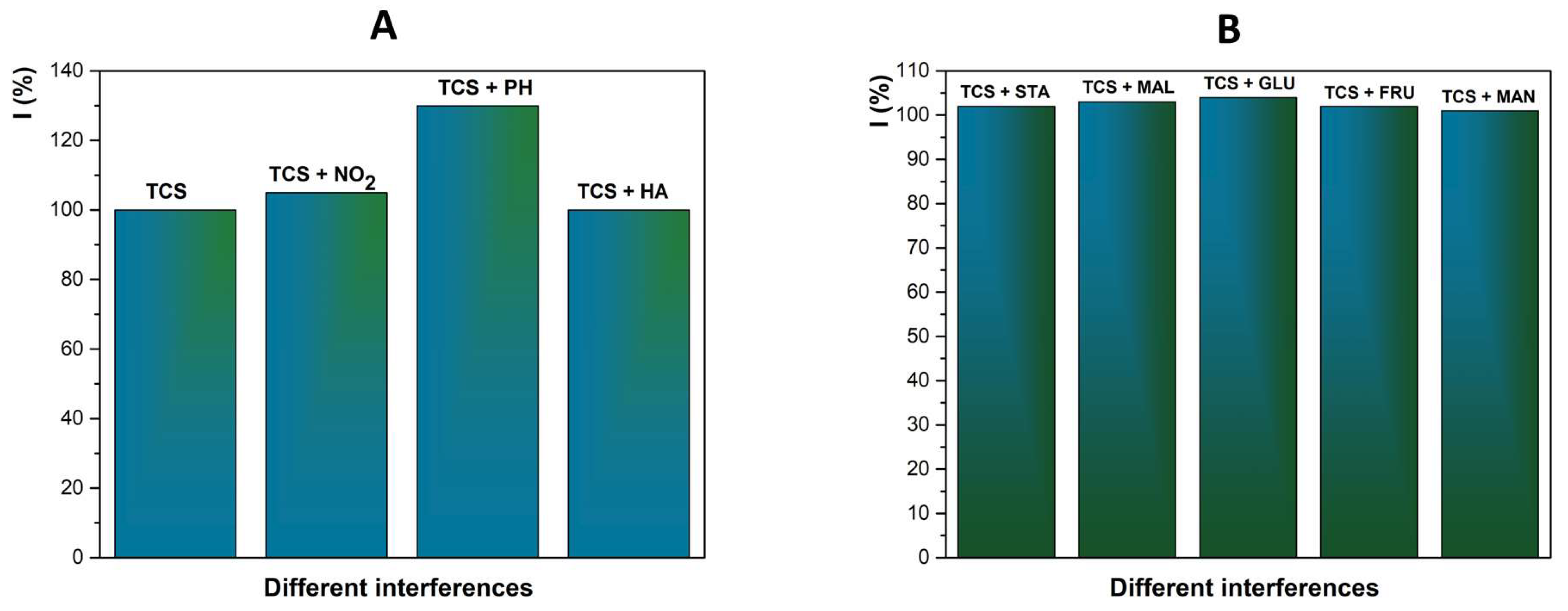
2.5. Interferences Studies
2.6. Real Sample
3. Materials and Methods
3.1. Chemicals and Apparatus
3.2. Preparation of TiO2/C
3.3. Preparation of Working Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agnihotri, A.S.; Varghese, A.; Nidhin, M. Transition metal oxides in electrochemical and bio sensing: A state-of-art review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Police, A.K.R.; Chennaiahgari, M.; Boddula, R.; Vattikuti, S.P.; Mandari, K.K.; Chan, B. Single-step hydrothermal synthesis of wrinkled graphene wrapped TiO2 nanotubes for photocatalytic hydrogen production and supercapacitor applications. Mater. Res. Bull. 2018, 98, 314–321. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Design and synthesis of ternary composite of polyaniline-sulfonated graphene oxide-TiO2 nanorods: A highly stable electrode material for supercapacitor. J. Solid State Electrochem. 2018, 22, 129–139. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Hybrid Material of PANI with TiO2-SnO2: Pseudocapacitor Electrode for Higher Performance Supercapacitors. ChemistrySelect 2017, 2, 65–73. [Google Scholar] [CrossRef]
- Taiwo, A.A.; Mustapha, S.; Oladejo, T.J.; Amoo, A.F.; Elabor, R. Occurrence, effects, detection, and photodegradation of triclosan and triclocarban in the environment: A review. Int. J. Environ. Anal. Chem. 2022, 27. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Arvand, M.; Ghodsi, N. Electrospun TiO2 nanofiber/graphite oxide modified electrode for electrochemical detection of l-DOPA in human cerebrospinal fluid. Sens. Actuators B Chem. 2014, 204, 393–401. [Google Scholar] [CrossRef]
- Chang, C.; Wang, Q.; Xue, Q.; Liu, F.; Hou, L.; Pu, S. Highly efficient detection of chloramphenicol in water using Ag and TiO2 nanoparticles modified laser-induced graphene electrode. Microchem. J. 2022, 173, 107037. [Google Scholar] [CrossRef]
- Kumar, N.; Bhadwal, A.S.; Mizaikoff, B.; Singh, S.; Kranz, C. Electrochemical detection and photocatalytic performance of MoS2/TiO2 nanocomposite against pharmaceutical contaminant: Paracetamol. Sens. Bio Sens. Res. 2019, 24, 100288. [Google Scholar] [CrossRef]
- Nasehi, P.; Moghaddam, M.S.; Rezaei-savadkouhi, N.; Alizadeh, M.; Yazdani, M.N.; Agheli, H. Monitoring of Bisphenol A in water and soft drink products using electrochemical sensor amplified with TiO2-SWCNTs and ionic liquid. Food Meas. 2022, 16, 2440–2445. [Google Scholar] [CrossRef]
- Qu, Y.; Min, H.; Wei, Y.; Xiao, F.; Shi, G.; Li, X.; Jin, L. Au–TiO2/Chit modified sensor for electrochemical detection of trace organophosphates insecticides. Talanta 2008, 76, 758–762. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Z.; Wang, X.; Yin, Z.; Tan, X.; Zhao, J. Facile preparation of MnO2–TiO2 nanotube arrays composite electrode for electrochemical detection of hydrogen peroxide. Talanta 2022, 244, 123407. [Google Scholar] [CrossRef]
- Azer, B.B.; Gulsaran, A.; Pennings, J.R.; Saritas, R.; Kocer, S.; Bennett, J.L.; Abhang, Y.D.; Pope, M.A.; Abdel-Rahman, E.; Yavuz, M. A Review: TiO2 based photoelectrocatalytic chemical oxygen demand sensors and their usage in industrial applications. J. Electroanal. Chem. 2022, 918, 116466. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Guaraldo, T.T.; Zanoni, M.V.B. Enhancement of Photoelectrocatalysis Efficiency by Using Nanostructured Electrodes. In Modern Electrochemical Methods in Nano, Surface and Corrosion Science; Aliofkhazraei, M., Ed.; InTechOpen: London, UK, 2014; ISBN 978-953-51-1586-1. [Google Scholar]
- Carp, O. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Miszczak, S.; Pietrzyk, B. Anatase–rutile transformation of TiO2 sol–gel coatings deposited on different substrates. Ceram. Int. 2015, 41, 7461–7465. [Google Scholar] [CrossRef]
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: London, UK, 2013; ISBN 978-1-4471-5060-2. [Google Scholar]
- Cui, A.; Ren, P.; Bai, Y.; Yu, H.; Meng, H. Nanoparticle size effect of Pt and TiO2 anatase/rutile phases “volcano-type” curve for HOR electrocatalytic activity at Pt/TiO2-CNx nanocatalysts. Appl. Surf. Sci. 2022, 584, 152644. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kowalczyk, Ł.; Tryba, B.; Kapica-Kozar, J.; Morawski, A.W. The Photocatalytic Performance of Benzene- Modified TiO2 Photocatalysts under UV-vis Light Irradiation. J. Adv. Oxid. Technol. 2015, 18, 204–210. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Zhang, Y.; Chen, G.; Hong, Z. One-step solvothermal preparation of TiO2/C composites and their visible-light photocatalytic activities. Appl. Surf. Sci. 2008, 254, 3762–3766. [Google Scholar] [CrossRef]
- Zakharchuk, I.; Komlev, A.A.; Soboleva, E.; Makarova, T.L.; Zherebtsov, D.A.; Galimov, D.M.; Lähderanta, E. Paramagnetic anatase titania/carbon nanocomposites. J. Nanophoton. 2017, 11, 32505. [Google Scholar] [CrossRef]
- Zherebtsov, D.A.; Galimov, D.M.; Dyachuk, V.V.; Mikhailov, G.G.; Uchaev, D.A.; Sergeeva, S.A. One-Pot Synthesis of Anatase/Carbon Nanocomposite. J. Nanoelectron. Optoelectron. 2013, 8, 221–222. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health Part B 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Allmyr, M.; Adolfsson-Erici, M.; McLachlan, M.S.; Sandborgh-Englund, G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 2006, 372, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.; Magalhâes-Mota, G.; Pires, F.; Sério, S.; Ribeiro, P.A.; Raposo, M. Detection of traces of triclosan in water. Appl. Surf. Sci. 2017, 421, 142–147. [Google Scholar] [CrossRef]
- Adolfsson-Erici, M.; Pettersson, M.; Parkkonen, J.; Sturve, J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 46, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Determining the distribution of triclosan and methyl triclosan in estuarine settings. Chemosphere 2014, 95, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peldszus, S.; Huck, P.M. Optimizing gas chromatographic–mass spectrometric analysis of selected pharmaceuticals and endocrine-disrupting substances in water using factorial experimental design. J. Chromatogr. A 2007, 1148, 65–77. [Google Scholar] [CrossRef]
- Hua, W.; Bennett, E.; Letcher, R. Triclosan in waste and surface waters from the upper Detroit River by liquid chromatography-electrospray-tandem quadrupole mass spectrometry. Environ. Int. 2005, 31, 621–630. [Google Scholar] [CrossRef]
- Liu, T.; Wu, D. High-performance liquid chromatographic determination of triclosan and triclocarban in cosmetic products. Int J. Cosmet. Sci. 2012, 34, 489–494. [Google Scholar] [CrossRef]
- Piccoli, A.; Fiori, J.; Andrisano, V.; Orioli, M. Determination of triclosan in personal health care products by liquid chromatography (HPLC). Il Farm. 2002, 57, 369–372. [Google Scholar] [CrossRef]
- Jevtić, S.; Stefanović, A.; Stanković, D.M.; Pergal, M.V.; Ivanović, A.T.; Jokić, A.; Petković, B.B. Boron-doped diamond electrode—A prestigious unmodified carbon electrode for simple and fast determination of bentazone in river water samples. Diam. Relat. Mater. 2018, 81, 133–137. [Google Scholar] [CrossRef]
- Knežević, S.; Ognjanović, M.; Dojčinović, B.; Antić, B.; Vranješ-Đurić, S.; Manojlović, D.; Stanković, D.M. Sensing Platform Based on Carbon Paste Electrode Modified with Bismuth Oxide Nanoparticles and SWCNT for Submicromolar Quantification of Honokiol. Food Anal. Methods 2022, 15, 856–867. [Google Scholar] [CrossRef]
- Petković, B.B.; Ognjanović, M.; Antić, B.; Viktorovich Avdin, V.; Manojlović, D.D.; Đurić, S.V.; Stanković, D.M. Easily Prepared CO3O4 Doped Porous Carbon Material Decorated with Single-wall Carbon Nanotubes Applied in Voltammetric Sensing of Antioxidant α-lipoic Acid. Electroanalysis 2021, 33, 446–454. [Google Scholar] [CrossRef]
- Stanković, D.M.; Milanović, Z.; Švorc, Ľ.; Stanković, V.; Janković, D.; Mirković, M.; Đurić, S.V. Screen printed diamond electrode as efficient “point-of-care” platform for submicromolar determination of cytostatic drug in biological fluids and pharmaceutical product. Diam. Relat. Mater. 2021, 113, 108277. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, A.; Jin, X.; Yu, H.; Zhang, Y.; Xia, S.; Liu, Z.; Zhang, G.; Dionysiou, D.D. Nitrogen-doped hollow carbon nanospheres as highly efficient electrocatalysts for detection of triclosan. J. Environ. Chem. Eng. 2021, 9, 106022. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.; Pollet, B.G.; Kalanur, S.S.; Shetti, N.P. Preparation and performance of WO3/rGO modified carbon sensor for enhanced electrochemical detection of triclosan. Electrochim. Acta 2022, 429, 141010. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Zhang, X.; Wu, K. Electrochemical Sensor for Rapid Detection of Triclosan Using a Multiwall Carbon Nanotube Film. J. Agric. Food Chem. 2009, 57, 9403–9407. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, Q. A facial electrochemical method for efficient triclosan detection constructed on dodecanethiol monolayers functioned Au nanoparticles-ErGO. Microchem. J. 2022, 175, 107144. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Koo, M.S.; Cho, K.; Yoon, J.; Choi, W. Photoelectrochemical Degradation of Organic Compounds Coupled with Molecular Hydrogen Generation Using Electrochromic TiO2 Nanotube Arrays. Environ. Sci. Technol. 2017, 51, 6590–6598. [Google Scholar] [CrossRef]
- Yildiz, A.; Lisesivdin, S.B.; Kasap, M.; Mardare, D. Electrical properties of TiO2 thin films. J. Non Cryst. Solids 2008, 354, 4944–4947. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Chen, Z.; Zuo, X. Innovative Electrochemical Sensor Using TiO2 Nanomaterials to Detect Phosphopeptides. Anal. Chem. 2021, 93, 10635–10643. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.K.; Shankar, S.S.; Sajina, N.; Padinjareveetil, A.K.; Pilankatta, R.; Kumar, V.B.S.; Mathew, B.; George, B.; Makvandi, P.; Černík, M.; et al. Fabrication of a Greener TiO2@Gum Arabic-Carbon Paste Electrode for the Electrochemical Detection of Pb2+ Ions in Plastic Toys. ACS Omega 2020, 5, 25390–25399. [Google Scholar] [CrossRef] [PubMed]
- Bertel, L.; Miranda, D.A.; García-Martín, J.M. Nanostructured Titanium Dioxide Surfaces for Electrochemical Biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, M.; Maulidiyah, M.; Salim, O.A.L.; Muzakkar, M.Z.; Umar, A.A. High performance cypermethrin pesticide detection using anatase TiO2-carbon paste nanocomposites electrode. Microchem. J. 2019, 145, 756–761. [Google Scholar] [CrossRef]
- Kear, G.; Walsh, F.C. The characteristics of a true tafel slope. Corros. Mater. 2005, 30, S1–S4. [Google Scholar]
- Silva, E.; Lopes, I.; Bruzaca, E.; Carvalho, P.A.; Tanaka, A.A. Triclosan: Electrochemistry, Spontaneous Degradation and Effects on Double-Stranded DNA. Braz. J. Anal. Chem. 2021, 8, 88–102. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315275161. [Google Scholar]
- Knežević, S.; Ostojić, J.; Ognjanović, M.; Savić, S.; Kovačević, A.; Manojlović, D.; Stanković, V.; Stanković, D. The environmentally friendly approaches based on the heterojunction interface of the LaFeO3/Fe2O3@g-C3N4 composite for the disposable and laboratory sensing of triclosan. Sci. Total Environ. 2023, 857, 159250. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Tian, Q.; Lun, L.; Zhao, P.; Yang, X.; Shen, J.; Jiang, B.; Zhou, Y.; Zhou, T. Application of carbon nanotubes and zwitterionic surfactant-modified acetylene black electrode for the determination of triclosan in household commodities. Int. J. Environ. Anal. Chem. 2022, 102, 987–1000. [Google Scholar] [CrossRef]
- Teixeira, P.R.; Machado, T.R.; Machado, F.; Sodré, F.F.; Silva, J.G.; Neto, B.A.D.; Paterno, L.G. Au nanoparticle-poly(ionic liquid) nanocomposite electrode for the voltammetric detection of triclosan in lake water and toothpaste samples. Microchem. J. 2020, 152, 104421. [Google Scholar] [CrossRef]
- Luyen, N.D.; Toan, T.T.T.; Trang, H.T.; Nguyen, V.T.; van Son, T.; Thanh, T.S.; Thanh, N.M.; Quy, P.T.; Khieu, D.Q. Electrochemical Determination of Triclosan Using ZIF-11/Activated Carbon Derived from the Rice Husk Modified Electrode. J. Nanomater. 2021, 2021, 8486962. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; He, Y.; Yan, Y.; Zhou, W.; Zhang, G.; Liu, D.; Ye, Z.; Qiu, F. An Electrochemical Sensor Based on Molecularly-Imprinted-Polymer-Modified Carbon Quantum Dots@hexagonal Boron Nitride Nanosheets Nanocomposites for Triclosan Determination. ChemistrySelect 2022, 7, e202201141. [Google Scholar] [CrossRef]




| Electrode | Linearity Range (µM) | Detection Limit (µM) | References |
|---|---|---|---|
| IS29@CPE | 0.1–15 | 0.07 | This work |
| N-HC-modified GCE | 0.09 to 20 | 0.03 | [36] |
| LaFeO3/Fe2O3@g-C3N4/SPE | 0.3–7 | 0.09 | [50] |
| AB/CNT/SB16 | 0.01–2 | 0.0062 | [51] |
| ITO/(Au-PIL/PDAC) | 10.0–60.0 | 0.098 | [52] |
| ZIF-11/activated carbon derived from rice husk | 0.1–8 | 0.076 | [53] |
| MIP-CQDs@HBNNS-NCs/GCE | 2 × 10−3–100 | 0.005 | [54] |
| Sample No. | Found (µM) | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0.00 | 1.00 | 1.05 | 105 |
| 2 | 0.00 | 3.00 | 3.08 | 103 |
| 3 | 0.00 | 5.00 | 4.93 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanković, V.; Manojlović, D.; Roglić, G.M.; Tolstoguzov, D.S.; Zherebtsov, D.A.; Uchaev, D.A.; Avdin, V.V.; Stanković, D.M. Synthesis and Application of Domestic Glassy Carbon TiO2 Nanocomposite for Electrocatalytic Triclosan Detection. Catalysts 2022, 12, 1571. https://doi.org/10.3390/catal12121571
Stanković V, Manojlović D, Roglić GM, Tolstoguzov DS, Zherebtsov DA, Uchaev DA, Avdin VV, Stanković DM. Synthesis and Application of Domestic Glassy Carbon TiO2 Nanocomposite for Electrocatalytic Triclosan Detection. Catalysts. 2022; 12(12):1571. https://doi.org/10.3390/catal12121571
Chicago/Turabian StyleStanković, Vesna, Dragan Manojlović, Goran M. Roglić, Dmitry S. Tolstoguzov, Dmitry A. Zherebtsov, Daniel A. Uchaev, Viacheslav V. Avdin, and Dalibor M. Stanković. 2022. "Synthesis and Application of Domestic Glassy Carbon TiO2 Nanocomposite for Electrocatalytic Triclosan Detection" Catalysts 12, no. 12: 1571. https://doi.org/10.3390/catal12121571
APA StyleStanković, V., Manojlović, D., Roglić, G. M., Tolstoguzov, D. S., Zherebtsov, D. A., Uchaev, D. A., Avdin, V. V., & Stanković, D. M. (2022). Synthesis and Application of Domestic Glassy Carbon TiO2 Nanocomposite for Electrocatalytic Triclosan Detection. Catalysts, 12(12), 1571. https://doi.org/10.3390/catal12121571








