Unraveling the Effect of MgAl/CuO Nanothermite on the Characteristics and Thermo-Catalytic Decomposition of Nanoenergetic Formulation Based on Nanostructured Nitrocellulose and Hydrazinium Nitro-Triazolone
Abstract
:1. Introduction
2. Results
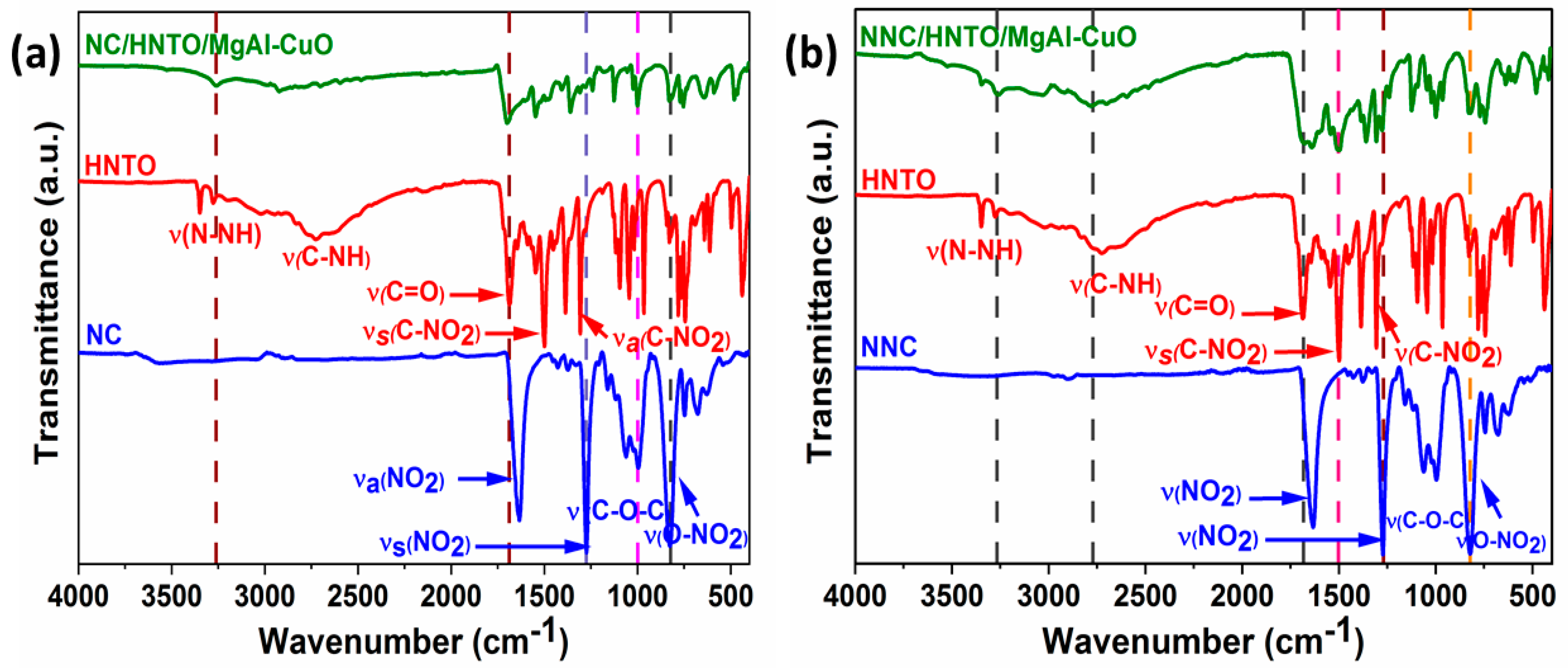
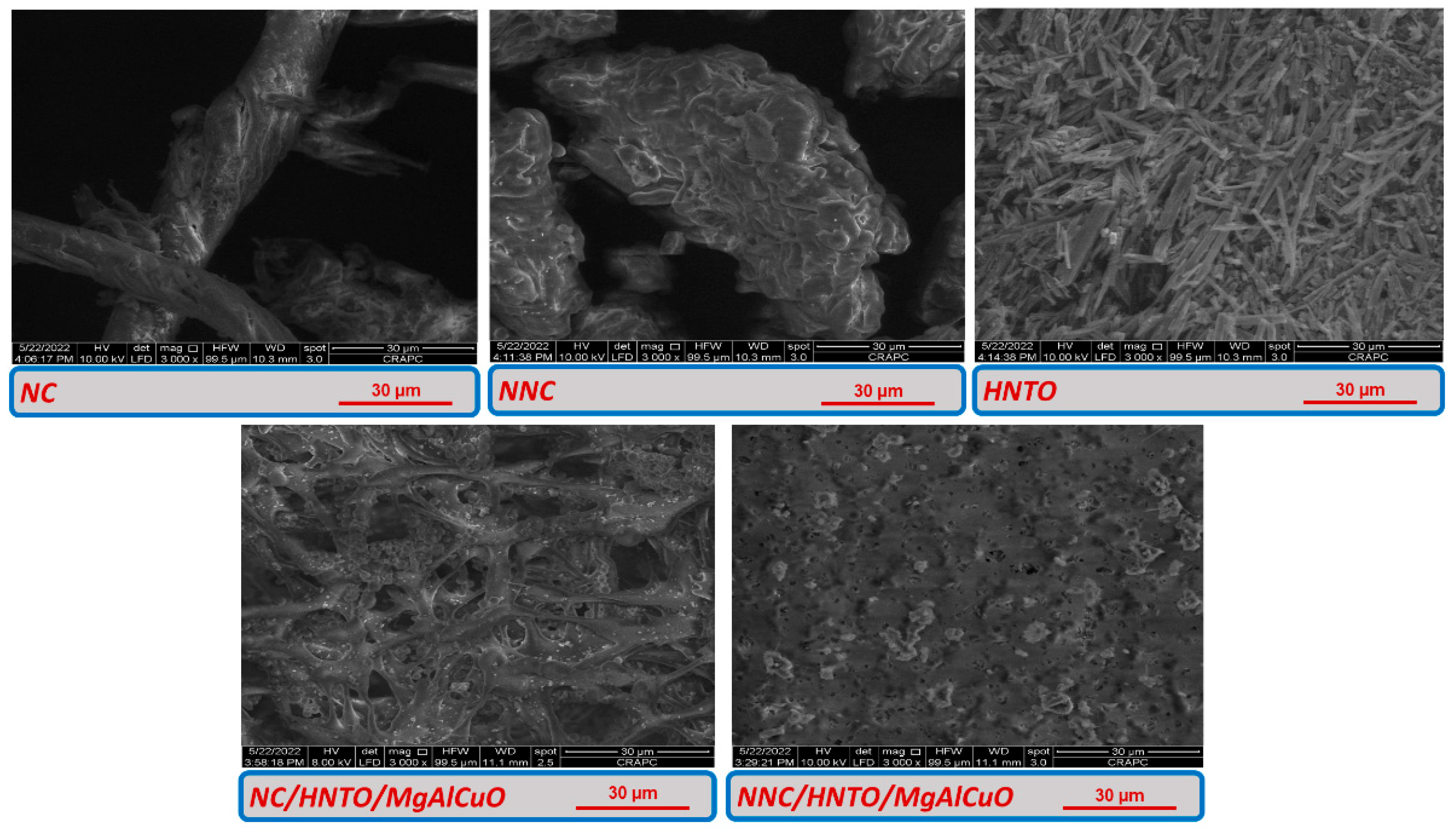
2.1. Chemical Structure and Morphology
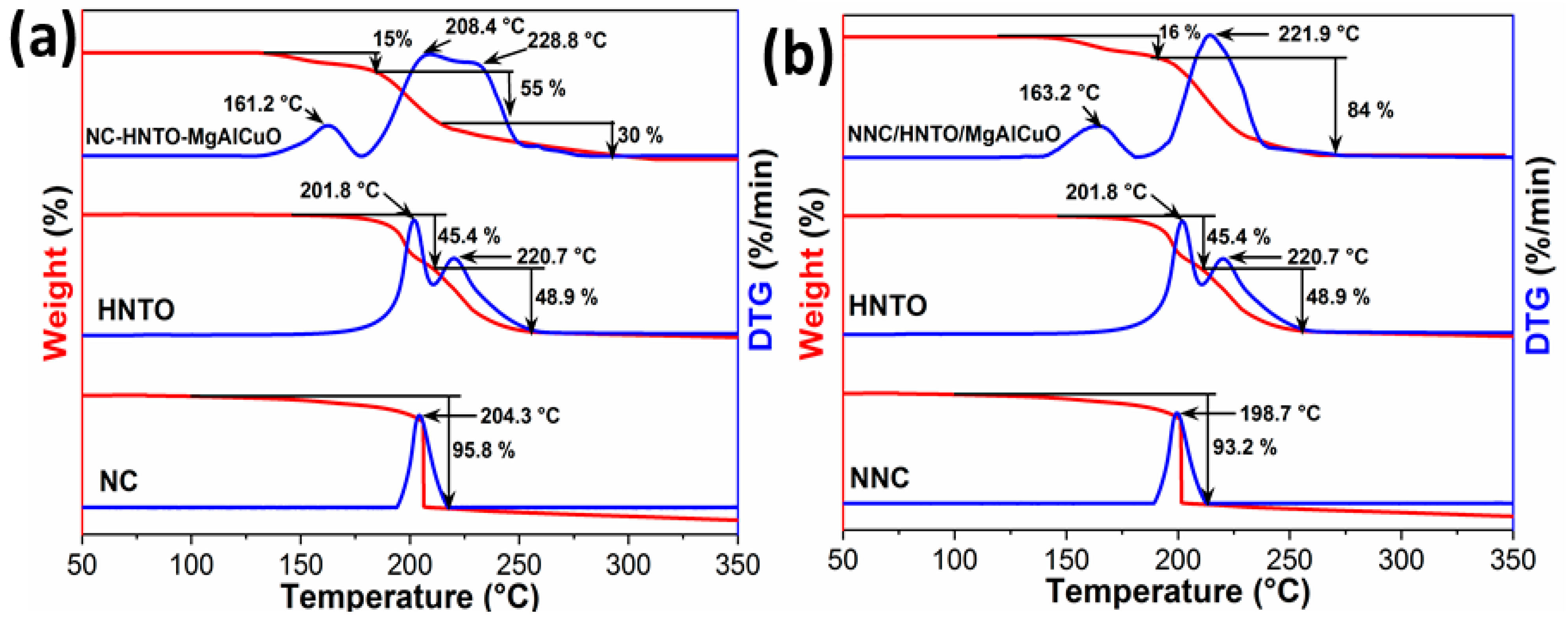
2.2. TGA Assessment
2.3. DSC Characterization
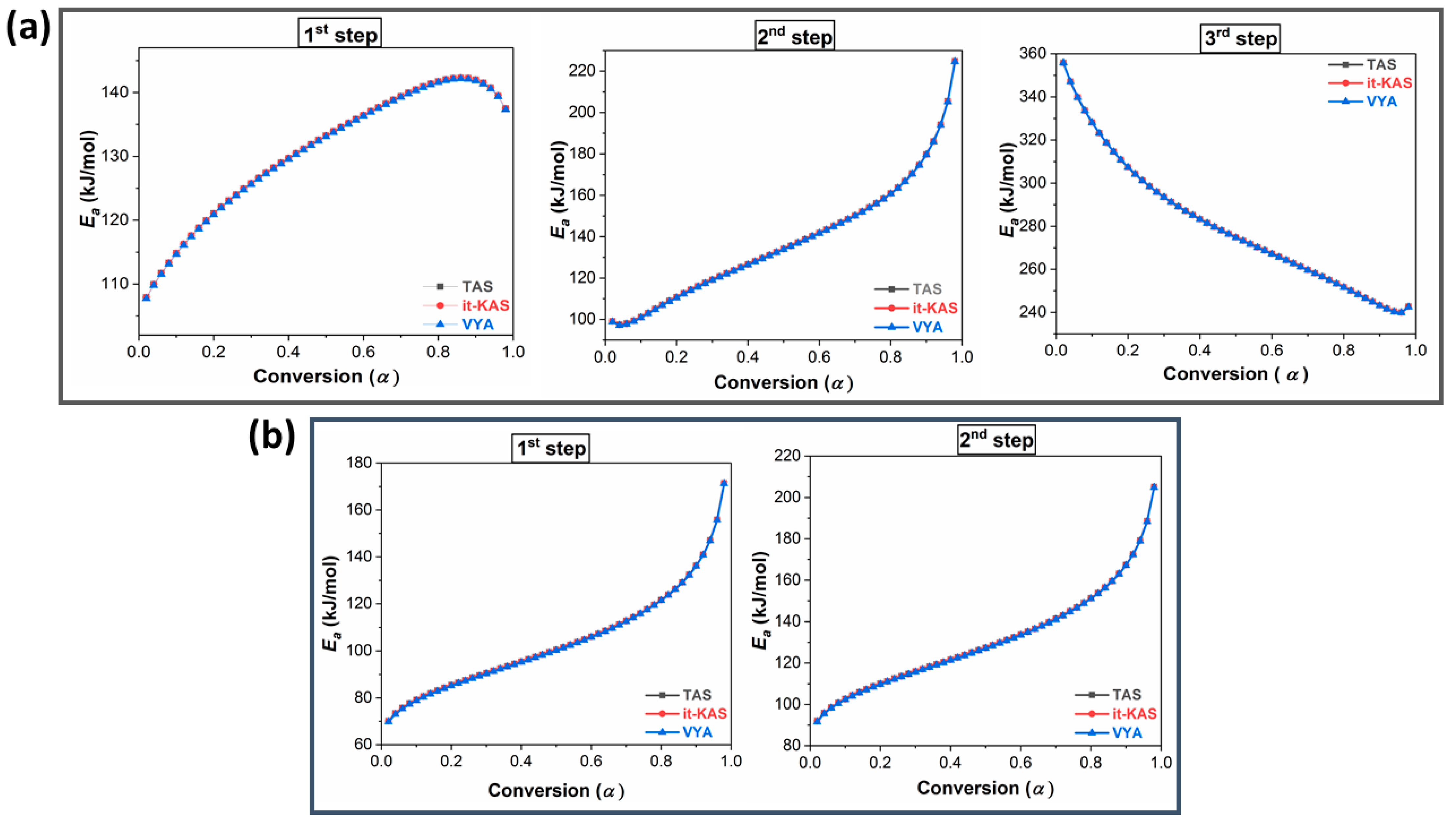
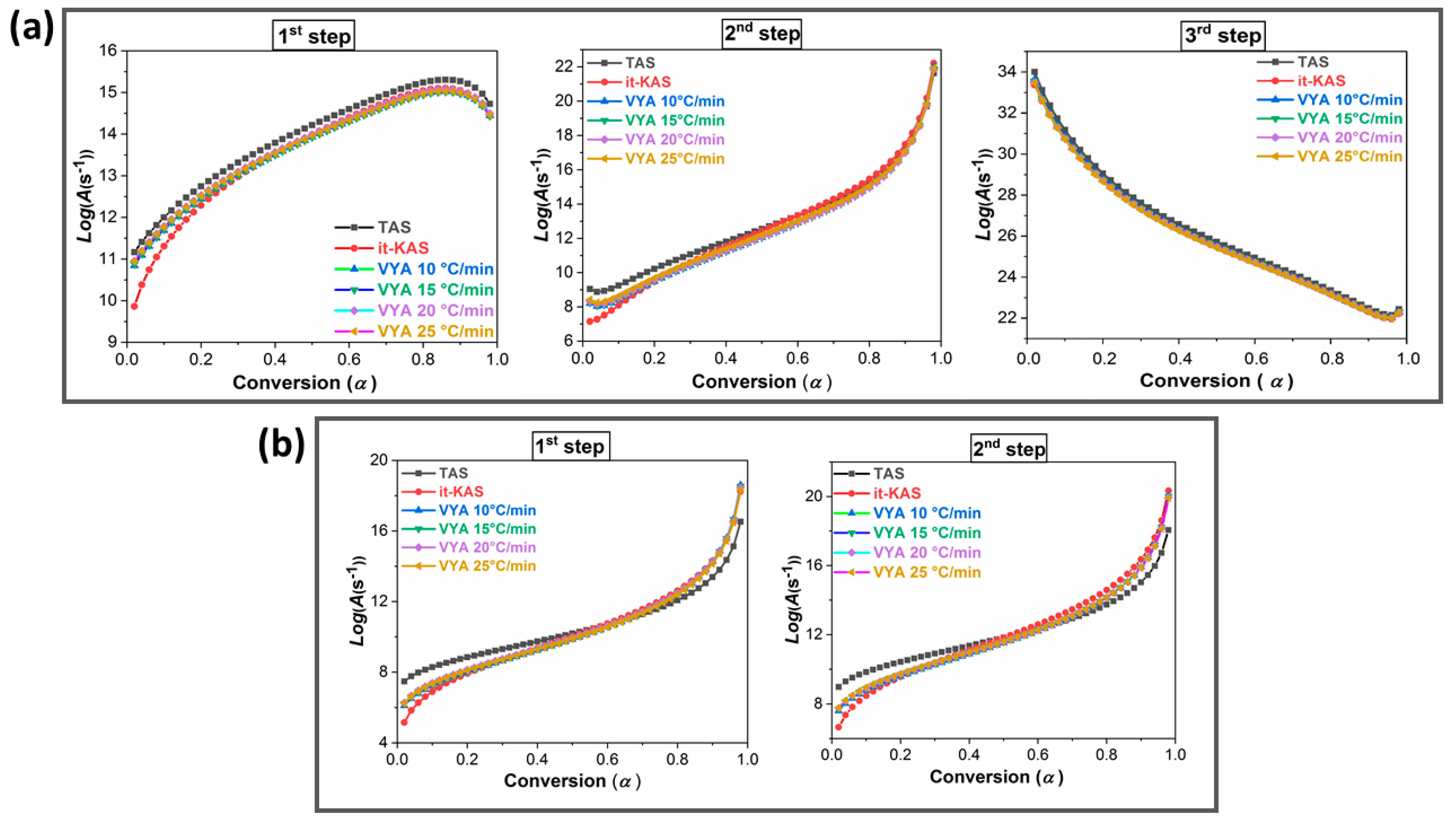
2.4. Determination of the Decomposition Kinetic Parameters
3. Experimental Section
3.1. Materials
3.2. Preparation of the Energetic Formulations
3.3. Characterization Techniques
3.4. Kinetic Decomposition Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trache, D.; DeLuca, L.T. Nanoenergetic Materials: Preparation, Properties, and Applications. Nanomaterials 2020, 10, 2347. [Google Scholar] [CrossRef] [PubMed]
- Kabra, S.; Gharde, S.; Gore, P.M.; Jain, S.; Khire, V.H.; Kandasubramanian, B. Recent trends in nanothermites: Fabrication, characteristics and applications. Nano Express 2020, 1, 032001. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F. Differentiation of stabilized nitrocellulose during artificial aging: Spectroscopy methods coupled with principal component analysis. J. Chemom. 2019, 33, e3163. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitrocellulose synthesis from miscanthus cellulose. Propellants Explos. Pyrotech. 2018, 43, 96–100. [Google Scholar] [CrossRef]
- Okada, K.; Saito, Y.; Akiyoshi, M.; Endo, T.; Matsunaga, T. Preparation and characterization of nitrocellulose nanofiber. Propellants Explos. Pyrotech. 2021, 46, 962–968. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Derradji, M.; Bessa, W.; Belgacemi, R. Cellulose nanoparticles: Extractions. In Cellulose Nanoparticles: Chemistry and Fundamentals; Royal Society of Chemistry: London, UK, 2021; pp. 113–148. [Google Scholar]
- Rani, A.; Reddy, R.; Sharma, U.; Mukherjee, P.; Mishra, P.; Kuila, A.; Sim, L.C.; Saravanan, P. A review on the progress of nanostructure materials for energy harnessing and environmental remediation. J. Nanostructure Chem. 2018, 8, 255–291. [Google Scholar] [CrossRef] [Green Version]
- Tarchoun, A.F.; Sayah, Z.B.D.; Trache, D.; Klapötke, T.M.; Belmerabt, M.; Abdelaziz, A.; Bekhouche, S. Towards investigating the characteristics and thermal kinetic behavior of emergent nanostructured nitrocellulose prepared using various sulfonitric media. J. Nanostructure Chem. 2022, 12, 963–977. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Kohsari, I.; Zandavar, H.; Foroutan Koudehi, M.; Mirsadeghi, S. Electrospinning and thermal characterization of nitrocellulose nanofibers containing a composite of diaminofurazan, aluminum nano-powder and iron oxide nanoparticles. Cellulose 2019, 26, 4405–4415. [Google Scholar] [CrossRef]
- Rozumov, E. Recent Advances in Gun Propellant Development: From Molecules to Materials. Energetic Mater. 2017, 25, 23–65. [Google Scholar]
- Ye, B.; An, C.; Wang, J.; Li, H.; Ji, W.; Gao, K. Preparation and characterization of RDX-based composite with glycidyl azide polymers and nitrocellulose. J. Propuls. Power 2016, 32, 1036–1040. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Li, X.; An, C. Preparation and properties of HMX/nitrocellulose nanocomposites. J. Propuls. Power 2015, 31, 757–761. [Google Scholar] [CrossRef]
- Trache, D.; Klapötke, T.M.; Maiz, L.; Abd-Elghany, M.; DeLuca, L.T. Recent advances in new oxidizers for solid rocket propulsion. Green Chem. 2017, 19, 4711–4736. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, H.; Pan, X.; Hua, M.; Jiang, J. Analysis and characterization of nitrocellulose as binder optimized by 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide. J. Therm. Anal. Calorim. 2021, 143, 113–126. [Google Scholar] [CrossRef]
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Solid Propellant Formulations: A Review of Recent Progress and Utilized Components. Materials 2021, 14, 6657. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, C.; Wang, Y.; Xu, W.; Xu, J.; Shen, Y.; Zhang, W.; Ye, Y.; Shen, R. From nanoparticles to on-chip 3D nanothermite: Electrospray deposition of reactive Al/CuO@ NC onto semiconductor bridge and its application for rapid ignition. Nanotechnology 2020, 31, 195712. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, W.; Wang, X.; Shen, F. Nitrification Progress of Nitrogen-Rich Heterocyclic Energetic Compounds: A Review. Molecules 2022, 27, 1465. [Google Scholar] [CrossRef]
- Srinivas, D.; Ghule, V.D.; Muralidharan, K.; Jenkins, H.D.B. Tetraanionic Nitrogen-Rich Tetrazole-Based Energetic Salts. Chem.-Asian J. 2013, 8, 1023–1028. [Google Scholar] [CrossRef]
- Yi, J.-H.; Zhao, F.-Q.; Gao, H.-X.; Xu, S.-Y.; Wang, M.-C. Preparation, characterization, non-isothermal reaction kinetics, thermodynamic properties, and safety performances of high nitrogen compound: Hydrazine 3-nitro-1,2,4-triazol-5-one complex. J. Hazard. Mater. 2008, 153, 261–268. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Tarchoun, A.F.; Boukeciat, H.; Trache, D. Insight into the Thermodynamic Properties of Promising Energetic HNTO· AN Co-Crystal: Heat Capacity, Combustion Energy, and Formation Enthalpy. Energies 2022, 15, 6722. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Gao, H.; Fu, W.; Li, Y.; Tang, L.; Zhou, Z. Promising hydrazinium 3-Nitro-1, 2, 4-triazol-5-one and its analogs. J. Mater. Sci. 2016, 51, 10849–10862. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly reactive metastable intermixed composites (MICs): Preparation and characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, G.; Shen, L.; Luo, Y. Application of Al/B/Fe2O3 nano thermite in composite solid propellant. Bull. Chem. React. Eng. Catal. 2016, 11, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhou, X.; Xu, J.; Ma, X.; Ye, Y.; Yang, G.; Zhang, K. In situ preparation of explosive embedded CuO/Al/CL20 nanoenergetic composite with enhanced reactivity. Chem. Eng. J. 2018, 354, 885–895. [Google Scholar] [CrossRef]
- Baer, M. Modeling heterogeneous energetic materials at the mesoscale. Thermochim. Acta 2002, 384, 351–367. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sullivan, K.; Piekiel, N.; Zhou, L.; Zachariah, M.R. Diffusive vs explosive reaction at the nanoscale. J. Phys. Chem. C 2010, 114, 9191–9195. [Google Scholar] [CrossRef]
- Yao, E.; Zhao, N.; Qin, Z.; Ma, H.; Li, H.; Xu, S.; An, T.; Yi, J.; Zhao, F. Thermal decomposition behavior and thermal safety of nitrocellulose with different shape CuO and Al/CuO nanothermites. Nanomaterials 2020, 10, 725. [Google Scholar] [CrossRef] [Green Version]
- Dolgachev, V.; Khaneft, A.; Mitrofanov, A. Ignition of organic explosive materials by a copper oxide film absorbing a laser pulse. Propellants Explos. Pyrotech. 2018, 43, 992–998. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K.; Mezroua, A.; Benziane, M. Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J. Therm. Anal. Calorim. 2016, 124, 1485–1496. [Google Scholar] [CrossRef]
- Dave, P.N.; Thakkar, R.; Sirach, R.; Chaturvedi, S. Effect of copper ferrite (CuFe2O4) in the thermal decomposition of modified nitrotriazolone. Mater. Adv. 2022, 3, 5019–5026. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Chelouche, S.; Derradji, M.; Bessa, W.; Mezroua, A. A promising energetic polymer from Posidonia oceanica brown algae: Synthesis, characterization, and kinetic modeling. Macromol. Chem. Phys. 2019, 220, 1900358. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D. Green synthesis of CuO nanoparticles using Malva sylvestris leaf extract with different copper precursors and their effect on nitrocellulose thermal behavior. J. Therm. Anal. Calorim. 2022, 147, 1–16. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Yan, S.; DeLisio, J.B.; Huang, C.; Zachariah, M.R. Electrospray formation of gelled nano-aluminum microspheres with superior reactivity. ACS Appl. Mater. Interfaces 2013, 5, 6797–6801. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, Z.; Luo, Y.; Ge, Z.; Du, F.; Chen, S.; Sun, J. Fabrication and properties of glycidyl azide polymer-modified nitrocellulose spherical powders. J. Therm. Anal. Calorim. 2017, 129, 1555–1562. [Google Scholar] [CrossRef]
- Yi, J.-H.; Zhao, F.-Q.; Ren, Y.-H.; Xu, S.-Y.; Ma, H.-X.; Hu, R.-Z. Thermal decomposition mechanism and quantum chemical investigation of hydrazine 3-nitro-1,2,4-triazol-5-one (HNTO). J. Therm. Anal. Calorim. 2010, 100, 623–627. [Google Scholar] [CrossRef]
- Chen, L.; He, W.; Liu, J. Safe fabrication, thermal decomposition kinetics, and mechanism of nanoenergetic composite NBC/CL-20. ACS Omega 2020, 5, 31407–31416. [Google Scholar] [CrossRef] [PubMed]
- Mezroua, A.; Hamada, R.A.; Brahmine, K.S.; Abdelaziz, A.; Tarchoun, A.F.; Boukeciat, H.; Bekhouche, S.; Bessa, W.; Benhammada, A.; Trache, D. Unraveling the role of ammonium perchlorate on the thermal decomposition behavior and kinetics of NC/DEGDN energetic composite. Thermochim. Acta 2022, 716, 179305. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D.; Kesraoui, M.; Chelouche, S. Hydrothermal synthesis of hematite nanoparticles decorated on carbon mesospheres and their synergetic action on the thermal decomposition of nitrocellulose. Nanomaterials 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Slimani, K.; Belouettar, B.e.; Abdelaziz, A.; Bekhouche, S.; Bessa, W. Valorization of esparto grass cellulosic derivatives for the development of promising energetic azidodeoxy biopolymers: Synthesis, characterization and isoconversional thermal kinetic analysis. Propellants Explos. Pyrotech. 2022, 47, e202100293. [Google Scholar] [CrossRef]
- Dobrynin, O.S.; Zharkov, M.N.; Kuchurov, I.V.; Fomenkov, I.V.; Zlotin, S.G.; Monogarov, K.A.; Meerov, D.B.; Pivkina, A.N.; Muravyev, N.V. Supercritical antisolvent processing of nitrocellulose: Downscaling to nanosize, reducing friction sensitivity and introducing burning rate catalyst. Nanomaterials 2019, 9, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, N.; Ma, H.; Yao, E.; Yu, Z.; An, T.; Zhao, F.; Yu, X. Influence of tailored CuO and Al/CuO nanothermites on the thermocatalytic degradation of nitrocellulose and combustion performance of AP/HTPB composite propellant. Cellulose 2021, 28, 8671–8691. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D. Thermal decomposition of energetic materials using TG-FTIR and TG-MS: A state-of-the-art review. Appl. Spectrosc. Rev. 2020, 55, 724–777. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Harrat, A.; Boukecha, W.O.; Hamouche, M.A.; Boukeciat, H.; Dourari, M. Elaboration, Characterization and Thermal Decomposition Kinetics of New Nanoenergetic Composite Based on Hydrazine 3-Nitro-1,2,4-triazol-5-one and Nanostructured Cellulose Nitrate. Molecules 2022, 27, 6945. [Google Scholar] [CrossRef] [PubMed]
- Elbasuney, S.; Yehia, M.; Hamed, A.; Mokhtar, M.; Gobara, M.; Saleh, A.; Elsaka, E.; El-Sayyad, G.S. Synergistic catalytic effect of thermite nanoparticles on HMX thermal decomposition. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2293–2305. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Abdelaziz, A.; Bekhouche, S.; Boukeciat, H.; Sahnoun, N. Making progress towards promising energetic cellulosic microcrystals developed from alternative lignocellulosic biomasses. J. Energetic Mater. 2022, 1–26. [Google Scholar] [CrossRef]
- Chen, L.; Cao, X.; Gao, J.; He, W.; Liu, J.; Wang, Y.; Zhou, X.; Shen, J.; Wang, B.; He, Y. Nitrated bacterial cellulose-based energetic nanocomposites as propellants and explosives for military applications. ACS Appl. Nano Mater. 2021, 4, 1906–1915. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, T.; Song, X.; Li, F. Electrospinning Preparation of NC/GAP/Submicron-HNS Energetic Composite Fiber and its Properties. ACS Omega 2019, 4, 14261–14271. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Liu, Y.; Huang, H.; Zhao, Y.; Song, K.; Wang, H.; Xie, W.; Cheng, Y.; Fan, X. Preparation and characterization of the Al/Fe2O3/RDX/NC nanocomposites by electrospray. J. Therm. Anal. Calorim. 2019, 137, 1615–1620. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Granado, L.; Sbirrazzuoli, N. Isoconversional computations for nonisothermal kinetic predictions. Thermochim. Acta 2021, 697, 178859. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of pre-exponential factors and of the mathematical functions f (α) or G (α) that describe the reaction mechanism in a model-free way. Thermochim. Acta 2013, 564, 59–69. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Selmani, A.; Saada, M.; Chelouche, S.; Mezroua, A.; Abdelaziz, A. New insensitive high-energy dense biopolymers from giant reed cellulosic fibers: Their synthesis, characterization, and non-isothermal decomposition kinetics. New J. Chem. 2021, 45, 5099–5113. [Google Scholar] [CrossRef]
- Bekhouche, S.; Trache, D.; Abdelaziz, A.; Tarchoun, A.F.; Chelouche, S.; Boudjellal, A.; Mezroua, A. Preparation and characterization of MgAl-CuO ternary nanothermite system by arrested reactive milling and its effect on the thermocatalytic decomposition of cellulose nitrate. Chem. Eng. J. 2022, 453, 139845. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Cao, X.; Gao, J.; Wang, Y.; Qin, Y.; Zhang, Y.; Zhang, J.; Jin, G.; Wang, M. Fabrication of nitrocellulose-based nanoenergetic composites, study on its structure, thermal decomposition kinetics, mechanism, and sensitivity. Nano Sel. 2021, 2, 2225–2236. [Google Scholar] [CrossRef]
- Hanafi, S.; Trache, D.; Meziani, R.; Boukciat, H.; Mezroua, A.; Tarchoun, A.F.; Derradji, M. Synthesis, characterization and thermal decomposition behavior of a novel HNTO/AN co-crystal as a promising rocket propellant oxidizer. Chem. Eng. J. 2021, 417, 128010. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Perez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2022, 719, 179384. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of pre-exponential factor and reaction mechanism in a model-free way. Thermochim. Acta 2020, 691, 178707. [Google Scholar] [CrossRef]
- Vyazovkin, S. Determining preexponential factor in model-free kinetic methods: How and why? Molecules 2021, 26, 3077. [Google Scholar] [CrossRef]
- Trache, D.; Maggi, F.; Palmucci, I.; DeLuca, L.T. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J. Therm. Anal. Calorim. 2018, 132, 1601–1615. [Google Scholar] [CrossRef]
- Achour, S.; Hamada, B.; Baroudi, S.; Abdelaziz, A.; Rezazgui, I.; Trache, D. Spectroscopic characterization andthermal decomposition kinetics of 1, 3-dibutyl-imidazolium bromide synthesized through a solvent-free and one-pot method. J. Mol. Liq. 2021, 339, 117266. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D.; Kesraoui, M.; Tarchoun, A.F.; Chelouche, S.; Mezroua, A. Synthesis and characterization of α-Fe2O3 nanoparticles from different precursors and their catalytic effect on the thermal decomposition of nitrocellulose. Thermochim. Acta 2020, 686, 178570. [Google Scholar] [CrossRef]



| Sample | Heating Rate (°C·mn−1) | Decomposition Stage | Tonset (°C) | Tpeak (°C) | ΔH (J·g−1) | ∆HT (J·g−1) |
|---|---|---|---|---|---|---|
| NC/HNTO/MgAl-CuO | 10 °C/min | 1st decomposition stage | 135.7 | 161.1 | 198.3 | 875.6 |
| 2nd decomposition stage | 189.4 | 206.6 | 589.7 | |||
| 3rd decomposition stage | 239.1 | 250.6 | 87.6 | |||
| 15 °C/min | 1st decomposition stage | 138.6 | 165.3 | 187.4 | 793.6 | |
| 2nd decomposition stage | 190.2 | 212.2 | 511.9 | |||
| 3rd decomposition stage | 237.27 | 253.9 | 71.9 | |||
| 20 °C/min | 1st decomposition stage | 141.7 | 168.4 | 178.7 | 825.8 | |
| 2nd decomposition stage | 192.4 | 216.2 | 608.3 | |||
| 3rd decomposition stage | 240.5 | 256.4 | 41.8 | |||
| 25 °C/min | 1st decomposition stage | 145.9 | 171.1 | 152.5 | 753.4 | |
| 2nd decomposition stage | 194.4 | 219.4 | 581.4 | |||
| 3rd decomposition stage | 246.5 | 258.3 | 19.51 | |||
| NNC/HNTO/MgAl-CuO | 10 °C/min | 1st decomposition stage | 134.7 | 154.1 | 212.5 | 1477.0 |
| 2nd decomposition stage | 187.2 | 204.7 | 1264.5 | |||
| 15 °C/min | 1st decomposition stage | 135.7 | 159.9 | 230.9 | 1520.0 | |
| 2nd decomposition stage | 185.9 | 210.1 | 1289.1 | |||
| 20 °C/min | 1st decomposition stage | 144.3 | 164.3 | 244.8 | 1418.2 | |
| 2nd decomposition stage | 187.6 | 214.8 | 1173.4 | |||
| 25 °C/min | 1st decomposition stage | 143.8 | 167.8 | 263.8 | 1423.4 | |
| 2nd decomposition stage | 193.8 | 217.9 | 1159.6 |
| Sample | Kinetic Method | Ea (kJ/mol) | Log(A) (s−1)) | g(α) | |
|---|---|---|---|---|---|
| NC-HNTO-MgAlCuO 1st step | TAS | 130.93 ± 15.22 | 13.93 ± 3.24 | A3/2 = [−ln(1 − α)]2/3 | |
| it-KAS | 130.90 ± 15.22 | 13.72 ± 3.19 | R1, F0, P1 = α | ||
| VYA/CE | β = 10 °C/min | 130.73 ± 15.19 | 13.64 ± 3.17 | / | |
| β = 15 °C/min | 13.64 ± 3.17 | / | |||
| β = 20 °C/min | 13.70 ± 3.19 | / | |||
| β = 25 °C/min | 13.67 ± 3.18 | / | |||
| NC-HNTO-MgAlCuO 2nd step | TAS | 137.94 ± 13.54 | 12.94 ± 1.41 | A3 = [−ln(1 − α)]1/3 | |
| it-KAS | 137.89 ± 13.55 | 12.96 ± 1.41 | A3/4 = [−ln(1 − α)]4/3 | ||
| VYA/CE | β = 10 °C/min | 137.68 ± 13.53 | 12.48 ± 1.40 | / | |
| β = 15 °C/min | 12.47 ± 1.40 | / | |||
| β = 20 °C/min | 12.50 ± 1.41 | / | |||
| β = 25 °C/min | 12.58 ± 1.42 | / | |||
| NC-HNTO-MgAlCuO 3rd step | TAS | 280.4 ± 18.84 | 26.28 ± 0.93 | A4 = [−ln(1 − α)]1/4 | |
| it-KAS | 280.36 ± 18.84 | 25.98 ± 0.92 | G7 = [1−(1 − α)1/2]1/2 | ||
| VYA/CE | β = 10 °C/min | 280.24 ± 18.83 | 26.06 ± 0.92 | / | |
| β = 15 °C/min | 26.04 ± 0.92 | / | |||
| β = 20 °C/min | 26.01 ± 0.92 | / | |||
| β = 25 °C/min | 25.98 ± 0.91 | / | |||
| NNC-HNTO-MgAlCuO 1st step | TAS | 104.41 ± 13.61 | 10.53 ± 1.78 | E1 = ln α | |
| it-KAS | 104.35 ± 13.60 | 10.59 ± 1.79 | R1, F0, P1 = α | ||
| VYA/CE | β = 10 °C/min | 104.13 ± 13.57 | 10.34 ± 1.75 | / | |
| β = 15 °C/min | 10.39 ± 1.76 | / | |||
| β = 20 °C/min | 10.40 ± 1.76 | / | |||
| β = 25 °C/min | 10.34 ± 1.75 | / | |||
| NNC-HNTO-MgAlCuO 2nd step | TAS | 131.51 ± 18.21 | 12.15 ± 2.11 | E1 = ln α | |
| it-KAS | 131.45 ± 18.20 | 12.85 ± 2.23 | A1= −ln(1 − α) | ||
| VYA/CE | β = 10 °C/min | 131.24 ± 18.17 | 12.80 ± 2.22 | / | |
| β = 15 °C/min | 12.83 ± 2.23 | / | |||
| β = 20 °C/min | 12.96 ± 2.25 | / | |||
| β = 25 °C/min | 12.84 ± 2.23 | / | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourari, M.; Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Bekhouche, S.; Harrat, A.; Boukeciat, H.; Matmat, N. Unraveling the Effect of MgAl/CuO Nanothermite on the Characteristics and Thermo-Catalytic Decomposition of Nanoenergetic Formulation Based on Nanostructured Nitrocellulose and Hydrazinium Nitro-Triazolone. Catalysts 2022, 12, 1573. https://doi.org/10.3390/catal12121573
Dourari M, Tarchoun AF, Trache D, Abdelaziz A, Bekhouche S, Harrat A, Boukeciat H, Matmat N. Unraveling the Effect of MgAl/CuO Nanothermite on the Characteristics and Thermo-Catalytic Decomposition of Nanoenergetic Formulation Based on Nanostructured Nitrocellulose and Hydrazinium Nitro-Triazolone. Catalysts. 2022; 12(12):1573. https://doi.org/10.3390/catal12121573
Chicago/Turabian StyleDourari, Mohammed, Ahmed Fouzi Tarchoun, Djalal Trache, Amir Abdelaziz, Slimane Bekhouche, Abdelatif Harrat, Hani Boukeciat, and Nawel Matmat. 2022. "Unraveling the Effect of MgAl/CuO Nanothermite on the Characteristics and Thermo-Catalytic Decomposition of Nanoenergetic Formulation Based on Nanostructured Nitrocellulose and Hydrazinium Nitro-Triazolone" Catalysts 12, no. 12: 1573. https://doi.org/10.3390/catal12121573







