Synthesis of Sulfonated Carbon from Discarded Masks for Effective Production of 5-Hydroxymethylfurfural
Abstract
:1. Introduction
2. Results and Discussion
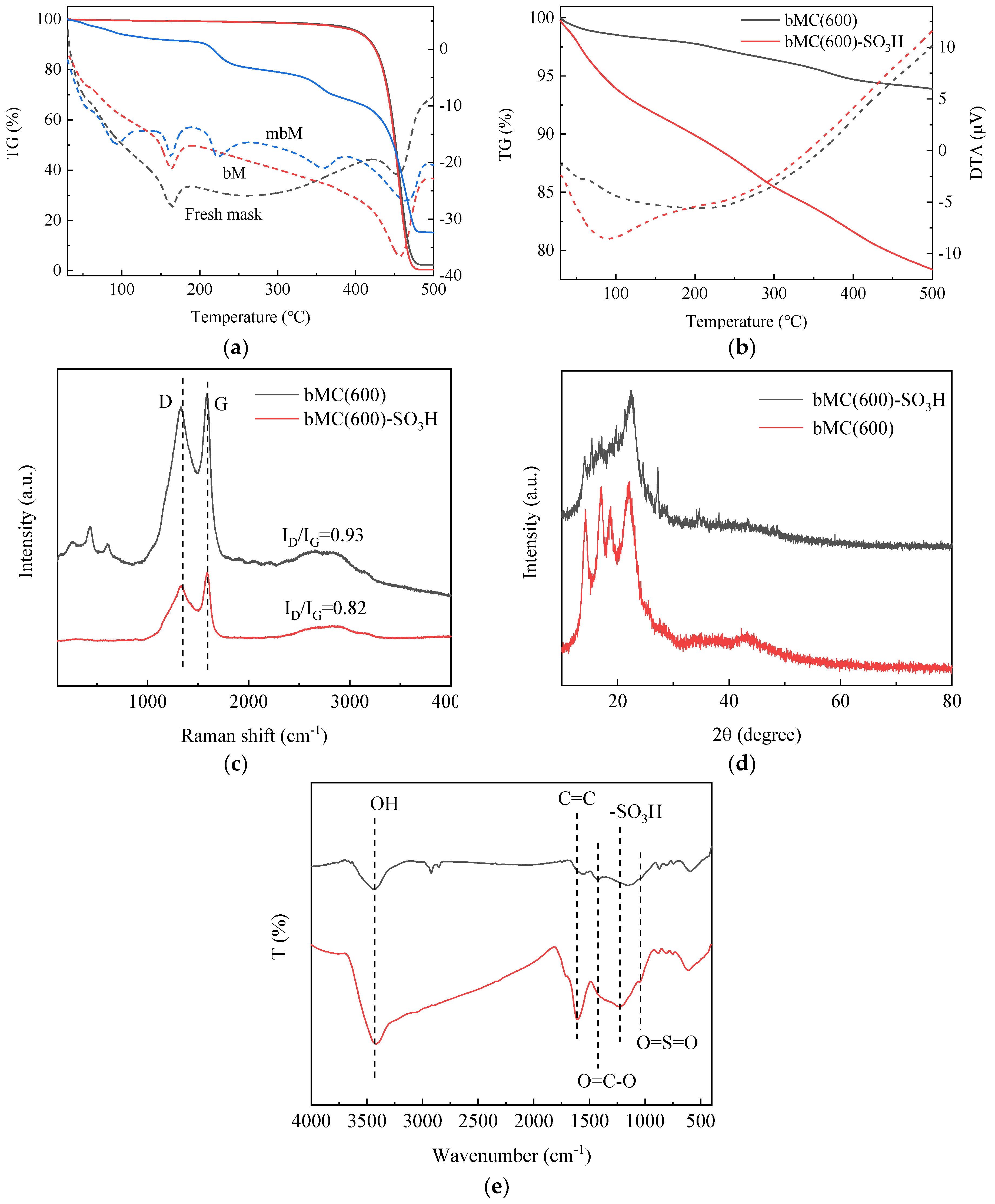
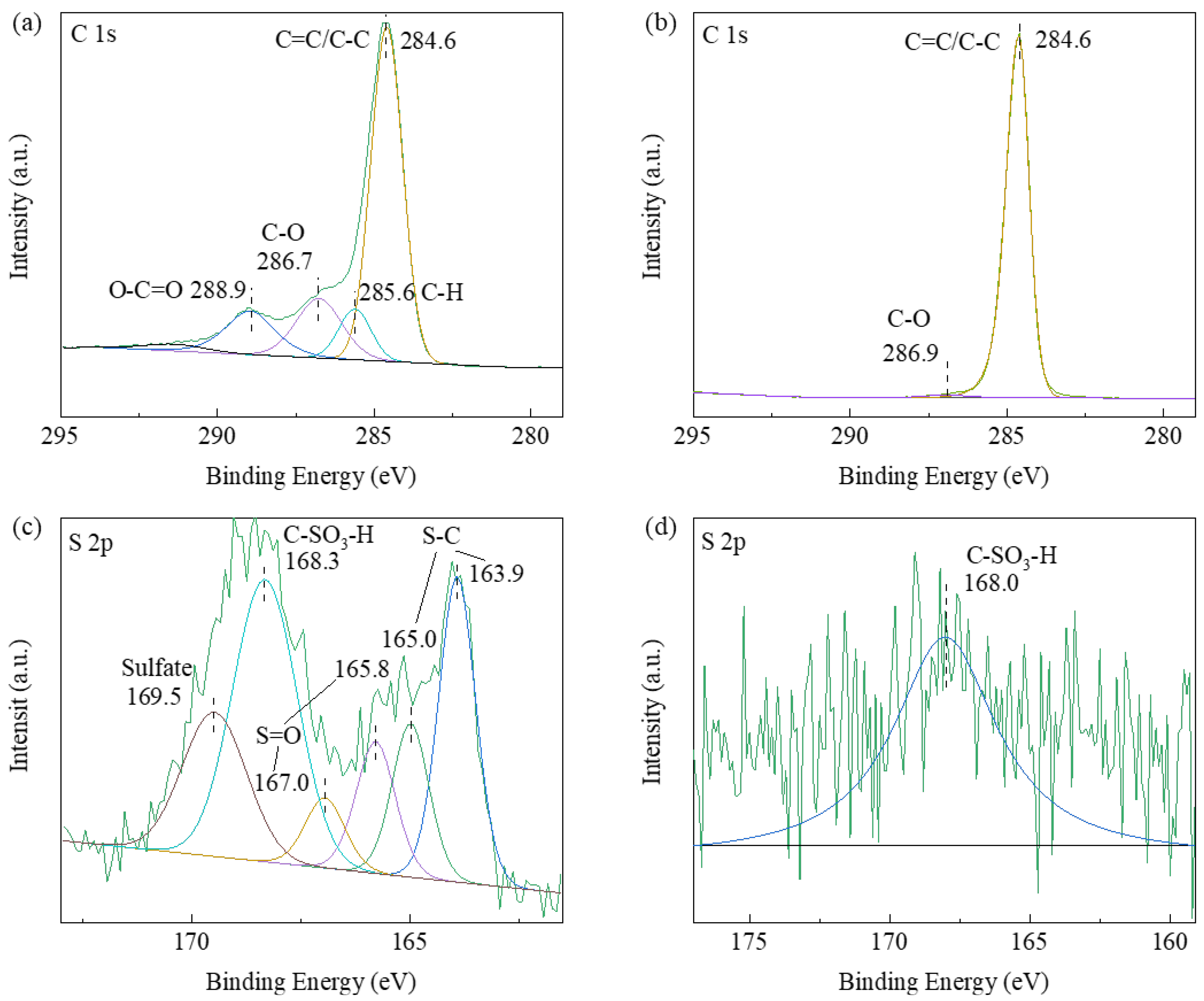
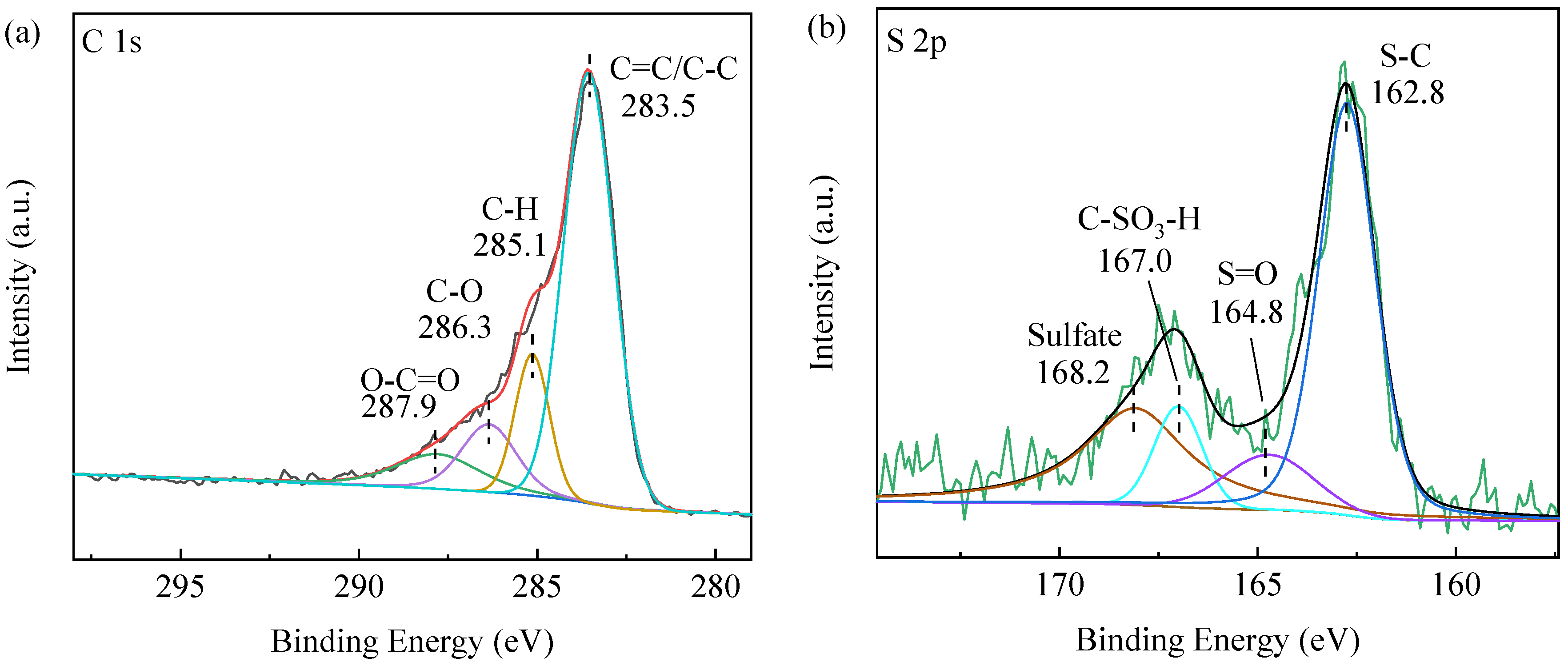
2.1. Catalyst Structural Analysis
2.2. Catalytic Performance
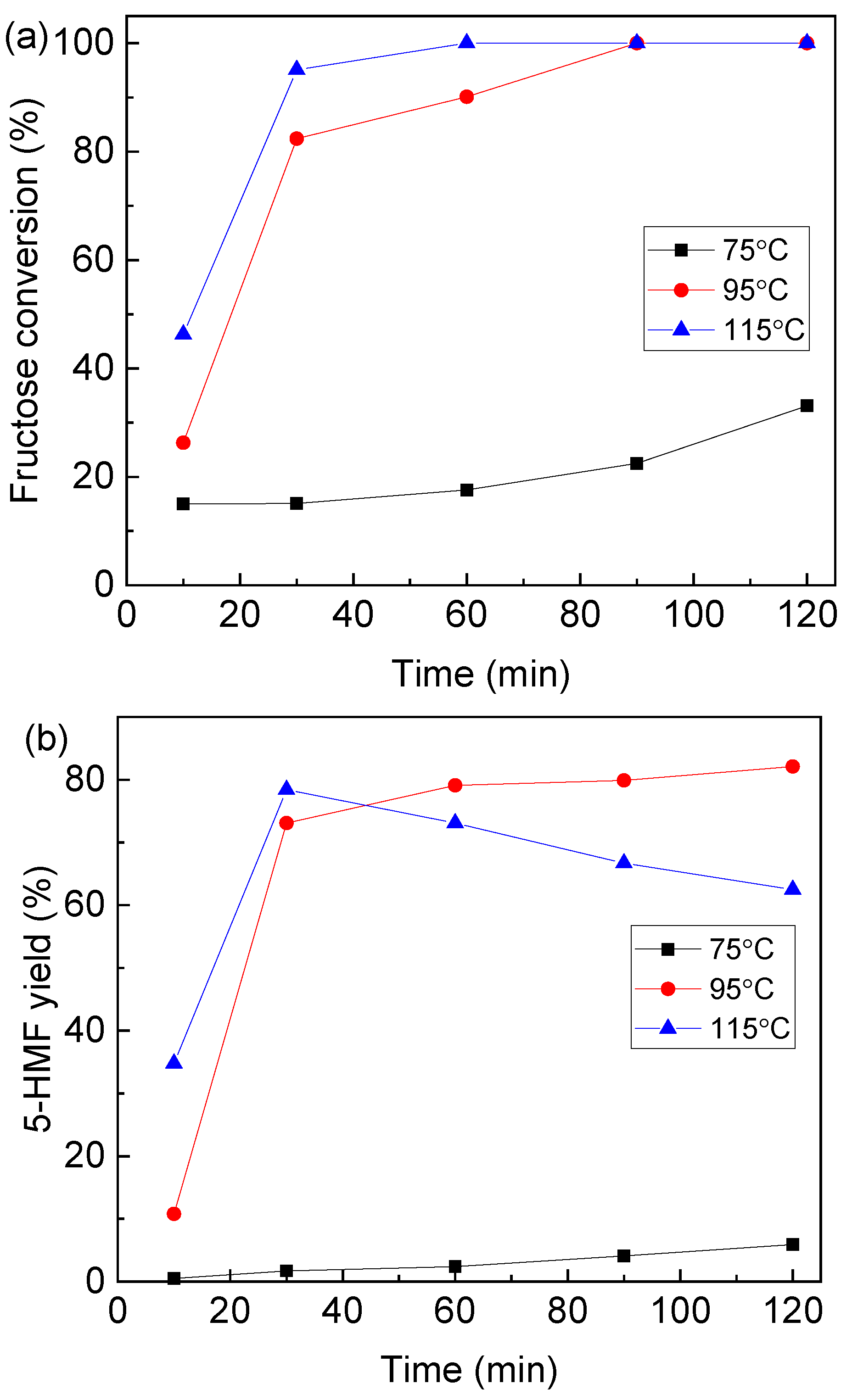
2.3. Effect of Reaction Temperature and Time
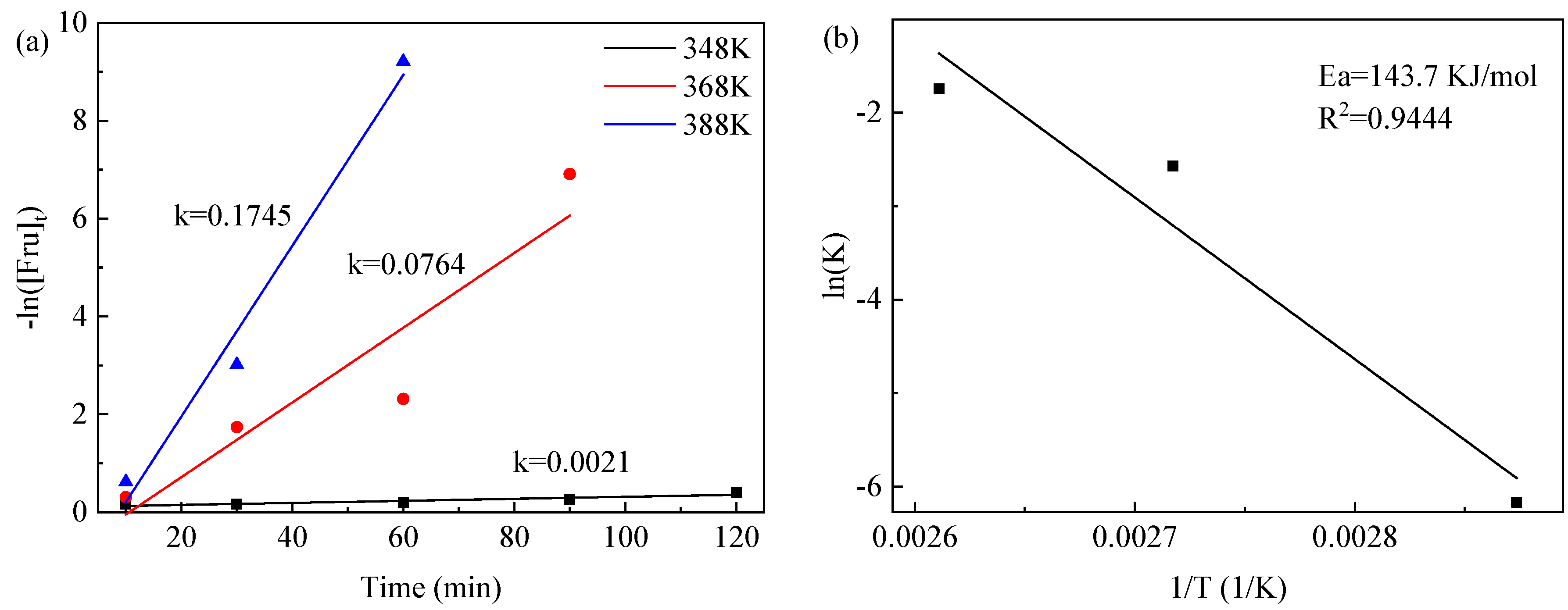
2.4. Kinetic Modeling Studies
- (1)
- All reactions are irreversible;
- (2)
- The main reaction is fructose to 5-HMF, which ignores other possible reactions;
- (3)
- All unidentified products are considered degradation products (humin);
- (4)
- All other intermediates had negligible concentrations.
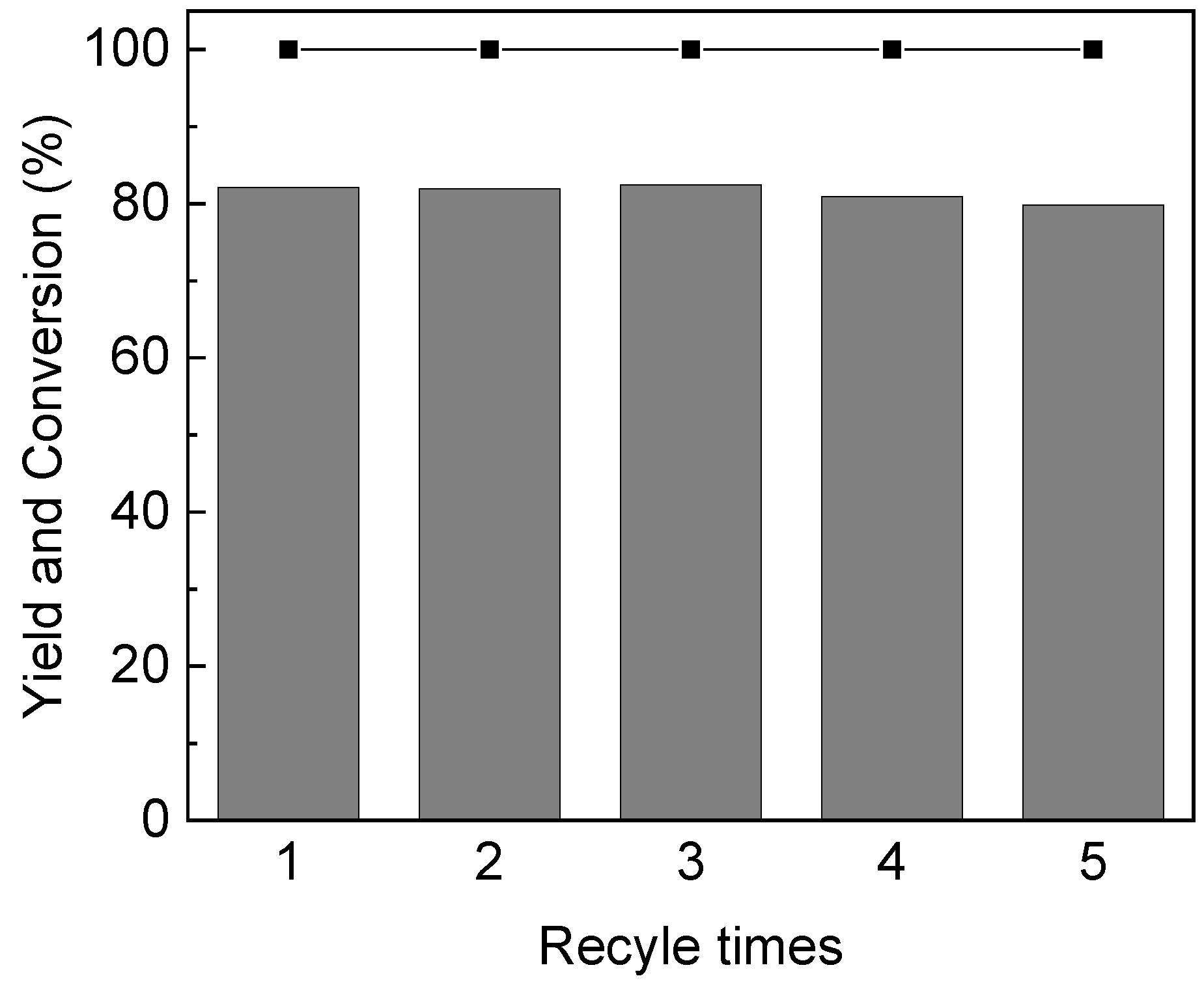
2.5. Catalyst Recycling
3. Material and Experimental
3.1. Materials
3.2. Catalyst Preparation and Characterization
3.3. Catalytic Evaluation and Product Analysis
3.4. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Fei, X.; Chen, D.; Qi, Y.; Xu, Q.; Liu, Y.; Zhang, Q.; Li, S.; Wang, T.; Qin, Y.; et al. Efficient catalytic upgrading of ethanol to higher alcohols via inhibiting C—C cleavage and promoting C—C coupling over biomass-derived NiZn@NC catalysts. ACS Catal. 2022, 12, 11573–11585. [Google Scholar] [CrossRef]
- Muhammad, S.; Bai, Y.; Liu, D.; Zhao, X. Organic acid catalyzed production of platform chemical 5-hydroxymethylfurfural from fructose: Process comparison and evaluation based on kinetic modeling. Arab. J. Chem. 2020, 13, 7430–7444. [Google Scholar]
- Nogueira, J.S.M.; Santana, V.T.; Henrique, P.V.; de Aguiar, L.G.; Silva, J.P.A.; Mussatto, S.I.; Carneiro, L.M. Production of 5-hydroxymethylfurfural from direct conversion of cellulose using heteropolyacid/Nb2O5 as catalyst. Catalysts 2020, 10, 1417. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Corsini, A.; Boschi, N.; Galletti, A.M.R. Smart valorization of waste biomass: Exhausted lemon peels, coffee silverskins and paper wastes for the production of levulinic acid. Chem. Eng. Trans. 2018, 65, 637–642. [Google Scholar]
- Guo, H.; Abe, Y.; Qi, X.; Smith, R.L., Jr. Bifunctional carbon Ni/NiO nanofiber catalyst based on 5-sulfosalicylic acid for conversion of C5/C6 carbohydrates into ethyl levulinate. React. Chem. Eng. 2020, 5, 1759–1767. [Google Scholar] [CrossRef]
- Guo, H.; Dowaki, T.; Shen, F.; Qi, X.; Smith, R.L. Critical Assessment of Reaction Pathways for Next-Generation Biofuels from Renewable Resources: 5-Ethoxymethylfurfural. ACS Sustain. Chem. Eng. 2022, 10, 9002–9021. [Google Scholar] [CrossRef]
- Bangalore, A.; Rahul, P.; Oinas, P.; Forssell, S. Techno-economic evaluation of a biorefinery to produce γ-valerolactone (GVL), 2-methyltetrahydrofuran (2-MTHF) and 5-hydroxymethylfurfural (5-HMF) from spruce. Renew. Energy 2022, 190, 396–407. [Google Scholar] [CrossRef]
- Tiwari, M.S.; Wagh, D.; Dicks, J.S.; Keogh, J.; Ansaldi, M.; Ranade, V.V.; Manyar, H.G. Solvent free upgrading of 5-hydroxymethylfurfural (HMF) with levulinic acid to HMF levulinate using tin exchanged tungstophosphoric acid supported on K-10 catalyst. ACS Org. Inorg. Au 2022. [Google Scholar] [CrossRef]
- Tan-Soetedjo, J.N.M.; van de Bovenkamp, H.H.; Abdilla, R.M.; Rasrendra, C.B.; van Ginkel, J.; Heeres, H.J. Experimental and kinetic modeling studies on the conversion of sucrose to levulinic acid and 5-hydroxymethylfurfural using sulfuric acid in water. Ind. Eng Chem. Res. 2017, 56, 13228–13239. [Google Scholar] [CrossRef]
- Román-Leshkov, J.A.D.Y. Solvent effects on fructose dehydration to 5-hydroxymethylfurfural in biphasic systems saturated with inorganic salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Wu, K.; Bi, C.; Cui, Q. Conversion of fructose into 5-hydroxymethylfurfural (HMF) and its derivatives promoted by inorganic salt in alcohol. Carbohydr. Res. 2012, 350, 20–24. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; He, C.; Dai, Y.; Jia, X.; Yang, Y. Efficient dehydration of fructose to 5-hydroxymethylfurfural over sulfonated carbon sphere solid acid catalysts. Catal. Today 2016, 264, 123–130. [Google Scholar] [CrossRef]
- Sampath, G.; Kannan, S. Fructose dehydration to 5-hydroxymethylfurfural: Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies. Catal. Commun. 2013, 37, 41–44. [Google Scholar] [CrossRef]
- Lyu, X.; Li, H.; Xiang, H.; Mu, Y.; Ji, N.; Lu, X.; Fan, X.; Gao, X. Energy efficient production of 5-hydroxymethylfurfural (5-HMF) over surface functionalized carbon superstructures under microwave irradiation. Chem. Eng. J. 2022, 428, 131143. [Google Scholar] [CrossRef]
- Antonetti, C.; Galletti, A.M.R.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Gao, Z.; Wang, L.; Zhang, J. Leaf-derived sulfonated carbon dots: Efficient and recoverable catalysts to synthesize 5-hydroxymethylfurfural from fructose. Mater. Today Chem. 2021, 20, 100423. [Google Scholar] [CrossRef]
- Khumho, R.; Yousatit, S.; Ngamcharussrivichai, C. Glucose conversion into 5-hydroxymethylfurfural over Niobium oxides supported on natural Rubber-derived carbon/silica nanocomposite. Catalysts 2021, 11, 887. [Google Scholar] [CrossRef]
- Guo, H.; Lian, Y.; Yan, L.; Qi, X.; Smith, R.L. Cellulose-derived superparamagnetic carbonaceous solid acid catalyst for cellulose hydrolysis in an ionic liquid or aqueous reaction system. Green Chem. 2013, 15, 2167. [Google Scholar] [CrossRef]
- Guo, H.; Ogawa, S.; Isoda, Y.; Shen, F.; Smith, R.L. Weak-acid biochar catalyst prepared from mechanochemically-activated biomass and humic acid for production of 5-hydroxymethylfurfural. Biochar 2022, 4, 42. [Google Scholar] [CrossRef]
- Dowaki, T.; Guo, H.; Smith, R.L. Lignin-derived biochar solid acid catalyst for fructose conversion into 5-ethoxymethylfurfural. Renew. Energy 2022, 199, 1534–1542. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar pyrolysis of waste biomass: Part 2 kinetic modeling and methodology of the determination of the kinetic parameters for solar pyrolysis of sewage sludge. Renew. Energy 2020, 153, 962–974. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Lubieniecki, B. Hydrothermal co-carbonization of sewage sludge and fuel additives: Combustion performance of hydrochar. Renew. Energy 2021, 178, 1046–1056. [Google Scholar] [CrossRef]
- McGaughy, K.; Reza, M.T. Hydrothermal carbonization of food waste: Simplified process simulation model based on experimental results. Biomass Convers. Biorefinery 2017, 8, 283–292. [Google Scholar] [CrossRef]
- Saqib, N.U.; Sarmah, A.K.; Baroutian, S. Effect of temperature on the fuel properties of food waste and coal blend treated under co-hydrothermal carbonization. Waste Manag. 2019, 89, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, G.; Sarrion, A.; Diaz, E.; Gori, R.; De la Rubia, M.A.; Mohedano, A.F. Improved energy recovery from food waste through hydrothermal carbonization and anaerobic digestion. Waste Manag. 2022, 142, 9–18. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Han, L.; Dong, M.; Zeng, Z.; Sun, L. Mask waste: A sustainable mask-based epoxy Resin/SiO2 composite for efficient purification of water-in-oil emulsions. ACS Appl. Polym. Mater. 2022, 4, 5180–5188. [Google Scholar] [CrossRef]
- Varghese, P.J.G.; David, D.A.; Karuth, A.; Manamkeri Jafferali, J.F.; Sabura-Begum, P.M.; George, J.J.; Rasulev, B.; Raghavan, P. Experimental and simulation studies on nonwoven polypropylene-nitrile rubber blend: Recycling of medical face masks to an engineering product. ACS Omega 2022, 7, 4791–4803. [Google Scholar] [CrossRef]
- Selvaranjan, K.; Navaratnam, S.; Rajeev, P.; Ravintherakumaran, N. Environmental challenges induced by extensive use of face masks during COVID-19: A review and potential solutions. Environ. Chall. 2021, 3, 100039. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. COVID-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Rong, Q.; Zhang, L.; Guo, S. Porous carbon materials derived from discarded COVID-19 masks via microwave solvothermal method for lithiumsulfur batteries. Sci. Total Environ. 2022, 817, 152995. [Google Scholar] [CrossRef]
- Lee, G.; Lee, M.E.; Kim, S.-S.; Joh, H.-I.; Lee, S. Efficient upcycling of polypropylene-based waste disposable masks into hard carbons for anodes in sodium ion batteries. J. Ind. Eng. Chem. 2022, 105, 268–277. [Google Scholar] [CrossRef]
- Dharmaraj, S.; Ashokkumar, V.; Hariharan, S.; Manibharathi, A.; Show, P.L.; Chong, C.T.; Ngamcharussrivichai, C. The COVID-19 pandemic face mask waste: A blooming threat to the marine environment. Chemosphere 2021, 272, 129601. [Google Scholar] [CrossRef]
- Hu, X.; Lin, Z. Transforming waste polypropylene face masks into S-doped porous carbon as the cathode electrode for supercapacitors. Ionics 2021, 27, 2169–2179. [Google Scholar] [CrossRef]
- Yu, R.; Wen, X.; Liu, J.; Wang, Y.; Chen, X.; Wenelska, K.; Mijowska, E.; Tang, T. A green and high-yield route to recycle waste masks into CNTs/Ni hybrids via catalytic carbonization and their application for superior microwave absorption. Appl. Catal. B Environ. 2021, 298, 120544. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Song, J.; Tang, T. Pressurized carbonization of mixed plastics into porous carbon sheets on magnesium oxide. RSC Adv. 2018, 8, 2469–2476. [Google Scholar] [CrossRef]
- Limani, N.; Marques, I.S.; Jarrais, B.; Fernandes, A.J.S.; Freire, C.; Fernandes, D.M. Cobalt phosphotungstate-based composites as bifunctional electrocatalysts for oxygen reactions. Catalysts 2022, 12, 357. [Google Scholar] [CrossRef]
- El Fergani, M.; Candu, N.; Tudorache, M.; Bucur, C.; Djelal, N.; Granger, P.; Coman, S.M. From useless humins by-product to Nb@graphite-like carbon catalysts highly efficient in HMF synthesis. Appl. Catal. A Gen. 2021, 618, 118130. [Google Scholar] [CrossRef]
- Alnassar, M.A.; Alshehri, A.; Narasimharao, K. Visible light active magnesium silicate-graphitic carbon nitride nanocomposites for methylene Blue degradation and Pb2+ adsorption. Catalysts 2022, 12, 1256. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; . Lin, X.; Wang, C. One-step synthesis of biomass-based sulfonated carbon catalyst by direct carbonization-sulfonation for organosolv delignification. Bioresour. Technol. 2021, 319, 124194. [Google Scholar]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, J.R.L. Efficient process for conversion of fructose to 5-hydroxymethylfurfural with ionic liquids. Green Chem. 2009, 11, 1327. [Google Scholar] [CrossRef]
- Bispo, C.; Vigier, K.D.; Sardo, M.; Bion, N.; Mafra, L.; Ferreira, P.; Jérôme, F. Catalytic dehydration of fructose to HMF over sulfonic acid functionalized periodic mesoporous organosilicas: Role of the acid density. Catal. Sci. Technol. 2014, 4, 2235–2240. [Google Scholar] [CrossRef]
- Nishimura, Y.; Suda, M.; Kuroha, M.; Kobayashi, H.; Nakajima, K.; Fukuoka, A. Synthesis of 5-hydroxymethylfurfural from highly concentrated aqueous fructose solutions using activated carbon. Carbohydr. Res. 2019, 486, 107826. [Google Scholar] [CrossRef] [PubMed]
- Motagamwala, A.H.; Huang, K.; Maravelias, C.T.; Dumesic, J.A. Solvent system for effective near-term production of hydroxymethylfurfural (HMF) with potential for long-term process improvement. Energy Environ. Sci. 2019, 12, 2212–2222. [Google Scholar] [CrossRef]
- Sajid, M.; Bai, Y.; Liu, D.; Zhao, X. Conversion of glucose to 5-hydroxymethylfurfural by co-catalysis of p-Toluenesulfonic acid (pTSA) and chlorides: A comparison based on kinetic modeling. Waste Biomass Valorization 2020, 12, 3271–3286. [Google Scholar] [CrossRef]
- Adib, F.; Bagreev, A.; Bandosz, T.J. Analysis of the relationship between H2S removal capacity and surface properties of unimpregnated activated carbons. Environ. Sci. Technol. 2000, 34, 686–692. [Google Scholar] [CrossRef]

| Entry | Catalyst | Acidity (mmol/g) | Conv. (%) | HMF yield (%) | Select. (%) | TOF (h−1) | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | - | - | 20.9 | 6.2 | 29.7 | - | * |
| 2 | bMC(600) | 1.2 | 22.0 | 8.2 | 37.3 | 2.03 | * |
| 3 | bMC(400)-SO3H | 3.4 | 40.5 | 25.3 | 62.5 | 1.32 | * |
| 4 | bMC(600)-SO3H | 4.2 | 90.1 | 79.1 | 87.8 | 2.38 | * |
| 5 a | bMC(600)-SO3H | 4.2 | 53.2 | 30.1 | 56.6 | 1.76 | * |
| 6 b | Amberlyst-15 | 4.7 | 100 | 82.2 | 82.2 | - | [43] |
| 7 c | Amberlytst-70 | 2.55 (meq/g) | 80.1 | 45.6 | 56.9 | - | [15] |
| 8 d | SBA-SO3H | 0.89 (H+ loading) | 96 | 55 | 57.3 | - | [42] |
| 9 e | CS-SO3H | 7.9 | ~85 | ~58 | ~62 | - | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Shen, F.; Yang, J.; Qiu, M.; Guo, H.; Qi, X. Synthesis of Sulfonated Carbon from Discarded Masks for Effective Production of 5-Hydroxymethylfurfural. Catalysts 2022, 12, 1567. https://doi.org/10.3390/catal12121567
Hao H, Shen F, Yang J, Qiu M, Guo H, Qi X. Synthesis of Sulfonated Carbon from Discarded Masks for Effective Production of 5-Hydroxymethylfurfural. Catalysts. 2022; 12(12):1567. https://doi.org/10.3390/catal12121567
Chicago/Turabian StyleHao, Hengyu, Feng Shen, Jirui Yang, Mo Qiu, Haixin Guo, and Xinhua Qi. 2022. "Synthesis of Sulfonated Carbon from Discarded Masks for Effective Production of 5-Hydroxymethylfurfural" Catalysts 12, no. 12: 1567. https://doi.org/10.3390/catal12121567
APA StyleHao, H., Shen, F., Yang, J., Qiu, M., Guo, H., & Qi, X. (2022). Synthesis of Sulfonated Carbon from Discarded Masks for Effective Production of 5-Hydroxymethylfurfural. Catalysts, 12(12), 1567. https://doi.org/10.3390/catal12121567










