Recent Advances in Ternary Metal Oxides Modified by N Atom for Photocatalysis
Abstract
:1. Introduction
2. The Influence of N
2.1. The Influence of N Doping
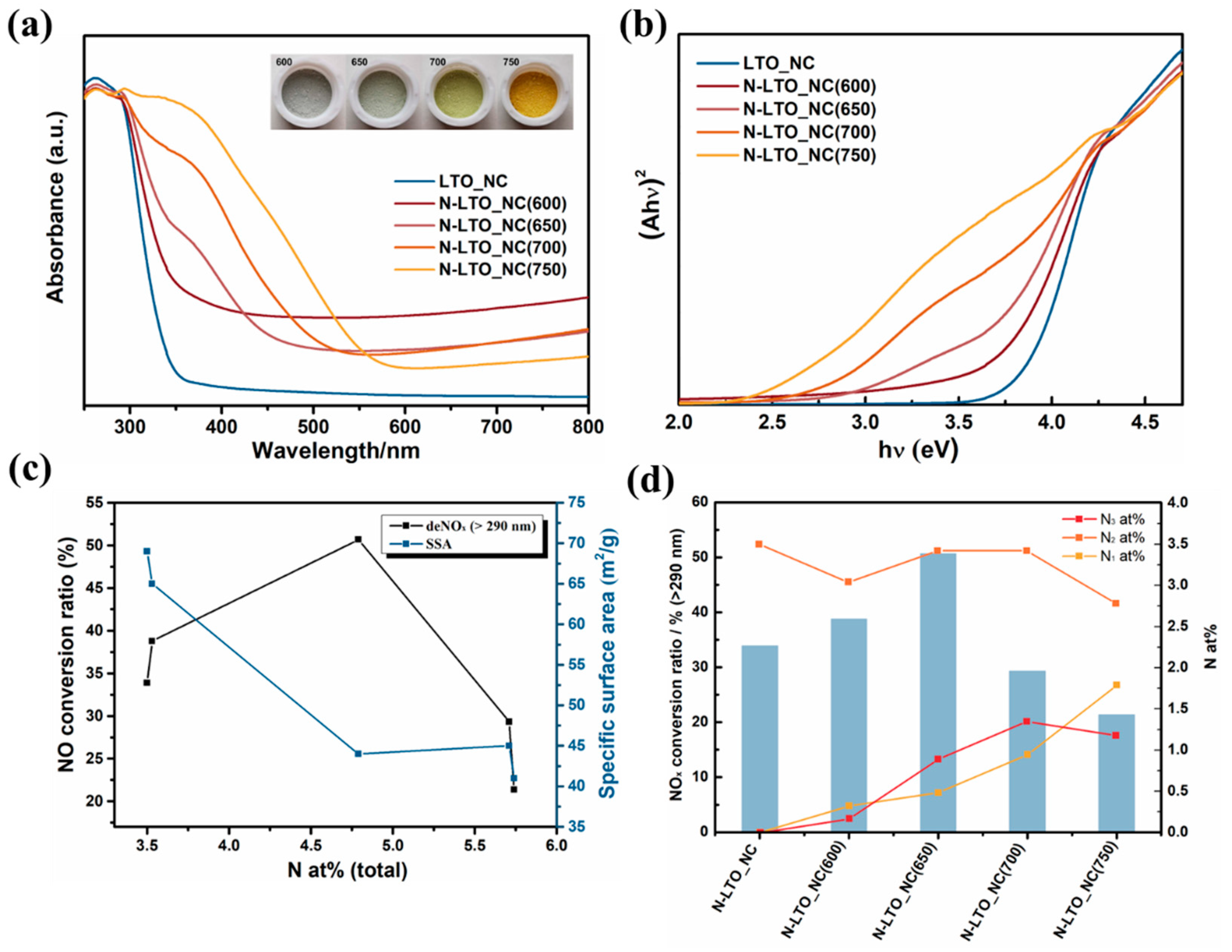
2.1.1. N Doping Concentration and Doping Site
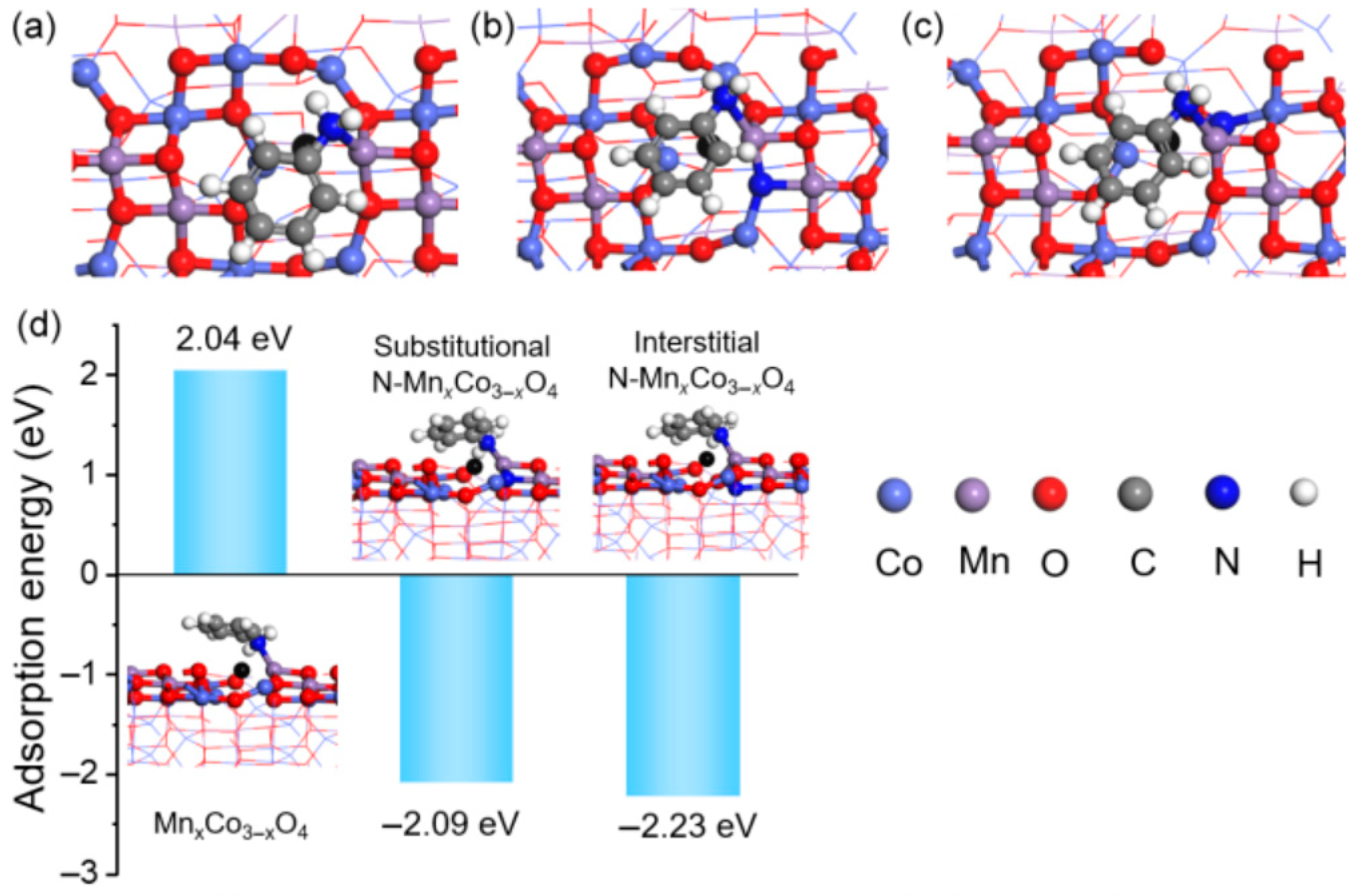
2.1.2. Defects Induced by N Doping
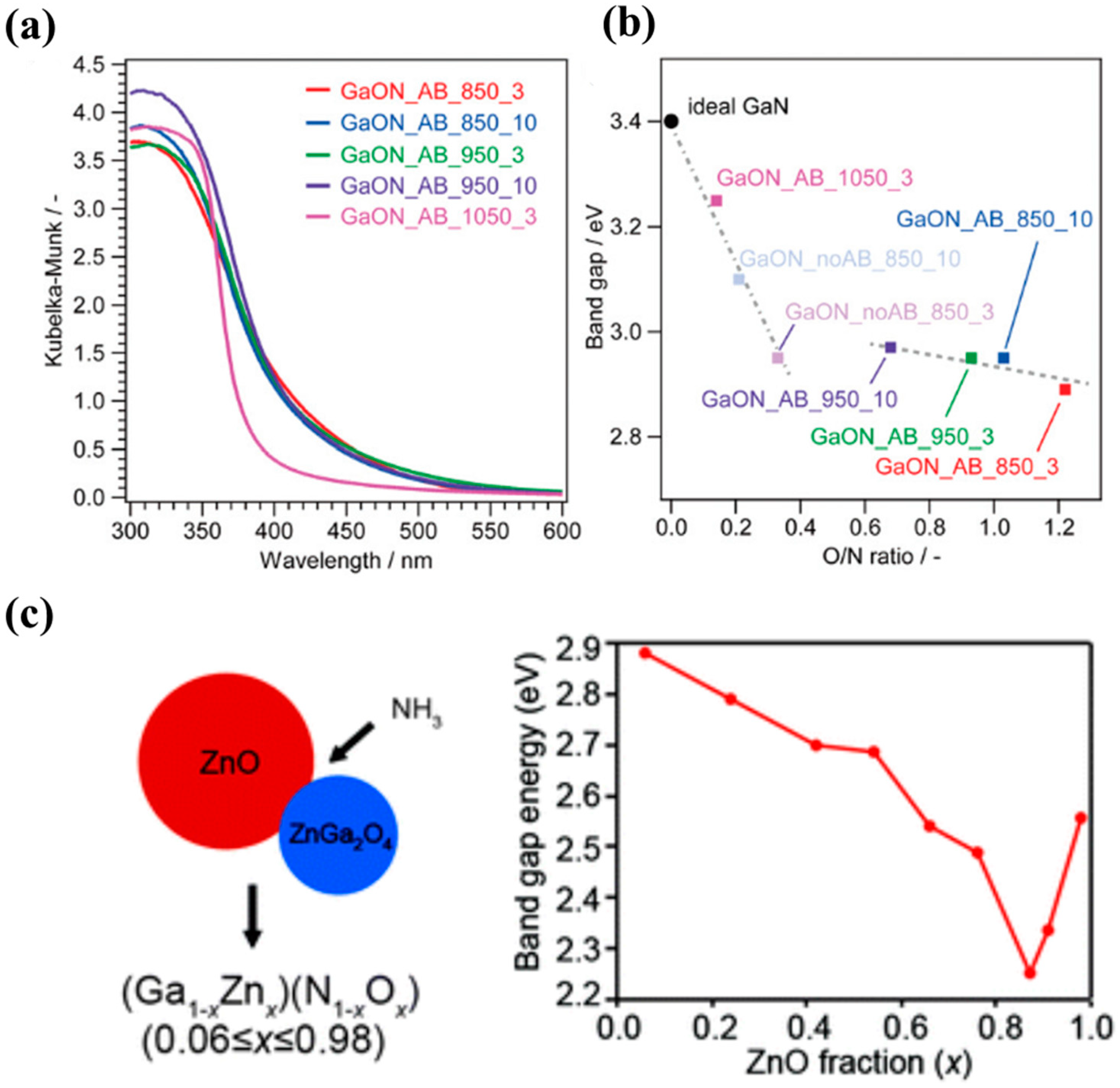
2.2. Phase Transformation by N Interaction
3. Recent Advances in Nitrogen Modified in TMOs
3.1. N-Doped La2Ti2O7 and LaTiO2N
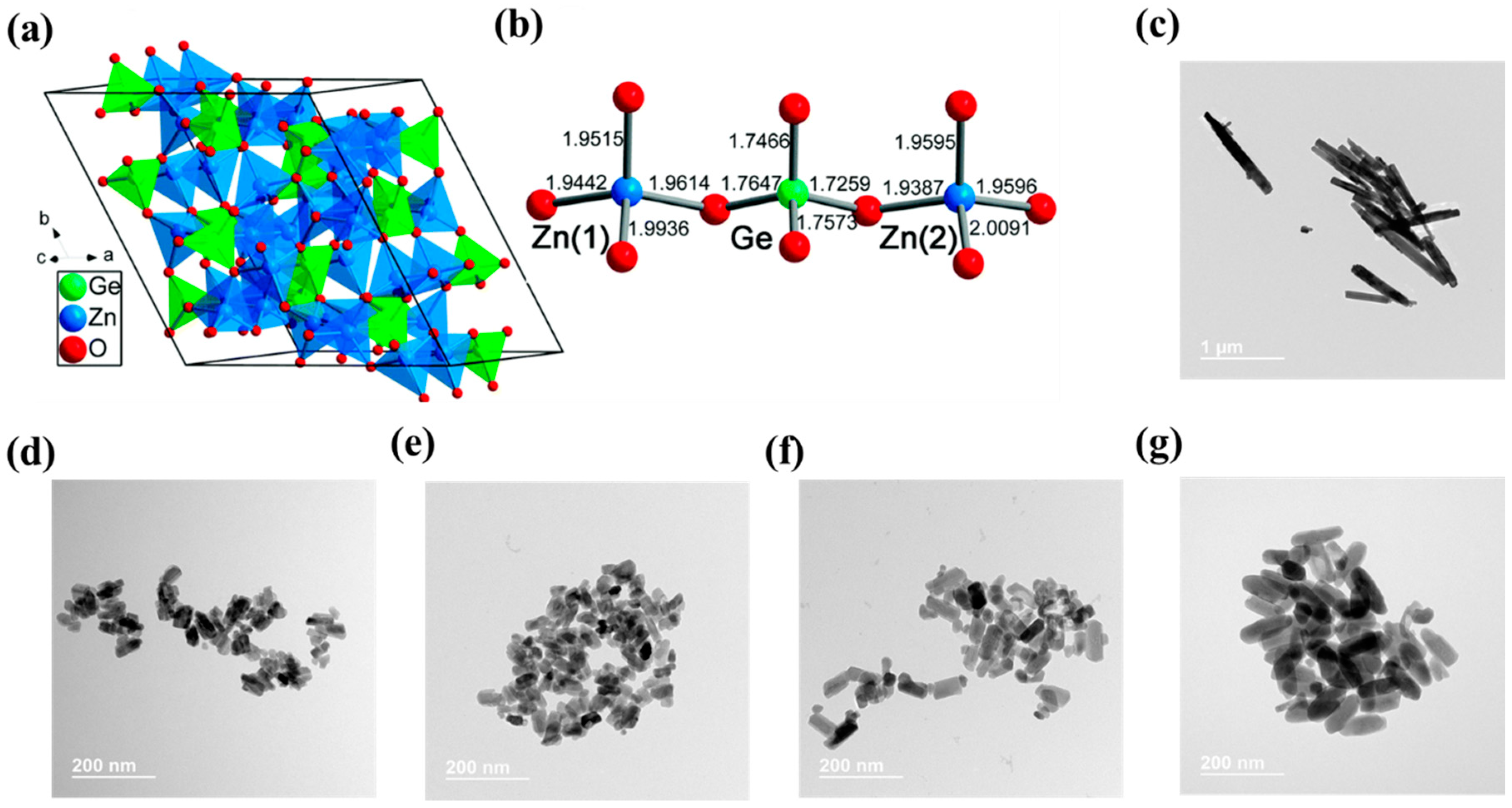
3.2. N-Doped Zn2GeO4 and (Zn1+xGe)(N2Ox)
4. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Yin, S. Creation of advanced optical responsive functionality of ceramics by green processes. J. Ceram. Soc. Jpn. 2015, 123, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Asakura, Y. Recent research progress on mixed valence state tungsten based materials. Tungsten 2019, 1, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Hasegawa, T. Morphology Control of Transition Metal Oxides by Liquid-Phase Process and Their Material Development. KONA Powder Part. J. 2022, 2023015. [Google Scholar] [CrossRef]
- Xue, Y.; Yin, S. Element doping: A marvelous strategy for pioneering the smart applications of VO2. Nanoscale 2022, 14, 11054–11097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, J.; Chang, H.; Asakura, Y.; Yin, S. Recent progress on mixed-anion type visible-light induced photocatalysts. Sci. China Technol. Sci. 2017, 60, 1447–1457. [Google Scholar] [CrossRef]
- Li, H.; Yin, S.; Wang, Y.; Sato, T. Current progress on persistent fluorescence-assisted composite photocatalysts. Funct. Mater. Lett. 2013, 6, 1330005. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates Jr, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Liu, B.; Wang, Y.; Sekino, T.; Lee, S.W.; Sato, T. UV, visible and near-infrared lights induced NOx destruction activity of (Yb, Er)-NaYF4/C-TiO2 composite. Sci. Rep. 2013, 3, 2918. [Google Scholar] [CrossRef] [Green Version]
- Komatsuda, S.; Asakura, Y.; Vequizo, J.J.M.; Yamakata, A.; Yin, S. Enhanced photocatalytic NOx decomposition of visible-light responsive F-TiO2/(N, C)-TiO2 by charge transfer between F-TiO2 and (N, C)-TiO2 through their doping levels. Appl. Catal. B 2018, 238, 358–364. [Google Scholar] [CrossRef]
- Noda, C.; Asakura, Y.; Shiraki, K.; Yamakata, A.; Yin, S. Synthesis of three-component C3N4/rGO/C-TiO2 photocatalyst with enhanced visible-light responsive photocatalytic deNOx activity. Chem. Eng. J. 2020, 390, 124616. [Google Scholar] [CrossRef]
- Gu, Z.; Cui, Z.; Wang, Z.; Qin, K.S.; Asakura, Y.; Hasegawa, T.; Tsukuda, S.; Hongo, K.; Maezono, R.; Yin, S. Carbon vacancies and hydroxyls in graphitic carbon nitride: Promoted photocatalytic NO removal activity and mechanism. Appl. Catal. B 2020, 279, 119376. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, J.P. Effects of dopant states on photoactivity in carbon-doped TiO2. J. Phys. Condens. Matter 2005, 17, L209. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wang, W.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Tanaka, S.; Asai, K. Visible light-induced degradation of methylene blue on S-doped TiO2. Chem. Lett. 2003, 32, 330–331. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven N-F-codoped TiO2 photocatalysts. 2. Optical characterization, photocatalysis, and potential application to air purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for visible light responses in anodic photocurrents at N-doped TiO2 film electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, A.; Cao, Y.; Xie, J.; Jia, W.; Jia, D. Interstitial N-doped SrSnO3 perovskite: Structural design, modification and photocatalytic degradation of dyes. New J. Chem. 2019, 43, 10965–10972. [Google Scholar] [CrossRef]
- Meng, F.; Hong, Z.; Arndt, J.; Li, M.; Zhi, M.; Yang, F.; Wu, N. Visible light photocatalytic activity of nitrogen-doped La2Ti2O7 nanosheets originating from band gap narrowing. Nano Res. 2012, 5, 213–221. [Google Scholar] [CrossRef]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Wang, C. K4Nb6O17/Fe3N/α-Fe2O3/C3N4 as an enhanced visible light-driven quaternary photocatalyst for acetamiprid photodegradation, CO2 reduction, and cancer cells treatment. Appl. Surf. Sci. 2021, 544, 148939. [Google Scholar] [CrossRef]
- Rhimi, B.; Padervand, M.; Jouini, H.; Ghasemi, S.; Bahnemann, D.W.; Wang, C. Recent progress in NOx photocatalytic removal: Surface/interface engineering and mechanistic understanding. J. Environ. Chem. Eng. 2022, 10, 108566. [Google Scholar] [CrossRef]
- Marschall, R.; Wang, L. Non-metal doping of transition metal oxides for visible-light photocatalysis. Catal. Today 2014, 225, 111–135. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.; Stout, J.; Gole, J.L. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Liu, G.; Yin, L.-C.; Wang, J.; Niu, P.; Zhen, C.; Xie, Y.; Cheng, H.-M. A red anatase TiO2 photocatalyst for solar energy conversion. Energy Environ. Sci. 2012, 5, 9603–9610. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.; Gopinath, C.S. Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO2 nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Kobayakawa, K.; Murakami, Y.; Sato, Y. Visible-light active N-doped TiO2 prepared by heating of titanium hydroxide and urea. J. Photochem. Photobiol. A 2005, 170, 177–179. [Google Scholar] [CrossRef]
- Wang, J.; Asakura, Y.; Hasegawa, T.; Yin, S. High-concentration N-doped La2Ti2O7 nanocrystals: Effects of nano-structuration and doping sites on enhancing the photocatalytic activity. Chem. Eng. J. 2021, 423, 130220. [Google Scholar] [CrossRef]
- Cao, J.; Hasegawa, T.; Asakura, Y.; Sun, P.; Yang, S.; Li, B.; Cao, W.; Yin, S. Synthesis and color tuning of titanium oxide inorganic pigment by phase control and mixed-anion co-doping. Adv. Powder Technol. 2022, 33, 103576. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Wang, J.; Saito, F.; Uchida, M. Synthesis of N-Doped ZnO by grinding and subsequent heating ZnO-urea mixture. Powder Technol. 2006, 162, 33–37. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Kim, T.-H.; Dhakal, D.; Lee, S.W. Mechanistic understanding of enhanced photocatalytic activity of N-doped BiVO4 towards degradation of ibuprofen: An experimental and theoretical approach. Mol. Catal. 2019, 470, 8–18. [Google Scholar] [CrossRef]
- Elghniji, K.; Ksibi, M.; Elaloui, E. Sol–gel reverse micelle preparation and characterization of N-doped TiO2: Efficient photocatalytic degradation of methylene blue in water under visible light. J. Ind. Eng. Chem. 2012, 18, 178–182. [Google Scholar] [CrossRef]
- Okato, T.; Sakano, T.; Obara, M. Suppression of photocatalytic efficiency in highly N-doped anatase films. Phys. Rev. B 2005, 72, 115124. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, K.; Sa, R.; Li, Q.; He, C.; Yi, Z. Mechanism of enhanced photocatalytic activities on N-doped La2Ti2O7: An insight from density-functional calculations. Int. J. Hydrog. Energy 2015, 40, 980–989. [Google Scholar] [CrossRef]
- Wang, J.; Yin, S.; Komatsu, M.; Zhang, Q.; Saito, F.; Sato, T. Preparation and characterization of nitrogen doped SrTiO3 photocatalyst. J. Photochem. Photobiol. A 2004, 165, 149–156. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Cui, P.; Yang, Y.; Ke, Q.; Ha, M.N.; Zhan, W.; Ruan, F.; Wan, C.; Lei, Z. Jahn-Teller distortion assisted interstitial nitrogen engineering: Enhanced oxygen dehydrogenation activity of N-doped MnxCo3−xO4 hierarchical micro-nano particles. Nano Res. 2021, 14, 2637–2643. [Google Scholar] [CrossRef]
- Fang, Z.; Bueken, B.; De Vos, D.E.; Fischer, R.A. Defect-engineered metal-organic frameworks. Angew. Chem. Int. Ed. 2015, 54, 7234–7254. [Google Scholar] [CrossRef]
- Kim, M.; Lee, B.; Ju, H.; Kim, J.Y.; Kim, J.; Lee, S.W. Oxygen-vacancy-introduced BaSnO3−δ photoanodes with tunable band structures for efficient solar-driven water splitting. Adv. Mater. 2019, 31, 1903316. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Seristatidou, E.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic activity of N-doped and N–F co-doped TiO2 and reduction of chromium (VI) in aqueous solution: An EPR study. Appl. Catal. B 2013, 132, 460–468. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Gong, X.-Q.; Selloni, A. Reactivity of anatase TiO2 nanoparticles: The role of the minority (001) surface. J. Phys. Chem. B 2005, 109, 19560–19562. [Google Scholar] [CrossRef]
- Selloni, A. Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef]
- Takata, T.; Pan, C.; Domen, K. Recent progress in oxynitride photocatalysts for visible-light-driven water splitting. Sci. Technol. Adv. Mater. 2015, 16, 033506. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Hayashi, K.; Maeda, K.; Attfield, J.P.; Hiroi, Z.; Rondinelli, J.M.; Poeppelmeier, K.R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojamberdiev, M.; Yubuta, K.; Vequizo, J.J.M.; Yamakata, A.; Oishi, S.; Domen, K.; Teshima, K. NH3-assisted flux growth of cube-like BaTaO2N submicron crystals in a completely ionized nonaqueous high-temperature solution and their water splitting activity. Cryst. Growth Des. 2015, 15, 4663–4671. [Google Scholar] [CrossRef]
- Asakura, Y.; Nishimura, Y.; Masubuchi, Y.; Yin, S. Utility of ZnGa2O4 nanoparticles obtained hydrothermally for preparation of GaN:ZnO solid solution nanoparticles and transparent films. Eur. J. Inorg. Chem. 2019, 2019, 1999–2005. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Yin, S. Morphology control of aluminum nitride (AlN) for a novel high-temperature hydrogen sensor. Int. J. Miner. Metall. Mater. 2020, 27, 1560–1567. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Domen, K. Development of cocatalysts for photocatalytic overall water splitting on (Ga1−xZnx)(N1−xOx) solid solution. Catal. Surv. Asia 2007, 11, 145–157. [Google Scholar] [CrossRef]
- Lee, Y.; Terashima, H.; Shimodaira, Y.; Teramura, K.; Hara, M.; Kobayashi, H.; Domen, K.; Yashima, M. Zinc germanium oxynitride as a photocatalyst for overall water splitting under visible light. J. Phys. Chem. C 2007, 111, 1042–1048. [Google Scholar] [CrossRef]
- Higashi, M.; Abe, R.; Takata, T.; Domen, K. Photocatalytic overall water splitting under visible light using ATaO2N (A = Ca, Sr, Ba) and WO3 in a IO3−/I− shuttle redox mediated system. Chem. Mater. 2009, 21, 1543–1549. [Google Scholar] [CrossRef]
- Ueda, K.; Kato, H.; Kobayashi, M.; Hara, M.; Kakihana, M. Control of valence band potential and photocatalytic properties of NaxLa1−xTaO1+2xN2−2x oxynitride solid solutions. J. Mater. Chem. A 2013, 1, 3667–3674. [Google Scholar] [CrossRef]
- Asakura, Y.; Inaguma, Y.; Ueda, K.; Masubuchi, Y.; Yin, S. Synthesis of gallium oxynitride nanoparticles through hydrothermal reaction in the presence of acetylene black and their photocatalytic NOx decomposition. Nanoscale 2018, 10, 1837–1844. [Google Scholar] [CrossRef]
- Lee, K.; Lu, Y.-G.; Chuang, C.-H.; Ciston, J.; Dukovic, G. Synthesis and characterization of (Ga1−xZnx)(N1−xOx) nanocrystals with a wide range of compositions. J. Mater. Chem. A 2016, 4, 2927–2935. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Li, J.; Liu, B.; Yang, W.; Jiang, Y.; Liu, L.; Zhang, X.; Xiong, C.; Jiang, X. Band-gap tailoring and visible-light-driven photocatalytic performance of porous (GaN)1−x(ZnO)x solid solution. Dalton Trans. 2017, 46, 2643–2652. [Google Scholar] [CrossRef]
- Sun, X.; Maeda, K.; Le Faucheur, M.; Teramura, K.; Domen, K. Preparation of (Ga1−xZnx)(N1−xOx) solid-solution from ZnGa2O4 and ZnO as a photo-catalyst for overall water splitting under visible light. Appl. Catal. A 2007, 327, 114–121. [Google Scholar] [CrossRef]
- Wang, J.; Asakura, Y.; Yin, S. Preparation of (Zn1+xGe)(N2Ox) nanoparticles with enhanced NOx decomposition activity under visible light irradiation by nitridation of Zn2GeO4 nanoparticles designed precisely. Nanoscale 2019, 11, 20151–20160. [Google Scholar] [CrossRef]
- Xiong, F.Q.; Wan, L.; Li, Y.; Thomas, T.; DiSalvo, F.J.; Yang, M. Crucial Role of Donor Density in the Performance of Oxynitride Perovskite LaTiO2N for Photocatalytic Water Oxidation. ChemSusChem 2017, 10, 930–937. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Fabrication of an efficient BaTaO2N photoanode harvesting a wide range of visible light for water splitting. J. Am. Chem. Soc. 2013, 135, 10238–10241. [Google Scholar] [CrossRef]
- Knez, M.; Scholz, R.; Nielsch, K.; Pippel, E.; Hesse, D.; Zacharias, M.; Gösele, U. Monocrystalline spinel nanotube fabrication based on the Kirkendall effect. Nat. Mater. 2006, 5, 627–631. [Google Scholar]
- Solís, R.R.; Bedia, J.; Rodríguez, J.J.; Belver, C. A review on alkaline earth metal titanates for applications in photocatalytic water purification. Chem. Eng. J. 2021, 409, 128110. [Google Scholar]
- Hwang, D.W.; Kim, H.G.; Lee, J.S.; Kim, J.; Li, W.; Oh, S.H. Photocatalytic hydrogen production from water over M-doped La2Ti2O7 (M = Cr, Fe) under visible light irradiation (λ > 420 nm). J. Phys. Chem. B 2005, 109, 2093–2102. [Google Scholar]
- Wang, Z.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water over Ag-modified La2Ti2O7. Appl. Catal. B 2015, 163, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Izawa, C.; Watanabe, T. Direct fabrication of La2Ti2O7 films on titanium metal substrate by hydrothermal reaction. Trans. Mater. Res. Soc. Japan 2011, 36, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xu, D.; Liu, J.; Li, K.; Wang, H. Preparation and photocatalytic properties of CdS/La2Ti2O7 nanocomposites under visible light. Chem. Eng. J. 2011, 168, 455–460. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Yamaguchi, A.; Yubuta, K.; Oishi, S.; Teshima, K. Fabrication of La2Ti2O7 crystals using an alkali-metal molybdate flux growth method and their nitridability to form LaTiO2N crystals under a high-temperature NH3 atmosphere. Inorg. Chem. 2015, 54, 3237–3244. [Google Scholar] [CrossRef]
- Onozuka, K.; Kawakami, Y.; Imai, H.; Yokoi, T.; Tatsumi, T.; Kondo, J. Perovskite-type La2Ti2O7 mesoporous photocatalyst. J. Solid State Chem. 2012, 192, 87–92. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Yan, X.; Addiego, C.; Jiang, L.; Wang, H.; Zhu, J. Origin of the enhanced piezoelectricity of vanadium-doped La2Ti2O7 ceramics. J. Phys. Chem. C 2021, 125, 26180–26187. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Hong, Z.; Zhi, M.; Sakla, A.; Xiang, C.; Wu, N. Photocatalytic generation of hydrogen with visible-light nitrogen-doped lanthanum titanium oxides. Catal. Today 2013, 199, 48–52. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, J.; Fujitsuka, M.; Majima, T. Graphitic-C3N4 hybridized N-doped La2Ti2O7 two-dimensional layered composites as efficient visible-light-driven photocatalyst. Appl. Catal. B 2017, 202, 191–198. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar]
- Wan, S.; Qi, F.; Jin, W.; Guo, X.; Liu, H.; Zhao, J.; Zhang, J.; Tang, C. Construction of ultrafine Ag3PO4 nanoparticle and La2Ti2O7nanosheet 0D/2D heterojunctions with improved photocatalytic performance. J. Alloys Compd. 2018, 740, 901–909. [Google Scholar] [CrossRef]
- Ao, Y.; Wang, K.; Wang, P.; Wang, C.; Hou, J. Synthesis of novel 2D-2D pn heterojunction BiOBr/La2Ti2O7 composite photocatalyst with enhanced photocatalytic performance under both UV and visible light irradiation. Appl. Catal. B 2016, 194, 157–168. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Wu, X.; Zhang, G. Vacancy mediated Z-scheme charge transfer in a 2D/2D La2Ti2O7/g-C3N4 nanojunction as a bifunctional photocatalyst for solar-to-energy conversion. J. Mater. Chem. A 2020, 8, 13241–13247. [Google Scholar]
- Shiraishi, Y.; Shiota, S.; Hirakawa, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Titanium dioxide/reduced graphene oxide hybrid photocatalysts for efficient and selective partial oxidation of cyclohexane. ACS Catal. 2017, 7, 293–300. [Google Scholar] [CrossRef]
- Ren, L.; Yi, X.; Tong, L.; Zhou, W.; Wang, D.; Liu, L.; Ye, J. Nitrogen-doped ultrathin graphene encapsulated Cu nanoparticles decorated on SrTiO3 as an efficient water oxidation photocatalyst with activity comparable to BiVO4 under visible-light irradiation. Appl. Catal. B 2020, 279, 119352. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-pot hydrothermal synthesis of Nitrogen-doped graphene as high-performance anode materials for lithium ion batteries. Sci. Rep. 2016, 6, 26146. [Google Scholar]
- Hua, Z.; Zhang, X.; Bai, X.; Lv, L.; Ye, Z.; Huang, X. Nitrogen-doped perovskite-type La2Ti2O7 decorated on graphene composites exhibiting efficient photocatalytic activity toward bisphenol A in water. J. Colloid Interface Sci. 2015, 450, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Cushing, S.K.; Li, J.; Hao, S.; Wu, N. Enhancement of solar hydrogen generation by synergistic interaction of La2Ti2O7 photocatalyst with plasmonic gold nanoparticles and reduced graphene oxide nanosheets. ACS Catal. 2015, 5, 1949–1955. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B 2011, 108, 100–107. [Google Scholar]
- Xia, M.; Yan, X.; Li, H.; Wells, N.; Yang, G. Well-designed efficient charge separation in 2D/2D N doped La2Ti2O7/ZnIn2S4 heterojunction through band structure/morphology regulation synergistic effect. Nano Energy 2020, 78, 105401. [Google Scholar]
- Moriya, Y.; Takata, T.; Domen, K. Recent progress in the development of (oxy) nitride photocatalysts for water splitting under visible-light irradiation. Coord. Chem. Rev. 2013, 257, 1957–1969. [Google Scholar] [CrossRef]
- Kasahara, A.; Nukumizu, K.; Hitoki, G.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Photoreactions on LaTiO2N under visible light irradiation. J. Phys. Chem. A 2002, 106, 6750–6753. [Google Scholar] [CrossRef]
- Zhang, F.; Yamakata, A.; Maeda, K.; Moriya, Y.; Takata, T.; Kubota, J.; Teshima, K.; Oishi, S.; Domen, K. Cobalt-modified porous single-crystalline LaTiO2N for highly efficient water oxidation under visible light. J. Am. Chem. Soc. 2012, 134, 8348–8351. [Google Scholar] [CrossRef]
- Maegli, A.E.; Pokrant, S.; Hisatomi, T.; Trottmann, M.; Domen, K.; Weidenkaff, A. Enhancement of photocatalytic water oxidation by the morphological control of LaTiO2N and cobalt oxide catalysts. J. Phys. Chem. C 2013, 118, 16344–16351. [Google Scholar] [CrossRef]
- Matsukawa, M.; Ishikawa, R.; Hisatomi, T.; Moriya, Y.; Shibata, N.; Kubota, J.; Ikuhara, Y.; Domen, K. Enhancing photocatalytic activity of LaTiO2N by removal of surface reconstruction layer. Nano Lett. 2014, 14, 1038–1041. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Ruan, X.; Song, H.; Lou, Z.; Ye, Z.; Zhu, L. Enhancing photocatalytic activity for visible-light-driven H2 generation with the surface reconstructed LaTiO2N nanostructures. Nano Energy 2015, 12, 775–784. [Google Scholar] [CrossRef]
- Inoue, Y. Photocatalytic water splitting by RuO2-loaded metal oxides and nitrides with d0- and d10-related electronic configurations. Energy Environ. Sci. 2009, 2, 364–386. [Google Scholar]
- Hu, J.; Song, E.; Ye, S.; Zhou, B.; Zhang, Q. Anomalous spontaneous-reduction of Mn7+/Mn4+ to Mn2+ and luminescence properties in Zn2GeO4:Mn. J. Mater. Chem. C 2017, 5, 3343–3351. [Google Scholar] [CrossRef]
- Huang, J.; Ding, K.; Hou, Y.; Wang, X.; Fu, X. Synthesis and photocatalytic activity of Zn2GeO4 nanorods for the degradation of organic pollutants in water. ChemSusChem 2008, 1, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wen, F.; Jiang, H.; Yang, J.; Ying, P.; Li, C. The synergistic effects of two co-catalysts on Zn2GeO4 on photocatalytic water splitting. Catal. Lett. 2010, 134, 78–86. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Shao, Z.; Wang, H. Morphology-dependent performance of Zn2GeO4 as a high-performance anode material for rechargeable lithium ion batteries. J. Mater. Chem. A 2015, 3, 15274–15279. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Z.; Ouyang, X.; Lin, Z. Core-shell Zn2GeO4 nanorods and their size-dependent photoluminescence properties. Nanoscale 2013, 5, 12335–12341. [Google Scholar] [CrossRef] [Green Version]
- Yi, R.; Feng, J.; Lv, D.; Gordin, M.L.; Chen, S.; Choi, D.; Wang, D. Amorphous Zn2GeO4 nanoparticles as anodes with high reversible capacity and long cycling life for Li-ion batteries. Nano Energy 2013, 2, 498–504. [Google Scholar] [CrossRef]
- Yan, S.; Wan, L.; Li, Z.; Zou, Z. Facile temperature-controlled synthesis of hexagonal Zn2GeO4 nanorods with different aspect ratios toward improved photocatalytic activity for overall water splitting and photoreduction of CO2. Chem. Commun. 2011, 47, 5632–5634. [Google Scholar] [CrossRef] [Green Version]
- Boppana, V.B.R.; Hould, N.D.; Lobo, R.F. Synthesis, characterization and photocatalytic properties of novel zinc germanate nano-materials. J. Solid State Chem. 2011, 184, 1054–1062. [Google Scholar]
- Yan, S.; Wang, J.; Zou, Z. An anion-controlled crystal growth route to Zn2GeO4 nanorods for efficient photocatalytic conversion of CO2 into CH4. Dalton Trans. 2013, 42, 12975–12979. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, R.; Qiao, Y.; Liu, W.; Wang, K.; Wang, X.; Wang, C. Preparation of self-assembled N-Zn2GeO4 nanocomposite and their photocatalytic properties. Int. J. Electrochem. Sci 2020, 15, 10243–10252. [Google Scholar] [CrossRef]
- Lan, N.; Tuan, D.; Tuan, T.; Kien, N.; Thang, C.; Tung, N.; Giang, N. Effect of Nitrogen Doping on the Structural and Optical Properties of Zn2GeO4 Phosphors. J. Appl. Spectrosc. 2022, 89, 652–657. [Google Scholar]
- Yang, K.; Dai, Y.; Huang, B. Study of the nitrogen concentration influence on N-doped TiO2 anatase from first-principles calculations. J. Phys. Chem. C 2007, 111, 12086–12090. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Czoska, A.; Paganini, M.; Giamello, E. Density functional theory and electron paramagnetic resonance study on the effect of N − F codoping of TiO2. Chem. Mater. 2008, 20, 3706–3714. [Google Scholar] [CrossRef]
- Li, Y.; Ding, K.; Cheng, B.; Zhang, Y.; Lu, Y. N, F-monodoping and N/F-codoping effects on the electronic structures and optical performances of Zn2GeO4. Phys. Chem. Chem. Phys. 2015, 17, 5613–5623. [Google Scholar]
- Lee, Y.; Teramura, K.; Hara, M.; Domen, K. Modification of (Zn1+xGe)(N2Ox) solid solution as a visible light driven photocatalyst for overall water splitting. Chem. Mater. 2007, 19, 2120–2127. [Google Scholar] [CrossRef]
- Wang, J.; Asakura, Y.; Yin, S. Synthesis of zinc germanium oxynitride nanotube as a visible-light driven photocatalyst for NOx decomposition through ordered morphological transformation from Zn2GeO4 nanorod obtained by hydrothermal reaction. J. Hazard. Mater. 2020, 396, 122709. [Google Scholar] [CrossRef]
- Sato, J.; Kobayashi, H.; Ikarashi, K.; Saito, N.; Nishiyama, H.; Inoue, Y. Photocatalytic activity for water decomposition of RuO2-dispersed Zn2GeO4 with d10 configuration. J. Phys. Chem. B 2004, 108, 4369–4375. [Google Scholar] [CrossRef]
- Yang, M.; Ji, Y.; Liu, W.; Wang, Y.; Liu, X. Facile microwave-assisted synthesis and effective photocatalytic hydrogen generation of Zn2GeO4 with different morphology. RSC Adv. 2014, 4, 15048–15054. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z. High-yield synthesis of ultralong and ultrathin Zn2GeO4 nanoribbons toward improved photocatalytic reduction of CO2 into renewable hydrocarbon fuel. J. Am. Chem. Soc. 2010, 132, 14385–14387. [Google Scholar] [CrossRef]
- Yu, L.; Zou, R.; Zhang, Z.; Song, G.; Chen, Z.; Yang, J.; Hu, J. A Zn2GeO4–ethylenediamine hybrid nanoribbon membrane as a recyclable adsorbent for the highly efficient removal of heavy metals from contaminated water. Chem. Commun. 2011, 47, 10719–10721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Ouyang, S.; Li, P.; Zhang, Y.; Xi, G.; Kako, T.; Ye, J. Ion-exchange synthesis of a micro/mesoporous Zn2GeO4 photocatalyst at room temperature for photoreduction of CO2. Chem. Commun. 2011, 47, 2041–2043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Tian, Z.; Chen, X.; Gao, J.; Zou, Z. Zn2GeO4 crystal splitting toward sheaf-like, hyperbranched nanostructures and photocatalytic reduction of CO2 into CH4 under visible light after nitridation. J. Mater. Chem. 2012, 22, 2033–2038. [Google Scholar] [CrossRef]
- Chen, H.; Yu, G.; Li, G.D.; Xie, T.; Sun, Y.; Liu, J.; Li, H.; Huang, X.; Wang, D.; Asefa, T. Unique electronic structure in a porous Ga-In bimetallic oxide nano-photocatalyst with atomically thin pore walls. Angew. Chem. Int. Ed. 2016, 55, 11442–11446. [Google Scholar] [CrossRef] [PubMed]
| Photocatalyst | N Doping Method | Bandgap Energy (eV) | N Amount (at. %) | Target Photocatalytic Reaction | Ref |
|---|---|---|---|---|---|
| N-LTO | NH3 treatment | 2.51 | 3.3 | Decomposition of methyl orange under UV and visible light | [23] |
| N-LTO | NH3 treatment | 2.84 | 4.79 | Decomposition of NOx under UV and visible light | [31] |
| N-LTO/rGO | NH3 treatment | 2.88 | 2.1 | Decomposition of bisphenol A under UV and visible light | [81] |
| N-LTO/g-C3N4 | Hydrothermal method + acid treatment | 2.65 | 5.8 | Degradation of MO and reductive generation of H2 under visible light | [73] |
| N-LTO/ZnIn2S4 | Hydrothermal method + acid treatment | 3.35 | - | H2 evolution reaction | [84] |
| Au@Pt-N-LTO/rGO | NH3 treatment | 2.25 | - | H2 evolution reaction | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hasegawa, T.; Asakura, Y.; Yin, S. Recent Advances in Ternary Metal Oxides Modified by N Atom for Photocatalysis. Catalysts 2022, 12, 1568. https://doi.org/10.3390/catal12121568
Wang J, Hasegawa T, Asakura Y, Yin S. Recent Advances in Ternary Metal Oxides Modified by N Atom for Photocatalysis. Catalysts. 2022; 12(12):1568. https://doi.org/10.3390/catal12121568
Chicago/Turabian StyleWang, Jingwen, Takuya Hasegawa, Yusuke Asakura, and Shu Yin. 2022. "Recent Advances in Ternary Metal Oxides Modified by N Atom for Photocatalysis" Catalysts 12, no. 12: 1568. https://doi.org/10.3390/catal12121568
APA StyleWang, J., Hasegawa, T., Asakura, Y., & Yin, S. (2022). Recent Advances in Ternary Metal Oxides Modified by N Atom for Photocatalysis. Catalysts, 12(12), 1568. https://doi.org/10.3390/catal12121568