A Review on Green Hydrogen Valorization by Heterogeneous Catalytic Hydrogenation of Captured CO2 into Value-Added Products
Abstract
:1. Introduction
2. Catalytic Hydrogenation of CO2 to Renewable Methane
3. Catalytic Hydrogenation of CO2 in Power-to-Liquid (P2L) Processes
3.1. Catalytic Hydrogenation of CO2 to Renewable Methanol
3.1.1. Hydrogenation CO2 to Methanol using Cu-Based Catalysts
3.1.2. CO2 Hydrogenation to Methanol by Noble or Rare Metal-Based Catalysts
3.1.3. CO2 Hydrogenation to Methanol over Mixed Oxide-Based Catalysts
| Metal Oxides | Conversion (%) | SCH3OH (%) | T (°C) | P (Atm.) | Ref. |
|---|---|---|---|---|---|
| NiO/In2O3 | 1–3 | 50–60 | 250 | 30 | [276] a |
| In2O3 | 1–10 | 45–95 | 240–330 | 30 | [277] b |
| InOx/ZrO2 | 0.5–2.5 | 70−80 | 250–300 | 50 | [278] a |
| ZnO/ZrO2 | 10 | 86–91 | 315–320 | 50 | [279] c |
| ZrO2/In2O3 | 0.4–5.0 | 85 | 220–300 | 50 | [280] a |
| GaxIn2−xO3 | 7–35 | 0.5–35 | 320–400 | 30 | [281] a |
| In2O3-ZrO2 | 3–11 | 53–91 | 255–300 | 40 | [282] |
| MaZrOx d | 4.3–12.4 | 80 | 250–300 | 50 | [283] |
| GaZnZrOx | 7.7–8.8 | 86–88 | 320 | 50 | [274] |
| In2O3/ZrO2 | e | e | 270–310 | 30–55 | [284] |
| In2O3 | 17 | 92.4 | 300 | 50 | [285] |
| In2O3/Support f | 0.1–6.0 | 5–40 | 220–300 | 1.0 | [286] a |
| In2O3/Support g | 1–20 | 5–51 | 260–360 | 30 | [287] |
| ZnO/ZrO2 | 9.2 | 50–95 | 320 | 30 | [288] |
| MnOx/Co3O4 | 3–57 | 2–22 | 250 | 10 | [289] |
| GaxIn2−xO3 | 7–38 | --- | 320–400 | 30 | [281] |
| ZnO/ZrO2 | 10 | 10–85 | 320 | 50 | [272] |
| Co3O4/In2O3 | 10 | 30–70 | 300 | 40 | [290] |
| ZnZrOx h | 1–18 | 30–90 | 200–360 | 45 | [291] |
| In2O3/ZrO2 | 3–8 | 65–90 | 300 | 50 | [292] |
| CoxOy/MgO | 7–35 | 8–30 | i | 1.0 | [293] |
| InNi3C0.5/ZrO2 | 25.7 | 90.2 | 325 | 60 | [294] a |
| In2O3/ZrO2 | 5–30 | --- | 320–400 | 20 | [295] |
| ZrZnOx/zeolite | 1–8 | 5–30 | 400 | 30 | [273] |
| In2O3/GO j | 1–14 | 5–100 | 200–450 | 30 | [296] |
| In2O3 | 4–18 | 20–85 | 260–360 | 40 | [297] |
3.1.4. Methanol Reaction Process for CO2 Hydrogenation to Fuels and Chemicals
3.2. One-Step Process for the Conversion of CO2 to Light Olefins
3.2.1. CO2 Hydrogenation in a One-Step Process over Bifunctional or Hybrid Catalysts
3.2.2. Modified Fischer-Tropsch Synthesis Route
4. Concluding Remarks, Challenges, and Research Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Streimikiene, D.; Balezentis, T.; Mardani, A.; Cavallaro, F.; Liao, H. A review of greenhouse gas emission profiles, dynamics, and climate change mitigation efforts across the key climate change players. J. Clean. Prod. 2019, 234, 1113–1133. [Google Scholar] [CrossRef]
- Teske, S.; Giurco, D.; Morris, T.; Nagrath, K.; Mey, F.; Briggs, C.; Dominish, E.; Florin, N. Achieving the Paris Climate Agreement Goals: Global and Regional 100% Renewable Energy Scenarios to Achieve the Paris Agreement Goals with Non-Energy GHG Pathways for +1.5 °C and +2 °C; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process analysis overview of ionic liquids on CO2 chemical capture. Chem. Eng. J. 2020, 390, 124509. [Google Scholar] [CrossRef]
- Anwar, M.; Fayyaz, A.; Sohail, N.; Khokhar, M.; Baqar, M.; Yasar, A.; Rasool, K.; Nazir, A.; Raja, M.; Rehan, M. CO2 utilization: Turning greenhouse gas into fuels and valuable products. J. Environ. Manag. 2020, 260, 110059. [Google Scholar] [CrossRef] [PubMed]
- Kleij, A.W.; North, M.; Urakawa, A. CO2 Catalysis; Wiley Online Library: New York, NY, USA, 2017; Volume 10, pp. 1036–1038. [Google Scholar]
- Sahoo, P.K.; Zhang, Y.; Das, S. CO2-promoted reactions: An emerging concept for the synthesis of fine chemicals and pharmaceuticals. ACS Catal. 2021, 11, 3414–3442. [Google Scholar] [CrossRef]
- Zahed, M.A.; Movahed, E.; Khodayari, A.; Zanganeh, S.; Badamaki, M. Biotechnology for carbon capture and fixation: Critical review and future directions. J. Environ. Manag. 2021, 293, 112830. [Google Scholar] [CrossRef]
- Chauvy, R.; Meunier, N.; Thomas, D.; De Weireld, G. Selecting emerging CO2 utilization products for short-to mid-term deployment. Appl. Energy 2019, 236, 662–680. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Roy, S.; Cherevotan, A.; Peter, S. Thermochemical CO2 hydrogenation to single carbon products: Scientific and technological challenges. ACS Energy Lett. 2018, 3, 1938–1966. [Google Scholar] [CrossRef] [Green Version]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Z.; Peng, S.H.; Sage, V.; Sun, Z. A critical perspective on CO2 conversions into chemicals and fuels. J. Nanosci. Nanotechnol. 2019, 19, 3097–3109. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Potrč, S.; Čuček, L.; Martin, M.; Kravanja, Z. Sustainable renewable energy supply networks optimization–The gradual transition to a renewable energy system within the European Union by 2050. Renew. Sustain. Energy Rev. 2021, 146, 111186. [Google Scholar] [CrossRef]
- Southall, E.; Lukashuk, L. Analysis of Liquid Organic Hydrogen Carrier Systems. Johns. Matthey Technol. Rev. 2022, 66, 271–284. [Google Scholar] [CrossRef]
- Safari, A.; Roy, J.; Assadi, M. Petroleum Sector-Driven Roadmap for Future Hydrogen Economy. Appl. Sci. 2021, 11, 10389. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Transformation of CO2 into liquid fuels and synthetic natural gas using green hydrogen: A comparative analysis. Fuel 2021, 291, 120111. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yu, J.C.-C.; Nguyen, V.-H.; Wu, J.C.-S.; Wang, W.-H.; Kočí, K. Reactor design for CO2 photo-hydrogenation toward solar fuels under ambient temperature and pressure. Catalysts 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Hong, X.; Thaore, V.B.; Karimi, I.A.; Farooq, S.; Wang, X.; Usadi, A.K.; Chapman, B.R.; Johnson, R.A. Techno-enviro-economic analyses of hydrogen supply chains with an ASEAN case study. Int. J. Hydrogen Energy 2021, 46, 32914–32928. [Google Scholar] [CrossRef]
- Cannone, S.F.; Lanzini, A.; Santarelli, M. A review on CO2 capture technologies with focus on CO2-enhanced methane recovery from hydrates. Energies 2021, 14, 387. [Google Scholar] [CrossRef]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar] [CrossRef]
- Sifat, N.S.; Haseli, Y. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, W.; Ren, X.; Shen, W.; Dong, L. Technology selection for sustainable hydrogen production: A multi-criteria assessment framework under uncertainties based on the combined weights and interval best-worst projection method. Int. J. Hydrogen Energy 2020, 45, 34396–34411. [Google Scholar] [CrossRef]
- Catalan, L.J.; Rezaei, E. Coupled hydrodynamic and kinetic model of liquid metal bubble reactor for hydrogen production by noncatalytic thermal decomposition of methane. Int. J. Hydrogen Energy 2020, 45, 2486–2503. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, J.; Liu, X.; Wang, H.; Zhang, W.; Yang, Q.; Ma, J.; Dong, X.; Yoo, S.J. Selective hydrogenation of CO2 to ethanol over cobalt catalysts. Angew. Chem. Int. Ed. 2018, 57, 6104–6108. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, X. Bifunctional {Pb10K2}–Organic Framework for High Catalytic Activity in Cycloaddition of CO2 with Epoxides and Knoevenagel Condensation. ACS Catal. 2022, 12, 10373–10383. [Google Scholar] [CrossRef]
- Lv, H.; Chen, H.; Fan, L.; Zhang, X. Nanocage-Based Tb3+-Organic Framework for Efficiently Catalyzing the Cycloaddition Reaction of CO2 with Epoxides and Knoevenagel Condensation. Inorg. Chem. 2022, 61, 15558–15568. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tan, L.; Tan, M.; Zhang, P.; Fang, Y.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics. ACS Catal. 2018, 9, 895–901. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Lonis, F.; Tola, V.; Cau, G. Renewable methanol production and use through reversible solid oxide cells and recycled CO2 hydrogenation. Fuel 2019, 246, 500–515. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Alarcón, A.; Guilera, J.; Díaz, J.A.; Andreu, T. Optimization of nickel and ceria catalyst content for synthetic natural gas production through CO2 methanation. Fuel Process. Technol. 2019, 193, 114–122. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 2021, 9, 105460. [Google Scholar] [CrossRef]
- Tada, S.; Nagase, H.; Fujiwara, N.; Kikuchi, R. What are the best active sites for CO2 methanation over Ni/CeO2? Energy Fuels 2021, 35, 5241–5251. [Google Scholar] [CrossRef]
- Hu, F.; Ye, R.; Lu, Z.-H.; Zhang, R.; Feng, G. Structure–Activity Relationship of Ni-Based Catalysts toward CO2 Methanation: Recent Advances and Future Perspectives. Energy Fuels 2022, 36, 1–156. [Google Scholar] [CrossRef]
- Mohd Ridzuan, N.D.; Shaharun, M.S.; Anawar, M.A.; Ud-Din, I. Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress. Catalysts 2022, 12, 469. [Google Scholar] [CrossRef]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B Environ. 2020, 264, 118494. [Google Scholar] [CrossRef]
- Feng, F.; Song, G.; Xiao, J.; Shen, L.; Pisupati, S.V. Carbon deposition on Ni-based catalyst with TiO2 as additive during the syngas methanation process in a fluidized bed reactor. Fuel 2019, 235, 85–91. [Google Scholar] [CrossRef]
- Alrafei, B.; Polaert, I.; Ledoux, A.; Azzolina-Jury, F. Remarkably stable and efficient Ni and Ni-Co catalysts for CO2 methanation. Catal. Today 2020, 346, 23–33. [Google Scholar] [CrossRef]
- Champon, I.; Bengaouer, A.; Chaise, A.; Thomas, S.; Roger, A.-C. Carbon dioxide methanation kinetic model on a commercial Ni/Al2O3 catalyst. J. CO2 Util. 2019, 34, 256–265. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La-Al2O3 catalysts: A competitive system for CO2 methanation. Appl. Catal. B Environ. 2019, 248, 286–297. [Google Scholar] [CrossRef]
- Liu, S.-S.; Jin, Y.-Y.; Han, Y.; Zhao, J.; Ren, J. Highly stable and coking resistant Ce promoted Ni/SiC catalyst towards high temperature CO methanation. Fuel Process. Technol. 2018, 177, 266–274. [Google Scholar] [CrossRef]
- Beierlein, D.; Haeussermann, D.; Pfeifer, M.; Schwarz, T.; Stoewe, K.; Traa, Y.; Klemm, E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive? Appl. Catal. B Environ. 2019, 247, 200–219. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Q. Lanthanum-modified MCF-derived nickel phyllosilicate catalyst for enhanced CO2 methanation: A comprehensive study. ACS Appl. Mater. Interfaces 2020, 12, 19587–19600. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Abate, S.; Perathoner, S.; Chen, S.; Centi, G. CO2 methanation over Ni catalysts based on ternary and quaternary mixed oxide: A comparison and analysis of the structure-activity relationships. Catal. Today 2018, 304, 181–189. [Google Scholar] [CrossRef]
- Romero-Sáez, M.D.; Dongil, A.B.; Benito, N.; Espinoza-González, R.; Escalona, N.; Gracia, F. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: A comparison between two impregnation strategies. Appl. Catal. B Environ. 2018, 237, 817–825. [Google Scholar] [CrossRef]
- Li, L.; Zeng, W.; Song, M.; Wu, X.; Li, G.; Hu, C. Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts 2022, 12, 244. [Google Scholar] [CrossRef]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.-E.; Yang, B.; Miao, Z.; Hu, X. Recent progresses in constructing the highly efficient Ni based catalysts with advanced low-temperature activity toward CO2 methanation. Front. Chem. 2020, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.H.; de Luna, G.S.; Angelucci, S.; Canciani, A.; Jones, W.; Decarolis, D.; Ospitali, F.; Aguado, E.R.; Rodríguez-Castellón, E.; Fornasari, G. Understanding structure-activity relationships in highly active La promoted Ni catalysts for CO2 methanation. Appl. Catal. B Environ. 2020, 278, 119256. [Google Scholar] [CrossRef]
- Liang, C.; Hu, X.; Wei, T.; Jia, P.; Zhang, Z.; Dong, D.; Zhang, S.; Liu, Q.; Hu, G. Methanation of CO2 over Ni/Al2O3 modified with alkaline earth metals: Impacts of oxygen vacancies on catalytic activity. Int. J. Hydrogen Energy 2019, 44, 8197–8213. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl. Catal. B Environ. 2019, 243, 262–272. [Google Scholar] [CrossRef]
- Sreedhar, I.; Varun, Y.; Singh, S.A.; Venugopal, A.; Reddy, B.M. Developmental trends in CO2 methanation using various catalysts. Catal. Sci. Technol. 2019, 9, 4478–4504. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [Green Version]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castello, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2020, 356, 419–432. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Wang, C.-C. Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts. Catalysts 2020, 10, 1112. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-based catalysts for CO2 methanation: A review. Nanomaterials 2020, 11, 28. [Google Scholar] [CrossRef]
- Polanski, J.; Lach, D.; Kapkowski, M.; Bartczak, P.; Siudyga, T.; Smolinski, A. Ru and Ni—Privileged Metal Combination for Environmental Nanocatalysis. Catalysts 2020, 10, 992. [Google Scholar] [CrossRef]
- Guilera, J.; del Valle, J.; Alarcón, A.; Díaz, J.A.; Andreu, T. Metal-oxide promoted Ni/Al2O3 as CO2 methanation micro-size catalysts. J. CO2 Util. 2019, 30, 11–17. [Google Scholar] [CrossRef]
- Baysal, Z.; Kureti, S. CO2 methanation on Mg-promoted Fe catalysts. Appl. Catal. B Environ. 2020, 262, 118300. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Brückner, A.; Behm, R.J. Encapsulation of Ru nanoparticles: Modifying the reactivity toward CO and CO2 methanation on highly active Ru/TiO2 catalysts. Appl. Catal. B Environ. 2020, 270, 118846. [Google Scholar] [CrossRef]
- Dreyer, J.A.; Li, P.; Zhang, L.; Beh, G.K.; Zhang, R.; Sit, P.H.-L.; Teoh, W.Y. Influence of the oxide support reducibility on the CO2 methanation over Ru-based catalysts. Appl. Catal. B Environ. 2017, 219, 715–726. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Moioli, E.; Chen, W.; Zhang, M.; Terlingen, B.J.; Zijlstra, B.; Filot, I.A.; Züttel, A.; Pidko, E.A.; Hensen, E.J. Efficient base-metal NiMn/TiO2 catalyst for CO2 methanation. Acs Catal. 2019, 9, 7823–7839. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zhao, G.; Yang, H.; Yuan, M.; An, X.; Zhou, H.; Qiao, Y.; Tian, Y. CO2 methanation over ordered mesoporous NiRu-doped CaO-Al2O3 nanocomposites with enhanced catalytic performance. Int. J. Hydrogen Energy 2018, 43, 239–250. [Google Scholar] [CrossRef]
- Ewald, S.; Kolbeck, M.; Kratky, T.; Wolf, M.; Hinrichsen, O. On the deactivation of Ni-Al catalysts in CO2 methanation. Appl. Catal. A Gen. 2019, 570, 376–386. [Google Scholar] [CrossRef]
- Men, Y.; Fang, X.; Gu, Q.; Singh, R.; Wu, F.; Danaci, D.; Zhao, Q.; Xiao, P.; Webley, P.A. Synthesis of Ni5Ga3 catalyst by Hydrotalcite-like compound (HTlc) precursors for CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2020, 275, 119067. [Google Scholar] [CrossRef]
- Ricca, A.; Truda, L.; Palma, V. Study of the role of chemical support and structured carrier on the CO2 methanation reaction. Chem. Eng. J. 2019, 377, 120461. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Laganà, M.; Ferraro, M.; Antonucci, V. High-temperature CO2 methanation over structured Ni/GDC catalysts: Performance and scale-up for Power-to-Gas application. Fuel Process. Technol. 2020, 202, 106365. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential role of the support for nickel-based CO2 methanation catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of support composition on the performance of nickel catalysts in CO2 methanation reaction. Catal. Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, S.; AlKhoori, A.A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable nickel catalysts supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation reaction. Appl. Catal. B Environ. 2021, 282, 119562. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Wu, X.; Wen, J.; Zhao, R.; Li, Z.; Tian, G.; Zhang, Q.; Ning, P.; Hao, J. Frustrated Lewis Pairs Boosting Low-Temperature CO2 Methanation Performance over Ni/CeO2 Nanocatalysts. ACS Catal. 2022, 12, 10587–10602. [Google Scholar] [CrossRef]
- Ye, R.-P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 2020, 268, 118474. [Google Scholar] [CrossRef]
- Cerda-Moreno, C.; Chica, A.; Keller, S.; Rautenberg, C.; Bentrup, U. Ni-sepiolite and Ni-todorokite as efficient CO2 methanation catalysts: Mechanistic insight by operando DRIFTS. Appl. Catal. B Environ. 2020, 264, 118546. [Google Scholar] [CrossRef]
- Miao, C.; Shang, K.; Liang, L.; Chen, S.; Ouyang, J. Efficient and Stable Ni/ZSM-5@ MCM-41 Catalyst for CO2 Methanation. ACS Sustain. Chem. Eng. 2022, 10, 12771–12782. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, L.; Zhu, Z.; Li, Z. La-promoted Ni/Mg-Al catalysts with highly enhanced low-temperature CO2 methanation performance. Int. J. Hydrogen Energy 2018, 43, 2197–2206. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castello, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B Environ. 2020, 265, 118538. [Google Scholar] [CrossRef]
- Bobadilla, L.; Garcilaso, V.; Centeno, M.; Odriozola, J. CO2 reforming of methane over Ni-Ru supported catalysts: On the nature of active sites by operando DRIFTS study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar]
- Renda, S.; Ricca, A.; Palma, V. Study of the effect of noble metal promotion in Ni-based catalyst for the Sabatier reaction. Int. J. Hydrogen Energy 2021, 46, 12117–12127. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Precursor salts influence in Ruthenium catalysts for CO2 hydrogenation to methane. Appl. Energy 2020, 279, 115767. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Ru–Ni/MgAl2O4 structured catalyst for CO2 methanation. Renew. Energy 2020, 161, 120–132. [Google Scholar] [CrossRef]
- Salomone, F.; Giglio, E.; Ferrero, D.; Santarelli, M.; Pirone, R.; Bensaid, S. Techno-economic modelling of a Power-to-Gas system based on SOEC electrolysis and CO2 methanation in a RES-based electric grid. Chem. Eng. J. 2019, 377, 120233. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Nohl, M.; Dittrich, L.; Foit, S.R.; de Haart, L.; Eichel, R.A.; Palkovits, R. Integrated Co-Electrolysis and Syngas Methanation for the Direct Production of Synthetic Natural Gas from CO2 and H2O. ChemSusChem 2021, 14, 2295–2302. [Google Scholar] [CrossRef]
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost benefits of optimizing hydrogen storage and methanation capacities for Power-to-Gas plants in dynamic operation. Appl. Energy 2020, 257, 113967. [Google Scholar] [CrossRef]
- Inkeri, E.; Tynjälä, T.; Karjunen, H. Significance of methanation reactor dynamics on the annual efficiency of power-to-gas-system. Renew. Energy 2021, 163, 1113–1126. [Google Scholar] [CrossRef]
- Park, S.; Choi, K.; Lee, C.; Kim, S.; Yoo, Y.; Chang, D. Techno-economic analysis of adiabatic four-stage CO2 methanation process for optimization and evaluation of power-to-gas technology. Int. J. Hydrogen Energy 2021, 46, 21303–21317. [Google Scholar] [CrossRef]
- Skorek-Osikowska, A. Thermodynamic and environmental study on synthetic natural gas production in power to gas approaches involving biomass gasification and anaerobic digestion. Int. J. Hydrogen Energy 2022, 47, 3284–3293. [Google Scholar] [CrossRef]
- Straka, P. A comprehensive study of Power-to-Gas technology: Technical implementations overview, economic assessments, methanation plant as auxiliary operation of lignite-fired power station. J. Clean. Prod. 2021, 311, 127642. [Google Scholar] [CrossRef]
- Fuentes, I.; Gracia, F. Fluid dynamic analytical model of CO2 methanation in a microreactor with potential application in Power-to-Gas technology. Chem. Eng. Sci. 2022, 251, 117465. [Google Scholar] [CrossRef]
- Giglio, E.; Pirone, R.; Bensaid, S. Dynamic modelling of methanation reactors during start-up and regulation in intermittent power-to-gas applications. Renew. Energy 2021, 170, 1040–1051. [Google Scholar] [CrossRef]
- Bailera, M.; Peña, B.; Lisbona, P.; Marín, J.; Romeo, L.M. Lab-scale experimental tests of power to gas-oxycombustion hybridization: System design and preliminary results. Energy 2021, 226, 120375. [Google Scholar] [CrossRef]
- Jensen, M.; Ottosen, L.; Kofoed, M. H2 gas-liquid mass transfer: A key element in biological Power-to-Gas methanation. Renew. Sustain. Energy Rev. 2021, 147, 111209. [Google Scholar] [CrossRef]
- Hodges, A.; Hoang, A.L.; Tsekouras, G.; Wagner, K.; Lee, C.-Y.; Swiegers, G.F.; Wallace, G.G. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 2022, 13, 1304. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Sarp, S.; Hernandez, S.G.; Chen, C.; Sheehan, S.W. Alcohol production from carbon dioxide: Methanol as a fuel and chemical feedstock. Joule 2021, 5, 59–76. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.; Lim, D.; Brigljević, B.; Cho, W.; Cho, H.-S.; Kim, C.-H.; Lim, H. Renewable methanol synthesis from renewable H2 and captured CO2: How can power-to-liquid technology be economically feasible? Appl. Energy 2020, 279, 115827. [Google Scholar] [CrossRef]
- Bowker, M. Methanol synthesis from CO2 hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A review of the methanol economy: The fuel cell route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Zhang, Y.; Wang, X.; Qiu, H. Catalytic Hydrogenation of CO2 to Methanol: A Review. Catalysts 2022, 12, 403. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R. Methanol synthesis from CO2: A review of the latest developments in heterogeneous catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laudenschleger, D.; Ruland, H.; Muhler, M. Identifying the nature of the active sites in methanol synthesis over Cu/ZnO/Al2O3 catalysts. Nat. Commun. 2020, 11, 3898. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Marcos, F.C.; Cavalcanti, F.M.; Petrolini, D.D.; Lin, L.; Betancourt, L.E.; Senanayake, S.D.; Rodriguez, J.A.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Effect of operating parameters on H2/CO2 conversion to methanol over Cu-Zn oxide supported on ZrO2 polymorph catalysts: Characterization and kinetics. Chem. Eng. J. 2022, 427, 130947. [Google Scholar] [CrossRef]
- Cui, Z.; Meng, S.; Yi, Y.; Jafarzadeh, A.; Li, S.; Neyts, E.C.; Hao, Y.; Li, L.; Zhang, X.; Wang, X. Plasma-Catalytic Methanol Synthesis from CO2 Hydrogenation over a Supported Cu Cluster Catalyst: Insights into the Reaction Mechanism. ACS Catal. 2022, 12, 1326–1337. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, M.; Fan, G.; Zheng, L.; Li, F. Efficient Role of Nanosheet-Like Pr₂O₃ Induced Surface-Interface Synergistic Structures over Cu-Based Catalysts for Enhanced Methanol Production from CO₂ Hydrogenation. ACS Appl. Mater. Interfaces 2022, 14, 2768–2781. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-Based Nanocatalysts for CO2 hydrogenation to methanol. Energy Fuels 2021, 35, 8558–8584. [Google Scholar] [CrossRef]
- Wang, Y.; Kattel, S.; Gao, W.; Li, K.; Liu, P.; Chen, J.G.; Wang, H. Exploring the ternary interactions in Cu–ZnO–ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1166. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wen, J.; Sun, Y.; Zhang, M.; Bao, Y.; Zhang, Y.; Liang, L.; Fu, M.; Wu, J.; Ye, D. CO2 hydrogenation to methanol over Cu/ZnO plate model catalyst: Effects of reducing gas induced Cu nanoparticle morphology. Chem. Eng. J. 2019, 374, 221–230. [Google Scholar] [CrossRef]
- Tada, S.; Otsuka, F.; Fujiwara, K.; Moularas, C.; Deligiannakis, Y.; Kinoshita, Y.; Uchida, S.; Honma, T.; Nishijima, M.; Kikuchi, R. Development of CO2-to-Methanol Hydrogenation Catalyst by Focusing on the Coordination Structure of the Cu Species in Spinel-Type Oxide Mg1–xCuxAl2O4. ACS Catal. 2020, 10, 15186–15194. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Fan, G.; Yang, L.; Li, F. Fabrication of Zr–Ce Oxide Solid Solution Surrounded Cu-Based Catalyst Assisted by a Microliquid Film Reactor for Efficient CO2 Hydrogenation to Produce Methanol. Ind. Eng. Chem. Res. 2021, 60, 16188–16200. [Google Scholar] [CrossRef]
- Yu, J.; Yang, M.; Zhang, J.; Ge, Q.; Zimina, A.; Pruessmann, T.; Zheng, L.; Grunwaldt, J.-D.; Sun, J. Stabilizing Cu+ in Cu/SiO2 catalysts with a shattuckite-like structure boosts CO2 hydrogenation into methanol. ACS Catal. 2020, 10, 14694–14706. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Lv, Y.; Liu, P.; Hao, F.; Xiong, W. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy. Fuel 2022, 314, 123035. [Google Scholar] [CrossRef]
- Cui, W.-G.; Li, Y.-T.; Yu, L.; Zhang, H.; Hu, T.-L. Zeolite-Encapsulated Ultrasmall Cu/ZnOx Nanoparticles for the Hydrogenation of CO2 to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 18693–18703. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Fujiwara, K.; Yamamura, T.; Nishijima, M.; Uchida, S.; Kikuchi, R. Flame spray pyrolysis makes highly loaded Cu nanoparticles on ZrO2 for CO2-to-methanol hydrogenation. Chem. Eng. J. 2020, 381, 122750. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Neja, S.Š.; Blaž, L. Correlation between synthesis pH, structure and Cu/MgO/Al2O3 heterogeneous catalyst activity and selectivity in CO2 hydrogenation to methanol. J. CO2 Util. 2018, 28, 189–199. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Likozar, B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity. Renew. Energy 2019, 140, 452–460. [Google Scholar] [CrossRef]
- Noh, G.; Lam, E.; Alfke, J.L.; Larmier, K.; Searles, K.; Wolf, P.; Copéret, C. Selective hydrogenation of CO2 to CH3OH on supported Cu nanoparticles promoted by isolated TiIV surface sites on SiO2. ChemSusChem 2019, 12, 968–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, E.; Noh, G.; Larmier, K.; Safonova, O.V.; Copéret, C. CO2 hydrogenation on Cu-catalysts generated from ZnII single-sites: Enhanced CH3OH selectivity compared to Cu/ZnO/Al2O3. J. Catal. 2021, 394, 266–272. [Google Scholar] [CrossRef]
- Fang, X.; Men, Y.; Wu, F.; Zhao, Q.; Singh, R.; Xiao, P.; Du, T.; Webley, P.A. Improved methanol yield and selectivity from CO2 hydrogenation using a novel Cu-ZnO-ZrO2 catalyst supported on Mg-Al layered double hydroxide (LDH). J. CO2 Util. 2019, 29, 57–64. [Google Scholar] [CrossRef]
- Koh, M.K.; Khavarian, M.; Chai, S.P.; Mohamed, A.R. The morphological impact of siliceous porous carriers on copper-catalysts for selective direct CO2 hydrogenation to methanol. Int. J. Hydrogen Energy 2018, 43, 9334–9342. [Google Scholar] [CrossRef]
- Lei, H.; Zheng, R.; Liu, Y.; Gao, J.; Chen, X.; Feng, X. Cylindrical shaped ZnO combined Cu catalysts for the hydrogenation of CO2 to methanol. RSC Adv. 2019, 9, 13696–13704. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Yu, J.; Liu, B.; Si, C.; Ban, H.; Cai, W.; Li, C.; Li, Z.; Fujimoto, K. Simple strategy synthesizing stable CuZnO/SiO2 methanol synthesis catalyst. J. Catal. 2019, 372, 163–173. [Google Scholar] [CrossRef]
- Tada, S.; Oshima, K.; Noda, Y.; Kikuchi, R.; Sohmiya, M.; Honma, T.; Satokawa, S. Effects of Cu precursor types on the catalytic activity of Cu/ZrO2 toward methanol synthesis via CO2 hydrogenation. Ind. Eng. Chem. Res. 2019, 58, 19434–19445. [Google Scholar] [CrossRef]
- Guo, H.; Li, Q.; Zhang, H.; Peng, F.; Xiong, L.; Yao, S.; Huang, C.; Chen, X. CO2 hydrogenation over acid-activated Attapulgite/Ce0.75Zr0.25O2 nanocomposite supported Cu-ZnO based catalysts. Mol. Catal. 2019, 476, 110499. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Chen, C.; Ayvalı, T.C.E.; Suo, H.; Zheng, J.; Teixeira, I.F.; Ye, L.; Zou, H.; O’Hare, D.; Tsang, S.C.E. CO2 hydrogenation to methanol over catalysts derived from single cationic layer CuZnGa LDH precursors. ACS Catal. 2018, 8, 4390–4401. [Google Scholar] [CrossRef]
- Prašnikar, A.; Dasireddy, V.D.; Likozar, B. Scalable combustion synthesis of copper-based perovskite catalysts for CO2 reduction to methanol: Reaction structure-activity relationships, kinetics, and stability. Chem. Eng. Sci. 2022, 250, 117423. [Google Scholar] [CrossRef]
- Li, H.-X.; Yang, L.-Q.-Q.; Chi, Z.-Y.; Zhang, Y.-L.; Li, X.-G.; He, Y.-L.; Reina, T.R.; Xiao, W.-D. CO2 Hydrogenation to Methanol Over Cu/ZnO/Al2O3 Catalyst: Kinetic Modeling Based on Either Single-or Dual-Active Site Mechanism. Catal. Lett. 2022, 152, 1–15. [Google Scholar] [CrossRef]
- Liu, T.; Hong, X.; Liu, G. In Situ Generation of the Cu@ 3D-ZrO x Framework Catalyst for Selective Methanol Synthesis from CO2/H2. ACS Catal. 2019, 10, 93–102. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Liu, J.; Zhang, J.; Zhang, F.; Zhou, T.; Rui, N.; Yao, S.; Deng, Y.; Yang, F. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 2020, 11, 5767. [Google Scholar] [CrossRef]
- Pori, M.; Arčon, I.; Lašič Jurković, D.; Marinšek, M.; Dražić, G.; Likozar, B.; Crnjak Orel, Z. Synthesis of a Cu/ZnO Nanocomposite by electroless plating for the catalytic conversion of CO2 to methanol. Catal. Lett. 2019, 149, 1427–1439. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Sushkevich, V.L.; Newton, M.A.; van Bokhoven, J.A. Copper–zinc alloy-free synthesis of methanol from carbon dioxide over Cu/ZnO/faujasite. ACS Catal. 2020, 10, 14240–14244. [Google Scholar] [CrossRef]
- Zhu, J.; Ciolca, D.; Liu, L.; Parastaev, A.; Kosinov, N.; Hensen, E.J. Flame synthesis of Cu/ZnO–CeO2 catalysts: Synergistic metal–support interactions promote CH3OH selectivity in CO2 hydrogenation. ACS Catal. 2021, 11, 4880–4892. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.S.; Naeem, A.; Tasleem, S.; Ahmad, P. Revalorization of CO2 for methanol production via ZnO promoted carbon nanofibers based Cu-ZrO2 catalytic hydrogenation. J. Energy Chem. 2019, 39, 68–76. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Gao, P.; Zhu, H.; Zhong, L.; Wang, H.; Wei, W.; Sun, Y. Preparation and CO2 hydrogenation catalytic properties of alumina microsphere supported Cu-based catalyst by deposition-precipitation method. J. CO2 Util. 2017, 17, 263–272. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, H.; Gao, P.; Li, X.; Zhang, J.; Liu, H.; Wang, H.; Wei, W.; Sun, Y. Slurry methanol synthesis from CO2 hydrogenation over micro-spherical SiO2 support Cu/ZnO catalysts. J. CO2 Util. 2018, 26, 642–651. [Google Scholar] [CrossRef]
- Li, S.; Guo, L.; Ishihara, T. Hydrogenation of CO2 to methanol over Cu/AlCeO catalyst. Catal. Today 2020, 339, 352–361. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Yang, B.; Guo, L. A highly active and selective mesostructured Cu/AlCeO catalyst for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 571, 51–60. [Google Scholar] [CrossRef]
- Diez-Ramirez, J.; Dorado, F.; de la Osa, A.R.; Valverde, J.L.; Sánchez, P. Hydrogenation of CO2 to methanol at atmospheric pressure over Cu/ZnO catalysts: Influence of the calcination, reduction, and metal loading. Ind. Eng. Chem. Res. 2017, 56, 1979–1987. [Google Scholar] [CrossRef]
- Ouyang, B.; Tan, W.; Liu, B. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation. Catal. Commun. 2017, 95, 36–39. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, C.; Baik, J.H.; Suh, Y.-W. Phases of Cu/Zn/Al/Zr precursors linked to the property and activity of their final catalysts in CO2 hydrogenation to methanol. Catal. Today 2020, 347, 70–78. [Google Scholar] [CrossRef]
- L’hospital, V.; Angelo, L.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.-C. Influence of the Zn/Zr ratio in the support of a copper-based catalyst for the synthesis of methanol from CO2. Catal. Today 2021, 369, 95–104. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, P.; Wang, X.; Song, F.; Bai, Y.; Zhang, M.; Wu, Y.; Xie, H.; Tan, Y. Effect of Vapor-phase-treatment to CuZnZr Catalyst on the Reaction Behaviors in CO2 Hydrogenation into Methanol. ChemCatChem 2019, 11, 1448–1457. [Google Scholar] [CrossRef]
- Yao, L.; Shen, X.; Pan, Y.; Peng, Z. Synergy between active sites of Cu-In-Zr-O catalyst in CO2 hydrogenation to methanol. J. Catal. 2019, 372, 74–85. [Google Scholar] [CrossRef]
- Chen, K.; Fang, H.; Wu, S.; Liu, X.; Zheng, J.; Zhou, S.; Duan, X.; Zhuang, Y.; Tsang, S.C.E.; Yuan, Y. CO2 hydrogenation to methanol over Cu catalysts supported on La-modified SBA-15: The crucial role of Cu–LaOx interfaces. Appl. Catal. B Environ. 2019, 251, 119–129. [Google Scholar] [CrossRef]
- Yan, Y.; Wong, R.J.; Ma, Z.; Donat, F.; Xi, S.; Saqline, S.; Fan, Q.; Du, Y.; Borgna, A.; He, Q. CO2 hydrogenation to methanol on tungsten-doped Cu/CeO2 catalysts. Appl. Catal. B Environ. 2022, 306, 121098. [Google Scholar] [CrossRef]
- Wang, X.; Alabsi, M.H.; Zheng, P.; Mei, J.; Ramirez, A.; Duan, A.; Xu, C.; Huang, K.-W. PdCu supported on dendritic mesoporous CexZr1−xO2 as superior catalysts to boost CO2 hydrogenation to methanol. J. Colloid Interface Sci. 2022, 611, 739–751. [Google Scholar] [CrossRef]
- Yamamura, T.; Tada, S.; Kikuchi, R.; Fujiwara, K.; Honma, T. Effect of Sm Doping on CO2-to-Methanol Hydrogenation of Cu/Amorphous-ZrO2 Catalysts. J. Phys. Chem. C 2021, 125, 15899–15909. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millan, E.; Pawelec, B.; García, R.; Fierro, J.; Navarro, R. Structure and activity of Cu/ZnO catalysts co-modified with aluminium and gallium for methanol synthesis. Catal. Today 2020, 355, 870–881. [Google Scholar] [CrossRef]
- Mota, N.; Guil-Lopez, R.; Pawelec, B.; Fierro, J.; Navarro, R. Highly active Cu/ZnO–Al catalyst for methanol synthesis: Effect of aging on its structure and activity. RSC Adv. 2018, 8, 20619–20629. [Google Scholar] [CrossRef] [Green Version]
- Deerattrakul, V.; Yigit, N.; Rupprechter, G.; Kongkachuichay, P. The roles of nitrogen species on graphene aerogel supported Cu-Zn as efficient catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 580, 46–52. [Google Scholar] [CrossRef]
- Fang, X.; Men, Y.; Wu, F.; Zhao, Q.; Singh, R.; Xiao, P.; Du, T.; Webley, P.A. Promoting CO2 hydrogenation to methanol by incorporating adsorbents into catalysts: Effects of hydrotalcite. Chem. Eng. J. 2019, 378, 122052. [Google Scholar] [CrossRef]
- Fang, X.; Men, Y.; Wu, F.; Zhao, Q.; Singh, R.; Xiao, P.; Du, T.; Webley, P.A. Moderate-pressure conversion of H2 and CO2 to methanol via adsorption enhanced hydrogenation. Int. J. Hydrogen Energy 2019, 44, 21913–21925. [Google Scholar] [CrossRef]
- Paris, C.; Karelovic, A.; Manrique, R.; Le Bras, S.; Devred, F.; Vykoukal, V.; Styskalik, A.; Eloy, P.; Debecker, D.P. CO2 hydrogenation to methanol with Ga-and Zn-doped mesoporous Cu/SiO2 catalysts prepared by the aerosol-assisted sol-gel process. ChemSusChem 2020, 13, 6409–6417. [Google Scholar] [PubMed]
- Jiang, X.; Jiao, Y.; Moran, C.; Nie, X.; Gong, Y.; Guo, X.; Walton, K.S.; Song, C. CO2 hydrogenation to methanol on PdCu bimetallic catalysts with lower metal loadings. Catal. Commun. 2019, 118, 10–14. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, Z.; Wu, D. CO2 hydrogenation to methanol over a highly active Cu–Ni/CeO2–nanotube catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar] [CrossRef]
- Sharma, S.K.; Khan, T.S.; Singha, R.K.; Paul, B.; Poddar, M.K.; Sasaki, T.; Bordoloi, A.; Samanta, C.; Gupta, S.; Bal, R. Design of highly stable MgO promoted Cu/ZnO catalyst for clean methanol production through selective hydrogenation of CO2. Appl. Catal. A Gen. 2021, 623, 118239. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Štefančič, N.S.; Huš, M.; Likozar, B. Effect of alkaline earth metal oxide (MO) Cu/MO/Al2O3 catalysts on methanol synthesis activity and selectivity via CO2 reduction. Fuel 2018, 233, 103–112. [Google Scholar] [CrossRef]
- Mureddu, M.; Ferrara, F.; Pettinau, A. Highly efficient CuO/ZnO/ZrO2@ SBA-15 nanocatalysts for methanol synthesis from the catalytic hydrogenation of CO2. Appl. Catal. B Environ. 2019, 258, 117941. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Tian, C.; Pan, Y.; Sun, X.; Zhang, J.; Wu, D. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. [Google Scholar] [CrossRef]
- Fujiwara, K.; Tada, S.; Honma, T.; Sasaki, H.; Nishijima, M.; Kikuchi, R. Influences of particle size and crystallinity of highly loaded CuO/ZrO2 on CO2 hydrogenation to methanol. AIChE J. 2019, 65, e16717. [Google Scholar] [CrossRef]
- Chang, K.; Wang, T.; Chen, J.G. Hydrogenation of CO2 to methanol over CuCeTiOx catalysts. Appl. Catal. B Environ. 2017, 206, 704–711. [Google Scholar] [CrossRef]
- Wang, G.; Mao, D.; Guo, X.; Yu, J. Methanol synthesis from CO2 hydrogenation over CuO-ZnO-ZrO2-MxOy catalysts (M = Cr, Mo and W). Int. J. Hydrogen Energy 2019, 44, 4197–4207. [Google Scholar] [CrossRef]
- Sadeghinia, M.; Ghaziani, A.N.K.; Rezaei, M. Component ratio dependent Cu/Zn/Al structure sensitive catalyst in CO2/CO hydrogenation to methanol. Mol. Catal. 2018, 456, 38–48. [Google Scholar] [CrossRef]
- Tada, S.; Watanabe, F.; Kiyota, K.; Shimoda, N.; Hayashi, R.; Takahashi, M.; Nariyuki, A.; Igarashi, A.; Satokawa, S. Ag addition to CuO-ZrO2 catalysts promotes methanol synthesis via CO2 hydrogenation. J. Catal. 2017, 351, 107–118. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. Effect of the nature of copper species on methanol synthesis from CO2 hydrogenation reaction over CuO/Ce0.4Zr0.6O2 catalyst. Mol. Catal. 2020, 493, 111105. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, X.; Ling, C.; Chen, S.; Wu, Z. High-performance Cu/ZnO catalysts prepared using a three-channel microreactor. Appl. Catal. A Gen. 2019, 570, 192–199. [Google Scholar] [CrossRef]
- Allam, D.; Bennici, S.; Limousy, L.; Hocine, S. Improved Cu-and Zn-based catalysts for CO2 hydrogenation to methanol. Comptes Rendus Chim. 2019, 22, 227–237. [Google Scholar] [CrossRef]
- Chang, K.; Wang, T.; Chen, J.G. Methanol synthesis from CO2 hydrogenation over CuZnCeTi mixed oxide catalysts. Ind. Eng. Chem. Res. 2019, 58, 7922–7928. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, D.; Wang, G.; Guo, X.; Yu, J. CO2 hydrogenation to methanol over CuOZnOTiO2ZrO2 catalyst prepared by a facile solid-state route: The significant influence of assistant complexing agents. Int. J. Hydrogen Energy 2019, 44, 14831–14841. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Wu, D. Enhanced CO2 hydrogenation to methanol over TiO2 nanotubes-supported CuO-ZnO-CeO2 catalyst. Appl. Catal. A Gen. 2019, 581, 58–66. [Google Scholar] [CrossRef]
- Chen, D.; Mao, D.; Wang, G.; Guo, X.; Yu, J. CO2 hydrogenation to methanol over CuO-ZnO-ZrO2 catalyst prepared by polymeric precursor method. J. Sol-Gel Sci. Technol. 2019, 89, 686–699. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced activity, selectivity and stability of a CuO-ZnO-ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem. Eng. J. 2018, 334, 1781–1791. [Google Scholar] [CrossRef]
- Ye, H.; Na, W.; Gao, W.; Wang, H. Carbon-Modified CuO/ZnO Catalyst with High Oxygen Vacancy for CO2 Hydrogenation to Methanol. Energy Technol. 2020, 8, 2000194. [Google Scholar] [CrossRef]
- Wang, G.; Mao, D.; Guo, X.; Yu, J. Enhanced performance of the CuO-ZnO-ZrO2 catalyst for CO2 hydrogenation to methanol by WO3 modification. Appl. Surf. Sci. 2018, 456, 403–409. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced catalytic performance of Zr modified CuO/ZnO/Al2O3 catalyst for methanol and DME synthesis via CO2 hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Sadeghinia, M.; Rezaei, M.; Kharat, A.N.; Jorabchi, M.N.; Nematollahi, B.; Zareiekordshouli, F. Effect of In2O3 on the structural properties and catalytic performance of the CuO/ZnO/Al2O3 catalyst in CO2 and CO hydrogenation to methanol. Mol. Catal. 2020, 484, 110776. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Kousi, K.; Kondarides, D.I. CO2 hydrogenation to methanol over La2O3-promoted CuO/ZnO/Al2O3 catalysts: A kinetic and mechanistic study. Catalysts 2020, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Phongamwong, T.; Chantaprasertporn, U.; Witoon, T.; Numpilai, T.; Poo-Arporn, Y.; Limphirat, W.; Donphai, W.; Dittanet, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over CuO–ZnO–ZrO2–SiO2 catalysts: Effects of SiO2 contents. Chem. Eng. J. 2017, 316, 692–703. [Google Scholar] [CrossRef]
- Liang, Y.; Mao, D.; Guo, X.; Yu, J.; Wu, G.; Ma, Z. Solvothermal preparation of CuO-ZnO-ZrO2 catalysts for methanol synthesis via CO2 hydrogenation. J. Taiwan Inst. Chem. Eng. 2021, 121, 81–91. [Google Scholar] [CrossRef]
- Poto, S.; van Berkel, D.V.; Gallucci, F.; d’Angelo, M.F.N. Kinetic modelling of the methanol synthesis from CO2 and H2 over a CuO/CeO2/ZrO2 catalyst: The role of CO2 and CO hydrogenation. Chem. Eng. J. 2022, 435, 134946. [Google Scholar] [CrossRef]
- Tada, S.; Satokawa, S. Effect of Ag loading on CO2-to-methanol hydrogenation over Ag/CuO/ZrO2. Catal. Commun. 2018, 113, 41–45. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Todaro, S.; Migliori, M.; Giordano, G.; Frusteri, F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 2020, 345, 175–182. [Google Scholar] [CrossRef]
- Tursunov, O.; Kustov, L.; Tilyabaev, Z. Methanol synthesis from the catalytic hydrogenation of CO2 over CuO–ZnO supported on aluminum and silicon oxides. J. Taiwan Inst. Chem. Eng. 2017, 78, 416–422. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. An investigation of Zr/Ce ratio influencing the catalytic performance of CuO/Ce1−xZrxO2 catalyst for CO2 hydrogenation to CH3OH. J. Energy Chem. 2020, 47, 18–28. [Google Scholar] [CrossRef]
- Poerjoto, A.J.; Ashok, J.; Dewangan, N.; Kawi, S. The role of lattice oxygen in CO2 hydrogenation to methanol over La1-xSrxCuO catalysts. J. CO2 Util. 2021, 47, 101498. [Google Scholar] [CrossRef]
- Min, L.; Wei, N.; YE, H.-C.; HUO, H.-H.; GAO, W.-G. Effect of additive on CuO-ZnO/SBA-15 catalytic performance of CO2 hydrogenation to methanol. J. Fuel Chem. Technol. 2019, 47, 1214–1225. [Google Scholar]
- Liu, X.; Zhan, G.; Wu, J.; Li, W.; Du, Z.; Huang, J.; Sun, D.; Li, Q. Preparation of integrated CuO/ZnO/OS nanocatalysts by using acid-etched oyster shells as a support for CO2 hydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 7162–7173. [Google Scholar] [CrossRef]
- Trifan, B.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Modifications in the composition of CuO/ZnO/Al2O3 catalyst for the synthesis of methanol by CO2 hydrogenation. Catalysts 2021, 11, 774. [Google Scholar] [CrossRef]
- Ortner, N.; Lund, H.; Armbruster, U.; Wohlrab, S.; Kondratenko, E.V. Factors affecting primary and secondary pathways in CO2 hydrogenation to methanol over CuZnIn/MZrOx (La, Ti or Y). Catal. Today 2022, 387, 47–53. [Google Scholar] [CrossRef]
- Song, H.-T.; Fazeli, A.; Kim, H.D.; Eslami, A.A.; Noh, Y.S.; Saeidabad, N.G.; Moon, D.J. Effect of lanthanum group promoters on Cu/(mixture of ZnO and Zn-Al-spinnel-oxides) catalyst for methanol synthesis by hydrogenation of CO and CO2 mixtures. Fuel 2021, 283, 118987. [Google Scholar] [CrossRef]
- Yang, B.; Liu, C.; Halder, A.; Tyo, E.C.; Martinson, A.B.; Seifert, S.n.; Zapol, P.; Curtiss, L.A.; Vajda, S. Copper cluster size effect in methanol synthesis from CO2. J. Phys. Chem. C 2017, 121, 10406–10412. [Google Scholar] [CrossRef]
- Sha, F.; Han, Z.; Tang, S.; Wang, J.; Li, C. Hydrogenation of Carbon Dioxide to Methanol over Non− Cu-based Heterogeneous Catalysts. ChemSusChem 2020, 13, 6160–6181. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, F.; Sun, K.; Liu, Z.; Xu, W.; Stavitski, E.; Senanayake, S.D.; Rodriguez, J.A.; Liu, C.-J. Hydrogenation of CO2 to methanol on a Auδ+–In2O3–x catalyst. ACS Catal. 2020, 10, 11307–11317. [Google Scholar] [CrossRef]
- Shen, C.; Sun, K.; Zhang, Z.; Rui, N.; Jia, X.; Mei, D.; Liu, C.-J. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: Experimental and theoretical studies. ACS Catal. 2021, 11, 4036–4046. [Google Scholar] [CrossRef]
- Docherty, S.R.; Phongprueksathat, N.; Lam, E.; Noh, G.; Safonova, O.V.; Urakawa, A.; Copéret, C. Silica-supported PdGa nanoparticles: Metal synergy for highly active and selective CO2-to-CH3OH hydrogenation. JACS Au 2021, 1, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Song, J.; Liu, S.; Yang, C.; Wang, G.; Tian, H.; Zhao, Z.-J.; Mu, R.; Gong, J. The role of Al doping in Pd/ZnO catalyst for CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2020, 263, 118367. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 2019, 10, 3377. [Google Scholar] [CrossRef] [Green Version]
- Men, Y.-L.; Liu, Y.; Wang, Q.; Luo, Z.-H.; Shao, S.; Li, Y.-B.; Pan, Y.-X. Highly dispersed Pt-based catalysts for selective CO2 hydrogenation to methanol at atmospheric pressure. Chem. Eng. Sci. 2019, 200, 167–175. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, J.; Wu, X.; Wang, W.; Chen, Y.; Zhang, M. Efficient hydrogenation of CO2 to methanol over Pd/In2O3/SBA-15 catalysts. J. CO2 Util. 2020, 36, 33–39. [Google Scholar] [CrossRef]
- Gallo, A.; Snider, J.L.; Sokaras, D.; Nordlund, D.; Kroll, T.; Ogasawara, H.; Kovarik, L.; Duyar, M.S.; Jaramillo, T.F. Ni5Ga3 catalysts for CO2 reduction to methanol: Exploring the role of Ga surface oxidation/reduction on catalytic activity. Appl. Catal. B Environ. 2020, 267, 118369. [Google Scholar] [CrossRef]
- Choi, H.; Oh, S.; Tran, S.B.T.; Park, J.Y. Size-controlled model Ni catalysts on Ga2O3 for CO2 hydrogenation to methanol. J. Catal. 2019, 376, 68–76. [Google Scholar] [CrossRef]
- Ting, K.W.; Toyao, T.; Siddiki, S.H.; Shimizu, K.-I. Low-temperature hydrogenation of CO2 to methanol over heterogeneous TiO2-Supported Re catalysts. ACS Catal. 2019, 9, 3685–3693. [Google Scholar] [CrossRef]
- Toyao, T.; Kayamori, S.; Maeno, Z.; Siddiki, S.H.; Shimizu, K.-I. Heterogeneous Pt and MoOx Co-Loaded TiO2 Catalysts for Low-Temperature CO2 Hydrogenation To Form CH3OH. ACS Catal. 2019, 9, 8187–8196. [Google Scholar] [CrossRef]
- Gothe, M.L.; Pérez-Sanz, F.J.; Braga, A.H.; Borges, L.R.; Abreu, T.F.; Bazito, R.C.; Gonçalves, R.V.; Rossi, L.M.; Vidinha, P. Selective CO2 hydrogenation into methanol in a supercritical flow process. J. CO2 Util. 2020, 40, 101195. [Google Scholar] [CrossRef]
- Wang, L.; Guan, E.; Wang, Y.; Wang, L.; Gong, Z.; Cui, Y.; Meng, X.; Gates, B.C.; Xiao, F.-S. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11, 1033. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Zhang, Z.; Shen, C.; Rui, N.; Liu, C.-J. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol. Green Energy Environ. 2022, 7, 807–817. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, P.; Zhang, Z.; Zhang, L.; Yang, G.; Han, B. Efficient hydrogenation of CO2 to methanol over supported subnanometer gold catalysts at low temperature. ChemCatChem 2017, 9, 3691–3696. [Google Scholar] [CrossRef]
- Vourros, A.; Garagounis, I.; Kyriakou, V.; Carabineiro, S.; Maldonado-Hódar, F.; Marnellos, G.; Konsolakis, M. Carbon dioxide hydrogenation over supported Au nanoparticles: Effect of the support. J. CO2 Util. 2017, 19, 247–256. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, K.; Wang, J.; Zhang, Z.; Liu, C. A highly active Au/In2O3-ZrO2 catalyst for selective hydrogenation of CO2 to methanol. Catalysts 2020, 10, 1360. [Google Scholar] [CrossRef]
- Wang, W.; Tongo, D.W.K.; Song, L.; Qu, Z. Effect of Au Addition on the Catalytic Performance of CuO/CeO2 Catalysts for CO2 Hydrogenation to Methanol. Top. Catal. 2021, 64, 446–455. [Google Scholar] [CrossRef]
- Rezvani, A.; Abdel-Mageed, A.M.; Ishida, T.; Murayama, T.; Parlinska-Wojtan, M.; Behm, R.J.r. CO2 reduction to methanol on Au/CeO2 catalysts: Mechanistic insights from activation/deactivation and SSITKA measurements. ACS Catal. 2020, 10, 3580–3594. [Google Scholar] [CrossRef]
- Wu, Q.; Shen, C.; Rui, N.; Sun, K.; Liu, C.-J. Experimental and theoretical studies of CO2 hydrogenation to methanol on Ru/In2O3. J. CO2 Util. 2021, 53, 101720. [Google Scholar] [CrossRef]
- Sagar, T.V.; Zavašnik, J.; Finšgar, M.; Novak Tušar, N.; Pintar, A. Evaluation of Au/ZrO2 Catalysts Prepared via Postsynthesis Methods in CO2 Hydrogenation to Methanol. Catalysts 2022, 12, 218. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Sha, F.; Tang, S.; Wang, J.; Li, C. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure. J. Catal. 2021, 396, 242–250. [Google Scholar] [CrossRef]
- Wang, J.; Sun, K.; Jia, X.; Liu, C.-J. CO2 hydrogenation to methanol over Rh/In2O3 catalyst. Catal. Today 2021, 365, 341–347. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Sun, K.; Xiong, S.; Zhang, Z.; Liu, C.-J. CO2 hydrogenation to methanol over Rh/In2O3–ZrO2 catalyst with improved activity. Green Chem. Eng. 2022, 3, 165–170. [Google Scholar] [CrossRef]
- Sun, K.; Rui, N.; Zhang, Z.; Sun, Z.; Ge, Q.; Liu, C.-J. A highly active Pt/In2O3 catalyst for CO2 hydrogenation to methanol with enhanced stability. Green Chem. 2020, 22, 5059–5066. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Jia, X.; Sun, K.; Wang, J.; Shen, C.; Liu, C.-J. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst. J. Energy Chem. 2020, 50, 409–415. [Google Scholar] [CrossRef]
- Dostagir, N.H.M.; Thompson, C.; Kobayashi, H.; Karim, A.M.; Fukuoka, A.; Shrotri, A. Rh promoted In2O3 as a highly active catalyst for CO 2 hydrogenation to methanol. Catal. Sci. Technol. 2020, 10, 8196–8202. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Sun, K.; Liu, C.-J. Improvement in the activity of Ni/In2O3 with the addition of ZrO2 for CO2 hydrogenation to methanol. Catal. Commun. 2022, 162, 106386. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Morales-Vidal, J.; Philipp, M.; Safonova, O.V.; López, N.; Stewart, J.A.; Ferré, D.C.; Pérez-Ramírez, J. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation. Nat. Commun. 2021, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Dai, J.; Li, W.; Tan, K.B.; Huang, Z.; Zhan, G.; Huang, J.; Li, Q. Pd supported on MIL-68 (In)-derived In2O3 nanotubes as superior catalysts to boost CO2 hydrogenation to methanol. ACS Catal. 2020, 10, 13275–13289. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Din, I.U.; Alotaibi, M.A.; Bagabas, A.; Naeem, A.; Alkhalifa, A. Low temperature green methanol synthesis by CO2 hydrogenation over Pd/SiO2 catalysts in slurry reactor. Inorg. Chem. Commun. 2022, 142, 109688. [Google Scholar] [CrossRef]
- Collins, S.E.; Baltanás, M.A.; Delgado, J.J.; Borgna, A.; Bonivardi, A.L. CO2 hydrogenation to methanol on Ga2O3-Pd/SiO2 catalysts: Dual oxide-metal sites or (bi) metallic surface sites? Catal. Today 2021, 381, 154–162. [Google Scholar] [CrossRef]
- Lee, K.; Anjum, U.; Araújo, T.P.; Mondelli, C.; He, Q.; Furukawa, S.; Pérez-Ramírez, J.; Kozlov, S.M.; Yan, N. Atomic Pd-promoted ZnZrOx solid solution catalyst for CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2022, 304, 120994. [Google Scholar] [CrossRef]
- Khobragade, R.; Roškarič, M.; Žerjav, G.; Košiček, M.; Zavašnik, J.; Van de Velde, N.; Jerman, I.; Tušar, N.N.; Pintar, A. Exploring the effect of morphology and surface properties of nanoshaped Pd/CeO2 catalysts on CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2021, 627, 118394. [Google Scholar] [CrossRef]
- Manrique, R.; Jiménez, R.; Rodríguez-Pereira, J.; Baldovino-Medrano, V.G.; Karelovic, A. Insights into the role of Zn and Ga in the hydrogenation of CO2 to methanol over Pd. Int. J. Hydrogen Energy 2019, 44, 16526–16536. [Google Scholar] [CrossRef]
- Richard, A.R.; Fan, M. Low-pressure hydrogenation of CO2 to CH3OH using Ni-In-Al/SiO2 catalyst synthesized via a phyllosilicate precursor. ACS Catal. 2017, 7, 5679–5692. [Google Scholar] [CrossRef]
- Cherevotan, A.; Raj, J.; Dheer, L.; Roy, S.; Sarkar, S.; Das, R.; Vinod, C.P.; Xu, S.; Wells, P.; Waghmare, U.V. Operando generated ordered heterogeneous catalyst for the selective conversion of CO2 to methanol. ACS Energy Lett. 2021, 6, 509–516. [Google Scholar] [CrossRef]
- Bavykina, A.; Yarulina, I.; Al Abdulghani, A.J.; Gevers, L.; Hedhili, M.N.; Miao, X.; Galilea, A.R.; Pustovarenko, A.; Dikhtiarenko, A.; Cadiau, A. Turning a methanation Co catalyst into an In–Co methanol producer. ACS Catal. 2019, 9, 6910–6918. [Google Scholar] [CrossRef]
- Snider, J.L.; Streibel, V.; Hubert, M.A.; Choksi, T.S.; Valle, E.; Upham, D.C.; Schumann, J.; Duyar, M.S.; Gallo, A.; Abild-Pedersen, F. Revealing the synergy between oxide and alloy phases on the performance of bimetallic In–Pd catalysts for CO2 hydrogenation to methanol. ACS Catal. 2019, 9, 3399–3412. [Google Scholar] [CrossRef]
- Li, M.M.J.; Zou, H.; Zheng, J.; Wu, T.S.; Chan, T.S.; Soo, Y.L.; Wu, X.P.; Gong, X.Q.; Chen, T.; Roy, K. Methanol synthesis at a wide range of H2/CO2 ratios over a Rh-In bimetallic catalyst. Angew. Chem. 2020, 132, 16173–16180. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F.; Daous, M.A.; Al-Zahrani, A.A.; Malik, A.S.; Driss, H.; Shterk, G.; Gascon, J. Optimizing Pd: Zn molar ratio in PdZn/CeO2 for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 584, 117185. [Google Scholar] [CrossRef]
- Malik, A.S.; Zaman, S.F.; Al-Zahrani, A.A.; Daous, M.A.; Driss, H.; Petrov, L.A. Selective hydrogenation of CO2 to CH3OH and in-depth DRIFT analysis for PdZn/ZrO2 and CaPdZn/ZrO2 catalysts. Catal. Today 2020, 357, 573–582. [Google Scholar] [CrossRef]
- Geng, F.; Zhan, X.; Hicks, J.C. Promoting Methanol Synthesis and Inhibiting CO2 Methanation with Bimetallic In–Ru Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 11891–11902. [Google Scholar] [CrossRef]
- Rasteiro, L.F.; De Sousa, R.A.; Vieira, L.H.; Ocampo-Restrepo, V.K.; Verga, L.G.; Assaf, J.M.; Da Silva, J.L.; Assaf, E.M. Insights into the alloy-support synergistic effects for the CO2 hydrogenation towards methanol on oxide-supported Ni5Ga3 catalysts: An experimental and DFT study. Appl. Catal. B Environ. 2022, 302, 120842. [Google Scholar] [CrossRef]
- Nie, X.; Jiang, X.; Wang, H.; Luo, W.; Janik, M.J.; Chen, Y.; Guo, X.; Song, C. Mechanistic understanding of alloy effect and water promotion for Pd-Cu bimetallic catalysts in CO2 hydrogenation to methanol. ACS Catal. 2018, 8, 4873–4892. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, X.; Boreriboon, N.; Wang, Z.; Song, C.; Cen, K. Effects of supports on bimetallic Pd-Cu catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 585, 117210. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Wang, X.; Wang, H.; Koizumi, N.; Chen, Y.; Guo, X.; Song, C. Origin of Pd-Cu bimetallic effect for synergetic promotion of methanol formation from CO2 hydrogenation. J. Catal. 2019, 369, 21–32. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Nie, X.; Koizumi, N.; Guo, X.; Song, C. CO2 hydrogenation to methanol on Pd-Cu bimetallic catalysts: H2/CO2 ratio dependence and surface species. Catal. Today 2018, 316, 62–70. [Google Scholar] [CrossRef]
- Wang, C.; Fang, Y.; Liang, G.; Lv, X.; Duan, H.; Li, Y.; Chen, D.; Long, M. Mechanistic study of Cu-Ni bimetallic catalysts supported by graphene derivatives for hydrogenation of CO2 to methanol. J. CO2 Util. 2021, 49, 101542. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Díaz, J.; Sánchez, P.; Dorado, F. Optimization of the Pd/Cu ratio in Pd-Cu-Zn/SiC catalysts for the CO2 hydrogenation to methanol at atmospheric pressure. J. CO2 Util. 2017, 22, 71–80. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Din, I.U.; Alotaibi, M.A. Effect of the Cu/Ni Ratio on the Activity of Zeolite Based Cu–Ni Bimetallic Catalysts for CO2 Hydrogenation to Methanol. Russ. J. Phys. Chem. A 2020, 94, 2563–2568. [Google Scholar] [CrossRef]
- Geng, F.; Bonita, Y.; Jain, V.; Magiera, M.; Rai, N.; Hicks, J.C. Bimetallic Ru–Mo phosphide catalysts for the hydrogenation of CO2 to methanol. Ind. Eng. Chem. Res. 2020, 59, 6931–6943. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, Y.; Pan, H.; Wu, L.; Zhang, W.; Cao, L.; Zhang, J.; Zheng, L.; Yao, T. Grain boundaries modulating active sites in RhCo porous nanospheres for efficient CO2 hydrogenation. Nano Res. 2018, 11, 2357–2365. [Google Scholar] [CrossRef]
- Hengne, A.M.; Samal, A.K.; Enakonda, L.R.; Harb, M.; Gevers, L.E.; Anjum, D.H.; Hedhili, M.N.; Saih, Y.; Huang, K.-W.; Basset, J.-M. Ni–Sn-supported ZrO2 catalysts modified by indium for selective CO2 hydrogenation to methanol. ACS Omega 2018, 3, 3688–3701. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Jiang, X.; Boreriboon, N.; Song, C.; Wang, Z.; Cen, K. CO2 hydrogenation to methanol over bimetallic Pd-Cu catalysts supported on TiO2-CeO2 and TiO2-ZrO2. Catal. Today 2021, 371, 150–161. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, Y.H.; Lee, D.-W.; Moon, D.-J.; Lee, K.-Y. Hydrogenation of CO2 to methanol over Pd–Cu/CeO2 catalysts. Mol. Catal. 2017, 434, 146–153. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, P.; Wang, H.; Sun, J.; Gao, P. Preparation of novel bimetallic CuZn-BTC coordination polymer nanorod for methanol synthesis from CO2 hydrogenation. Mater. Chem. Phys. 2018, 215, 211–220. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Guo, Q.; Guo, X.; Da Costa, P.; Mao, D. Ultrasmall bimetallic Cu/ZnOx nanoparticles encapsulated in UiO-66 by deposition–precipitation method for CO2 hydrogenation to methanol. Fuel 2022, 324, 124694. [Google Scholar] [CrossRef]
- Qi, T.; Zhao, Y.; Chen, S.; Li, W.; Guo, X.; Zhang, Y.; Song, C. Bimetallic metal organic framework-templated synthesis of a Cu-ZnO/Al2O3 catalyst with superior methanol selectivity for CO2 hydrogenation. Mol. Catal. 2021, 514, 111870. [Google Scholar] [CrossRef]
- Alotaibi, M.A.; Din, I.U.; Alharthi, A.I.; Bakht, M.A.; Centi, G.; Shaharun, M.S.; Naeem, A. Green methanol synthesis by catalytic CO2 hydrogenation, deciphering the role of metal-metal interaction. Sustain. Chem. Pharm. 2021, 21, 100420. [Google Scholar] [CrossRef]
- Duma, Z.G.; Dyosiba, X.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M. Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks. Catalysts 2022, 12, 401. [Google Scholar] [CrossRef]
- Qiu, R.; Ding, Z.; Xu, Y.; Yang, Q.; Sun, K.; Hou, R. CuPd bimetallic catalyst with high Cu/Pd ratio and its application in CO2 hydrogenation. Appl. Surf. Sci. 2021, 544, 148974. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.; Williams, C.K. PdIn intermetallic nanoparticles for the hydrogenation of CO2 to methanol. Appl. Catal. B Environ. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Sun, K.; Jia, X.; Ye, J.; Liu, C.-J. Advances in Studies of Structural Effect of the Supported Ni Catalyst for CO2 Hydrogenation: From Nanoparticle to Single Atom Catalyst. J. Mater. Chem. A 2022, 10, 5792–5812. [Google Scholar] [CrossRef]
- Niu, J.; Liu, H.; Jin, Y.; Fan, B.; Qi, W.; Ran, J. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis. Int. J. Hydrogen Energy 2022, 47, 9183–9200. [Google Scholar] [CrossRef]
- Shen, C.; Bao, Q.; Xue, W.; Sun, K.; Zhang, Z.; Jia, X.; Mei, D.; Liu, C.-J. Synergistic effect of the metal-support interaction and interfacial oxygen vacancy for CO2 hydrogenation to methanol over Ni/In2O3 catalyst: A theoretical study. J. Energy Chem. 2022, 65, 623–629. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M. Recent advances on the rational design of non-precious metal oxide catalysts exemplified by CuOx/CeO2 binary system: Implications of size, shape and electronic effects on intrinsic reactivity and metal-support interactions. Catalysts 2020, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Li, Z.; Xiong, W.; Zhang, Y.; Ye, A.; Huang, W. Structural evolution and catalytic performance in CO2 hydrogenation reaction of ZnO-ZrO2 composite oxides. Appl. Surf. Sci. 2022, 587, 152884. [Google Scholar] [CrossRef]
- Ticali, P.; Salusso, D.; Ahmad, R.; Ahoba-Sam, C.; Ramirez, A.; Shterk, G.; Lomachenko, K.A.; Borfecchia, E.; Morandi, S.; Cavallo, L. CO2 hydrogenation to methanol and hydrocarbons over bifunctional Zn-doped ZrO2/zeolite catalysts. Catal. Sci. Technol. 2021, 11, 1249–1268. [Google Scholar] [CrossRef]
- Sha, F.; Tang, C.; Tang, S.; Wang, Q.; Han, Z.; Wang, J.; Li, C. The promoting role of Ga in ZnZrOx solid solution catalyst for CO2 hydrogenation to methanol. J. Catal. 2021, 404, 383–392. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Fan, G.; Yang, L.; Li, F. Unveiling the roles of Fe-Co interactions over ternary spinel-type ZnCoxFe2-xO4 catalysts for highly efficient CO2 hydrogenation to produce light olefins. J. Catal. 2021, 400, 355–366. [Google Scholar] [CrossRef]
- Zhu, J.; Cannizzaro, F.; Liu, L.; Zhang, H.; Kosinov, N.; Filot, I.A.; Rabeah, J.; Bruckner, A.; Hensen, E.J. Ni–In Synergy in CO2 Hydrogenation to Methanol. ACS Catal. 2021, 11, 11371–11384. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Wu, D. Mixed-phase indium oxide as a highly active and stable catalyst for the hydrogenation of CO2 to CH3OH. Ind. Eng. Chem. Res. 2021, 60, 3532–3542. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Cao, C.; Chen, T.-B.; Ding, X.; Huang, H.; Shen, L.; Cao, X.; Zhu, M.; Xu, J.; Gao, J. Unraveling highly tunable selectivity in CO2 hydrogenation over bimetallic In-Zr oxide catalysts. ACS Catal. 2019, 9, 8785–8797. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [Green Version]
- Frei, M.S.; Mondelli, C.; Cesarini, A.; Krumeich, F.; Hauert, R.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Role of zirconia in indium oxide-catalyzed CO2 hydrogenation to methanol. ACS Catal. 2019, 10, 1133–1145. [Google Scholar] [CrossRef]
- Akkharaphatthawon, N.; Chanlek, N.; Cheng, C.K.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Tuning adsorption properties of GaxIn2−xO3 catalysts for enhancement of methanol synthesis activity from CO2 hydrogenation at high reaction temperature. Appl. Surf. Sci. 2019, 489, 278–286. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Lobo, R.F. Direct conversion of CO2 into methanol over promoted indium oxide-based catalysts. Appl. Catal. A Gen. 2019, 583, 117144. [Google Scholar] [CrossRef]
- Wang, J.; Tang, C.; Li, G.; Han, Z.; Li, Z.; Liu, H.; Cheng, F.; Li, C. High-Performance MaZrOx (Ma = Cd, Ga) Solid-Solution Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 10253–10259. [Google Scholar] [CrossRef]
- Araújo, T.P.; Hergesell, A.H.; Faust-Akl, D.; Büchele, S.; Stewart, J.A.; Mondelli, C.; Pérez-Ramírez, J. Methanol Synthesis by Hydrogenation of Hybrid CO2−CO Feeds. ChemSusChem 2021, 14, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Qin, B.; Yang, Y.; Wang, H.; Cai, J.; Han, Y.; Li, S.; Gao, P.; Sun, Y. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci. Adv. 2020, 6, eaaz2060. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.Y.R.; Manavi, N.; Zhou, Z.; Wang, L.-C.; Diao, W.; Karakalos, S.; Liu, B.; Stowers, K.J.; Zhou, M.; Luo, H. Mechanistic understanding of support effect on the activity and selectivity of indium oxide catalysts for CO2 hydrogenation. Chem. Eng. J. 2021, 426, 131767. [Google Scholar] [CrossRef]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Influence of Mn and Mg oxides on the performance of In2O3 catalysts for CO2 hydrogenation to methanol. Chem. Phys. Lett. 2022, 786, 139173. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yang, C.; Yi, Y.; Wang, X.; Liu, F.; Cao, J.; Pan, H. A novel microreaction strategy to fabricate superior hybrid zirconium and zinc oxides for methanol synthesis from CO2. Appl. Catal. A Gen. 2020, 595, 117507. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.Y.; Ding, Y.; Yu, Z. Mesoporous manganese-cobalt oxide spinel catalysts for CO2 hydrogenation to methanol. J. CO2 Util. 2019, 32, 146–154. [Google Scholar] [CrossRef]
- Li, L.; Yang, B.; Gao, B.; Wang, Y.; Zhang, L.; Ishihara, T.; Qi, W.; Guo, L. CO2 hydrogenation selectivity shift over In-Co binary oxides catalysts: Catalytic mechanism and structure-property relationship. Chin. J. Catal. 2022, 43, 862–876. [Google Scholar] [CrossRef]
- Xu, D.; Hong, X.; Liu, G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover. J. Catal. 2021, 393, 207–214. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, F.; Ma, J.; Yang, C.; Wang, X.; Cao, J. Catalytic roles of In2O3 in ZrO2-based binary oxides for CO2 hydrogenation to methanol. Mol. Catal. 2022, 525, 112354. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Wang, Y.; Oulego, P.; Rothenberg, G.; Tu, X.; Shiju, N.R. CO2 hydrogenation at atmospheric pressure and low temperature using plasma-enhanced catalysis over supported cobalt oxide catalysts. ACS Sustain. Chem. Eng. 2020, 8, 17397–17407. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhao, G.; Shi, X.-R.; Chen, P.; Liu, Y.; Lu, Y. Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0.5 toward efficient CO2 hydrogenation to methanol. Sci. Adv. 2021, 7, eabi6012. [Google Scholar] [CrossRef]
- Numpilai, T.; Kidkhunthod, P.; Cheng, C.K.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. CO2 hydrogenation to methanol at high reaction temperatures over In2O3/ZrO2 catalysts: Influence of calcination temperatures of ZrO2 support. Catal. Today 2021, 375, 298–306. [Google Scholar] [CrossRef]
- Shi, Y.; Su, W.; Kong, L.; Wang, J.; Lv, P.; Hao, J.; Gao, X.; Yu, G. The homojunction formed by h-In2O3 (110) and c-In2O3 (440) promotes carbon dioxide hydrogenation to methanol on graphene oxide modified In2O3. J. Colloid Interface Sci. 2022, 623, 1048–1062. [Google Scholar] [CrossRef]
- Yang, B.; Li, L.; Jia, Z.; Liu, X.; Zhang, C.; Guo, L. Comparative study of CO2 hydrogenation to methanol on cubic bixbyite-type and rhombohedral corundum-type indium oxide. Chin. Chem. Lett. 2020, 31, 2627–2633. [Google Scholar] [CrossRef]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. CO2 hydrogenation to methanol over Pd/MnO/In2O3 catalyst. J. Environ. Chem. Eng. 2022, 10, 106965. [Google Scholar] [CrossRef]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Solid-State Synthesis of Pd/In2O3 Catalysts for CO2 Hydrogenation to Methanol. Catal. Lett. 2022, 1–8. [Google Scholar] [CrossRef]
- Pechenkin, A.; Potemkin, D.; Rubtsova, M.; Snytnikov, P.; Plyusnin, P.; Glotov, A. CuO-In2O3 Catalysts Supported on Halloysite Nanotubes for CO2 Hydrogenation to Dimethyl Ether. Catalysts 2021, 11, 1151. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Liu, T.; Liu, G.; Hong, X. Fabrication of PdZn alloy catalysts supported on ZnFe composite oxide for CO2 hydrogenation to methanol. J. Colloid Interface Sci. 2021, 597, 260–268. [Google Scholar] [CrossRef]
- Duyar, M.S.; Gallo, A.; Regli, S.K.; Snider, J.L.; Singh, J.A.; Valle, E.; McEnaney, J.; Bent, S.F.; Rønning, M.; Jaramillo, T.F. Understanding selectivity in CO2 hydrogenation to methanol for mop nanoparticle catalysts using in situ techniques. Catalysts 2021, 11, 143. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, G.; Yang, L.; Li, F. Tuning surface-interface structures of ZrO2 supported copper catalysts by in situ introduction of indium to promote CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2020, 605, 117805. [Google Scholar] [CrossRef]
- Stangeland, K.; Chamssine, F.; Fu, W.; Huang, Z.; Duan, X.; Yu, Z. CO2 hydrogenation to methanol over partially embedded Cu within Zn-Al oxide and the effect of indium. J. CO2 Util. 2021, 50, 101609. [Google Scholar] [CrossRef]
- Vu, T.T.N.; Desgagnés, A.; Iliuta, M.C. Efficient approaches to overcome challenges in material development for conventional and intensified CO2 catalytic hydrogenation to CO, methanol, and DME. Appl. Catal. A Gen. 2021, 617, 118119. [Google Scholar] [CrossRef]
- Millán Ordóñez, E.; Mota, N.; Guil-López, R.; Garcia Pawelec, B.; Fierro, J.L.G.; Navarro Yerga, R.M. Direct Synthesis of Dimethyl Ether on Bifunctional Catalysts Based on Cu–ZnO (Al) and Supported H3PW12O40: Effect of Physical Mixing on Bifunctional Interactions and Activity. Ind. Eng. Chem. Res. 2021, 60, 18853–18869. [Google Scholar] [CrossRef]
- Liu, Z.; An, X.; Song, M.; Wang, Z.; Wei, Y.; Mintova, S.; Giordano, G.; Yan, Z. Dry gel assisting crystallization of bifunctional CuO–ZnO–Al2O3/SiO2–Al2O3 catalysts for CO2 hydrogenation. Biomass Bioenergy 2022, 163, 106525. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, L.; Liu, Y.; Zhao, Z. Cu-embedded porous Al2O3 bifunctional catalyst derived from metal–organic framework for syngas-to-dimethyl ether. Chin. Chem. Lett. 2022, 33, 2906–2910. [Google Scholar] [CrossRef]
- Temvuttirojn, C.; Chuasomboon, N.; Numpilai, T.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Development of SO42−–ZrO2 acid catalysts admixed with a CuO-ZnO-ZrO2 catalyst for CO2 hydrogenation to dimethyl ether. Fuel 2019, 241, 695–703. [Google Scholar] [CrossRef]
- Mureddu, M.; Lai, S.; Atzori, L.; Rombi, E.; Ferrara, F.; Pettinau, A.; Cutrufello, M.G. Ex-LDH-based catalysts for CO2 conversion to methanol and dimethyl ether. Catalysts 2021, 11, 615. [Google Scholar] [CrossRef]
- Pechenkin, A.; Potemkin, D.; Badmaev, S.; Smirnova, E.; Cherednichenko, K.; Vinokurov, V.; Glotov, A. CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes. Green Process. Synth. 2021, 10, 594–605. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Z.; Sun, K.; Huang, W. Effects of Sn on the catalytic performance for one step syngas to DME in slurry reactor. New J. Chem. 2021, 45, 3783–3789. [Google Scholar] [CrossRef]
- Ateka, A.; Sánchez-Contador, M.; Portillo, A.; Bilbao, J.; Aguayo, A.T. Kinetic modeling of CO2+ CO hydrogenation to DME over a CuO-ZnO-ZrO2@ SAPO-11 core-shell catalyst. Fuel Process. Technol. 2020, 206, 106434. [Google Scholar] [CrossRef]
- Ren, S.; Shoemaker, W.R.; Wang, X.; Shang, Z.; Klinghoffer, N.; Li, S.; Yu, M.; He, X.; White, T.A.; Liang, X. Highly active and selective Cu-ZnO based catalyst for methanol and dimethyl ether synthesis via CO2 hydrogenation. Fuel 2019, 239, 1125–1133. [Google Scholar] [CrossRef]
- Godini, H.R.; Kumar, S.R.; Tadikamalla, N.; Gallucci, F. Performance analysis of hybrid catalytic conversion of CO2 to DiMethyl ether. Int. J. Hydrogen Energy 2022, 47, 11341–11358. [Google Scholar] [CrossRef]
- Suwannapichat, Y.; Numpilai, T.; Chanlek, N.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Direct synthesis of dimethyl ether from CO2 hydrogenation over novel hybrid catalysts containing a CuZnOZrO2 catalyst admixed with WOx/Al2O3 catalysts: Effects of pore size of Al2O3 support and W loading content. Energy Convers. Manag. 2018, 159, 20–29. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Thuriot-Roukos, J.; Wojcieszak, R.; Khodakov, A.Y. Structure–performance correlations in the hybrid oxide-supported copper–zinc SAPO-34 catalysts for direct synthesis of dimethyl ether from CO2. J. Mater. Sci. 2022, 57, 3268–3279. [Google Scholar] [CrossRef]
- Fang, X.; Jia, H.; Zhang, B.; Li, Y.; Wang, Y.; Song, Y.; Du, T.; Liu, L. A novel in situ grown Cu-ZnO-ZrO2/HZSM-5 hybrid catalyst for CO2 hydrogenation to liquid fuels of methanol and DME. J. Environ. Chem. Eng. 2021, 9, 105299. [Google Scholar] [CrossRef]
- Feng, W.-H.; Yu, M.-M.; Wang, L.-J.; Miao, Y.-T.; Shakouri, M.; Ran, J.; Hu, Y.; Li, Z.; Huang, R.; Lu, Y.-L. Insights into bimetallic oxide synergy during carbon dioxide hydrogenation to methanol and dimethyl ether over GaZrO x oxide catalysts. ACS Catal. 2021, 11, 4704–4711. [Google Scholar] [CrossRef]
- Ateka, A.; Ereña, J.; Pérez-Uriarte, P.; Aguayo, A.T.; Bilbao, J. Effect of the content of CO2 and H2 in the feed on the conversion of CO2 in the direct synthesis of dimethyl ether over a CuOZnOAl2O3/SAPO-18 catalyst. Int. J. Hydrogen Energy 2017, 42, 27130–27138. [Google Scholar] [CrossRef]
- Şeker, B.; Dizaji, A.K.; Balci, V.; Uzun, A. MCM-41-supported tungstophosphoric acid as an acid function for dimethyl ether synthesis from CO2 hydrogenation. Renew. Energy 2021, 171, 47–57. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, K.; Pant, K.K.; Parikh, J.K. Unravelling synergetic interaction over tandem Cu-ZnO-ZrO2/hierarchical ZSM5 catalyst for CO2 hydrogenation to methanol and DME. Fuel 2022, 318, 123641. [Google Scholar] [CrossRef]
- Tan, K.B.; Tian, P.; Zhang, X.; Tian, J.; Zhan, G.; Huang, J.; Li, Q. Green synthesis of microspherical-confined nano-Pd/In2O3 integrated with H-ZSM-5 as bifunctional catalyst for CO2 hydrogenation into dimethyl ether: A carbonized alginate templating strategy. Sep. Purif. Technol. 2022, 297, 121559. [Google Scholar] [CrossRef]
- Liu, C.; Kang, J.; Huang, Z.-Q.; Song, Y.-H.; Xiao, Y.-S.; Song, J.; He, J.-X.; Chang, C.-R.; Ge, H.-Q.; Wang, Y. Gallium nitride catalyzed the direct hydrogenation of carbon dioxide to dimethyl ether as primary product. Nat. Commun. 2021, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2021, 58, 55–77. [Google Scholar] [CrossRef]
- Ateka, A.; Rodriguez-Vega, P.; Ereña, J.; Aguayo, A.; Bilbao, J. A review on the valorization of CO2. Focusing on the thermodynamics and catalyst design studies of the direct synthesis of dimethyl ether. Fuel Process. Technol. 2022, 233, 107310. [Google Scholar] [CrossRef]
- Weber, D.; He, T.; Wong, M.; Moon, C.; Zhang, A.; Foley, N.; Ramer, N.J.; Zhang, C. Recent Advances in the Mitigation of the Catalyst Deactivation of CO2 Hydrogenation to Light Olefins. Catalysts 2021, 11, 1447. [Google Scholar] [CrossRef]
- Ma, Z.; Porosoff, M.D. Development of tandem catalysts for CO2 hydrogenation to olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, J.; Wang, F. An economic analysis of twenty light olefin production pathways. J. Energy Chem. 2021, 56, 193–202. [Google Scholar] [CrossRef]
- Gogate, M.R. Methanol-to-olefins process technology: Current status and future prospects. Pet. Sci. Technol. 2019, 37, 559–565. [Google Scholar] [CrossRef]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Sedighi, M.; Mohammadi, M. CO2 hydrogenation to light olefins over Cu-CeO2/SAPO-34 catalysts: Product distribution and optimization. J. CO2 Util. 2020, 35, 236–244. [Google Scholar] [CrossRef]
- Tada, S.; Kinoshita, H.; Ochiai, N.; Chokkalingam, A.; Hu, P.; Yamauchi, N.; Kobayashi, Y.; Iyoki, K. Search for solid acid catalysts aiming at the development of bifunctional tandem catalysts for the one-pass synthesis of lower olefins via CO2 hydrogenation. Int. J. Hydrogen Energy 2021, 46, 36721–36730. [Google Scholar] [CrossRef]
- Dokania, A.; Ould-Chikh, S.; Ramirez, A.; Cerrillo, J.L.; Aguilar, A.; Russkikh, A.; Alkhalaf, A.; Hita, I.; Bavykina, A.; Shterk, G. Designing a Multifunctional Catalyst for the Direct Production of Gasoline-Range Isoparaffins from CO2. JACS Au 2021, 1, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Gao, P.; Liu, Z.; Chen, X.; Yang, C.; Wang, H.; Zhong, L.; Li, S.; Sun, Y. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, Y.; Hu, Z.; Fu, Y.; Fang, X.; Jiang, Q.; Chen, Z.; Zhu, W.; Liu, Z. Insights into effects of ZrO2 crystal phase on syngas-to-olefin conversion over ZnO/ZrO2 and SAPO-34 composite catalysts. Chin. J. Catal. 2022, 43, 877–884. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Y.; Jiao, F.; Pan, X.; Jiang, Q.; Cai, J.; Li, Y.; Tong, W.; Xu, C.; Qu, S. Steering the reaction pathway of syngas-to-light olefins with coordination unsaturated sites of ZnGaOx spinel. Nat. Commun. 2022, 13, 2742. [Google Scholar] [CrossRef]
- Portillo, A.; Ateka, A.; Ereña, J.; Bilbao, J.; Aguayo, A. Role of Zr loading into In2O3 catalysts for the direct conversion of CO2/CO mixtures into light olefins. J. Environ. Manag. 2022, 316, 115329. [Google Scholar] [CrossRef]
- Portillo, A.; Ateka, A.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Conditions for the joint conversion of CO2 and syngas in the direct synthesis of light olefins using In2O3–ZrO2/SAPO-34 catalyst. Ind. Eng. Chem. Res. 2021, 61, 10365–10376. [Google Scholar] [CrossRef]
- Gao, P.; Dang, S.; Li, S.; Bu, X.; Liu, Z.; Qiu, M.; Yang, C.; Wang, H.; Zhong, L.; Han, Y. Direct production of lower olefins from CO2 conversion via bifunctional catalysis. ACS Catal. 2018, 8, 571–578. [Google Scholar] [CrossRef]
- Mou, J.; Fan, X.; Liu, F.; Wang, X.; Zhao, T.; Chen, P.; Li, Z.; Yang, C.; Cao, J. CO2 hydrogenation to lower olefins over Mn2O3-ZnO/SAPO-34 tandem catalysts. Chem. Eng. J. 2021, 421, 129978. [Google Scholar] [CrossRef]
- Numpilai, T.; Chanlek, N.; Poo-Arporn, Y.; Wannapaiboon, S.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Kongkachuichay, P.; Chareonpanich, M.; Rupprechter, G. Pore size effects on physicochemical properties of Fe-Co/K-Al2O3 catalysts and their catalytic activity in CO2 hydrogenation to light olefins. Appl. Surf. Sci. 2019, 483, 581–592. [Google Scholar] [CrossRef]
- Guo, L.; Cui, Y.; Li, H.; Fang, Y.; Prasert, R.; Wu, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Selective formation of linear-alpha olefins (LAOs) by CO2 hydrogenation over bimetallic Fe/Co-Y catalyst. Catal. Commun. 2019, 130, 105759. [Google Scholar] [CrossRef]
- Numpilai, T.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Optimization of synthesis condition for CO2 hydrogenation to light olefins over In2O3 admixed with SAPO-34. Energy Convers. Manag. 2019, 180, 511–523. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, G.; Tian, J.; Tan, K.B.; Guo, M.; Han, Y.; Fu, T.; Huang, J.; Li, Q. Direct CO2 Hydrogenation to Light Olefins over ZnZrOx Mixed with Hierarchically Hollow SAPO-34 with Rice Husk as Green Silicon Source and Template. Appl. Catal. B Environ. 2022, 315, 121572. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Qu, Y.; Liu, H.; Tang, C.; Miao, S.; Feng, Z.; An, H.; Li, C. Highly selective conversion of carbon dioxide to lower olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Qin, Z.; Yan, W.; Dong, M.; Li, J.; Wang, J.; Fan, W. Enhancement of light olefin production in CO2 hydrogenation over In2O3-based oxide and SAPO-34 composite. J. Catal. 2020, 391, 459–470. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Wu, D.; Zhang, J.; Ma, Q.; Gao, X.; Lai, X.; Xia, H.; Fan, S.; Zhao, T.-S. Hydrogenation of CO2 to light olefins on CuZnZr@(Zn-) SAPO-34 catalysts: Strategy for product distribution. Fuel 2019, 239, 44–52. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mohammadi, M.; Sedighi, M. Sustainable production of light olefins from greenhouse gas CO2 over SAPO-34 supported modified cerium oxide. Microporous Mesoporous Mater. 2020, 297, 110029. [Google Scholar] [CrossRef]
- Li, J.; Yu, T.; Miao, D.; Pan, X.; Bao, X. Carbon dioxide hydrogenation to light olefins over ZnO-Y2O3 and SAPO-34 bifunctional catalysts. Catal. Commun. 2019, 129, 105711. [Google Scholar] [CrossRef]
- Ramirez, A.; Chowdhury, A.D.; Caglayan, M.; Rodriguez-Gomez, A.; Wehbe, N.; Abou-Hamad, E.; Gevers, L.; Ould-Chikh, S.; Gascon, J. Coated sulfated zirconia/SAPO-34 for the direct conversion of CO2 to light olefins. Catal. Sci. Technol. 2020, 10, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, A.; Jiang, X.; Janik, M.J.; Qiu, J.; Liu, Z.; Guo, X.; Song, C. The anti-sintering catalysts: Fe–Co–Zr polymetallic fibers for CO2 hydrogenation to C2=–C4=–rich hydrocarbons. J. CO2 Util. 2018, 23, 219–225. [Google Scholar] [CrossRef]
- Tong, M.; Chizema, L.G.; Chang, X.; Hondo, E.; Dai, L.; Zeng, Y.; Zeng, C.; Ahmad, H.; Yang, R.; Lu, P. Tandem catalysis over tailored ZnO-ZrO2/MnSAPO-34 composite catalyst for enhanced light olefins selectivity in CO2 hydrogenation. Microporous Mesoporous Mater. 2021, 320, 111105. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, L.; Meng, F.; Wang, L.; Zhang, R.; Yang, G.; Li, Z. Boosting CO2 hydrogenation performance for light olefin synthesis over GaZrOx combined with SAPO-34. Appl. Catal. B Environ. 2022, 305, 121042. [Google Scholar] [CrossRef]
- Tian, H.; Yao, J.; Zha, F.; Yao, L.; Chang, Y. Catalytic activity of SAPO-34 molecular sieves prepared by using palygorskite in the synthesis of light olefins via CO2 hydrogenation. Appl. Clay Sci. 2020, 184, 105392. [Google Scholar] [CrossRef]
- Numpilai, T.; Kahadit, S.; Witoon, T.; Ayodele, B.V.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to light olefins over In2O3/SAPO-34 and Fe-Co/K-Al2O3 composite catalyst. Top. Catal. 2021, 64, 316–327. [Google Scholar] [CrossRef]
- Wang, P.; Zha, F.; Yao, L.; Chang, Y. Synthesis of light olefins from CO2 hydrogenation over (CuO-ZnO)-kaolin/SAPO-34 molecular sieves. Appl. Clay Sci. 2018, 163, 249–256. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Ibegbu, A.J. Production of light olefins by catalytic hydrogenation of CO2 over Y2O3/Fe-Co modified with SAPO-34. Appl. Catal. A Gen. 2022, 643, 118784. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, J.; Wang, R.; Zhong, Q. FeZnK/SAPO-34 Catalyst for Efficient Conversion of CO2 to Light Olefins. Catal. Lett. 2022, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Liu, R.; Liu, W.; Gao, X.; Tan, Y.; Zhang, Z.; Tu, W. CO2 hydrogenation to olefins on supported iron catalysts: Effects of support properties on carbon-containing species and product distribution. Fuel 2022, 324, 124649. [Google Scholar] [CrossRef]
- Rimaz, S.; Kosari, M.; Zarinejad, M.; Ramakrishna, S. A comprehensive review on sustainability-motivated applications of SAPO-34 molecular sieve. J. Mater. Sci. 2022, 57, 1–39. [Google Scholar]
- Dugkhuntod, P.; Wattanakit, C. A comprehensive review of the applications of hierarchical zeolite nanosheets and nanoparticle assemblies in light olefin production. Catalysts 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Usman, M. Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes 2022, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, G.; Zhu, J.; Ding, F.; Zhang, A.; Song, C.; Guo, X. Boosting light olefin selectivity in CO2 hydrogenation by adding Co to Fe catalysts within close proximity. Catal. Today 2021, 371, 142–149. [Google Scholar] [CrossRef]
- Goud, D.; Gupta, R.; Maligal-Ganesh, R.; Peter, S.C. Review of catalyst design and mechanistic studies for the production of olefins from anthropogenic CO2. ACS Catal. 2020, 10, 14258–14282. [Google Scholar] [CrossRef]
- Tian, H.; He, H.; Jiao, J.; Zha, F.; Guo, X.; Tang, X.; Chang, Y. Tandem catalysts composed of different morphology HZSM-5 and metal oxides for CO2 hydrogenation to aromatics. Fuel 2022, 314, 123119. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. Reaction coupling as a promising methodology for selective conversion of syngas into hydrocarbons beyond Fischer-Tropsch synthesis. Sci. China Chem. 2017, 60, 1382–1385. [Google Scholar] [CrossRef]
- Winter, L.R.; Gomez, E.; Yan, B.; Yao, S.; Chen, J.G. Tuning Ni-catalyzed CO2 hydrogenation selectivity via Ni-ceria support interactions and Ni-Fe bimetallic formation. Appl. Catal. B Environ. 2018, 224, 442–450. [Google Scholar] [CrossRef]
- Azhari, N.J.; Nurdini, N.; Mardiana, S.; Ilmi, T.; Fajar, A.T.; Makertihartha, I.; Kadja, G.T. Zeolite-based catalyst for direct conversion of CO2 to C2+ hydrocarbon: A review. J. CO2 Util. 2022, 59, 101969. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Zhang, C.; Han, S.J.; Park, H.-G.; Kim, Y.T.; Yang, S.; Jun, K.-W.; Kim, S.K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. [Google Scholar] [CrossRef]
- Kim, H.D.; Song, H.-T.; Fazeli, A.; Eslami, A.A.; Noh, Y.S.; Saeidabad, N.G.; Lee, K.-Y.; Moon, D.J. CO/CO2 hydrogenation for the production of lighter hydrocarbons over SAPO-34 modified hybrid FTS catalysts. Catal. Today 2020, 388–389, 410–416. [Google Scholar] [CrossRef]
- Liu, B.; Geng, S.; Zheng, J.; Jia, X.; Jiang, F.; Liu, X. Unravelling the New Roles of Na and Mn Promoter in CO2 Hydrogenation over Fe3O4-Based Catalysts for Enhanced Selectivity to Light α-Olefins. ChemCatChem 2018, 10, 4718–4732. [Google Scholar] [CrossRef]
- Khangale, P.R.; Meijboom, R.; Jalama, K. CO2 hydrogenation to liquid hydrocarbons via modified Fischer–Tropsch over alumina-supported cobalt catalysts: Effect of operating temperature, pressure and potassium loading. J. CO2 Util. 2020, 41, 101268. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Jiang, X.; Wang, J.; Song, C.; Guo, X. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons. Catal. Today 2021, 371, 162–170. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Han, S.J.; Park, H.-G.; Lee, H.; An, K.; Jun, K.-W.; Kim, S.K. Atomically Alloyed Fe–Co Catalyst derived from a N-coordinated Co single-atom structure for CO2 hydrogenation. ACS Catal. 2021, 11, 2267–2278. [Google Scholar] [CrossRef]
- Yao, B.; Xiao, T.; Makgae, O.A.; Jie, X.; Gonzalez-Cortes, S.; Guan, S.; Kirkland, A.I.; Dilworth, J.R.; Al-Megren, H.A.; Alshihri, S.M. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Li, X.; Lin, T.; An, Y.; Zhong, L.; Sun, Y. Fischer-Tropsch to olefins over CO2C-based catalysts: Effect of thermal pretreatment of SiO2 support. Appl. Catal. A Gen. 2021, 623, 118283. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, B.; Geng, S.; Xu, Y.; Liu, X. Hydrogenation of CO2 into hydrocarbons: Enhanced catalytic activity over Fe-based Fischer–Tropsch catalysts. Catal. Sci. Technol. 2018, 8, 4097–4107. [Google Scholar] [CrossRef]
- Gong, K.; Lin, T.; An, Y.; Wang, X.; Yu, F.; Wu, B.; Li, X.; Li, S.; Lu, Y.; Zhong, L. Fischer-Tropsch to olefins over CoMn-based catalysts: Effect of preparation methods. Appl. Catal. A Gen. 2020, 592, 117414. [Google Scholar] [CrossRef]
- Kwok, K.M.; Chen, L.; Zeng, H.C. Design of hollow spherical Co@ hsZSM5@ metal dual-layer nanocatalysts for tandem CO2 hydrogenation to increase C2+ hydrocarbon selectivity. J. Mater. Chem. A 2020, 8, 12757–12766. [Google Scholar] [CrossRef]
- Liu, R.; Ma, Z.; Sears, J.D.; Juneau, M.; Neidig, M.L.; Porosoff, M.D. Identifying correlations in Fischer-Tropsch synthesis and CO2 hydrogenation over Fe-based ZSM-5 catalysts. J. CO2 Util. 2020, 41, 101290. [Google Scholar] [CrossRef]
- Guo, L.; Sun, S.; Li, J.; Gao, W.; Zhao, H.; Zhang, B.; He, Y.; Zhang, P.; Yang, G.; Tsubaki, N. Boosting liquid hydrocarbons selectivity from CO2 hydrogenation by facilely tailoring surface acid properties of zeolite via a modified Fischer-Tropsch synthesis. Fuel 2021, 306, 121684. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B Environ. 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 hydrogenation to hydrocarbons on Cu-promoted Fe-based catalysts: Dependence on Cu–Fe interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, G.; Ding, S.; Sheikh, M.C.; Long, D.; Yoneyama, Y.; Tsubaki, N. Design of ultra-active iron-based Fischer-Tropsch synthesis catalysts over spherical mesoporous carbon with developed porosity. Chem. Eng. J. 2018, 334, 714–724. [Google Scholar] [CrossRef]
- Qadir, M.I.; Bernardi, F.; Scholten, J.D.; Baptista, D.L.; Dupont, J. Synergistic CO2 hydrogenation over bimetallic Ru/Ni nanoparticles in ionic liquids. Appl. Catal. B Environ. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Chiang, F.-K.; Dugulan, A.I.; Song, Y.; Pestman, R.; Zhang, K.; Yao, J.; Feng, B.; Miao, P. Synthesis of stable and low-CO2 selective ε-iron carbide Fischer-Tropsch catalysts. Sci. Adv. 2018, 4, eaau2947. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, D.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Highly selective conversion of CO2 to light olefins via Fischer-Tropsch synthesis over stable layered K–Fe–Ti catalysts. Appl. Catal. A Gen. 2019, 573, 32–40. [Google Scholar] [CrossRef]
- Song, F.; Yong, X.; Wu, X.; Zhang, W.; Ma, Q.; Zhao, T.; Tan, M.; Guo, Z.; Zhao, H.; Yang, G. FeMn@ HZSM-5 capsule catalyst for light olefins direct synthesis via Fischer-Tropsch synthesis: Studies on depressing the CO2 formation. Appl. Catal. B Environ. 2022, 300, 120713. [Google Scholar] [CrossRef]
- Cui, M.; Qian, Q.; Zhang, J.; Wang, Y.; Bediako, B.B.A.; Liu, H.; Han, B. Liquid fuel synthesis via CO2 hydrogenation by coupling homogeneous and heterogeneous catalysis. Chem 2021, 7, 726–737. [Google Scholar] [CrossRef]
- Straß-Eifert, A.; Sheppard, T.L.; Becker, H.; Friedland, J.; Zimina, A.; Grunwaldt, J.D.; Güttel, R. Cobalt-based Nanoreactors in Combined Fischer-Tropsch Synthesis and Hydroprocessing: Effects on Methane and CO2 Selectivity. ChemCatChem 2021, 13, 5216–5227. [Google Scholar] [CrossRef]
- Sirikulbodee, P.; Ratana, T.; Sornchamni, T.; Phongaksorn, M.; Tungkamani, S. Catalytic performance of Iron-based catalyst in Fischer–Tropsch synthesis using CO2 containing syngas. Energy Procedia 2017, 138, 998–1003. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Jia, L.; Sun, C.; Chen, B.; Liu, R.; Tan, Y.; Tu, W. Effects of the reducing gas atmosphere on performance of FeCeNa catalyst for the hydrogenation of CO2 to olefins. Chem. Eng. J. 2022, 428, 131388. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Yan, J.; Qiao, X.; Guan, Q.; Li, W. N-doped ordered mesoporous carbon (N-OMC) confined Fe3O4-FeCx heterojunction for efficient conversion of CO2 to light olefins. Appl. Catal. B Environ. 2021, 299, 120639. [Google Scholar] [CrossRef]
- Guilera, J.; Díaz-López, J.A.; Berenguer, A.; Biset-Peiró, M.; Andreu, T. Fischer-Tropsch synthesis: Towards a highly-selective catalyst by lanthanide promotion under relevant CO2 syngas mixtures. Appl. Catal. A Gen. 2022, 629, 118423. [Google Scholar] [CrossRef]
- Jo, H.; Khan, M.K.; Irshad, M.; Arshad, M.W.; Kim, S.K.; Kim, J. Unraveling the role of cobalt in the direct conversion of CO2 to high-yield liquid fuels and lube base oil. Appl. Catal. B Environ. 2022, 305, 121041. [Google Scholar] [CrossRef]
- Ahmad, E.; Upadhyayula, S.; Pant, K.K. Biomass-derived CO2 rich syngas conversion to higher hydrocarbon via Fischer-Tropsch process over Fe–Co bimetallic catalyst. Int. J. Hydrogen Energy 2019, 44, 27741–27748. [Google Scholar]
- Bashiri, N.; Omidkhah, M.R.; Godini, H.R. Direct conversion of CO2 to light olefins over FeCo/XK-ϒAl2O3 (X = La, Mn, Zn) catalyst via hydrogenation reaction. Res. Chem. Intermed. 2021, 47, 5267–5289. [Google Scholar] [CrossRef]
- Gong, K.; Wei, Y.; Dai, Y.; Lin, T.; Yu, F.; An, Y.; Wang, X.; Sun, F.; Jiang, Z.; Zhong, L. Carbon-encapsulated metallic Co nanoparticles for Fischer-Tropsch to olefins with low CO2 selectivity. Appl. Catal. B Environ. 2022, 316, 121700. [Google Scholar] [CrossRef]
- He, Z.; Cui, M.; Qian, Q.; Zhang, J.; Liu, H.; Han, B. Synthesis of liquid fuel via direct hydrogenation of CO2. Proc. Natl. Acad. Sci. USA 2019, 116, 12654–12659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, T.; Wang, J.; Ding, F.; Zhang, A.; Li, W.; Guo, X.; Song, C. CO2 hydrogenation to hydrocarbons over alumina-supported iron catalyst: Effect of support pore size. J. CO2 Util. 2017, 19, 202–208. [Google Scholar] [CrossRef]
- Numpilai, T.; Witoon, T.; Chanlek, N.; Limphirat, W.; Bonura, G.; Chareonpanich, M.; Limtrakul, J. Structure–activity relationships of Fe-Co/K-Al2O3 catalysts calcined at different temperatures for CO2 hydrogenation to light olefins. Appl. Catal. A Gen. 2017, 547, 219–229. [Google Scholar] [CrossRef]
- Bradley, M.J.; Ananth, R.; Willauer, H.D.; Baldwin, J.W.; Hardy, D.R.; Williams, F.W. The effect of copper addition on the activity and stability of iron-based CO2 hydrogenation catalysts. Molecules 2017, 22, 1579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, H.; Yu, G.; He, S.; Kang, J.; Liu, Z.; Cheng, K.; Zhang, Q.; Wang, Y. Selective hydrogenation of CO2 and CO into olefins over Sodium-and Zinc-Promoted iron carbide catalysts. J. Catal. 2021, 395, 350–361. [Google Scholar] [CrossRef]
- Feng, K.; Tian, J.; Guo, M.; Wang, Y.; Wang, S.; Wu, Z.; Zhang, J.; He, L.; Yan, B. Experimentally unveiling the origin of tunable selectivity for CO2 hydrogenation over Ni-based catalysts. Appl. Catal. B Environ. 2021, 292, 120191. [Google Scholar] [CrossRef]
- Sibi, M.G.; Khan, M.K.; Verma, D.; Yoon, W.; Kim, J. High-yield synthesis of BTEX over Na–FeAlOx/Zn–HZSM-5@ SiO2 by direct CO2 conversion and identification of surface intermediates. Appl. Catal. B Environ. 2022, 301, 120813. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Tang, X.; Yin, H.; Kang, J.; Zhang, Q.; Wang, Y. Zn and Na promoted Fe catalysts for sustainable production of high-valued olefins by CO2 hydrogenation. Fuel 2022, 309, 122105. [Google Scholar] [CrossRef]
- Chaipraditgul, N.; Numpilai, T.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Wattanakit, C.; Limtrakul, J.; Witoon, T. Tuning interaction of surface-adsorbed species over Fe/K-Al2O3 modified with transition metals (Cu, Mn, V, Zn or Co) on light olefins production from CO2 hydrogenation. Fuel 2021, 283, 119248. [Google Scholar] [CrossRef]
- Garona, H.A.; Cavalcanti, F.M.; de Abreu, T.F.; Schmal, M.; Alves, R.M. Evaluation of Fischer-Tropsch synthesis to light olefins over Co-and Fe-based catalysts using artificial neural network. J. Clean. Prod. 2021, 321, 129003. [Google Scholar] [CrossRef]
- Hodala, J.L.; Moon, D.J.; Reddy, K.R.; Reddy, C.V.; Kumar, T.N.; Ahamed, M.I.; Raghu, A.V. Catalyst design for maximizing C5+ yields during Fischer-Tropsch synthesis. Int. J. Hydrogen Energy 2021, 46, 3289–3301. [Google Scholar] [CrossRef]
- Shiba, N.C.; Yao, Y.; Liu, X.; Hildebrandt, D. Recent developments in catalyst pretreatment technologies for cobalt based Fisher–Tropsch synthesis. Rev. Chem. Eng. 2021, 38, 503–538. [Google Scholar] [CrossRef]
- Lu, F.; Chen, X.; Lei, Z.; Wen, L.; Zhang, Y. Revealing the activity of different iron carbides for Fischer-Tropsch synthesis. Appl. Catal. B Environ. 2021, 281, 119521. [Google Scholar] [CrossRef]
- Gong, W.; Ye, R.-P.; Ding, J.; Wang, T.; Shi, X.; Russell, C.K.; Tang, J.; Eddings, E.G.; Zhang, Y.; Fan, M. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission. Appl. Catal. B Environ. 2020, 278, 119302. [Google Scholar] [CrossRef]
- Lin, T.; Liu, P.; Gong, K.; An, Y.; Yu, F.; Wang, X.; Zhong, L.; Sun, Y. Designing silica-coated CoMn-based catalyst for Fischer-Tropsch synthesis to olefins with low CO2 emission. Appl. Catal. B Environ. 2021, 299, 120683. [Google Scholar] [CrossRef]
- Kirsch, H.; Lochmahr, N.; Staudt, C.; Pfeifer, P.; Dittmeyer, R. Production of CO2-neutral liquid fuels by integrating Fischer-Tropsch synthesis and hydrocracking in a single micro-structured reactor: Performance evaluation of different configurations by factorial design experiments. Chem. Eng. J. 2020, 393, 124553. [Google Scholar] [CrossRef]
- Li, M.; Noreen, A.; Fu, Y.; Amoo, C.C.; Jiang, Y.; Sun, X.; Lu, P.; Yang, R.; Xing, C.; Wang, S. Direct conversion of syngas to gasoline ranged olefins over Na impellent Fe@ NaZSM-5 catalyst. Fuel 2022, 308, 121938. [Google Scholar] [CrossRef]
- Yang, J.; Rodriguez, C.L.; Qi, Y.; Ma, H.; Holmen, A.; Chen, D. The effect of co-feeding ethene on Fischer-Tropsch synthesis to olefins over Co-based catalysts. Appl. Catal. A Gen. 2020, 598, 117564. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Qing, M.; Liu, C.-L.; Dong, W.-S. Direct synthesis of lower olefins from syngas via Fischer–Tropsch synthesis catalyzed by a dual-bed catalyst. Mol. Catal. 2020, 485, 110824. [Google Scholar] [CrossRef]
- Shiba, N.C.; Yao, Y.; Forbes, R.P.; Okoye-Chine, C.G.; Liu, X.; Hildebrandt, D. Role of CoO-Co nanoparticles supported on SiO2 in Fischer-Tropsch synthesis: Evidence for enhanced CO dissociation and olefin hydrogenation. Fuel Process. Technol. 2021, 216, 106781. [Google Scholar] [CrossRef]
- Ghosh, I.K.; Iqbal, Z.; van Heerden, T.; van Steen, E.; Bordoloi, A. Insights into the unusual role of chlorine in product selectivity for direct hydrogenation of CO/CO2 to short-chain olefins. Chem. Eng. J. 2021, 413, 127424. [Google Scholar] [CrossRef]
- Yang, S.; Lee, S.; Kang, S.C.; Han, S.J.; Jun, K.-W.; Lee, K.-Y.; Kim, Y.T. Linear α-olefin production with Na-promoted Fe–Zn catalysts via Fischer–Tropsch synthesis. RSC Adv. 2019, 9, 14176–14187. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yan, Q.; Han, J.; Cao, B.; Street, J.; Yu, F. Fischer–Tropsch synthesis of olefin-rich liquid hydrocarbons from biomass-derived syngas over carbon-encapsulated iron carbide/iron nanoparticles catalyst. Fuel 2017, 193, 369–384. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jia, G.; Zhao, C.; Xing, Y. Efficient Fischer-Tropsch to light olefins over iron-based catalyst with low methane selectivity and high olefin/paraffin ratio. Fuel 2021, 288, 119572. [Google Scholar] [CrossRef]
- Lu, P.; Riswan, M.; Chang, X.; Zhu, K.; Hondo, E.; Nyako, A.; Xing, C.; Du, C.; Chen, S. Hydrogenation of CO2 to light olefins on ZZ/MnSAPO-34@ Si-2: Effect of silicalite-2 seeds on the acidity and catalytic activity. Fuel 2022, 330, 125470. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Gao, Y.; Zhu, Y.; Wan, H. Efficient production of renewable hydrocarbon fuels using waste CO2 and green H2 by integrating Fe-based Fischer-Tropsch synthesis and olefin oligomerization. Energy 2022, 248, 123616. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Song, C.; Guo, X. Catalytic conversion of carbon dioxide to methanol: Current status and future perspective. Front. Energy Res. 2021, 8, 621119. [Google Scholar] [CrossRef]
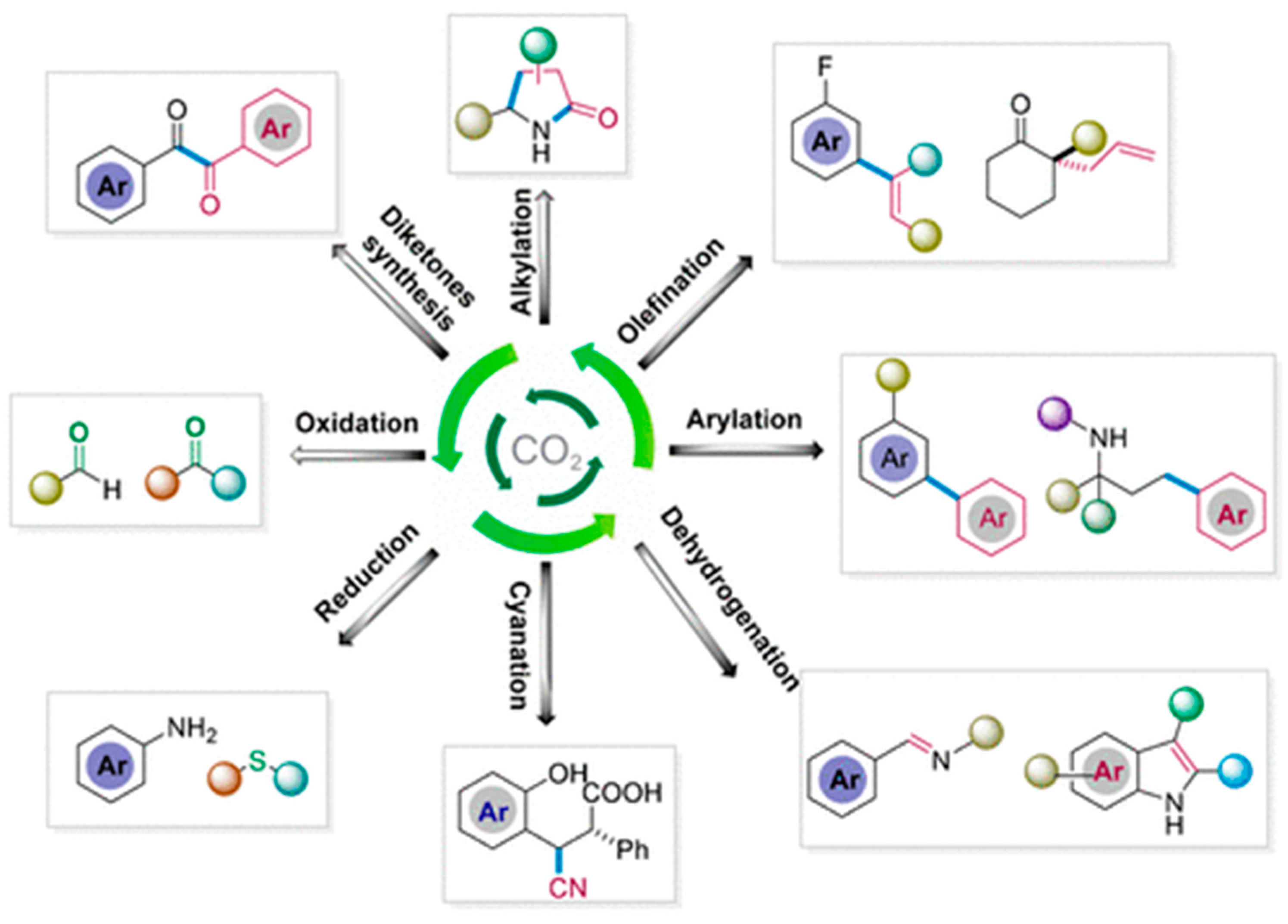
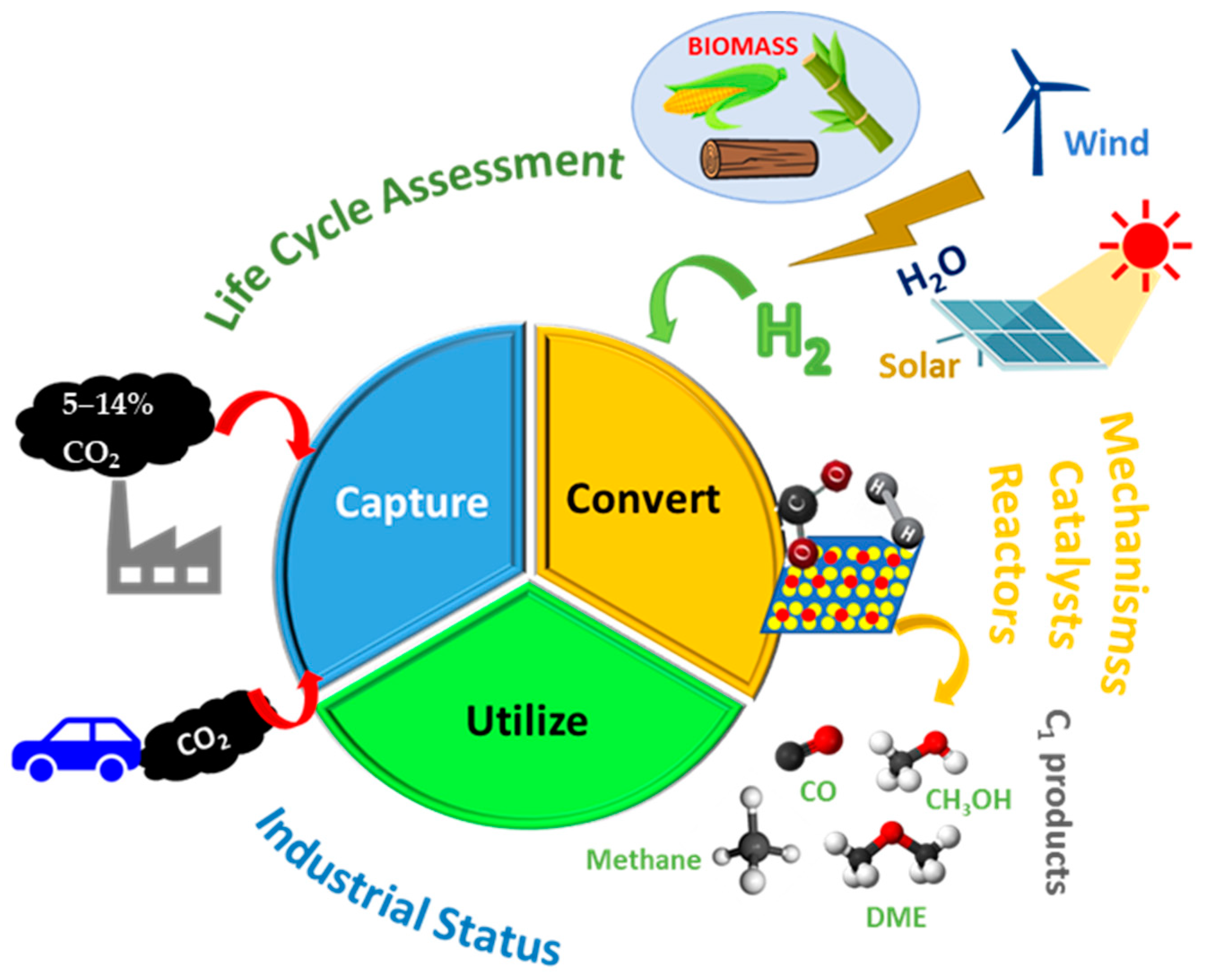
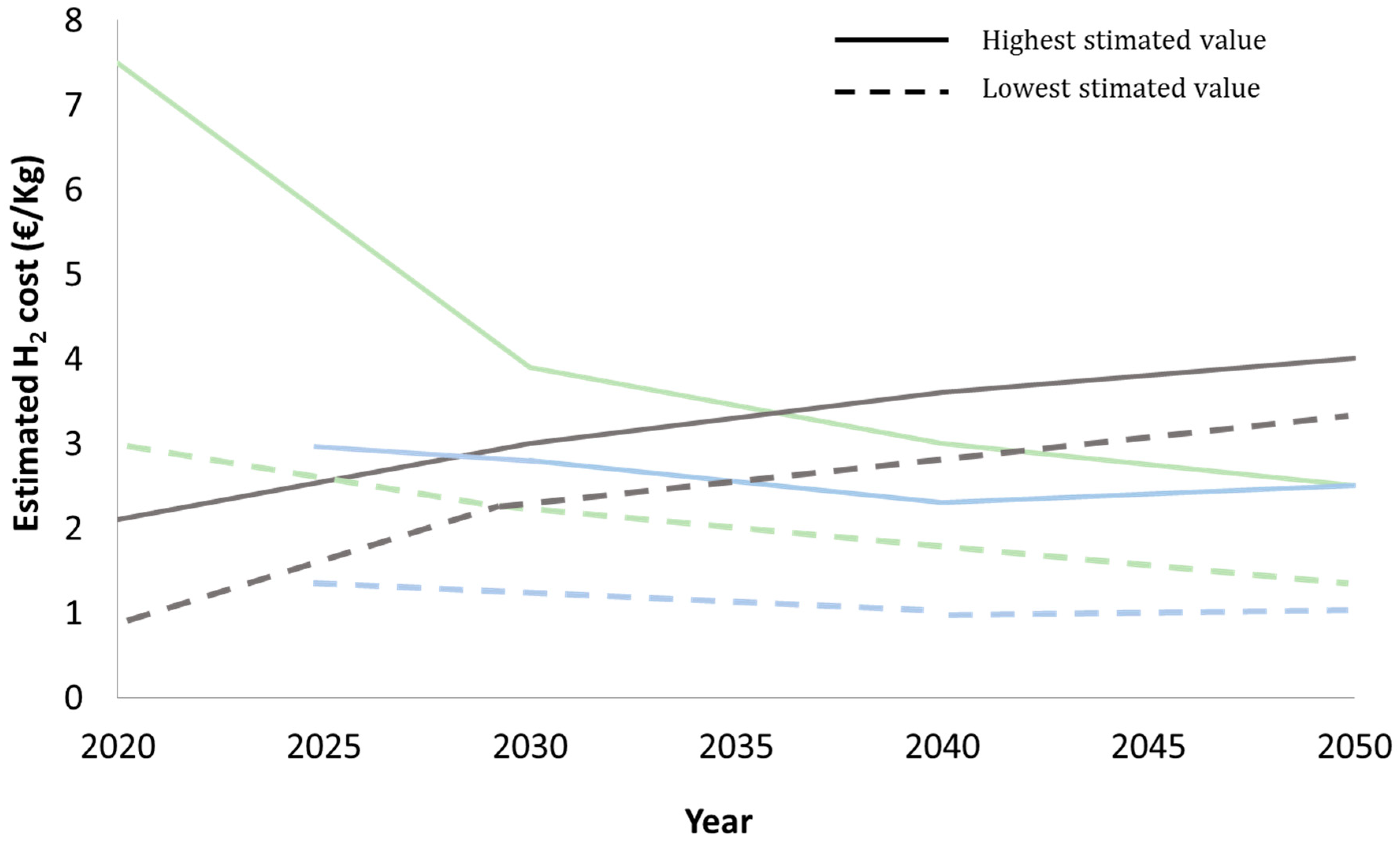





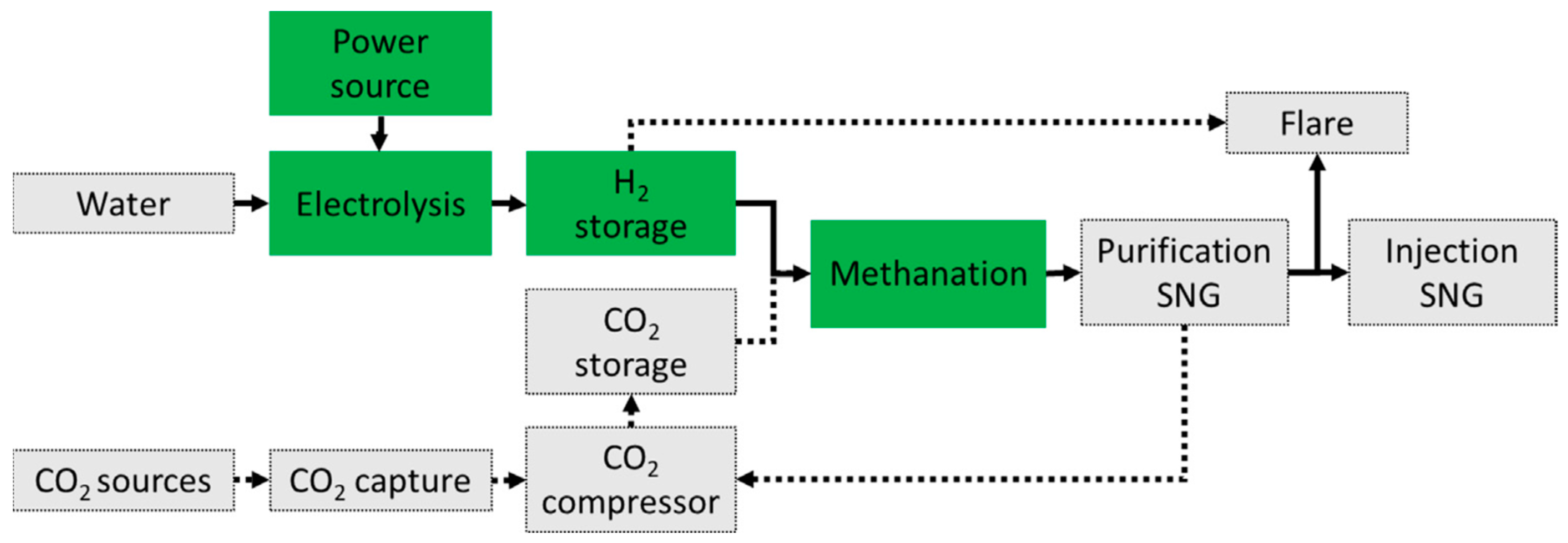
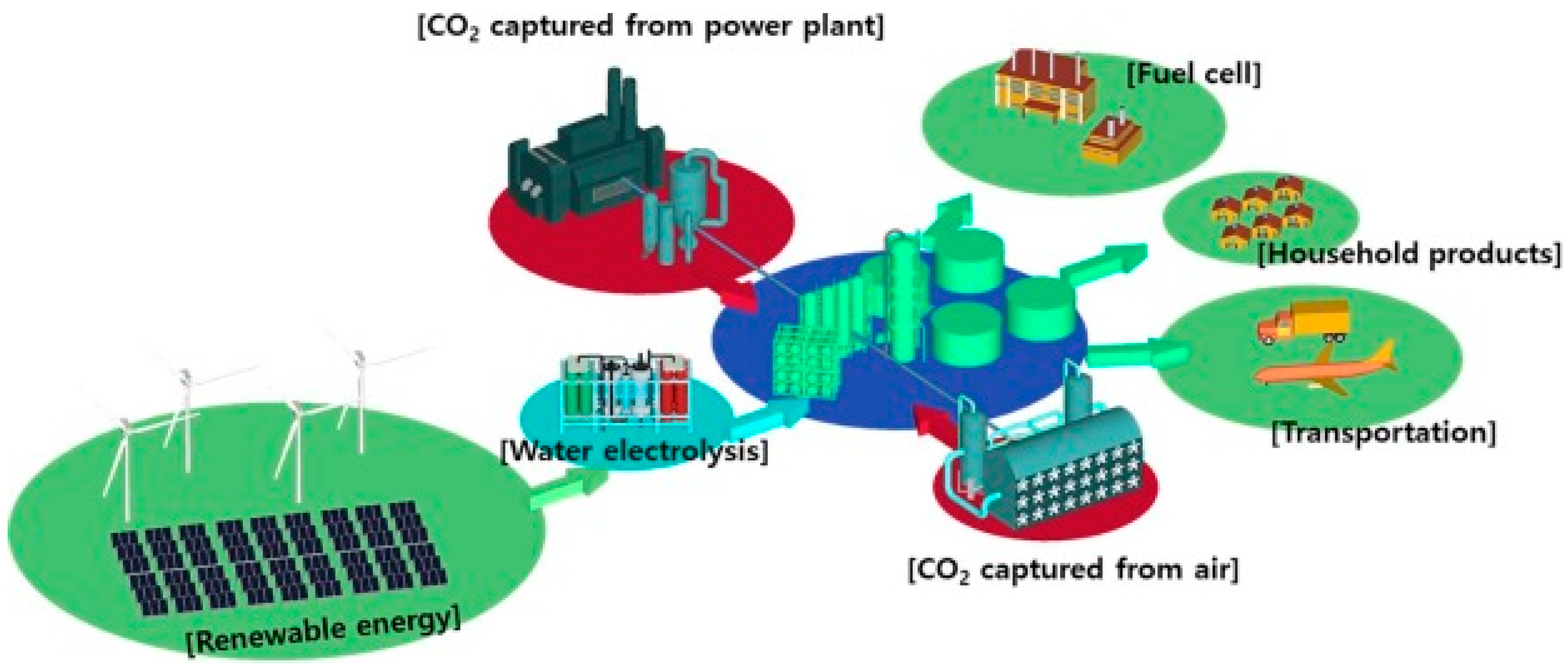
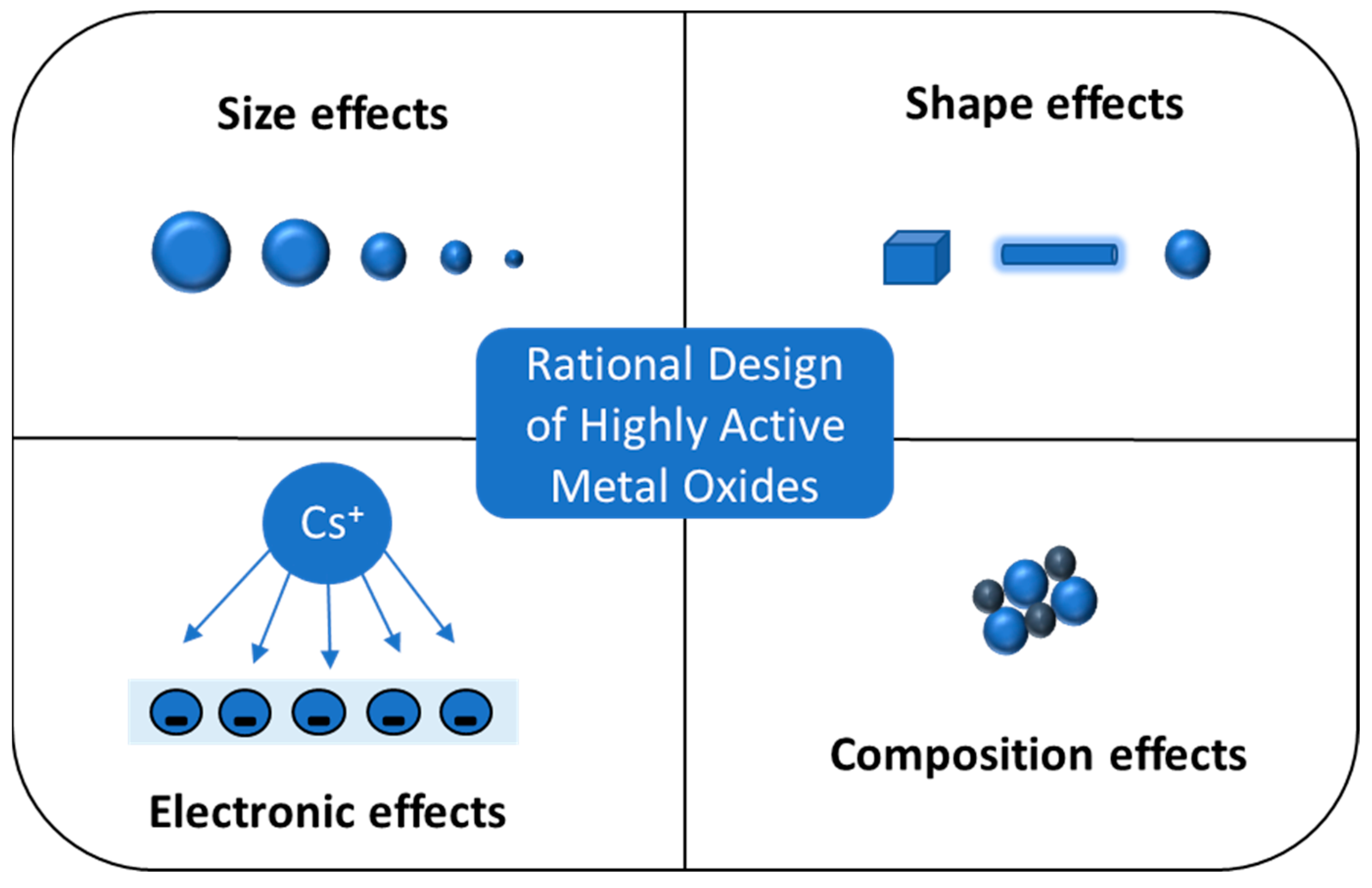
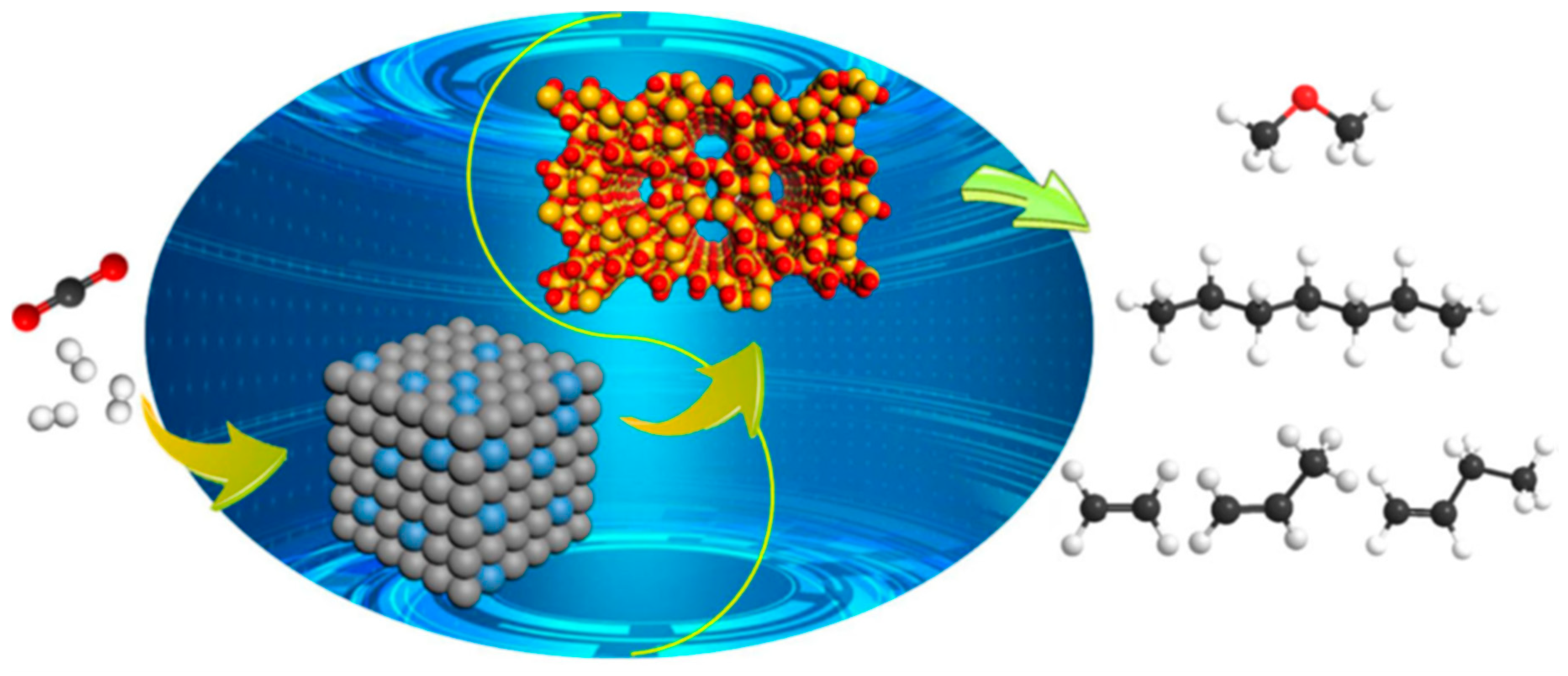
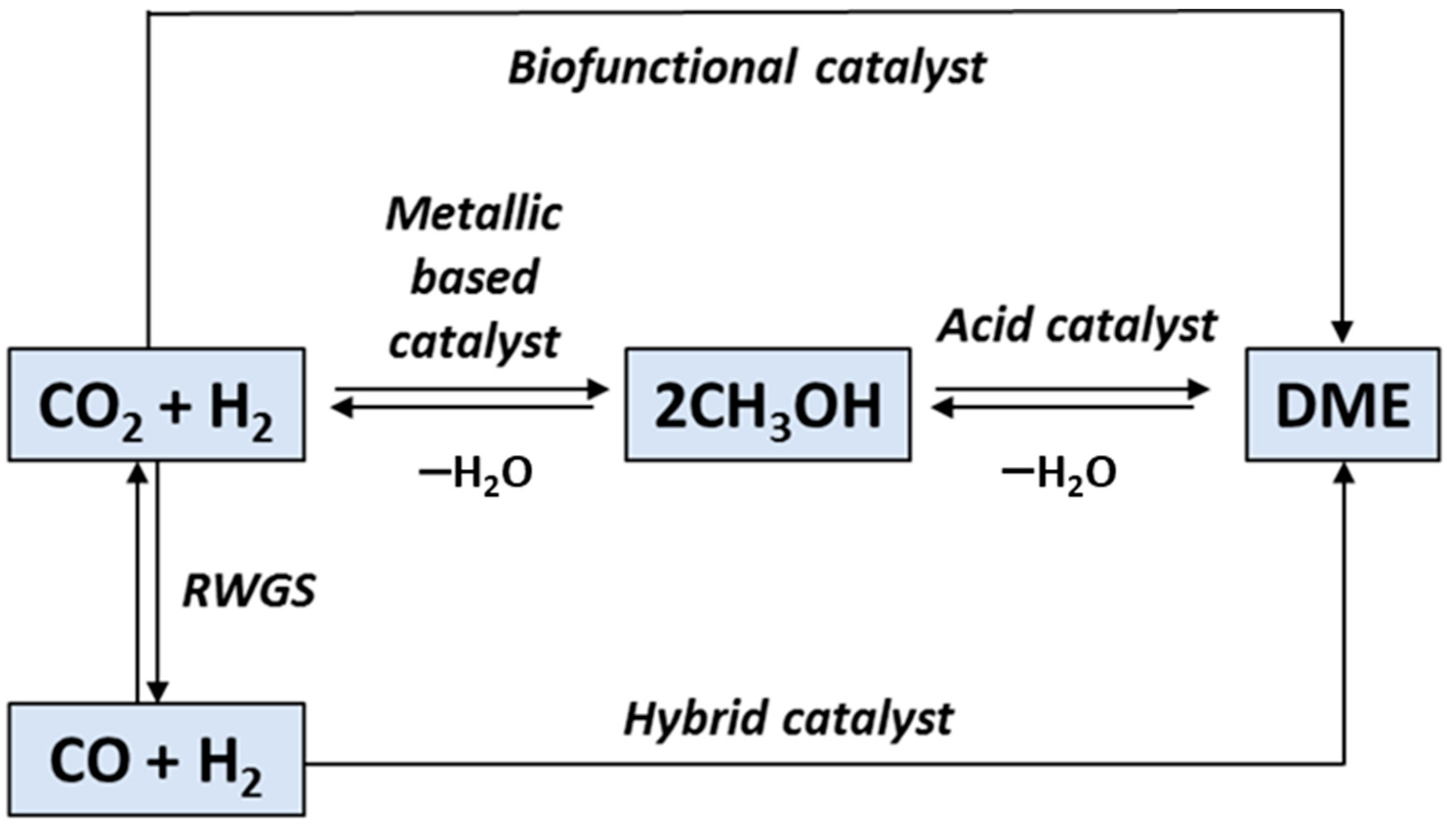

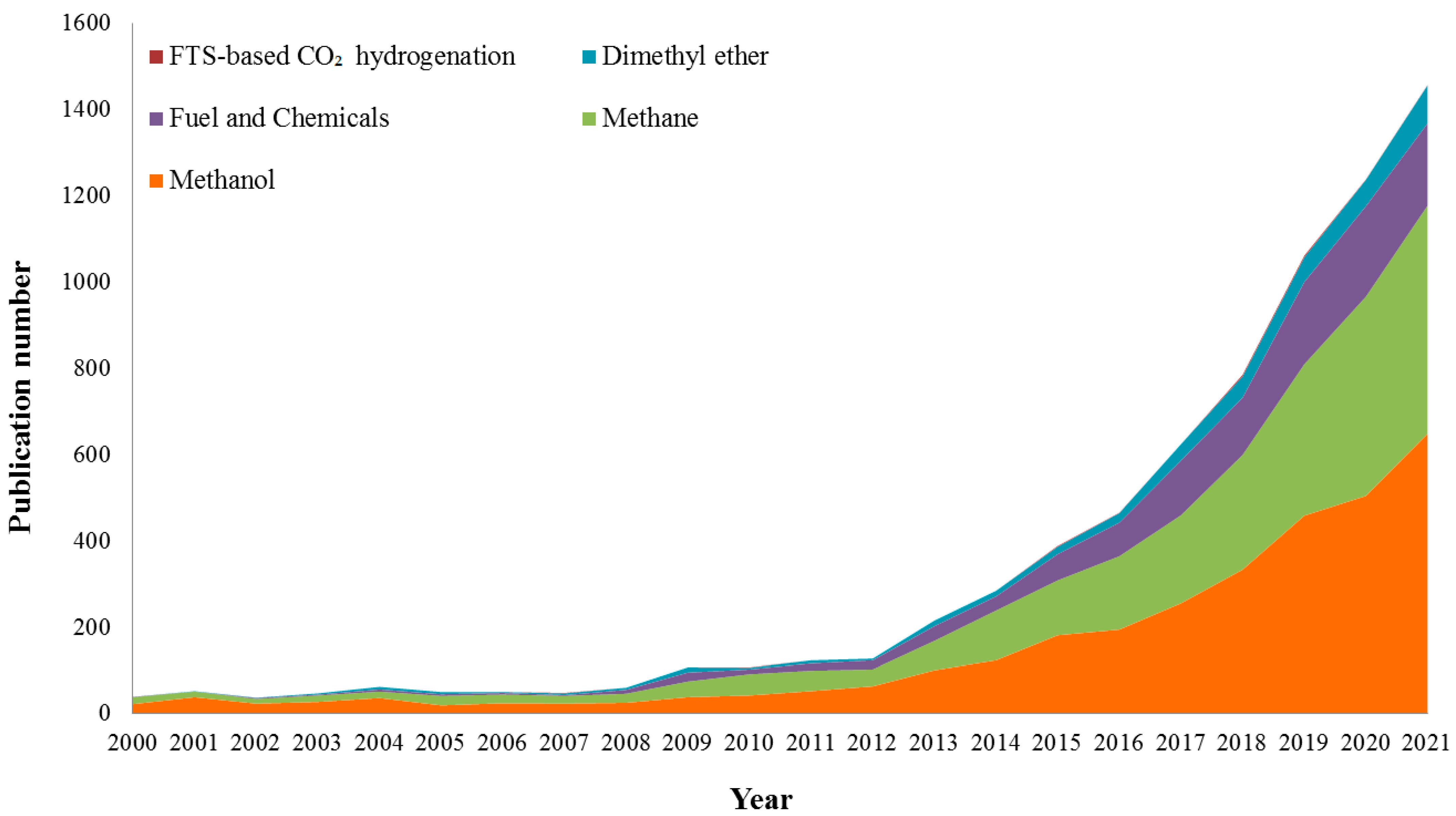
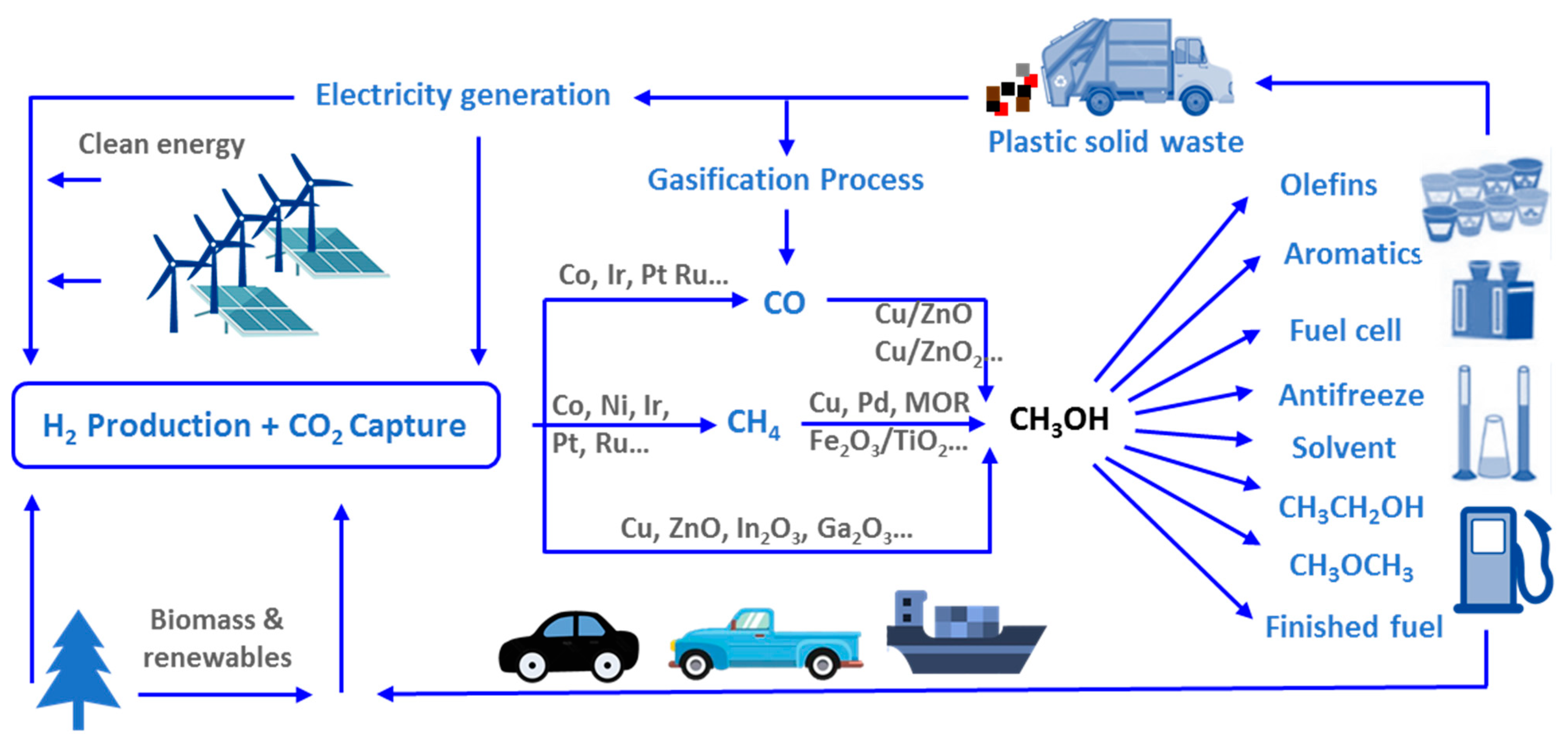
| Hydrogen | Brown | Grey | Blue | Green |
|---|---|---|---|---|
| Feedstock | Coal | Natural Gas | Natural Gas | Renewable electricity |
| Carbon Capture | Gasification No CCS | Steam methane reforming No CCS | Advanced gas reforming CCS | Electrolysis |
| GHG: Emissions (tonCO2/tonH2) | Highest 19 | High emissions 11 | Low emissions 0.2 | Potential for zero GHG emissions |
| Estimated Cost (per kg H2) | $1.2–$2.1 | $1–$2.1 | $1.5–$2.9 | $3–$7.5 |
| Metal | Promoter | Catalyst Support | Conversion (%) | T (°C) | P (atm) * | Ref. |
|---|---|---|---|---|---|---|
| Ni | CeO2 | γ-Al2O3 | >60 | 300 | 0–10 | [34] |
| Ru | --- | TiO2 | >60 | 210–300 | 1.0 | [66] |
| Ru | --- | Al2O3, CeO2, MnOx, ZnO | 25–80 | 400 | 1.5 | [67] |
| Ni | --- | CeO2, Al2O3, Y2O3 | 60–80 | 250−500 | 1.0 | [42] |
| Ni | TiO2 | Al2O3 | 40 | 550 | 3.0 | [43] |
| Ni | Mn | TiO2 | 95 | 350 | --- | [68] |
| Ni, Ni-Co | --- | Al2O3 | 90 | 350–400 | 1.0 | [44] |
| Ni | --- | CeO2-ZrO2 | 55–99.8 | 200–350 | --- | [49] |
| Ni | --- | ZrO2 | 72 | 300 | --- | [69] |
| Ni | --- | Al2O3 | 5–75 | 350–450 | 1.0 | [45] |
| Ni | Ce | SiC | 80–95 | 600 | 10–15 | [47] |
| Ni | La | Mg-Al | 61 | 250 | 1.0 | [50] |
| Ni | La | γ-Al2O3 | 4–14 | 377–400 | 1.0 | [46] |
| Ni-Ru | --- | CaO-Al2O3 | 84 | 380–550 | 1.0 | [70] |
| Ni | CeO2 | Al2O3-ZrO2-TiO2 | 83 | 300–400 | 5.0 | [51] |
| Ni-ZrO2 | --- | Carb. Nanotubes | 10–50 | 200–500 | 1.0 | [52] |
| Ni | --- | γ-Al2O3 | 66–92 | 250–350 | ---- | [71] |
| Ni/Al2O3 | --- | 3D-copper | 3–45 | 300–500 | 1.0 | [72] |
| Ni | --- | Al2O3, CeO2, CeO2-ZrO2 | 60–80 | 200–450 | 1.0 | [73] |
| Ni/GDC | --- | Ceramic monolith, Open-cell foam | 70–52 | 300–600 | 1.0 | [74] |
| Ni | --- | Attapulgite | 60–80 | 200–600 | 1.0 | [75] |
| Ni | --- | Al2O3, CeO2, ZrO2 | 70–90 | 200–600 | 1.9 | [76] |
| Ni | Sm2O3, Pr2O3, MgO | CeO2 | 55 | 200−500 | 1.0 | [77] |
| Ni | --- | CeO2 nanocatalyst | 84 | 220 | 1.0 | [78] |
| Ni | ---- | CeO2 | 81 | 250 | 1.0 | [79] |
| Ni | La | Hydrotalcite-Al2O3 | 60–80 | 225–425 | 1.0 | [55] |
| Ni | --- | Sepiolite, Todorokite | 70–100 | 250–450 | 1.0 | [80] |
| Ni | --- | ZSM-5@MCM-41 | 80 | 400 | 1.0 | [81] |
| Ni | La | MgO, Al2O3 | 60–80 | 200–400 | 1.0 | [82] |
| Ni-Ru | --- | Al2O3 | 70–85 | 200–500 | 1.0 | [60] |
| Ni | --- | Al2O3 | 30 | 400 | 15.8 | [48] |
| Ni | Mg, Ca, Sr, Ba | Al2O3 | 40–60 | 200–600 | 1.0 | [56] |
| Ni | --- | Al2O3, CeO2 | 60–80 | 200–500 | 1.0 | [83] |
| Ni | --- | Al2O3-ZrO2 | 60–77 | 160–460 | 1.0 | [57] |
| Ni-Ru | --- | Al2O3 | 40–85 | 250–550 | 1.0 | [61] |
| Ni-Ru | --- | MgO-Al2O3 | 40–65 | 650–550 | 1.0 | [84] |
| Ni | Pt, Ru, Rh | CeO2, CeZrO4, CeO2/SiO2 | 65–70 | 200–400 | 1.0 | [85] |
| Ni-Ru | CeO2-ZrO2 | 50–80 | 200–450 | 1.0 | [86] | |
| Ni-Ru | MgAl2O4 | 55–70 | 200–400 | 1.0 | [87] | |
| Ni | CeO2, La2O3, Sm2O3, Y2O3, ZrO2 | Al2O3 | 75–95 | 200–300 | 5.0 | [64] |
| Catalyst Support | Conversion (%) | SCH3OH (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|
| ZnO/Al2O3 | 60–90 | 50–80 | 210–250 | 60 | [109] |
| ZnO/ZrO2 | 50–80 | 65–85 | 220 | 30 | [117] a |
| ZnO/ZrO2 | 20–60 | 30–90 | 200–300 | 1–40 | [113] b |
| Al2O3 | 50–70 | 50–65 | 180−300 | 1–360 | [114] a |
| ZnO | 2–9 | 45–95 | 220–300 | 30 | [118] |
| Al2O3/MgO | 0–25 | 20–30 | 150–250 | 10 | [119] |
| ZrO2/CeO2 | 3–10 | 40–82 | 220−260 | 30 | [120] c |
| SiO2 | 5 | 79 | 190−250 | 30 | [121] |
| ZrO2/ZnO | 30–70 | 30–70 | 190–250 | 10–30 | [122] |
| Na-ZSM-5/ZnOx | 2–12 | 25–100 | 200–300 | 30 | [123] |
| CuO/ZrO2 | 5–15 | 15–70 | 230 | 10 | [124] |
| Al2O3/MgO | 20–35 | 5–35 | 200–400 | 20 | [125] |
| Al2O3/ZnO | 5–35 | 5–70 | 200–400 | 20 | [126] |
| SiO2/TiIV Surf. | 4–18 | 49–85 | 230 | 25.0 | [127] |
| SiO2/ZnII Surf | 1–5 | 48–86 | 230 | 50.0 | [128] |
| ZnO/ZrO2/Mg-Al (LDH) | 1–7 | 50–100 | 200–300 | 30.0 | [129] |
| ZnO/MnO/SBA-15 silica | 4–8 | 100 | 180 | 40.0 | [130] |
| ZnO | 9–13 | 65–80 | 240 | 30.0 | [131] |
| ZnO/SiO2 | 90–100 | 65–100 | 250 | 40.0 | [132] |
| ZrO2 | 2–7 | 30–70 | 350 | 10.0 | [133] |
| ZnO/Attapulgite | 12–18 | 7–25 | 320 | 6.0 | [134] |
| ZnGa/LDH nanosheet | 17–20 | 30–50 | 270 | 50.0 | [135] |
| Sr-Perovskite | 1–16 | 36–63 | 200–280 | 20–50 | [136] |
| CexZryOz | 5–16 | 45–95 | 200–300 | 30 | [137] |
| ZrOx | 13.1 | 78.8 | 260 | 45 | [138] c |
| ZrO2 | 1.0–5.0 | 68–75 | 220 | 30 | [139] |
| ZnO | 1.0–25.0 | 10–90 | 200–300 | 20 | [140] |
| ZnO/Faujasite | 2.0 | 27–35 | 240−260 | 15 | [141] a,c |
| ZnO/CeO2 | 1.0–3.5 | 20–70 | 250 | 30 | [142] c |
| ZnO/ZrO2/C-nanofibers | 8–14 | 78–92 | 180 | 30 | [143] |
| ZnO/Al2O3 | 10–28 | 33–85 | 240 | 40 | [144] |
| ZnO/SiO2 | 8–14 | 50–59 | 220 | 30 | [145] |
| AlCeO | 2–24 | 12–95 | 200–280 | 30 | [146] |
| AlCeO | 6–22 | 25–97 | 200–280 | 30 | [147] |
| ZnO | 1–14 | 1–60 | 150–300 | 1.0 | [148] |
| CeO2 | 1–7 | 20–90 | 240–300 | 20 | [149] |
| Al2O3/ZrO2/ZnO | <7.0 | 43–59 | 230 | 30 | [150] |
| ZnO/ZrO2 | 19.6 | 50 | 280 | 50 | [151] |
| ZnO/ZrO2 | 9–15 | 87–98 | 250 | 50 | [152] |
| Al2O3 | 50 | - | 325 | 1.25 | [153] |
| Promoter | Catalyst Support | Conversion (%) | SCH3OH (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|
| Pr2O3 | ZnO | 80–100 | 75–100 | 200–260 | 30 | [115] |
| In2O3 | ZrO2 | 1–6 | 60–90 | 210–290 | 10–250 | [153] |
| CeO2 | Al2O3 | 16–23 | 59–94 | 220–280 | 40 | [147] |
| LaOx | Silica SBA-15 | 45–85 | 45–81 | 220–280 | 30 | [154] |
| W | CeO2 | 13 | 87 | 250 | 35 | [155] |
| Pd | Ce0.3Zr0.7O2 | 15–25 | 90–95 | 250 | 50 | [156] a |
| Sm2O3 | ZrO2 | 8–14 | 50–80 | 230 | 10 | [157] |
| Al + Ga | ZnO | 16–18 | 99 | 250 | 30 | [158] |
| Al | ZnO | 1–17 | 99 | 250 | 30 | [159] b |
| Zn | Graphene | 18–20 | 50–80 | 250 | 15.0 | [160] |
| Hydrotalcite | ZnO-Al2O3 | 6 | 64–73 | 250 | 15–30 | [161] |
| ZnO-ZrO2 | Hydrotalcite | 3–6 | 35–65 | 250 | 25.0 | [162] |
| Zn, Ga | SiO2 | 0.5–5.0 | 10–80 | 220–280 | 8.0 | [163] |
| Pd | SiO2 | 6.6–3.7 | 12–30 | 300 | 41 | [164] |
| Pd | CexZr1-xO2 | 13–20 | 10–25 | 250–300 | 30–60 | [156] |
| Ni | CeO2-nanotube | 2–18 | 75–86 | 220–300 | 20–40 | [165] |
| ZnO | Al2O3 | 10–20 | 50–100 | 160–250 | 10–25 | [137] a |
| MgO | ZnO | 4–16 | 25–100 | 200–300 | 30 | [166] |
| MgO, CaO, SrO, BaO, ZnO | Al2O3 | 2–9 | 10–100 | 200–400 | 20 | [167] |
| Promoter | Catalyst Support | Conversion (%) | SCH3OH (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|
| ZrO2 | 5–15 | 15–70 | 230 | 10 | [124] | |
| ZnO/ZrO2 | SBA-15 silica | 10–25 | 20–35 | 250 | 30 | [168] |
| ZnO/ZrO2 | Mg-Al (LDH) | 1–7 | 50–100 | 200–300 | 30.0 | [129] |
| In2O3 | CuO | 5–12 | 50–90 | 220–280 | 5–30 | [169] |
| CuO/ZrO2 | 2–12 | 20–70 | 230 | 10.0 | [170] | |
| CuO/CeO2/TiO2 | 1.5–6.5 | 28–52 | 190–235 | 30 | [171] | |
| MoO3/WO3/Cr2O3 | CuO/ZnO/ZrO2 | 18–20 | 40–48 | 240 | 40 | [172] |
| CuO/ZnO/Al2O3 | 10–16 | >99 | 250 | 50 | [173] | |
| Ag | CuO/ZrO2 | 4–8 | 25–45 | 270 | 10 | [174] |
| CuO/Ce0.4Zr0.6O2 | 7–13 | 72–96 | 220–280 | 30 | [175] | |
| CuO/ZnO | 38 | 70 | 270 | 50 | [176] | |
| CuO/ZnO/CeO2 | 14–20 | 95–98 | 240 | 1.0 | [177] a | |
| Cu/Zn/Ce/TiOx | 4–7 | 25–45 | 275 | 30 | [178] | |
| CuO/ZnO/TiO2/Zr | 3–25 | 15–85 | 200–280 | 30 | [179] | |
| CuO/ZnO/CeO2 | TiO2 nanotubes | 10–20 | 25–80 | 220–300 | 30 | [180] |
| CuO/ZnO/ZrO2 | 9–17 | 40–54 | 300–600 | 30 | [181] | |
| Graphene oxide | CuO/ZnO/ZrO2 | 2–25 | 10–76 | 200–280 | 20 | [182] |
| Carbon | CuO/ZnO | 8–24 | 18–60 | 230–290 | 30 | [183] |
| WO3 | CuO-ZnO-ZrO2 | 5–20 | 42–64 | 240 | 30 | [184] |
| ZrO2/Al2O3 | CuO/ZnO | 20–25 | 40–95 | 200−260 | 27.6 | [185] |
| In2O3, Pd | CuO/ZnO/Al2O3 | 7–16 | 99–100 | 250 | 50 | [186] |
| La2O3 | CuO/ZnO/Al2O3 | 1–18 | 5–100 | 160–260 | 1.0 | [187] |
| SiO2 | CuO/ZnO/ZrO2 | 2–5 | 10–70 | 200–280 | 20 | [188] |
| CuO/ZnO/ZrO2 | 4–15 | 45–85 | 240 | 30 | [189] | |
| CuO/CeO2/ZrO2 | 5–20 | 2–8 | 200–260 | 30 | [190] b | |
| Ag | CuO/ZrO2 | 1–7 | 30–70 | 230 | 10 | [191] |
| Zeolite | CuO/ZnO/ZrO2 | 5–20 | 5–12 | 260 | 30 | [192] |
| CuO-ZnO | Al2O3, SiO2 | 2–14 | 46–59 | 250, 270 | 30, 50 | [193] |
| Ce1-xZrxO2 | 2–15 | 10–95 | 200–300 | 30 | [194] | |
| LaxSr1-xCuO | Perovskite | 1–16 | 4–55 | 250–300 | 30 | [195] |
| ZrO2, MnO2 | CuO-ZnO/SBA-15 | 8–9 | 10–25 | 250 | 30 | [196] |
| CuO/ZnO | Oyster Shells | 1–2 | 50–70 | 250 | 30 | [197] |
| Pd | CuO/ZnO/Al2O3 | 1–10 | 10–90 | 180–240 | 50 | [198] |
| La, Ti or Y | CuZnIn/MZrOx | 2–6 | 40–80 | 225 | 20 | [199] |
| La, Ce, or Sm | CuZnO/Zn-AlOx | 25 | 54 | 250 | 40 | [200] |
| Noble Metal | Promoter | Catalyst Support | Conversion (%) | SCH3OH (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|---|
| Au | In2O3 | 5–13 | 60–100 | 250–300 | 50 | [203] a | |
| Ir | In2O3 | 18 | 70 | 300 | 50 | [204] | |
| Pd | Ga | SiO2 | 1–5 | 81 | 230 | 25 | [205] |
| Pd | CeO2 | 2–18 | 4–100 | 200–280 | 10–250 | [206] | |
| Pd | Al | ZnO | 2–14 | 15–70 | 250 | 30 | [207] |
| Pd | In2O3 | 3 | 100 | 280 | 50 | [208] | |
| Pt | In2O3 | 37 | 63 | 30.0 | 1.0 | [209] | |
| Pd | In2O3/SBA-15 | 13 | 83 | 260 | 50 | [210] | |
| Ni5Ga3 | SiO2 | 3–35 | 11–16 | 200–300 | 1.0 | [211] | |
| Ni | Ga2O3 | 0.5–1 | 10–100 | 160–300 | 5.0 | [212] | |
| Re | TiO2 | 1–2 | 82 | 150 | 50 | [213] | |
| Ti | MoOx/TiO2 | 80 | 70 | 150 | 50 | [214] | |
| ReOx | TiO2 | 18 | 98 | 200 | [215] | ||
| Co | SiO2 | 2–14 | 10–80 | 260–320 | 20 | [216] | |
| Ag | In2O3 | 5–30 | 75–100 | 200–275 | 50 | [217] | |
| Au | ZrO2 | 5–9 | 40–70 | 140–220 | 30 | [218] | |
| Au | MxOy b | 5–45 | 10–95 | 200–350 | 1.0 | [219] | |
| Au | In2O3-ZrO2 | 2–15 | 65–100 | 200–300 | 50 | [220] | |
| Au | CuO/CeO2 | 4–10 | 30 | 200–300 | 30 | [221] | |
| Au | CeO2 | 1∓2 | 5–45 | 240 | 5∓50 | [222] | |
| Ru | In2O3 | 1–30 | 70–97 | 200–300 | 50 | [223] | |
| Au | ZrO2 | 4–6 | 48–75 | 240 | 40 | [224] | |
| Au | ZnO-ZrO2 | 4.5–6 | 82–95 | 320 | 55 | [225] | |
| Rh | In2O3 | 1–17 | 56–100 | 250–300 | 50 | [226] | |
| Rh | In2O3–ZrO2 | 1–18 | 65–100 | 250–300 | 50 | [227] | |
| Pt | In2O3 | 1–15 | 54–100 | 225–300 | 50 | [228] a | |
| Pd | In2O3 | 1–20 | 70–100 | 200–300 | 50 | [229] | |
| Ni | In2O3 | 1–18 | 60–100 | 200–300 | 50 | [230] | |
| Au | In2O3 | 2–14 | 70–100 | 225–300 | 50 | [203] a | |
| Rh | In2O3 | 4–10 | 60–80 | 270–320 | 50 | [231] a | |
| Ni | ZrO2 | In2O3 | 1–18 | 43–100 | 200–300 | 50 | [232] |
| Ni | In2O3 | 6–15 | 30–80 | 280 | 50 | [233] | |
| Pd | In2O3 | 10 | 72 | 295 | 30 | [234] a | |
| Pd | SiO2 | --- | 64–71 | 200 | 30 | [235] | |
| Pd | Ga2O3 | SiO2 | 10 | 17–65 | 220–250 | 30 | [236] |
| Pd | ZnZrOx | 4–35 | 5–90 | 200–400 | 50 | [237] | |
| Pd | CeO2 | 2–10 | 40–78 | 200–260 | 50 | [238] | |
| Pd | SiO2 | 1–20 | 1–28 | 220–280 | 8.0 | [239] |
| Metal | Catalyst Support | Conversion (%) | SCH3OH (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|
| Ni/In/Al | SiO2 | 1.6–3.8 | 1–12 | 210–290 | 1.0 | [240] a |
| Ni/In | SiO2-SBA-15 | 1–17 | 1–90 | 300 | 5−50 | [241] |
| Co/In | In2O3 | 19 | 69 | 300 | 50 | [242] |
| In/Pd | SiO2 | 2–5 | 61 | 300 | 40 | [243] |
| Rh/In | Al2O3 | 1–10 | 5–90 | 270 | 45 | [244] a |
| Pd/Zn | CeO2 | 8–17 | 65–98 | 220–270 | 20 | [245] |
| Ca/Pd/Zn | ZrO2 | 2–10 | 97–100 | 220–270 | 20–30 | [246] a |
| In/Ru | SiO2 | 1–5 | 20–85 | 200−240 | 34 | [247] |
| Ni/Ga | SiO2, CeO2, ZrO2 | 1–6 | 5–30 | 180–270 | 1–30 | [248] a |
| Pd/Cu | SiO2 | 1.6–2.8 | 18–27 | 300 | 30–50 | [249] |
| Pd/Cu | MxOy b | 7–16 | 28–34 | 300 | 40 | [250] a |
| Pd/Cu | SiO2 | 3–7 | 12–40 | 300 | 40 | [251] |
| Pd/Cu | SiO2 | 3–6 | 12–40 | 300 | 40 | [252] |
| Cu/Ni | Graphene | 7.87 | 98.7 | 225 | 40 | [253] |
| Pd/Cu/Zn | SiC | 1–11 | 10–100 | 150–300 | 1.0 | [254] |
| Cu/Ni | Mordenite | 100 | 30–60 | 220 | 30 | [255] |
| Ru/Mo | Ru−Mo Phosphide | 0.5–4.5 | 5–75 | 180–220 | 65–72 | [256] |
| Rh/Co | nanospheres | 100 | 96 | 150 | 24 | [257] |
| Pd/Zn/Al | ZnO, Al2O3 | 0.5–4.0 | 15–70 | 250 | 30 | [207] |
| Ni/Sn | InZrO2 | 1–5 | 55–100 | 225–275 | 25 | [258] |
| Pd/Cu | TiO2-MO2 c | 7–16 | 25–40 | 250 | 40 | [259] |
| Ni/Ga | Hydrotalcite | 2–3.5 | 60–100 | 200–300 | 30 | [72] |
| Pd/Cu | CeO2 | 2–17 | 24–84 | 190–270 | 30 | [260] a |
| Cu/Zn | Coord. polymer | 13–20 | 25–59 | 220–260 | 40 | [261] |
| Cu/Zn | UiO-66 (Zr) MOF | 12–22 | 28–54 | 220–300 | 30 | [262] |
| Cu/ZnO | Al2O3 | 5–11 | 64–87 | 220–260 | 30 | [263] a |
| Ag/Cu | Mordenite | ---- | 48–61 | 230 | 30 | [264] |
| Cu/Zn | UiO-66 (Zr) MOF | 25–30 | 15–24 | 230 | 50 | [265] |
| Cu/Pd | SiO2 | 2–32 | 1–4 | 220–360 | 40 | [266] a |
| Pd/In | Unsupported nanoparticles | <3.0 | 25–90 | 190–270 | 50 | [267] |
| Metal Catalyst | Acid Catalyst | Conversion (%) | SCO (%) | SCH3OH (%) | SDME (%) | T (°C) | P (Atm.) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CZZA a | HZSM-5 | 25–28 | 20–80 | 5–7 | 10–70 | 220–280 | 27.6 | [185] |
| CuO/ZnO/ZrO2 | Zeolite | 2–20 | 15–20 | 5–12 | 1–30 | 260 | 30 | [192] |
| CuO/ZnO/Al2O3 | SiO2-Al2O3 | 5–9 | 49–65 | 11–17 | 22–35 | 260 | 30 | [307] |
| Cu-BTC MOF b | Al2O3 | 2–26 | 15–25 | 5–50 | 14–90 | 260 | 30 | [308] |
| CuO/ZnO/ZrO2 | Zr(SO4)2 | 14–17 | 60–80 | 7–20 | 14–28 | 260 | 20 | [309] |
| CuZnAlZrCe | ZSM-5 | 13–19 | 59–63 | 9–11 | 26–33 | 250 | 30 | [310] |
| In2O3 | HNT c | 1–5 | 0.0 | 20–80 | 10–70 | 200–300 | 10–40 | [311] |
| CZA/HPW d | TiO2 | 5–22 | 14–17 | 5–99 | 7–59 | 250 | 30 | [306] |
| CuZnAlSi/Sn | ------ | 10 | 50–60 | 5–9 | 80–85 | 280 | 40 | [312] |
| CuO/ZnO/ZrO2 | SAPO-11 | 40–50 | 0.0 | 10–50 | 50–90 | 250–325 | 10–50 | [313] |
| CZA e | HZSM-5 | 25 | 25–28 | 7.0 | 65–70 | 220–280 | 21–42 | [314] |
| CuO/ZnO | HZSM-5 | 15–35 | 10–30 | 5–88 | 10–80 | 200–260 | 15–20 | [315] |
| CuZnOZrO2 | WOx/Al2O3 | 10–20 | 64–69 | 8–17 | 15–28 | 300 | 20 | [316] |
| Cu/ZnO/MOx f | SAPO-34 | 5–20 | 50–90 | 19–24 | 25–31 | 200–260 | 10 | [317] |
| Cu/ZnO/ZrO2 | HZSM-5 | 1–11 | 9–90 | 6–18 | 5–75 | 200–330 | 30 | [318] |
| GaZrOx | ------ | 1–9 | 10–88 | 10–100 | 10–25 | 240–380 | 30 | [319] |
| CuO/ZnO/Al2O3 | SAPO-18 | 1–8 | 5–8 | 3–15 | 85–90 | 250–350 | 20–40 | [320] |
| CuO/ZnO/Al2O3 | MCM-41-TPA g | 2–7 | 23–65 | 18–50 | 18–25 | 220–250 | 45 | [321] |
| Cu/ZnO/ZrO2 | ZSM5 | 8–11 | 15–49 | 24–26 | 38–58 | 240 | 30 | [322] |
| nano-Pd/In2O3 | H-ZSM-5 | 6–11 | 40–45 | 17–19 | 36–42 | 280–300 | 30 | [323] |
| Gallium nitride | 1–25 | 40–82 | 18–42 | 0–80 | 300–450 | 20 | [324] |
| Metal Catalyst | Acid Catalyst | Conversion (%) | SCO (%) | SC2-C4 (%) | SC5 (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| In2O3 | HZSM-5 | 12–15 | 45–50 | 20–25 | 79 | 340 | 30 | [331] |
| Cu/CeO2 | SAPO-34 | 4–20 | 30–75 | 30–65 | 4–9 | 300–500 | 20 | [332] |
| ZnZrOx | Zeolites a | 18–24 | ----- | 1.5–2.8 | 2–9 | 325–400 | 10 | [333] |
| InCo | Zn-zeolite beta | 8.0 | 6 | 8 | 85 | 300 | 50 | [334] |
| In2O3/ZrO2 | SAPO-34 | 17–26 | 64–70 | 65–82 | 2–5 | 380 | 30 | [335] |
| ZnO/ZrO2 | SAPO-34 | 42–45 b | 4–22 | 76–85 | 2–3 | 375 | 15 | [336] |
| ZnGaOx spinel | SAPO-34 | 7–50 | ----- | 15–76 | 5–7 | 400 | 40 | [337] |
| In2O3/ZrO2 | SAPO-34 | 15–21 c | ----- | 3–6 | ---- | 400 | 30 | [338] |
| In2O3/ZrO2 | SAPO-34 | 23–25 | 2–6 | 2–7 | ---- | 400 | 30 | [339] |
| In-Zr | SAPO-34 | 35 | ----- | 93 | ---- | 400 | 30 | [340] |
| Mn2O3-ZnO | SAPO-34 | 9–30 | 50–91 | 86–92 | 3–13 | 380 | 30 | [341] |
| Fe/Co | K-Al2O3 | 37–42 | 12–16 | 67 | 17–21 | 320 | 20 | [342] |
| Fe5C2 | Zeolite d | 8–56 | 1–49 | 12–93 e | 1–56 | 300 | 10 | [343] |
| In2O3 | SAPO-34 | 18–35 | 18–37 | 16–34 | ---- | 340–400 | 10–25 | [344] |
| ZnZrOx | SAPO-34 | 9–14 | 40–43 | 82–83 | ---- | 380 | 30 | [345] |
| In2O3/ZrO2 | SAPO–34 | 29–38 | 45–90 | 68–85 | 3–5 | 400 | 10–30 | [331] |
| ZnZrO | SAPO-34 | 10–15 | ----- | 80.0 | 1–3 | 330–380 | 20 | [346] |
| InCeOx/InCrOx | SAPO-34 | 5–20 | 15–60 | 70–90 | 3–7 | 300–350 | 10–35 | [347] |
| CuZnZr(CZZ) | SAPO-34 | 10–20 | 57–86 | 70–88 | 0.5–5 | 400 | 20 | [348] |
| NiCu/CeO2 | SAPO-34 | 12–20 | 55–85 | 62–79 | 2–4 | 350–450 | 20 | [349] |
| ZnO/Y2O3 | SAPO-34 | 6–28 | 75–97 | 90–94 | 1–5 | 390 | 40 | [350] |
| ZrS/Fe2O3@KO2 | SAPO-34 | 46–48 | 24–27 | 42–55 | 25–38 | 375 | 30 | [351] |
| 10K13Fe2Co100Zr | Polymetallic fibers | 10–48 | ----- | 70–80 | ---- | 400 | 30 | [352] |
| ZnO/ZrO2 | MnSAPO-34 f | 15–21 | ----- g | 90–99 | 0.4–7.6 | 380 | 20 | [353] |
| GaZrOx | SAPO-34 | 5–12 | 50–60 | 92–95 | 1–3 | 370–410 | 30 | [354] |
| CuO/ZnO/Al2O3 | SAPO-34 | 50–56 | 4–10 | 50–56 | ---- h | 250–450 | 30 | [355] |
| In2O3 | SAPO-34 i | 27–51 | 3–75 | 50–92 | 5–20 | 360 | 25 | [356] |
| CuO/ZnO | kaolin/SAPO-34 | 33–58 | 7–10 | 78–81 | ---- j | 400 | 30 | [357] |
| Y2O3/Fe/Co | SAPO-34 | 7–18 | 31–35 | 75–85 | 1–3 | 300–400 | 10–25 | [358] |
| FeZnK | SAPO-34 | 42–50 | 14–20 | 54–61 | 8–25 | 280–360 | 15 | [359] |
| FeNa | Supports k | 19–33 | 10–60 | 17–73 | ---- | 320 | 20 | [360] |
| Metal Catalysts | Alkali Metal | Support | Conversion (%) | SCO (%) | SCH4 (%) | SC2-C5 (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fe | --- | Carbon | 14–52 | 5–49 | 3–8 | 5–38 | 300 | 25 | [373] |
| Co | --- | SAPO-34 | ---- | 64–74 | 24–28 | 65–70 | 220 | 20 | [374] |
| Fe3O4/Mn | Na | ----- | 22–30 | 14–32 | 12–36 | 64–88 | 320 | 5.0 | [375] |
| Co | K | Al2O3 | 15–97 | 1–34 | 2–33 | 2–57 | 200–350 | 1–50 | [376] |
| Fe5C2 | --- | ----- | 41–50 | 3–10 | 20–46 | 51–70 | 320 | 30 | [377] |
| Fe/Co | K | ----- | 32–58 | 2–10 | 8–36 | 62–82 | 300 | 25 | [378] |
| Fe/Mn | K | ----- | 38.2 | 5.6 | 10.4 | 22.3 | 300 | 10 | [379] |
| Co/Mn | Na | SiO2 | 45–47 | 18–20 | 2.0 | 52–54 | 260–270 | 50 | [380] |
| Fe/Co (Ru) | K | ----- | 30–57 | 2–16 | 7–30 | 54–84 | 450 | 2.0 | [381] |
| Co3O4/MnO2 | --- | ----- | 42–48 | 2–39 | 4–23 | 90–96 | 270 | 1.0 | [382] |
| Co/Pt | --- | ZSM-5 | 10–28 | ---- | 52–100 | 10–48 | 200–500 | 1–30 | [383] |
| Fe | Na | ZSM-5 | 18–22 | 28–32 | 22–41 | 30–54 | 450 | 20 | [384] |
| Fe/C | K | X-ZSM-5 a | 34–36 | 18–20 | 10–15 | 85–89 | 320 | 20 | [385] |
| CuFeO2 | --- | ---- | 13–18 | 28–32 | 1–60 | 40–95 | 300 | 10 | [386] |
| Cu/Fe | --- | Al2O3 | 35–42 | 23–42 | 28–38 | 51–91 | 300–400 | 30 | [387] |
| Fe | SMC b | 8–45 | 16–86 | 5–11 | 70–89 | 260 | 10 | [388] | |
| Ru/Ni(NPs) c | ---- | 2–30 | 0–47 | 1–100 | 7–76 | 150 | 2–8.5 | [389] | |
| Raney-Fe, Fe | ---- | SiO2 | 4–12 | 14–27 | 5–22 | 22–78 | 220–265 | 20 | [390] |
| Fe/Ti d | K | ---- | 30–35 | 38–85 | 23–25 | 72–75 | 320 | 20 | [391] |
| FeMn | --- | HZSM-5 | 28–40 | 64–68 | ----- | 58–69 | 280 | 10 | [392] |
| RuCl3/Ru | --- | ---- | ---- | 0–85 | 14–100 | 17.5 | 180 | 50 | [393] |
| Co, CoO, Co3O4 | ---- | SixAlyOz | 3–35 | 0–12 | 6–31 | 15–93 | 220–260 | 20 | [394] |
| Fe3O4 | ---- | SiO2 | 5–7 | 2–5 | 57–93 | 7–43 | 220 | 1.0 | [395] |
| Fe3O4/FexCy | Na | 36–46 | 8–11 | 36–60 | 16–52 | 320 | 20 | [396] | |
| Fe3O4/FeCx | Mesop. C | 15–54 | 5–31 | 13–75 | 25–87 | 320 | 30 | [397] | |
| Co/Ce/La | ---- | Al2O3 | 13 | 49–52 | 15 | 99–100 | 230 | 20 | [398] |
| Co@CoOx/Co2C-Mn | Na | 1–62 | 1–92 | 45–70 | 29–54 | 230–310 | 40 | [399] | |
| Fe-/Co | ---- | SiO2 | 4–20 | 11–13 | 26–45 | 43–70 | 260–280 | 20 | [400] |
| FeCo X,(X: La, Mn, Zn) | K | Al2O3 | 65–100 | 30–38 | 0–58 | 44–100 | 300 | 10 | [401] |
| Co | ---- | C/SiO2 e | 2–8 | 6–14 | 21–32 | 62–70 | 250 | 5 | [402] |
| Co6/MnOx | ---- | ------- | 15 | 0–0.7 | ----- | 0–99 | 200 | 8 | [403] |
| Fe2O3 | K | Al2O3 | 40–47 | 19–33 | 20–31 | 40–50 | 400 | 30 | [404] |
| Fe-Co | K | Al2O3 | 37–49 | 9–29 | 14–23 | 58–68 | 320–360 | 20–30 | [405] |
| Fe-Cu | K | ----- | 24–41 | 6–16 | 5–10 | 79–88 | 250–340 | 20 | [406] |
| X-Fe5C2/ZnO | Na | ----- | 2–28 | 15–36 | 10–16 | 68–89 | 280–370 | 25 | [407] |
| Ni | MgAl2O4 | 6–70 | 2–96 | 3–98 | ---- | 330–400 | 1.0 | [408] | |
| FeAlOx | Na | HZSM-5/SiO2 | 29–48 | 8–18 | 10–35 | 47–88 f | 335–400 | 35 | [409] |
| Fe-Zn | K | SAPO | 43–48 | 14–18 | 15–40 | 36–57 | 320 | 15 | [359] |
| Fe-Zn | Na | ---- | 15–39 | 14–30 | 12–48 | 52–88 | 340 | 25 | [410] |
| ZnCoxFe2-xO4 | SiO2 | 24–52 | 6–16 | 16–21 | 36.1 | 260–340 | 25 | [275] | |
| Fe (Cu, Mn, V, Zn, Co) | K | Al2O3 | 29–40 | 10–20 | 15–22 | 65–74 | 340 | 20 | [411] |
| Metal Catalysts | Alkali Metal | Support | Conversion (%) | SCO2 (%) | SCH4 (%) | SC2-C5 (%) | T (°C) | P (Atm) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| α-Fe2O3 | SiO2, Al2O3 | 18–65 | 25–36 | 16–19 | 81–94 | 280 | 10 | [415] | |
| Fe-Mn, Cu | SiO2 | 75–96 | 23–45 | 15–19 | 80–85 | 200 | 20 | [416] | |
| CoMnAlOx a | ---- | SiO2 | 5–14 | 9–48 | 2–24 | 45–85 b | 260 | 10 | [417] |
| Co-Re, Pt-ZSM-5 | ---- | Al2O3 | 5–75 | ---- | ----- | 24–43 c | 225–255 | 20–30 | [418] |
| Fe | Na | ZSM-5 | 24–87 | 26–42 | 16–41 | 57–85 | 300 | 10 | [419] |
| Co, Re | ---- | Al2O3, CNT d | 2–4 | ---- | 42–56 | 44–58 | 210 | 1.9 | [420] |
| Fe-Zn | Na | Zeolites e | 47–44 | 88–95 | 11–16 | 75–80 | 360 | 1.0 | [421] |
| CoO-Co | SiO2, TiO2, Al2O3 | 24–75 | ---- | ---- | 45–83 | 210 | 20 | [422] | |
| Fe-Cu | K | 65–90 | 16 | 19–37 | 63–81 | 340 | 15 | [423] | |
| Fe1Zn1.2Ox | Na | 38–95 | 31–37 | 15–19 | 85–87 | 340 | 20 | [424] | |
| Fe, Fe3C | Carbon | 80–90 | 10–14 | 7–9 | 88–90 | 250–350 | 34–85 | [425] | |
| Fe | K | Al2O3 | 7–90 | 18–70 | 7–8 | 12–74 | 300–420 | 20 | [426] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; López-Tenllado, F.J.; Romero, A.A.; Luna, D. A Review on Green Hydrogen Valorization by Heterogeneous Catalytic Hydrogenation of Captured CO2 into Value-Added Products. Catalysts 2022, 12, 1555. https://doi.org/10.3390/catal12121555
Estevez R, Aguado-Deblas L, Bautista FM, López-Tenllado FJ, Romero AA, Luna D. A Review on Green Hydrogen Valorization by Heterogeneous Catalytic Hydrogenation of Captured CO2 into Value-Added Products. Catalysts. 2022; 12(12):1555. https://doi.org/10.3390/catal12121555
Chicago/Turabian StyleEstevez, Rafael, Laura Aguado-Deblas, Felipa M. Bautista, Francisco J. López-Tenllado, Antonio A. Romero, and Diego Luna. 2022. "A Review on Green Hydrogen Valorization by Heterogeneous Catalytic Hydrogenation of Captured CO2 into Value-Added Products" Catalysts 12, no. 12: 1555. https://doi.org/10.3390/catal12121555
APA StyleEstevez, R., Aguado-Deblas, L., Bautista, F. M., López-Tenllado, F. J., Romero, A. A., & Luna, D. (2022). A Review on Green Hydrogen Valorization by Heterogeneous Catalytic Hydrogenation of Captured CO2 into Value-Added Products. Catalysts, 12(12), 1555. https://doi.org/10.3390/catal12121555











