Size Effect of Cu Particles on Interface Formation in Cu/ZnO Catalysts for Methanol Synthesis
Abstract
1. Introduction
2. Results
2.1. Structural Characterization of As-Prepared Cu/ZnO Catalyst
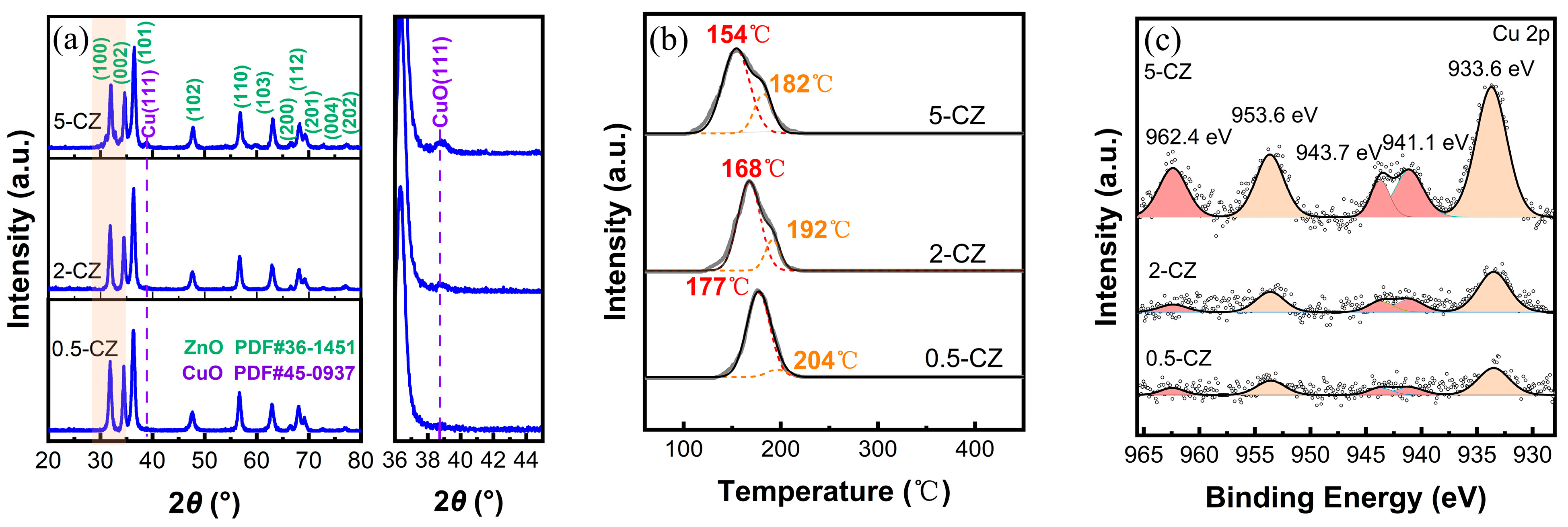
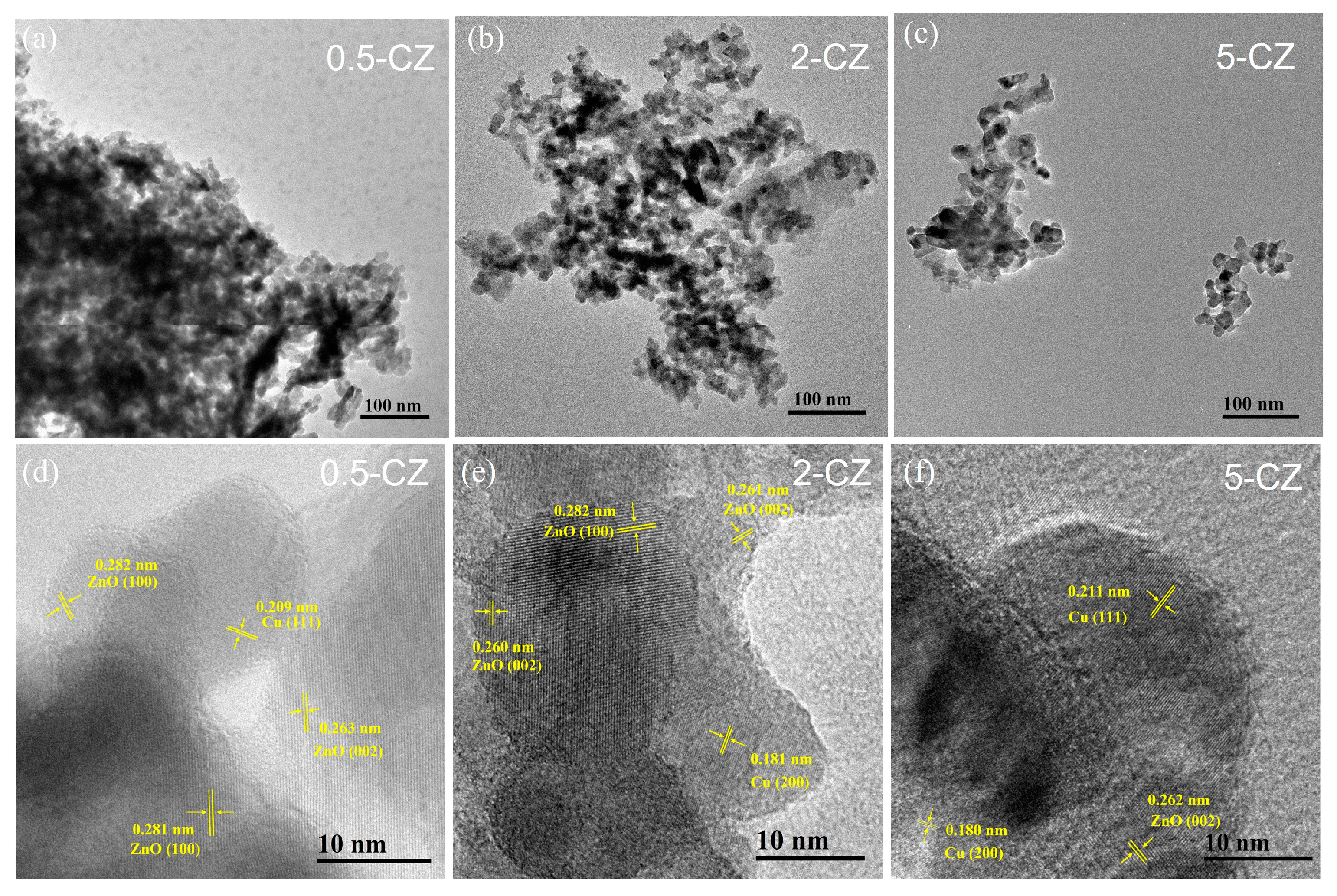
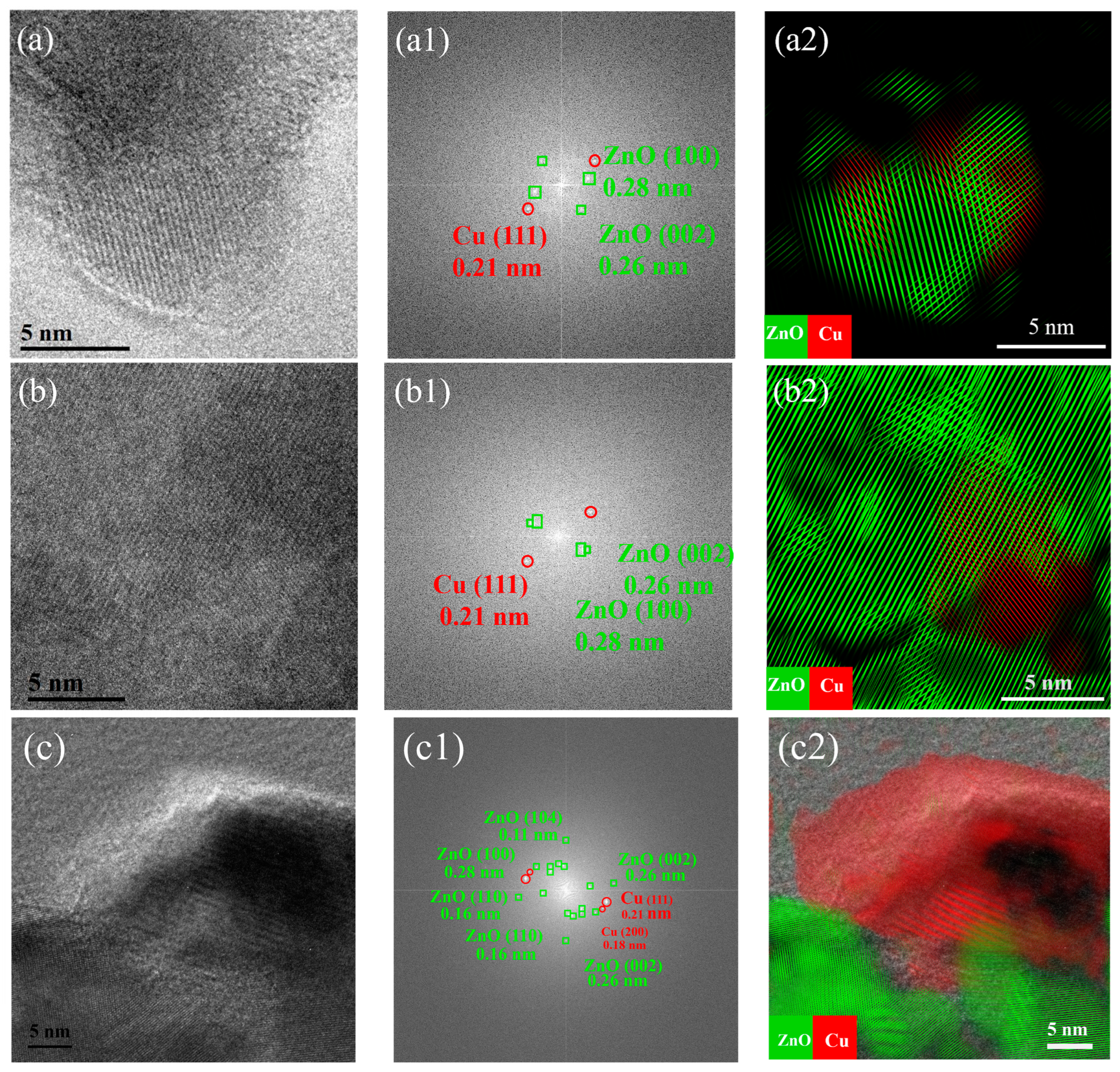
2.2. Structural Characterization of Cu/ZnO Catalyst after H2 Reduction
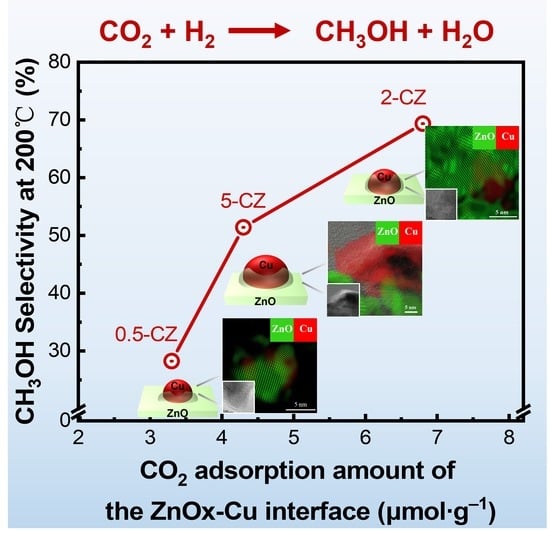
2.3. Catalytic Activity and CO2 Adsorption Ability of Cu-ZnO Catalysts
3. Materials and Methods
3.1. Chemicals and Starting Materials
3.2. Preparation of Catalysts
3.3. Catalyst Characterization
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chai, R.; Liu, Y.; Wang, J.; Liu, Q.; Rui, Z. CO2 utilization and sequestration in Reservoir: Effects and mechanisms of CO2 electrochemical reduction. Appl. Energy 2022, 323, 119584. [Google Scholar] [CrossRef]
- Lang, X.D.; He, L.N. Integration of CO2 reduction with subsequent carbonylation: Towards extending chemical utilization of CO2. ChemSusChem 2018, 11, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Hong, T.; Kim, J. Development of a CO2 emission benchmark for achieving the national CO2 emission reduction target by 2030. Energy Build. 2018, 158, 86–94. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. (Eds.) Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997; Volume 2, pp. 427–440. [Google Scholar]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The applications of morphology controlled ZnO in catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Zeng, Z.; Liao, F.; Hong, X.; Tsang, S.C.E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J. Catal. 2016, 343, 157–167. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.-K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.-X.; Lu, J. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413. [Google Scholar] [CrossRef]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Lunkenbein, T.; Schumann, J.; Behrens, M.; Schlögl, R.; Willinger, M.G. Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal–support interactions. Angew. Chem. 2015, 127, 4627–4631. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Lv, Y.; Liu, P.; Hao, F.; Xiong, W.; Luo, H.a. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy. Fuel 2022, 314, 123035. [Google Scholar] [CrossRef]
- Van Den Berg, R.; Prieto, G.; Korpershoek, G.; Van Der Wal, L.I.; Van Bunningen, A.J.; Lægsgaard-Jørgensen, S.; De Jongh, P.E.; De Jong, K.P. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 2016, 7, 13057. [Google Scholar] [CrossRef] [PubMed]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu–ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki Jr, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active sites and structure–activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2013, 468, 442–452. [Google Scholar] [CrossRef]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef]
- Raoufi, D.; Raoufi, T. The effect of heat treatment on the physical properties of sol–gel derived ZnO thin films. Appl. Surf. Sci. 2009, 255, 5812–5817. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. The role of copper particle size in low pressure methanol synthesis via CO2 hydrogenation over Cu/ZnO catalysts. Catal. Sci. Technol. 2015, 5, 869–881. [Google Scholar] [CrossRef]
- Xie, B.; Kumar, P.; Tan, T.H.; Esmailpour, A.A.; Aguey-Zinsou, K.-F.; Scott, J.; Amal, R. Doping-mediated metal–support interaction promotion toward light-assisted methanol production over Cu/ZnO/Al2O3. ACS Catal. 2021, 11, 5818–5828. [Google Scholar] [CrossRef]
- Liang, M.; Kang, W.; Xie, K. Comparison of reduction behavior of Fe2O3, ZnO and ZnFe2O4 by TPR technique. J. Nat. Gas Chem. 2009, 18, 110–113. [Google Scholar] [CrossRef]
- Melian-Cabrera, I.; Granados, M.L.; Fierro, J. Pd-modified Cu–Zn catalysts for methanol synthesis from CO2/H2 mixtures: Catalytic structures and performance. J. Catal. 2002, 210, 285–294. [Google Scholar] [CrossRef]
- Guo, H.; Li, S.; Peng, F.; Zhang, H.; Xiong, L.; Huang, C.; Wang, C.; Chen, X. Roles investigation of promoters in K/Cu–Zn catalyst and higher alcohols synthesis from CO2 hydrogenation over a novel two-stage bed catalyst combination system. Catal. Lett. 2015, 145, 620–630. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, G.; Zhang, H.; Shen, Y.; Zhang, X.; Zhang, Y.; Li, J. Bridging Mo2C–C and highly dispersed copper by incorporating N-functional groups to greatly enhance the catalytic activity and durability for carbon dioxide hydrogenation. J. Mater. Chem. A 2018, 6, 15510–15516. [Google Scholar] [CrossRef]
- da Silva, R.J.; Pimentel, A.F.; Monteiro, R.S.; Mota, C.J. Synthesis of methanol and dimethyl ether from the CO2 hydrogenation over Cu· ZnO supported on Al2O3 and Nb2O5. J. CO2 Util. 2016, 15, 83–88. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.-L.; Fan, K. The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: New insights on the synergetic effect between Cu0 and Cu+. J. Phys. Chem. C 2009, 113, 11003–11013. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Rudolph, J.; Jacob, C.R. Revisiting the dependence of Cu K-edge X-ray absorption spectra on oxidation state and coordination environment. Inorg. Chem. 2018, 57, 10591–10607. [Google Scholar] [CrossRef]
- Kau, L.S.; Spira-Solomon, D.J.; Penner-Hahn, J.E.; Hodgson, K.O.; Solomon, E.I. X-ray absorption edge determination of the oxidation state and coordination number of copper. Application to the type 3 site in Rhus vernicifera laccase and its reaction with oxygen. J. Am. Chem. Soc. 1987, 109, 6433–6442. [Google Scholar] [CrossRef]
- Moen, A.; Nicholson, D.G.; Rønning, M. Studies on the pre-edge region of the X-ray absorption spectra of copper (I) oxide and the diamminecopper (I) ion. J. Chem. Soc. Faraday Trans. 1995, 91, 3189–3194. [Google Scholar] [CrossRef]
- Meitzner, G.; Iglesia, E. New insights into methanol synthesis catalysts from X-ray absorption spectroscopy. Catal. Today 1999, 53, 433–441. [Google Scholar] [CrossRef]
- Yilmaz, M.; Handoko, A.D.; Parkin, I.P.; Sankar, G. Probing the electronic and geometric structures of photoactive electrodeposited Cu2O films by X-ray absorption spectroscopy. J. Catal. 2020, 389, 483–491. [Google Scholar] [CrossRef]
- Gaur, A.; Klysubun, W.; Nair, N.N.; Shrivastava, B.; Prasad, J.; Srivastava, K. XAFS study of copper (II) complexes with square planar and square pyramidal coordination geometries. J. Mol. Struct. 2016, 1118, 212–218. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Moribe, S.; Kanamori, Y.; Kakudate, M.; Takezawa, N. Preparation of a coprecipitated Cu/ZnO catalyst for the methanol synthesis from CO2—Effects of the calcination and reduction conditions on the catalytic performance. Appl. Catal. A Gen. 2001, 207, 121–128. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, P.; Xiao, L.; Liang, J.; Zhang, B.; Zhao, H.; Kosol, R.; Ma, Q.; Chen, J.; Peng, X. Structure–Performance Correlations over Cu/ZnO Interface for Low-Temperature Methanol Synthesis from Syngas Containing CO2. ACS Appl. Mater. Interfaces 2021, 13, 8191–8205. [Google Scholar] [CrossRef]
- Liao, F.; Huang, Y.; Ge, J.; Zheng, W.; Tedsree, K.; Collier, P.; Hong, X.; Tsang, S.C. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2 to CH3OH. Angew. Chem. 2011, 123, 2210–2213. [Google Scholar] [CrossRef]
- Lei, H.; Nie, R.; Wu, G.; Hou, Z. Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology. Fuel 2015, 154, 161–166. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Lu, D.; Li, C.; Fang, P.; Wang, X. The synthesis of Cu/plate-like ZnO nanostructures and their self-assembly mechanism. Solid State Sci. 2016, 55, 69–76. [Google Scholar] [CrossRef]
- Yurieva, T.M.; Plyasova, L.M.; Zaikovskii, V.I.; Minyukova, T.P.; Bliek, A.; van den Heuvel, J.C.; Davydova, L.P.; Molina, I.Y.; Demeshkina, M.P.; Khassin, A.A. In situ XRD and HRTEM studies on the evolution of the Cu/ZnO methanol synthesis catalyst during its reduction and re-oxidation. Phys. Chem. Chem. Phys. 2004, 6, 4522–4526. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Abliz, A.; Huang, C.-W.; Wang, J.; Xu, L.; Liao, L.; Xiao, X.; Wu, W.-W.; Fan, Z.; Jiang, C.; Li, J. Rational design of ZnO: H/ZnO bilayer structure for high-performance thin-film transistors. ACS Appl. Mater. Interfaces 2016, 8, 7862–7868. [Google Scholar] [CrossRef]
- Pal, S.; Gogurla, N.; Das, A.; Singha, S.; Kumar, P.; Kanjilal, D.; Singha, A.; Chattopadhyay, S.; Jana, D.; Sarkar, A. Clustered vacancies in ZnO: Chemical aspects and consequences on physical properties. J. Phys. D Appl. Phys. 2018, 51, 105107. [Google Scholar] [CrossRef]
- Dong, X.; Li, F.; Zhao, N.; Tan, Y.; Wang, J.; Xiao, F. A study on the order of calcination and liquid reduction over Cu-based catalyst for synthesis of methanol from CO2/H2. Catal. Lett. 2017, 147, 1235–1242. [Google Scholar] [CrossRef]
- Witoon, T.; Kachaban, N.; Donphai, W.; Kidkhunthod, P.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J. Tuning of catalytic CO2 hydrogenation by changing composition of CuO–ZnO–ZrO2 catalysts. Energy Convers. Manag. 2016, 118, 21–31. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]

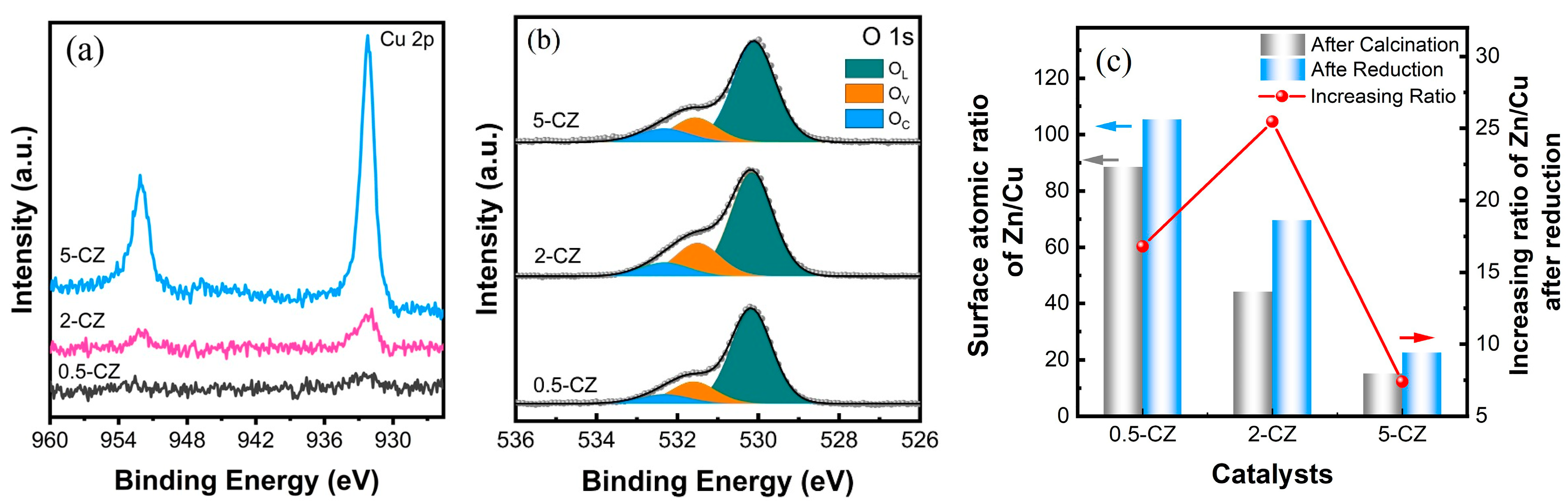

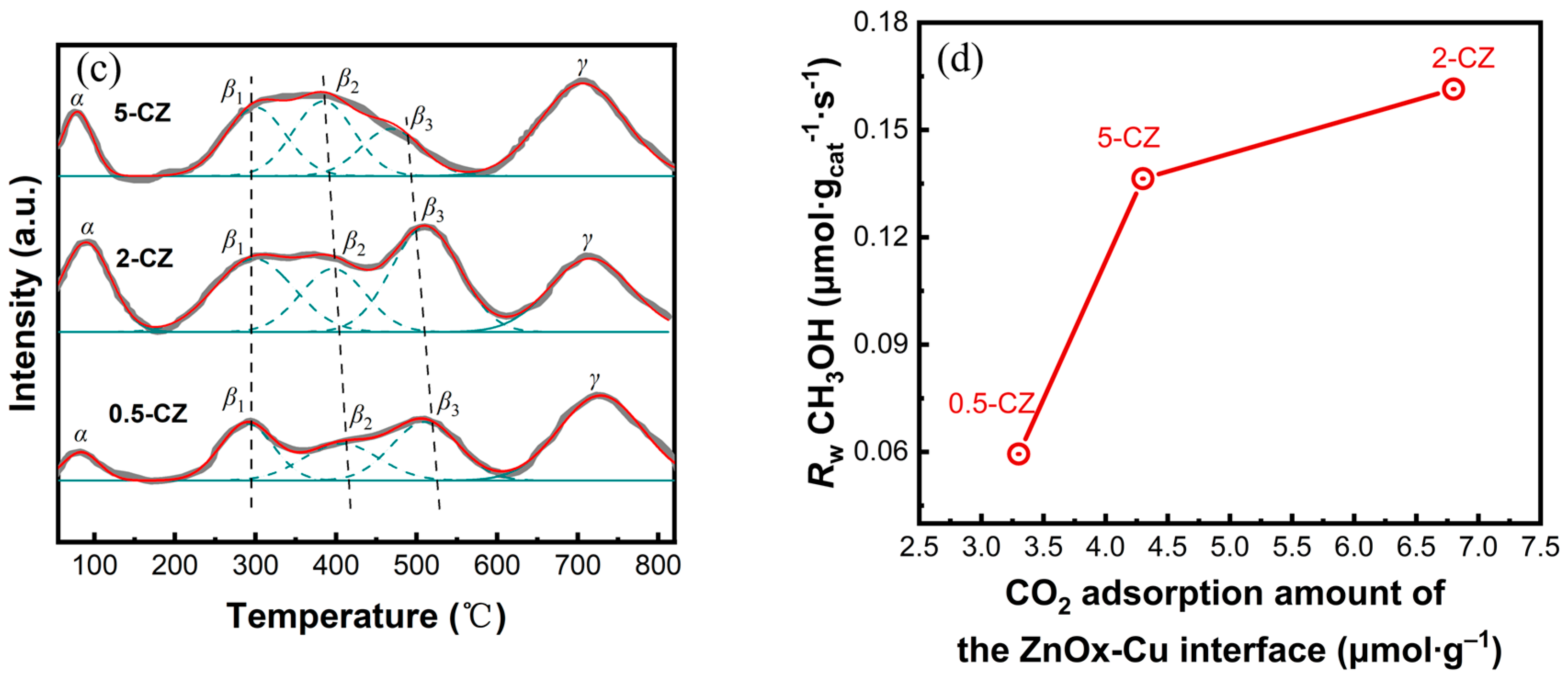
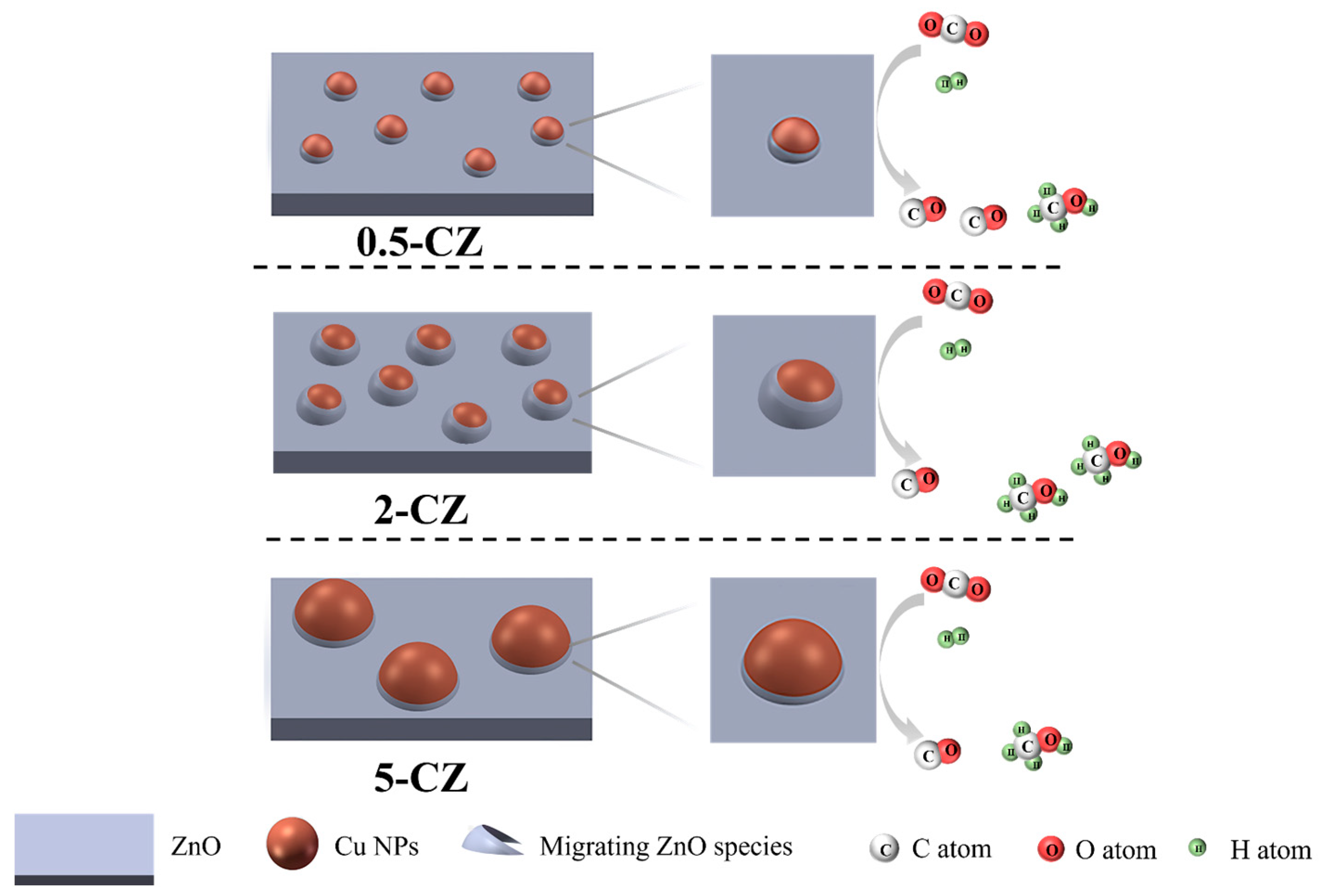
| Sample | Pore Volume a/(cm3·g−1) | SBET b/(m2·g−1) | Pore Size/(nm) | dCuO c/(nm) | dZnO d/(nm) |
|---|---|---|---|---|---|
| 0.5-CZ | 0.282 | 42.6 | 25.8 | 8.4 | 11.3 |
| 2-CZ | 0.226 | 33.6 | 17.3 | 15.0 | 17.7 |
| 5-CZ | 0.127 | 25.4 | 8.7 | 20.1 | 20.8 |
| Sample | Total Amount of Basic Sites/(μmol∙g–1) | Peak Position/(°C) | |||
|---|---|---|---|---|---|
| β | γ | β1 | β2 | β3 | |
| 0.5-CZ | 4.7 | 6.4 | 289 | 420 | 516 |
| 2-CZ | 8.7 | 3.8 | 279 | 413 | 515 |
| 5-CZ | 8.1 | 4.9 | 286 | 376 | 498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhang, L.; Wu, Z.; Huang, C.; Chen, K.; Wang, H.; Yang, F. Size Effect of Cu Particles on Interface Formation in Cu/ZnO Catalysts for Methanol Synthesis. Catalysts 2023, 13, 1190. https://doi.org/10.3390/catal13081190
Zhao L, Zhang L, Wu Z, Huang C, Chen K, Wang H, Yang F. Size Effect of Cu Particles on Interface Formation in Cu/ZnO Catalysts for Methanol Synthesis. Catalysts. 2023; 13(8):1190. https://doi.org/10.3390/catal13081190
Chicago/Turabian StyleZhao, Lirong, Lunjia Zhang, Zhaoxuan Wu, Chaojie Huang, Kuncheng Chen, Hui Wang, and Fan Yang. 2023. "Size Effect of Cu Particles on Interface Formation in Cu/ZnO Catalysts for Methanol Synthesis" Catalysts 13, no. 8: 1190. https://doi.org/10.3390/catal13081190
APA StyleZhao, L., Zhang, L., Wu, Z., Huang, C., Chen, K., Wang, H., & Yang, F. (2023). Size Effect of Cu Particles on Interface Formation in Cu/ZnO Catalysts for Methanol Synthesis. Catalysts, 13(8), 1190. https://doi.org/10.3390/catal13081190