Efficient and Stable Rice Husk Bioderived Silica Supported Cu2S-FeS for One Pot Esterification and Transesterification of a Malaysian Palm Fatty Acid Distillate
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterisation
2.2. One-Step Esterification of PFAD
2.3. Biodiesel Production Reaction Optimisation
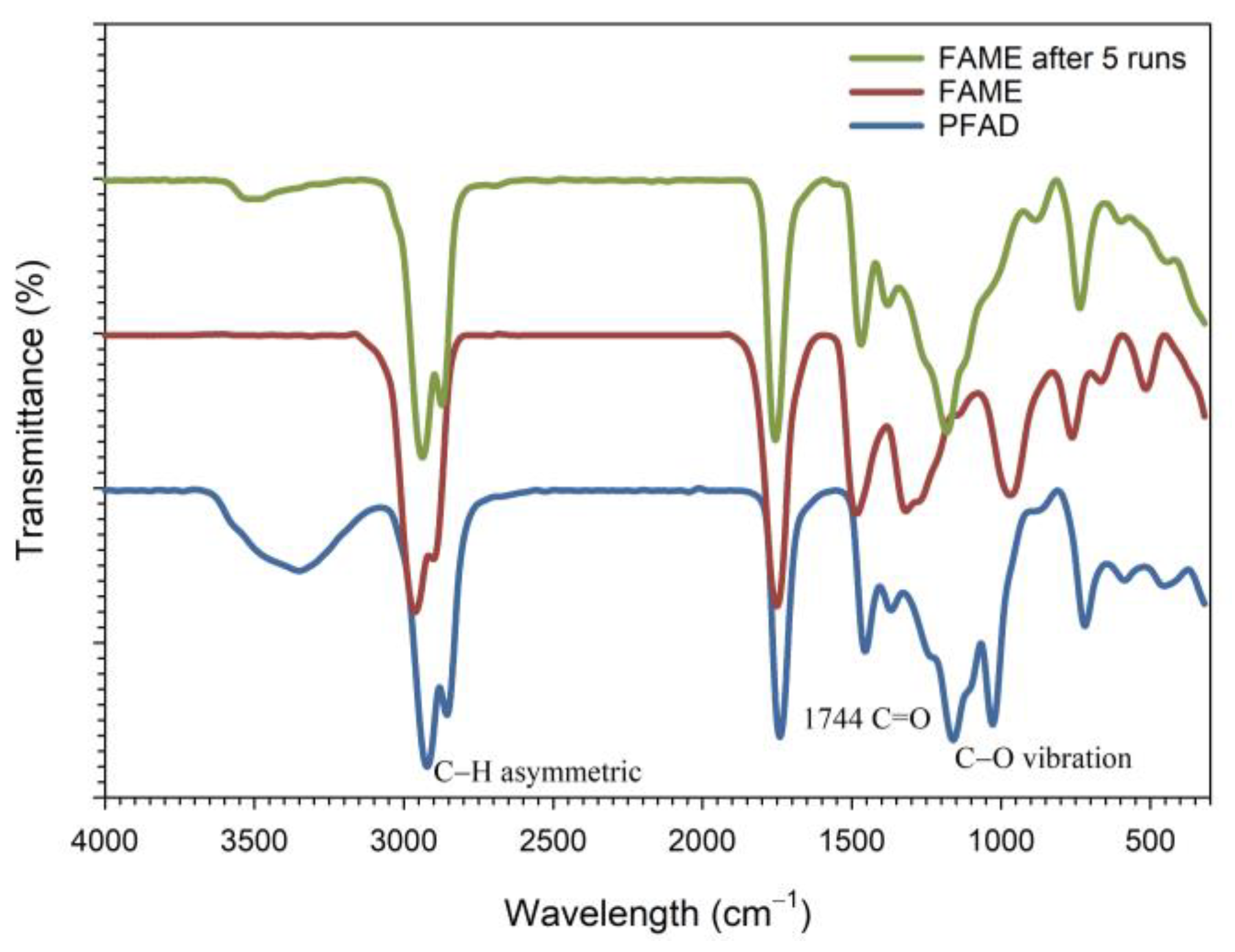
2.4. Catalyst Reusability
| Entry | Catalyst | Substrates | Reaction Conditions | Rate Constant (k) | Biodiesel Yield (%) | Reusability (Yield of the Last Run (%)) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | SO42−/ZrO2-SiO2(Et)-IL-3 | Soybean oil + MeOH | T = 150 °C, CA = 5 wt.%, M:O = 18:1 | - | 98.99 | 5 (95.22) | [48] |
| 2 | SO2−4/TiO2-SiO2 | waste oil + MeOH | T = 120 °C, CA = 10 wt.%, M:O = 20:1, 3 h | - | 88 | 2 (74.3) | [49] |
| Oleic acid + MeOH | T = 120 °C, CA = 10 wt.%, M:O = 20:1, 3 h | - | 93.4 | - | |||
| 3 | La-PW-SiO2/SWCNTs | Oleic acid + MeOH | T = 65 °C, CA = 1.5 wt.%, M:O = 15:1, | - | 93.1 | 6 (88.7) | [50] |
| 4 | SO2−4/TiO2-SiO2 | Waste cooking oil | T = 200 °C, CA = 3 wt.%, M:O = 9:1, 5 h | - | 92 | - | [51] |
| 5 | P-C-750-S-210 | Waste Oil | T = 220 °C, CA = 4 wt.%, M:O = 21:1, 5 h | 0.0166 (mol−1·min−1) | 50% (conversion) | - | [52] |
| 6 | H2SO4 and NaOH | Jatropha curcas oil | T = 65 °C, CA = 1 wt.%, M:O = 3:10, 6 h | 0.0031 (min−1) and 0.008 min−1 | 21.2% and 90.1% | - | [53] |
| 7 | CaO | Sunflower oil | T = 80 °C, CA = 1 wt.%, M:O = 6:1, 15 bar 5.5 h | 59.22 × 10−3·min−1 | 91% | - | [54] |
| 8 | Mg0.81Al | Palm oil | T = 60 °C, CA = 1 wt.%, M:O = 6:1, 3 h | 1.60 × 10−6 | 74.8 | - | [55] |
| 9 | Cu2S(8%)-FeS(10%)/SiO2 | PFAD | T = 70 °C, CA = 2 wt.%, M:O = 15:1, 3 h | 4.03 × 10−6 (S−1) | 98% (conversion) | 5 (82.73) | This work |
2.5. Heterogeneous Acid Catalysts Mechanism for Biodiesel Production
3. Materials and Methods
3.1. Preparation of SiO2
3.2. Preparation of Cu/SiO2 and Fe/SiO2 Supported Catalyst
3.3. Preparation of CuS-FeS/SiO2 Supported Catalyst
3.4. Catalyst Characterisation
3.5. Esterification Reaction of PFAD
3.6. Mass Transfer Limitations and Mole Balance Catalyst Evaluation
3.7. Catalyst Reusability and Leaching Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juera-Ong, P.; Pongraktham, K.; Oo, Y.M.; Somnuk, K. Reduction in Free Fatty Acid Concentration in Sludge Palm Oil Using Heterogeneous and Homogeneous Catalysis: Process Optimization, and Reusable Heterogeneous Catalysts. Catalysts 2022, 12, 1007. [Google Scholar] [CrossRef]
- Núñez, F.; Chen, L.; Wang, J.A.; Flores, S.O.; Salmones, J.; Arellano, U.; Noreña, L.E.; Tzompantzi, F. Bifunctional Co3O4/ZSM-5 Mesoporous Catalysts for Biodiesel Production via Esterification of Unsaturated Omega-9 Oleic Acid. Catalysts 2022, 12, 900. [Google Scholar] [CrossRef]
- Tabassum, N.; Pothu, R.; Pattnaik, A.; Boddula, R.; Balla, P.; Gundeboyina, R.; Challa, P.; Rajesh, R.; Perugopu, V.; Mameda, N.; et al. Heterogeneous Catalysts for Conversion of Biodiesel-Waste Glycerol into High-Added-Value Chemicals. Catalysts 2022, 12, 767. [Google Scholar] [CrossRef]
- Sarwer, A.; Hussain, M.; Al-Muhtaseb, A.H.; Inayat, A.; Rafiq, S.; Khurram, M.S.; Ul-Haq, N.; Shah, N.S.; Alaud Din, A.; Ahmad, I.; et al. Suitability of Biofuels Production on Commercial Scale from Various Feedstocks: A Critical Review. ChemBioEng Rev. 2022, 9, 423–441. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Junaid Khalil, M.; Arham Khan, M.; Azad Alam, M.; Masood, F.; Bazli, L.; Chelliapan, S.; et al. Current trends in hydrogen production, storage and applications in India: A review. Sustain. Energy Technol. Assess. 2022, 53, 102677. [Google Scholar] [CrossRef]
- Janaswamy, S.; Yadav, M.P.; Hoque, M.; Bhattarai, S.; Ahmed, S. Cellulosic fraction from agricultural biomass as a viable alternative for plastics and plastic products. Ind. Crops Prod. 2022, 179, 114692. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin Mijan, N.; Mansir, N.; Razali, S.Z.; Yunus, R.; Taufiq-Yap, Y.H. Combustion and Emission Performance of CO/NOx/SOxfor Green Diesel Blends in a Swirl Burner. ACS Omega 2021, 6, 408–415. [Google Scholar] [CrossRef]
- Awogbemi, O.; Vandi, D.; Kallon, V.; Von Kallon, D.V. Application of Tubular Reactor Technologies for the Acceleration of Biodiesel Production. Bioengineering 2022, 9, 347. [Google Scholar] [CrossRef]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.C.; Fouquet, E.; Felpin, F.X. Recyclable heterogeneous palladium catalysts in pure water: Sustainable developments in Suzuki, Heck, Sonogashira and Tsuji-Trost reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-Yap, Y.H. A short review on catalyst, feedstock, modernised process, current state and challenges on biodiesel production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Obeas, L.K.; Yunus, R.; Razali, S.Z.; Islam, A.; Hin Taufiq-Yap, Y. In-situ operando and ex-situ study on light hydrocarbon-like-diesel and catalyst deactivation kinetic and mechanism study during deoxygenation of sludge oil. Chem. Eng. J. 2022, 429, 132206. [Google Scholar] [CrossRef]
- Adzahar, N.A.; Asikin-Mijan, N.; Saiman, M.I.; Alsultan, G.A.; Mastuli, M.S.; Shamsuddin, M.R.; Taufiq-Yap, Y.H. Chemoselective decarboxylation of ceiba oil to diesel-range alkanes over a red mud based catalyst under H2-free conditions. RSC Adv. 2022, 12, 16903–16917. [Google Scholar] [CrossRef] [PubMed]
- Karis, D.; Cain, R.; Young, K.; Shand, A.; Holm, T.; Springer, E. Non-fuel uses for fatty acid methyl esters. Biofuels Bioprod. Biorefining 2022, 16, 1893–1908. [Google Scholar] [CrossRef]
- Xiao, X.; Bergstrom, H.; Saenger, R.; Johnson, B.; Sun, R.; Peterson, A. The role of oxygen vacancies in biomass deoxygenation by reducible zinc/zinc oxide catalysts. Catal. Sci. Technol. 2018, 8, 1819–1827. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Hemanth, G.; Prashanth, B.; Benerjee, N.; Choudhuri, T. Mrityunjay Dual fuel mode operation and its emission characteristics in diesel engine with producer gas as primary fuel and jatropha biodiesel as pilot fuel. Int. J. Mech. Eng. Technol. 2017, 8, 138–147. [Google Scholar]
- Osatiashtiani, A.; Lee, A.F.; Granollers, M.; Brown, D.R.; Olivi, L.; Morales, G.; Melero, J.A.; Wilson, K. Hydrothermally stable, conformal sulfated zirconia monolayer catalysts for glucose conversion to 5-HMF. ACS Catal. 2015, 5, 4345–4352. [Google Scholar] [CrossRef]
- Jiang, T.; Budarin, V.L.; Shuttleworth, P.S.; Ellis, G.; Parlett, C.M.A.; Wilson, K.; Macquarrie, D.J.; Hunt, A.J. Green preparation of tuneable carbon-silica composite materials from wastes. J. Mater. Chem. A 2015, 3, 14148–14156. [Google Scholar] [CrossRef]
- Kumar, S.; Saroha, B.; Kumar, G.; Lathwal, E.; Kumar, S.; Parshad, B.; Kumari, M.; Kumar, N.; Mphahlele-Makgwane, M.M.; Makgwane, P.R. Recent Developments in Nanocatalyzed Green Synthetic Protocols of Biologically Potent Diverse O-Heterocycles—A Review. Catalysts 2022, 12, 657. [Google Scholar] [CrossRef]
- Alam, M.M.; Hossain, M.A.; Hossain, M.D.; Johir, M.A.H.; Hossen, J.; Rahman, M.S.; Zhou, J.L.; Hasan, A.T.M.K.; Karmakar, A.K.; Ahmed, M.B. The potentiality of rice husk-derived activated carbon: From synthesis to application. Processes 2020, 8, 203. [Google Scholar] [CrossRef]
- Moayedi, H.; Aghel, B.; Abdullahi, M.M.; Nguyen, H.; Safuan, A.; Rashid, A. Applications of Rice Husk Ash as Green and Sustainable Biomass; Elsevier: Amsterdam, The Netherlands, 2019; Volume 237, p. 117851. [Google Scholar]
- Akhter, F.; Soomro, S.A.; Jamali, A.R.; Chandio, Z.A.; Siddique, M.; Ahmed, M. Rice husk ash as green and sustainable biomass waste for construction and renewable energy applications: A review. Biomass Convers. Biorefinery 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Embong, N.H.; Hindryawati, N.; Bhuyar, P.; Govindan, N.; Rahim, M.H.A.; Maniam, G.P. Enhanced biodiesel production via esterification of palm fatty acid distillate (PFAD) using rice husk ash (NiSO4)/SiO2 catalyst. Appl. Nanosci. 2021, 1–9. [Google Scholar] [CrossRef]
- Shah, I.; Adnan, R.; Alsultan, A.G.; Taufiq-Yap, Y.H. Catalytic conversion of waste cooking oil into biodiesel using functionally advanced recyclable iron-impregnated activated carbon materials. J. Dispers. Sci. Technol. 2020, 43, 1245–1260. [Google Scholar] [CrossRef]
- Kader, S.S.; Jusoh, M.; Zakaria, Z.Y. Palm fatty acid distillate-based biodiesel with sulfonated chicken and cow bone catalyst. Mater. Today Proc. 2022, 57, 1053–1060. [Google Scholar] [CrossRef]
- Ngaini, Z.; Jamil, N.; Wahi, R.; Shahrom, F.D.; Ahmad, Z.A.; Farooq, S. Convenient Conversion of Palm Fatty Acid Distillate to Biodiesel via Rice Husk Ash Catalyst. Bioenergy Res. 2022, 15, 1316–1326. [Google Scholar] [CrossRef]
- Chanakaewsomboon, I.; Phoungthong, K.; Palamanit, A.; Seechamnanturakit, V.; Cheng, C.K. Biodiesel produced using potassium methoxide homogeneous alkaline catalyst: Effects of various factors on soap formation. Biomass Convers. Biorefinery 2021, 1–11. [Google Scholar] [CrossRef]
- Xu, H.; Lee, U.; Wang, M. Life-cycle energy use and greenhouse gas emissions of palm fatty acid distillate derived renewable diesel. Renew. Sustain. Energy Rev. 2020, 134, 110144. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Go, A.W.; Alivio, R.K.O.; Santoso, S.P.; Angkawijaya, A.E.; Soetaredjo, F.E.; Agapay, R.C. Non-isothermal (trans)esterification of linoleic acid and soybean oil deodorizer distillate with methanol under subcritical conditions. Renew. Energy 2022, 197, 528–544. [Google Scholar] [CrossRef]
- Esan, A.O.; Smith, S.M.; Ganesan, S. Dimethyl carbonate assisted catalytic esterification of palm fatty acid distillate using catalyst derived from spent bleaching clay. J. Clean. Prod. 2022, 337, 130574. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Taufiq-Yap, Y.H. Effective catalytic deoxygenation of waste cooking oil over nanorods activated carbon supported CaO. Key Eng. Mater. 2016, 707, 175–181. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.M.; Zhen, Q.; Bashir, S.; et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H. A new route for the synthesis of La-Ca oxide supported on nano activated carbon via vacuum impregnation method for one pot esterification-transesterification reaction. Chem. Eng. J. 2016, 304, 61–71. [Google Scholar] [CrossRef]
- Shuit, S.H.; Ng, E.P.; Tan, S.H. A facile and acid-free approach towards the preparation of sulphonated multi-walled carbon nanotubes as a strong protonic acid catalyst for biodiesel production. J. Taiwan Inst. Chem. Eng. 2015, 52, 100–108. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Alejandro-Martín, S.; Acaricia, A.M.; Cerda-Barrera, C.; Pérez, H.D. Influence of chemical surface characteristics of ammonium-modified chilean zeolite on oak catalytic pyrolysis. Catalysts 2019, 9, 465. [Google Scholar] [CrossRef]
- Pauzi, N.N.P.N.; Ramli, N.A.S.; Chung-Hung, C.; Ahmad Hazmi, A.S.; Ikram, R.; Mohamed Jan, B. Non-catalytic esterification of palm fatty acid distillate with 2-ethyl hexanol for high purity production of biolubricant ester. Biofuels Bioprod. Biorefining 2022, 16, 1583–1598. [Google Scholar] [CrossRef]
- Carlucci, C. An Overview on the Production of Biodiesel Enabled by Continuous Flow Methodologies. Catalysts 2022, 12, 717. [Google Scholar] [CrossRef]
- Ding, J.; Xia, Z.; Lu, J. Esterification and deacidification of a waste cooking oil (TAN 68.81 mg KOH/g) for biodiesel production. Energies 2012, 5, 2683–2691. [Google Scholar] [CrossRef]
- Song, J.; Hou, M.; Jiang, T.; Han, B.; Li, X.; Liu, G.; Yang, G. Enhancing reaction rate of transesterification of glycerol monostearate and methanol by CO2. J. Phys. Chem. A 2007, 111, 12007–12010. [Google Scholar] [CrossRef]
- Wu, Z.; Xiong, Z.; Liu, R.; He, C.; Liu, Y.; Pan, Z.; Yao, G.; Lai, B. Pivotal roles of N-doped carbon shell and hollow structure in nanoreactor with spatial confined Co species in peroxymonosulfate activation: Obstructing metal leaching and enhancing catalytic stability. J. Hazard. Mater. 2022, 427, 128204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Y.; Huang, Y.; Song, L.; Chen, H.; Zhu, S.; Tang, C. Enhanced adsorption of phosphate on orange peel-based biochar activated by Ca/Zn composite: Adsorption efficiency and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129728. [Google Scholar] [CrossRef]
- Li, S.; Luo, S.; Wan, S.; Wang, P.; Zhou, G.; Wang, W.; Chen, R. Enhanced Performance of nZVI/MXene@CNTs for Rapid As(III) Removal from Aqueous Solutions. Appl. Sci. 2022, 12, 8206. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.; Wei, G.; Gu, Y.; Shi, H. A critical assessment of the roles of water molecules and solvated ions in acid-base-catalyzed reactions at solid-water interfaces. Chin. J. Catal. 2022, 43, 1964–1990. [Google Scholar] [CrossRef]
- Rahman, M.W.; Mondal, A.K.; Hasan, M.S.; Sultana, M. Biodiesel production from a non-edible source of royna (Aphanamixis polystachya) oil. Energy. Sustain. Soc. 2022, 12, 33. [Google Scholar] [CrossRef]
- Mansir, N.; Mohd Sidek, H.; Teo, S.H.; Mijan, N.A.; Ghassan Alsultan, A.K.; Ng, C.H.; Shamsuddin, M.R.; Taufiq-Yap, Y.H. Catalytically active metal oxides studies for the conversion technology of carboxylic acids and bioresource based fatty acids to ketones: A review. Bioresour. Technol. Rep. 2022, 17, 100988. [Google Scholar] [CrossRef]
- Fan, M.; Liu, H.; Zhang, P. Ionic liquid on the acidic organic-inorganic hybrid mesoporous material with good acid-water resistance for biodiesel production. Fuel 2018, 215, 541–550. [Google Scholar] [CrossRef]
- Shao, G.N.; Sheikh, R.; Hilonga, A.; Lee, J.E.; Park, Y.H.; Kim, H.T. Biodiesel production by sulfated mesoporous titania-silica catalysts synthesized by the sol-gel process from less expensive precursors. Chem. Eng. J. 2013, 215–216, 600–607. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, X.; Huo, Y.; Tan, Y.; Zhang, C.; Zou, L. Construction of a Brönsted-Lewis solid acid catalyst La-PW-SiO2/SWCNTs based on electron withdrawing effect of La(III) on π bond of SWCNTs for biodiesel synthesis from esterification of oleic acid and methanol. Chin. J. Chem. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2-MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Liao, Y.; Wang, J. Reaction kinetics of biodiesel synthesis from waste oil using a carbon-based solid acid catalyst. Chin. J. Chem. Eng. 2011, 19, 163–168. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Biodiesel production from Jatropha curcas oil. Renew. Sustain. Energy Rev. 2010, 14, 3140. [Google Scholar] [CrossRef]
- Vujicic, D.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Kapil, A.; Wilson, K.; Lee, A.F.; Sadhukhan, J. Kinetic modeling studies of heterogeneously catalyzed biodiesel synthesis reactions. Ind. Eng. Chem. Res. 2011, 50, 4818–4830. [Google Scholar] [CrossRef]
- Le Guen, M.J.; Hill, S.; Smith, D.; Theobald, B.; Gaugler, E.; Barakat, A.; Mayer-Laigle, C. Influence of Rice Husk and Wood Biomass Properties on the Manufacture of Filaments for Fused Deposition Modeling. Front. Chem. 2019, 7, 735. [Google Scholar] [CrossRef] [PubMed]
- Abdul Kapor, N.Z.; Maniam, G.P.; Rahim, M.H.A.; Yusoff, M.M. Palm fatty acid distillate as a potential source for biodiesel production-a review. J. Clean. Prod. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Kefas, H.M.; Yunus, R.; Rashid, U.; Taufiq-Yap, Y.H. Enhanced biodiesel synthesis from palm fatty acid distillate and modified sulfonated glucose catalyst via an oscillation flow reactor system. J. Environ. Chem. Eng. 2019, 7, 102993. [Google Scholar] [CrossRef]
- Islam, A.; Teo, S.H.; Awual, M.R.; Taufiq-Yap, Y.H. Improving the hydrogen production from water over MgO promoted Ni–Si/CNTs photocatalyst. J. Clean. Prod. 2019, 238, 117887. [Google Scholar] [CrossRef]
- Alsultan, A.; Asikin-Mijan, N.; Obeas, L.; Islam, A.; Mansir, N.; Teo, S.; Razali, S.; Nassar, M.; Mohamad, S.; Taufiq-Yap, Y. Selective Deoxygenation of Sludge Palm Oil into Diesel Range Fuel over Mn-Mo Supported on Activated Carbon Catalyst. Catalysts 2022, 12, 566. [Google Scholar] [CrossRef]
- Melero, J.A.; Sánchez-Vázquez, R.; Vasiliadou, I.A.; Martínez Castillejo, F.; Bautista, L.F.; Iglesias, J.; Morales, G.; Molina, R. Municipal sewage sludge to biodiesel by simultaneous extraction and conversion of lipids. Energy Convers. Manag. 2015, 103, 111–118. [Google Scholar] [CrossRef]




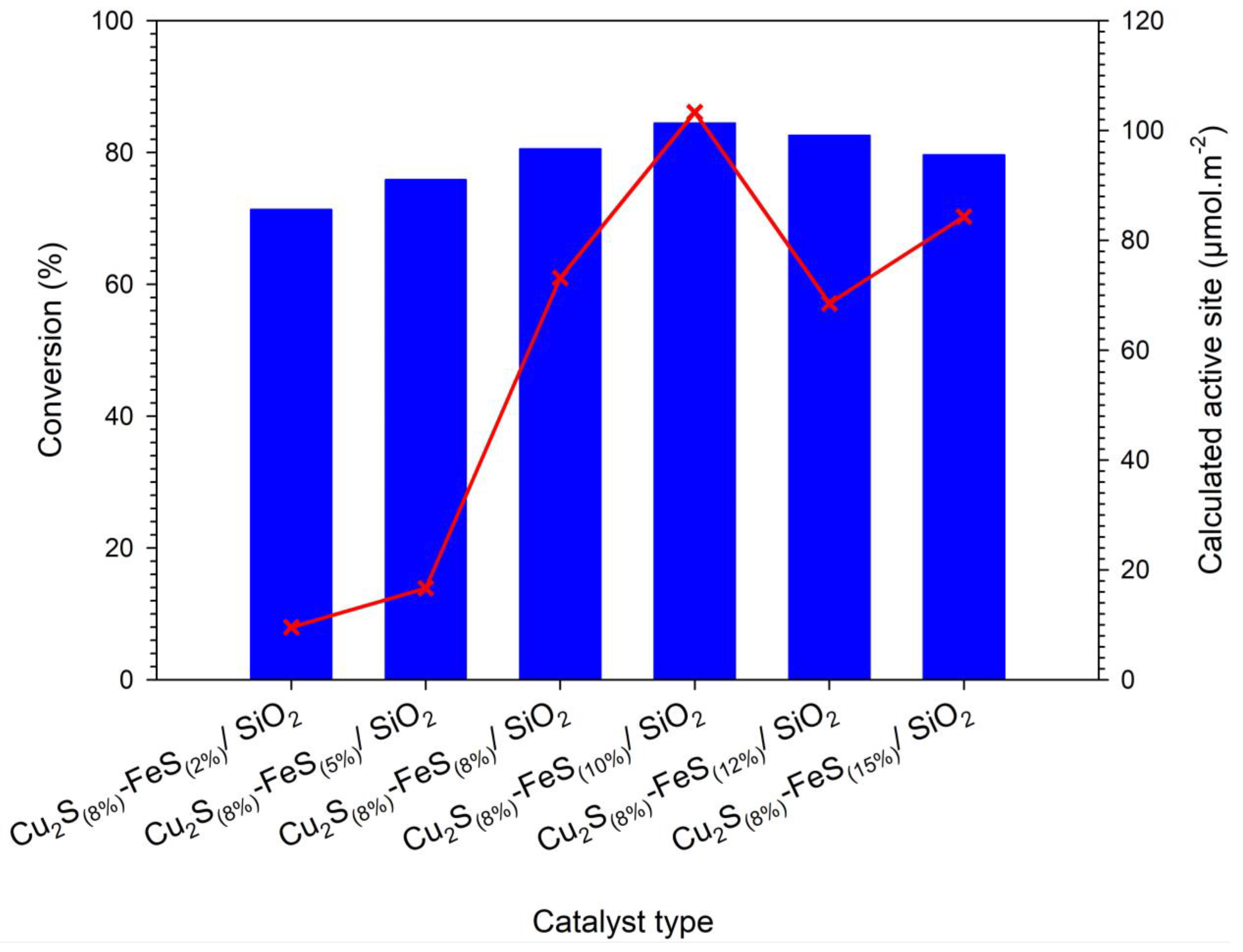



| Catalyst | XRD a | BET b | TPD-NH3 c | Calculated Active Site (µmol·m−2) | Mass Transfer Limitations | ||
|---|---|---|---|---|---|---|---|
| Crystallite Size a (nm) | Surface Area b (m2·g−1) | Pore Volume b (cm3·g−1) | Amount of NH3 Adsorbed c (µmol·g−1) | Measured Rate (umol·g−1·min−1) | Reaction Rate Constant k (mole·S−1) | ||
| SiO2 | 13 | 68 | 0.72 | 152.06 | 2.23 | 132.59 | 3.54 × 10−7 |
| Cu/SiO2 | 17 | 62 | 0.71 | 198.33 | 3.19 | 293.55 | 2.59 × 10−7 |
| Fe/SiO2 | 18 | 58 | 0.70 | 204.81 | 3.53 | 363.68 | 2.79 × 10−7 |
| Cu2S(2%)/SiO2 | 26 | 50 | 0.69 | 252.14 | 5.04 | 407.55 | 2.34 × 10−7 |
| Cu2S(5%)/SiO2 | 30 | 44 | 0.65 | 437.99 | 9.95 | 458.58 | 2.18 × 10−7 |
| Cu2S(8%)/SiO2 | 30 | 42 | 0.62 | 2590.4 | 61.6 | 480. 75 | 3.49 × 10−7 |
| Cu2S(10%)/SiO2 | 35 | 50 | 0.64 | 1879.68 | 37.5 | 533. 87 | 2.90 × 10−7 |
| Cu2S(12%)/SiO2 | 38 | 37 | 0.60 | 1744.12 | 47.1 | 586.98 | 2.32 × 10−7 |
| Cu2S(15%)/SiO2 | 41 | 33 | 0.57 | 1503.28 | 45.55 | 548. 61 | 2.23 × 10−7 |
| Cu2S(8%)-FeS(2%)/SiO2 | 31 | 42 | 0.63 | 402.32 | 9.57 | 667.30 | 3.45 × 10−7 |
| Cu2S(8%)-FeS(5%)/SiO2 | 36 | 42 | 0.60 | 698.86 | 16.63 | 760.735 | 3.82 × 10−7 |
| Cu2S(8%)-FeS(8%)/SiO2 | 38 | 41 | 0.59 | 2999.21 | 73.15 | 813. 66 | 4.71 × 10−7 |
| Cu2S(8%)-FeS(10%)/SiO2 | 46 | 40 | 0.57 | 4133.29 | 103.33 | 2639.92 | 4.03 × 10−6 |
| Cu2S(8%)-FeS(12%)/SiO2 | 48 | 35 | 0.30 | 2398.64 | 68.53 | 869.41 | 5.77 × 10−7 |
| Cu2S(8%)-FeS(15%)/SiO2 | 51 | 33 | 0.26 | 2782.91 | 84.33 | 910. 88 | 3.97 × 10−7 |
| Properties | Method | Present Study | Ref a | Ref b |
|---|---|---|---|---|
| FFA content (%) | AOCS Cd 3d-63 | 0.71 | 86.3 | 90.24 |
| Saponification value (mg KOH/g) | AOCS Tr 1a-64 | 3.32 | 149.74 | 207.69 |
| Molecular weight (g mol−1) a | 193.2 | 270.11 | ||
| Fatty acid composition (wt.%) | ||||
| Myristic (C14:0) | 0.05 | 1.9 | 1.03 | |
| Palmitic (C16:0) | 0.32 | 45.7 | 48.02 | |
| Stearic (C18:0) | 1.01 | 4.3 | 3.42 | |
| Oleic (C18:1) | 1.32 | 40.2 | 41.01 | |
| Linoleic (C18:2) | 0.71 | 7.9 | 6.52 | |
| Σ Saturated | 63.24 | 51.9 | 52.44 | |
| Σ Unsaturated | 36.76 | 48.1 | 47.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalushi, M.Y.; Abdulkreem-Alsultan, G.; Asikin-Mijan, N.; bin Saiman, M.I.; Tan, Y.P.; Taufiq-Yap, Y.H. Efficient and Stable Rice Husk Bioderived Silica Supported Cu2S-FeS for One Pot Esterification and Transesterification of a Malaysian Palm Fatty Acid Distillate. Catalysts 2022, 12, 1537. https://doi.org/10.3390/catal12121537
Albalushi MY, Abdulkreem-Alsultan G, Asikin-Mijan N, bin Saiman MI, Tan YP, Taufiq-Yap YH. Efficient and Stable Rice Husk Bioderived Silica Supported Cu2S-FeS for One Pot Esterification and Transesterification of a Malaysian Palm Fatty Acid Distillate. Catalysts. 2022; 12(12):1537. https://doi.org/10.3390/catal12121537
Chicago/Turabian StyleAlbalushi, Mohammed Yousuf, G. Abdulkreem-Alsultan, N. Asikin-Mijan, Mohd Izham bin Saiman, Yen Ping Tan, and Y. H. Taufiq-Yap. 2022. "Efficient and Stable Rice Husk Bioderived Silica Supported Cu2S-FeS for One Pot Esterification and Transesterification of a Malaysian Palm Fatty Acid Distillate" Catalysts 12, no. 12: 1537. https://doi.org/10.3390/catal12121537
APA StyleAlbalushi, M. Y., Abdulkreem-Alsultan, G., Asikin-Mijan, N., bin Saiman, M. I., Tan, Y. P., & Taufiq-Yap, Y. H. (2022). Efficient and Stable Rice Husk Bioderived Silica Supported Cu2S-FeS for One Pot Esterification and Transesterification of a Malaysian Palm Fatty Acid Distillate. Catalysts, 12(12), 1537. https://doi.org/10.3390/catal12121537








