A Continuous Fixed Bed Adsorption Process for Fez City Urban Wastewater Using Almond Shell Powder: Experimental and Optimization Study
Abstract
1. Introduction
2. Results and Discussion
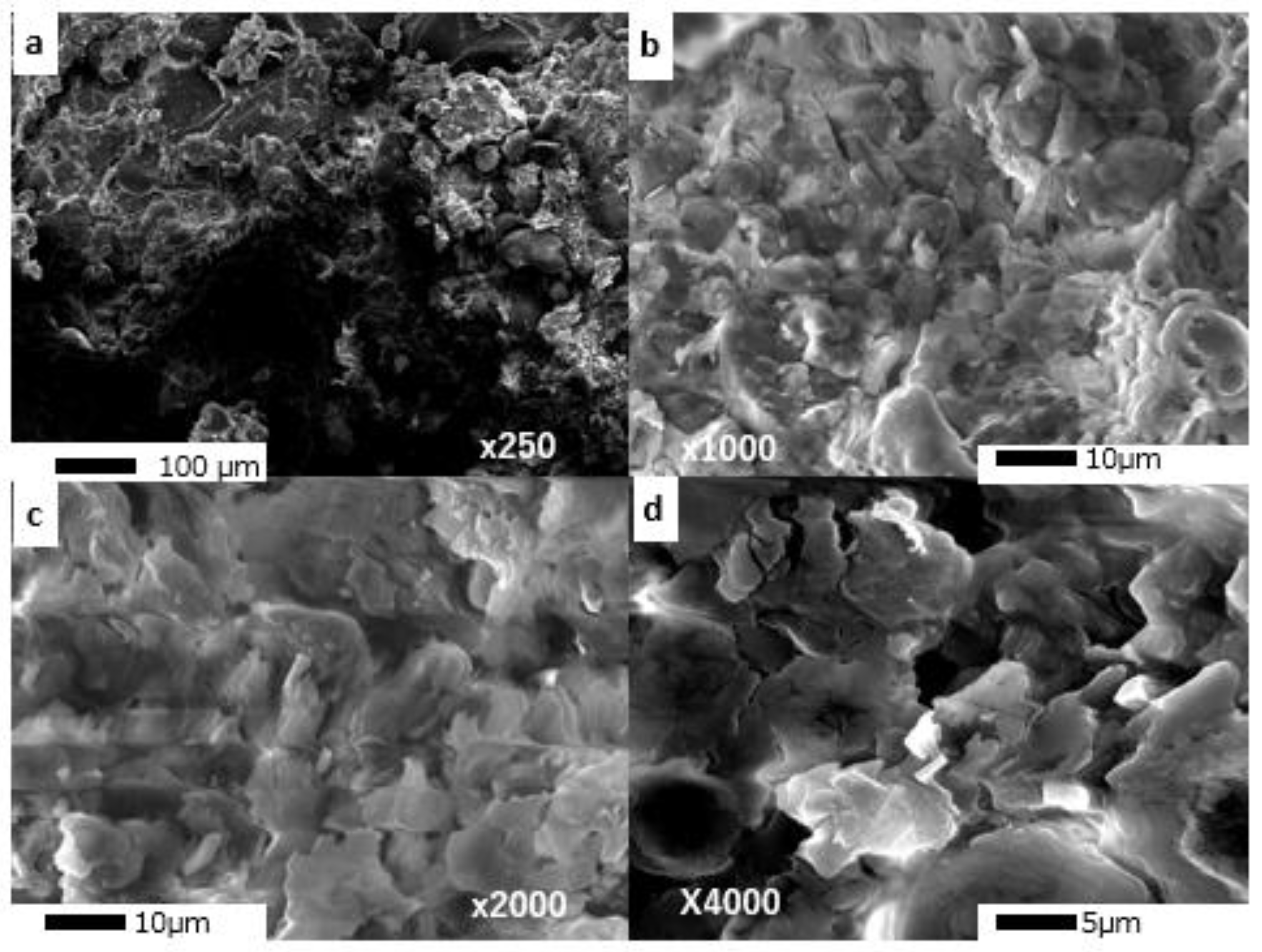
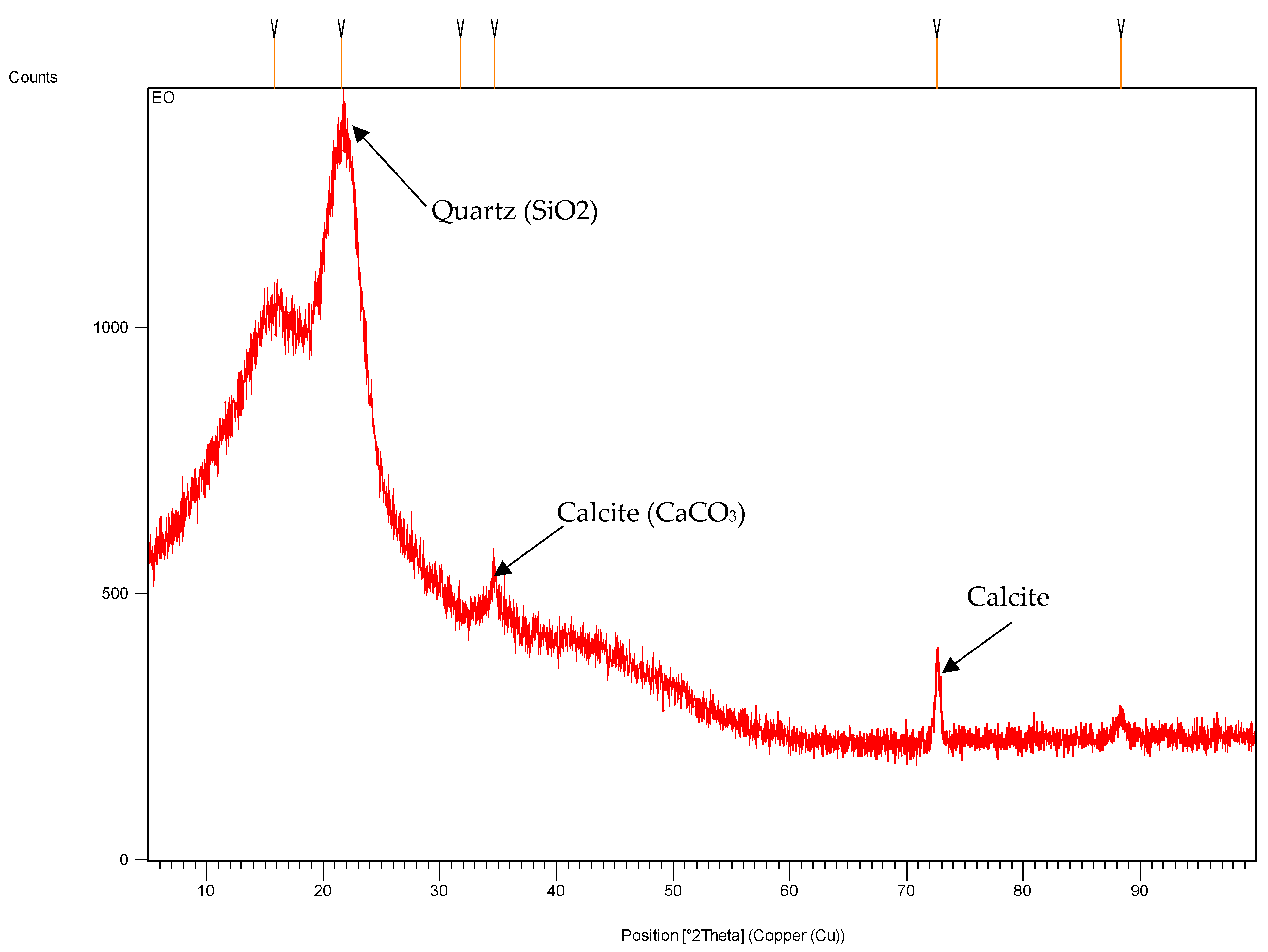
2.1. Characterization of Almond Shell
2.2. Fixed Bed Column Adsorption
2.3. Effect of Almond Shell Particle Size on the Solution pH
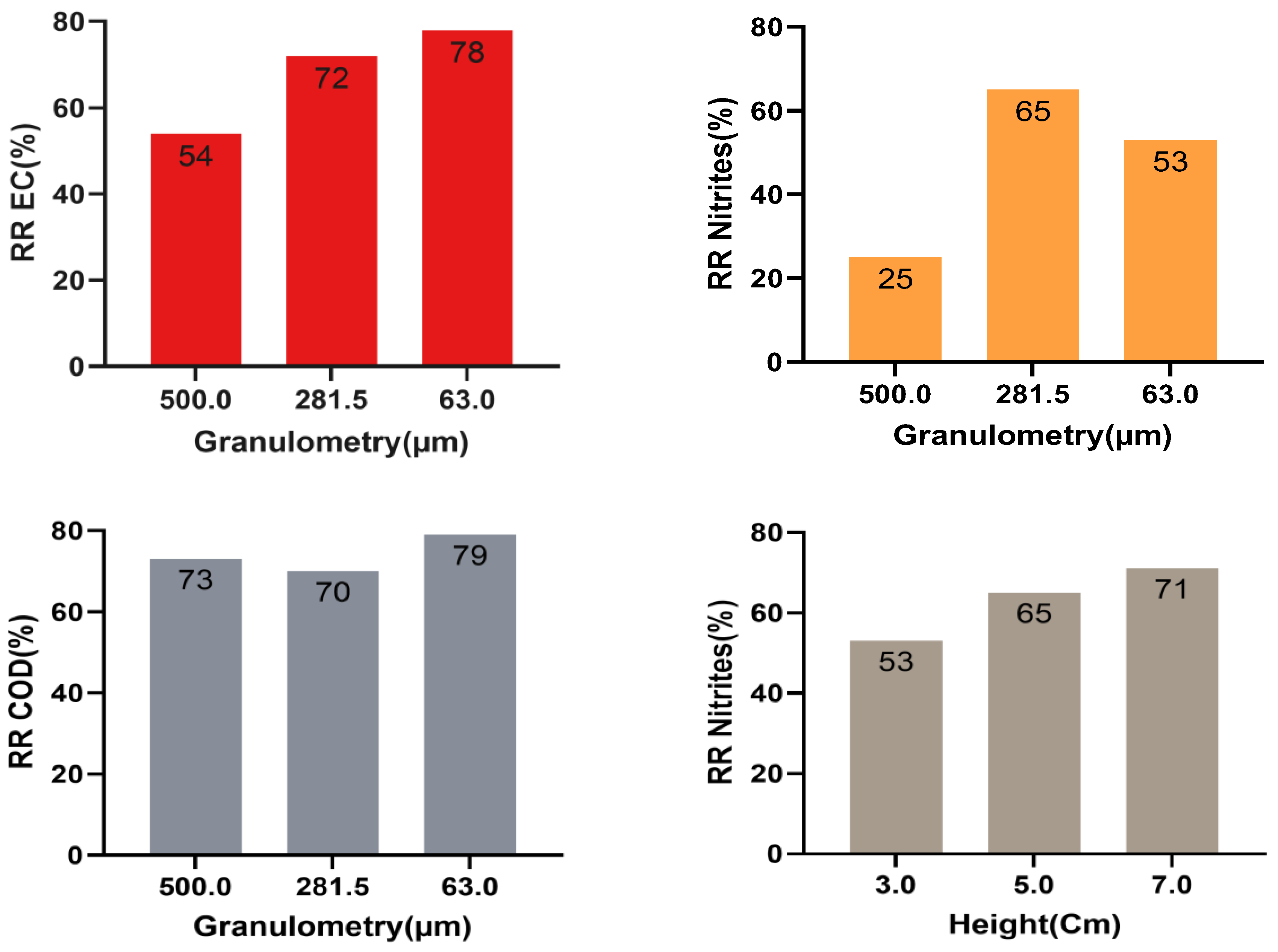
2.4. Effect of Almond Shell Particle Size on COD Reduction
2.5. Effect of the Granulometry of Almond Shell on the Decrease of Electrical Conductivity
2.6. Effect of Particle Size on Nitrite Removal
2.7. Effect of Almond Shell Size on Effluent Absorbance
2.8. Effect of Height on Parametric Reduction
2.9. Continuous Adsorption Modelling Using the Experimental Design Technique
2.9.1. Box-Behnken and Statistical Analysis
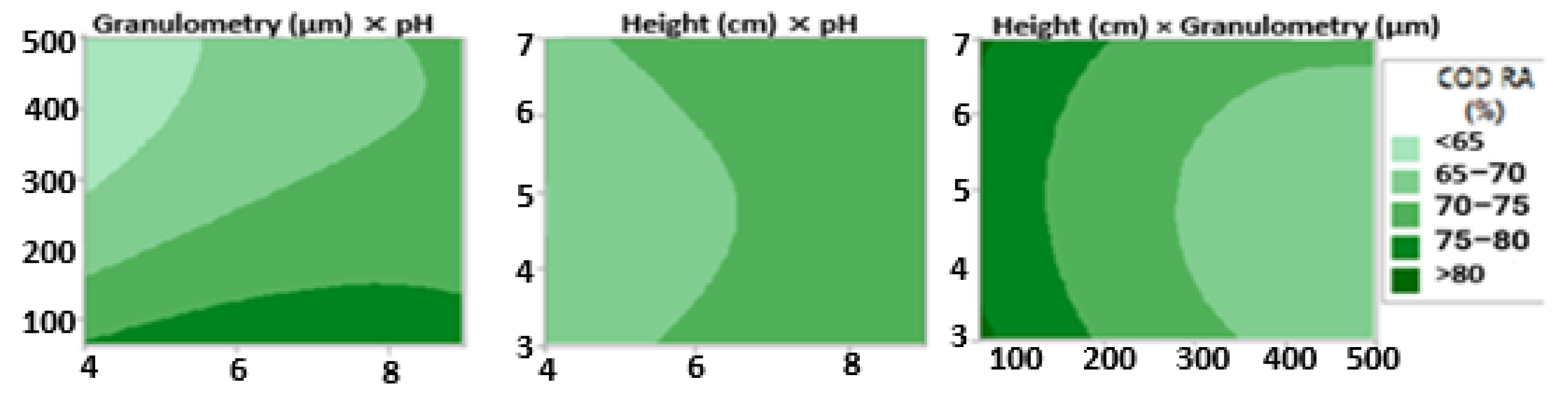
2.9.2. Effects of Variables on the Parametric Reduction Percent
3. Materials and Methods
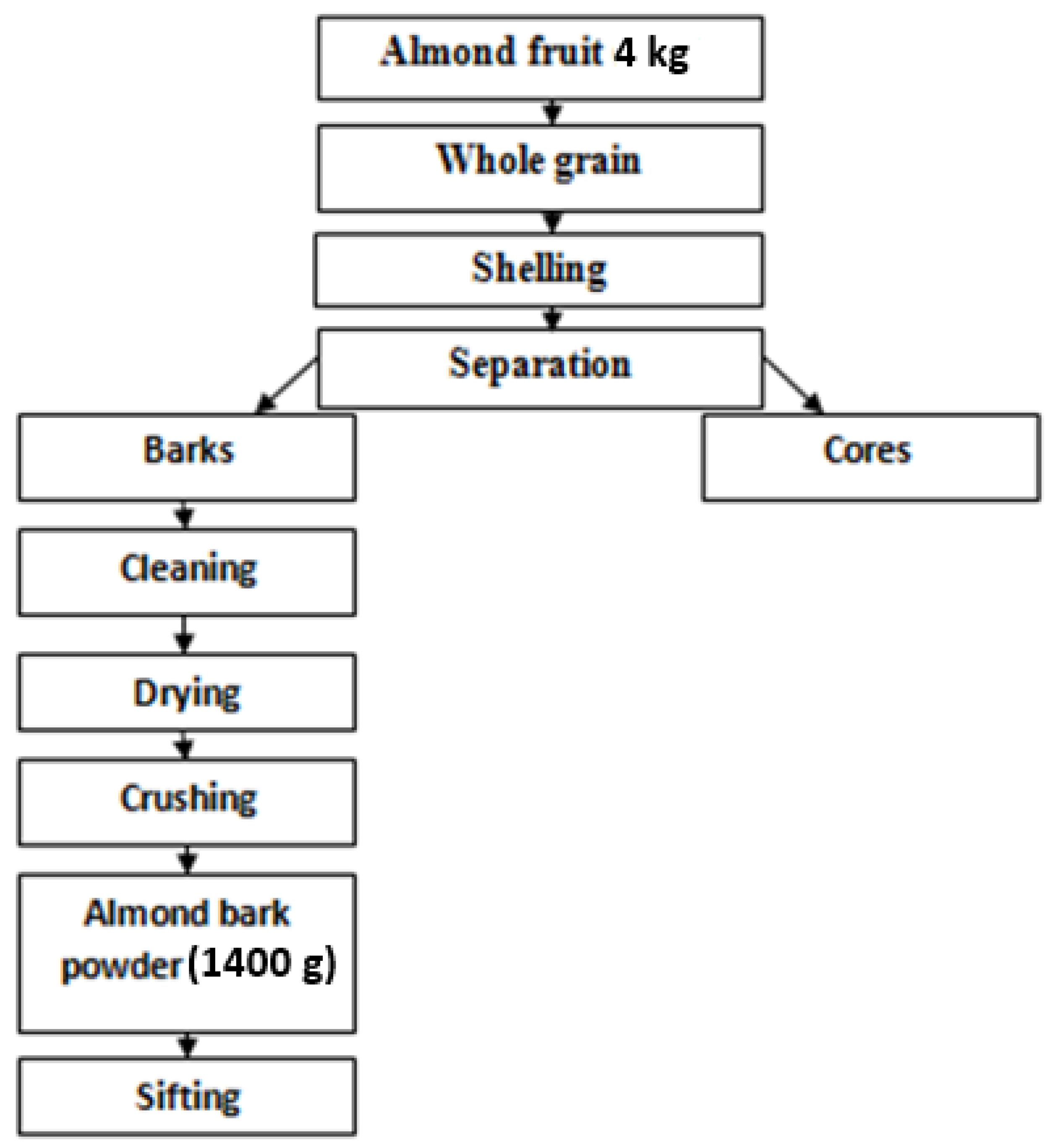
3.1. Materials
3.2. Methods
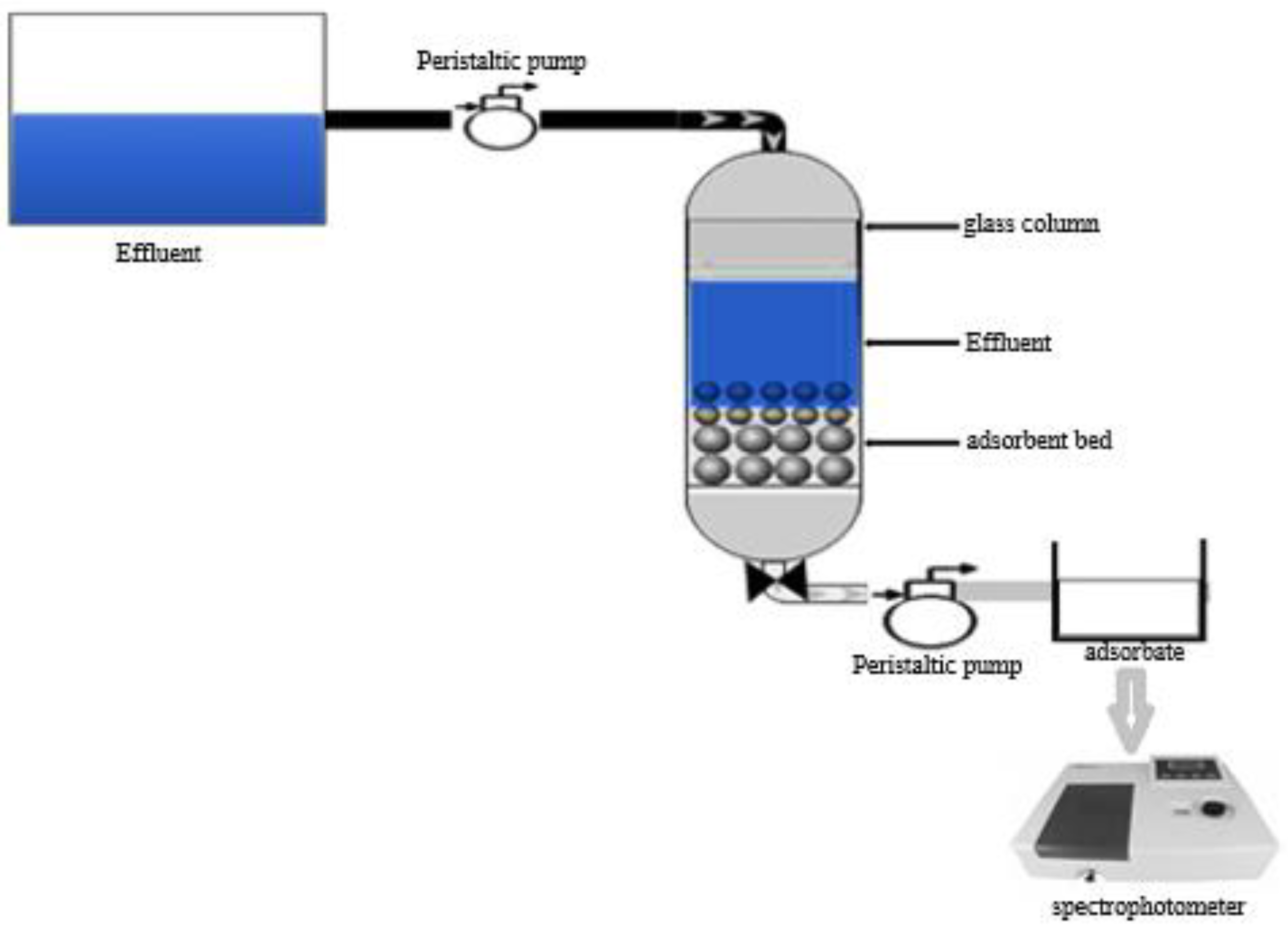
3.2.1. Fixed Bed Column Adsorption Treatment
3.2.2. Optimization of Continuous Adsorption Using the Design of Experiments Technique
3.2.3. Fixed Bed Column Adsorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ait Kadi, M.; Ziyad, A. Integrated water resources management in morocco. Water Resour. Dev. Manag. 2018, 20, 143–163. [Google Scholar]
- El Mouhri, G.; Merzouki, M.; Kachkoul, R.; Belhassan, H.; Miyah, Y.; Amakdouf, H.; Elmountassir, R.; Lahrichi, A. Fixed-bed adsorption of tannery wastewater pollutants using bottom ash: An optimized process. Surf. Interfaces 2020, 22, 100868. [Google Scholar] [CrossRef]
- Melhaoui, R.; Miyah, Y.; Kodad, S.; Houmy, N.; Addi, M.; Abid, M.; Mihamou, A.; Serghini-Caid, H.; Lairini, S.; Tijani, N.; et al. On the Suitability of Almond Shells for the Manufacture of a Natural Low-Cost Bioadsorbent to Remove Brilliant Green: Kinetics and Equilibrium Isotherms Study. Study. Sci. World J. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tulun, Ş.; Şimşek, İ.; Bahadır, T.; Çelebi, H. Investigation of removal of anthocyanin in turnip juice wastewater by using different adsorbents. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Yahya, M.D.; Obayomi, K.S.; Abdulkadir, M.B.; Iyaka, Y.A.; Olugbenga, A.G. Characterization of cobalt ferrite-supported activated carbon for removal of chromium and lead ions from tannery wastewater via adsorption equilibrium. Water Sci. Eng. 2020, 13, 202–213. [Google Scholar] [CrossRef]
- Jawad, A.H.; Malek, N.N.A.; Abdulhameed, A.S.; Razuan, R. Synthesis of magnetic chitosan-fly ash/Fe3O4 composite for adsorption of reactive Orange 16 dye: Optimization by Box–Behnken design. J. Polym. Environ. 2020, 28, 1068–1082. [Google Scholar] [CrossRef]
- Gaga, Y.; Benmessaoud, S.; Kara, M.; Assouguem, A.; Al-Ghamdi, A.A.; Al-Hemaid, F.M.; Elshikh, M.S.; Ullah, R.; Banach, A.; Bahhou, J. New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment. Water 2022, 14, 3319. [Google Scholar] [CrossRef]
- Elmountassir, R.; Bennani, B.; Miyah, Y.; Fegousse, A.; el Mouhri, G.; Oumokhtar, B.; Khatouf, M.; Elkarrach, K.; Touimi, G.B.; Lahrichi, A. Microbiological and Physicochemical Characterization of Hospital Effluents before and after Treatment with Two Types of Sawdust. J. Chem. 2019, 2019, 327510. [Google Scholar] [CrossRef]
- Aliyu, A. Synthesis, electron microscopy properties and adsorption studies of Zinc(II) ions (Zn2+) onto as-prepared Carbon Nanotubes (CNTs) using Box-Behnken Design (BBD). Sci. African 2019, 3, e00069. [Google Scholar] [CrossRef]
- Arida, C.V.J.; De Luna, M.D.G.; Futalan, C.M. Optimization of As(V) removal using chitosan-coated bentonite from groundwater using Box—Behnken design effects of adsorbent mass, flow rate, and initial concentration. Desalination Water Treat. 2015, 57, 3994. [Google Scholar] [CrossRef]
- Sithole, N.T.; Ntuli, F.; Okonta, F. Fixed bed column studies for decontamination of acidic mineral effluent using porous fly ash-basic oxygen furnace slag based geopolymers. Miner. Eng. 2018, 154, 106397. [Google Scholar] [CrossRef]
- Elmansouri, I.; Lahkimi, A.; Mansour, O.; Elouadrhiri, F.; Chaouch, M.; Eloutassi, N.; Elkhamar, F.; Bekkari, H. Study of the Operation of an Industrial Water Treatment Plant of the Northern Soft Drink CompanFez, Morocco. Ecol. Eng. Environ. Technol. 2022, 23, 227–232. [Google Scholar] [CrossRef]
- Dutta, J.; Kandhari, D. Efficacy of Almond Shells for Removal of Dye From Waste Water. EM Int. 2019, 37, 25–31. [Google Scholar]
- Yahya, M.D.; Abubakar, H.; Obayomi, K.S.; Iyaka, Y.A.; Suleiman, B. Results in Engineering Simultaneous and continuous biosorption of Cr and Cu (II) ions from industrial tannery effluent using almond shell in a fixed bed column. Results Eng. 2020, 6, 100113. [Google Scholar] [CrossRef]
- El Mouhri, G.; Merzouki, M.; Belhassan, H.; Miyah, Y.; Amakdouf, H.; Elmountassir, R.; Lahrichi, A. Continuous Adsorption Modeling and Fixed Bed Column Studies: Adsorption of Tannery Wastewater Pollutants Using Beach Sand. J. Chem. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Cemalgil, S.; Onat, O.; Tanaydın, M.K.; Etli, S. Effect of waste textile dye adsorbed almond shell on self-compacting mortar. Constr. Build. Mater. 2021, 300, 123978. [Google Scholar] [CrossRef]
- Berrada, S.; Squalli, F.Z.; Squalli, H.T.; Hannin, M.; El Oualti, A.; El Ouali Lalami, A. Recycling of the effluents of the haemodialysis service of the Al Ghassani hospital in the city of Fez: Characterization before and after treatment. J. Mater. Environ. Sci. 2014, 5, 2265–2277. [Google Scholar]
- Kumari, U.; Biswas, S.; Meikap, B.C. Environmental Technology & Innovation Defluoridation characteristics of a novel adsorbent developed from ferroalloy electric arc furnace slag: Batch, column study and treatment of industrial wastewater. Environ. Technol. Innov. 2020, 18, 100782. [Google Scholar]
- Prasanna, L.; Rao, K.; Chang, Y. Chemosphere Process optimization and modeling of lead removal using iron oxide nanocomposites generated from bio-waste mass. Chemosphere 2020, 243, 125257. [Google Scholar]
- Peter, C.; Olufemi, G.; Zhang, Q.; Zhu, G.; Wang, C.; Guo, Q. Aqueous scavenging of polycyclic aromatic hydrocarbons using epichlorohydrin, diphenyl diisocyanate modified starch: Pollution remediation approach. Arab. J. Chem. 2019, 12, 2760–2773. [Google Scholar]
- Elassassi, Z.; Ougrad, I.; Bedoui, I.; Kara, M.; El Bouch, M.; Assouguem, A.; Fadli, M.; Almeer, R.; Mohamed, H.R.H.; Peluso, I.; et al. Spatial and Temporal Variations of the Water Quality of the Tiflet River, Province of Khemisset, Morocco. Water 2022, 14, 1829. [Google Scholar] [CrossRef]
- Jafari, A.; Mahvi, A.H.; Nasseri, S.; Rashidi, A.; Nabizadeh, R.; Rezaee, R. Environmental Health Ultrafiltration of natural organic matter from water by vertically aligned carbon nanotube membrane. J. Environ. Health Sci. Eng. 2015, 13, 1–9. [Google Scholar]
- Sugashini, S.; Begum, K.M.M.S. Column Adsorption Studies for the Removal of Cr (VI) Ions by thylamine Modied Chitosan Carbonized Riceus Composite Beads with Modelling and Optimization. J. Chem. 2013, 2013, 460971. [Google Scholar] [CrossRef]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; El Hamri, R.; Taitai, A. Applied Surface Science Removal of fluoride from aqueous solution by adsorption on Apatitic tricalcium phosphate using Box—Behnken design and desirability function. Appl. Surf. Sci. 2012, 258, 4402–4410. [Google Scholar] [CrossRef]
- Reske, G.D.; da Rosa, B.C.; Visioli, L.J.; Dotto, G.L.; De Castilhos, F. Intensification of Ni(II) adsorption in a fixed bed column through subcritical conditions. Chem. Eng. Process. Process Intensif. 2020, 149, 107863. [Google Scholar] [CrossRef]
- Dwivedi, N.; Balomajumder, C.; Mondal, P. Study for the treatment of Cyanide bearing Wastewater using Bioadsorbent Prunus Amygdalus (Almond shell): Effect of pH, adsorbent dose, Contact Time, Temperature, and initial Cyanide concentration. Int. Res. J. Environ. Sci. 2014, 3, 23–30. [Google Scholar]
- Akazdam, S.; Chafi, M.; Yassine, W.; Sebbahi, L.; Gourich, B.; Barka, N. Adsorption on NaOH treated eggshells: Batch and fixed bed column study using Response Surface Methodology Decolourization of Cationic and Anionic Dyes from Aqueous Solution by Adsorption on NaOH Treated Eggshells: Batch and Fixed Bed Column Study using response surface methodology. J. Mater. Env. Sci. 2017, 8, 784–800. [Google Scholar]
- Estevinho, B.N.; Ribeiro, E.; Alves, A.; Santos, L. A preliminary feasibility study for pentachlorophenol column sorption by almond shell residues. Chem. Eng. J. 2008, 136, 188–194. [Google Scholar] [CrossRef]
- Elmansouri, I.; Lahkimi, A.; Benaabou, M.; Chaouch, M.; Eloutassi, N.; Bekkari, H. Contribution to the Treatment of Urban Wastewater in the City of Fez by Coagulation and Flocculation Using a Biodegradable Reagent. J. Ecol. Eng. 2022, 23, 77–85. [Google Scholar] [CrossRef]
- Adachi, A.; Ouadrhiri, F.E.; Kara, M.; El Manssouri, I.; Assouguem, A.; Almutairi, M.H.; Bayram, R.; Mohamed, H.R.H.; Peluso, I.; Eloutassi, N.; et al. Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization. Catalysts 2022, 12, 665. [Google Scholar] [CrossRef]
- Rodier, J.; Legube, B.; Merle, N. Water Analysis, 9th ed.; DUNOD: Paris, France, 2009; p. 1600. [Google Scholar]
- Azoulay, K.; Bencheikh, I.; Moufti, A.; Dahchour, A.; Mabrouki, J.; El, S. Comparative study between static and dynamic adsorption efficiency of dyes by the mixture of palm waste using the central composite design. Chem. Data Collect. 2020, 27, 100385. [Google Scholar]
- Magdziarz, A.; Dalai, A.K.; Koziński, J.A. Chemical composition, character and reactivity of renewable fuel ashes. Fuel 2016, 176, 135–145. [Google Scholar] [CrossRef]
- Shi, R.; Li, J.; Jiang, J.; Mehmood, K.; Liu, Y.; Xu, R.; Qian, W. Characteristics of biomass ashes from different materials and their ameliorative effects on acid soils. JES 2016, 55, 294–302. [Google Scholar] [CrossRef] [PubMed]







| Ord Essai | Granulometry (µm) | Height (cm) | pH | COD RP (%) | EC RP (%) | RP Nitrite (%) |
|---|---|---|---|---|---|---|
| 1 | 281.5 | 7 | 9.0 | 73 | 73 | 43 |
| 2 | 63.0 | 3 | 6.5 | 79 | 78 | 53 |
| 3 | 281.5 | 5 | 6.5 | 70 | 72 | 65 |
| 4 | 63.0 | 7 | 6.5 | 82 | 79 | 71 |
| 5 | 500.0 | 5 | 4.0 | 60 | 45 | 21 |
| 6 | 281.5 | 5 | 6.5 | 70 | 72 | 65 |
| 7 | 281.5 | 7 | 4.0 | 66 | 72 | 37 |
| 8 | 63.0 | 5 | 9.0 | 78 | 78 | 56 |
| 9 | 500.0 | 3 | 6.5 | 67 | 48 | 25 |
| 10 | 281.5 | 3 | 4.0 | 68 | 63 | 56 |
| 11 | 281.5 | 5 | 6.5 | 70 | 72 | 65 |
| 12 | 500.0 | 7 | 6.5 | 73 | 54 | 25 |
| 13 | 500.0 | 5 | 9.0 | 70 | 52 | 15 |
| 14 | 281.5 | 3 | 9.0 | 75 | 76 | 43 |
| 15 | 63.0 | 5 | 4.0 | 76 | 64 | 53 |
| Source | F-Value | p-Value | Remarks |
|---|---|---|---|
| Model | 10.39 | 0.009 | Significant |
| pH (A) | 18.57 | 0.008 | Significant |
| Granulometry (µm) (B) | 55.63 | 0.001 | Significant |
| height (cm) © | 0.69 | 0.445 | Not significant |
| pH × pH (AA) | 2.85 | 0.152 | Not significant |
| Granulometry (mm) × Granulometry (mm) (BB) | 6.71 | 0.049 | Significant |
| height (cm) × height (cm) (CC) | 4.58 | 0.085 | Not significant |
| pH × Granulometry (mm) (AB) | 3.52 | 0.120 | Not significant |
| pH × height (cm) (AC) | 0.00 | 1.000 | Not significant |
| Granulometry (mm) × height (cm) (BC) | 0.49 | 0.513 | Not significant |
| Source | Value of F | Value of p | Remarks |
|---|---|---|---|
| Model | 54.67 | 0.000 | Significant |
| pH (A) | 40.83 | 0.001 | Significant |
| Granulometry (µm) (B) | 333.33 | 0.000 | Significant |
| height (cm) (C) | 5.63 | 0.064 | Not Significant |
| pH × pH (AA) | 8.86 | 0.031 | Significant |
| Granulometry (mm) × Granulometry (mm) (BB) | 84.25 | 0.000 | Significant |
| height (cm) × height (cm) (CC) | 3.94 | 0.104 | Not Significant |
| pH × Granulometry (mm) (AB) | 3.27 | 0.131 | Not Significant |
| pH × height (cm) (AC) | 9.60 | 0.027 | Significant |
| Granulometry (mm) × height (cm) (BC) | 1.67 | 0.253 | Not Significant |
| Source | Value of F | Value of p | Remarks |
|---|---|---|---|
| Model | 14.11 | 0.005 | Significant |
| pH (A) | 0.36 | 0.574 | Not Significant |
| Granulometry (µm) (B) | 77.95 | 0.000 | Significant |
| height (cm) (C) | 0.00 | 0.954 | Not Significant |
| pH × pH (AA) | 20.15 | 0.006 | Significant |
| Granulometry (mm) × Granulometry (mm) (BB) | 23.98 | 0.004 | Significant |
| hauteur(cm) × height (cm) (CC) | 4.50 | 0.087 | Not Significant |
| pH × Granulometry (mm) (AB) | 0.58 | 0.479 | Not Significant |
| pH × height (cm) (AC) | 2.60 | 0.167 | Not Significant |
| Granulometry (mm) × height (cm) (BC) | 2.34 | 0.187 | Not Significant |
| Stations | Geographical Coordinates |
|---|---|
| Oued Elmehraz (Station 1) | Y02°97′23″ N X99°09′13″ W |
| Dokkarat (Station 2) | Y04°73′47″ N X02°45′03″W |
| Oued Fès (Station 3) | Y05°88′85″ N X98°99′48″ W |
| Medina Fès (Station 4) | Y06°65′80″ N X97°06′98″ W |
| Ain Nokbi (Station 5) | Y06°66′12″ N X95°25′04″ W |
| Parameters | Values | Methods |
|---|---|---|
| Absorbance | 4.98 | Spectrophotometer UV-Visible, type Hach lange DR 3900 |
| COD (mg of O2/L) | 1068 | COD meter type hanna HI839800 |
| BOD5 (mg of O2/L) | 260 | BOD metre type OXITOP |
| pH | 4.6 | Multiparameter HANNA HI 9829 |
| Nitrite (mg/L) | 32 | Sulphosalicylic acid method |
| Dissolved oxygen (mg of O2/L) | 1.5 | Multiparameter HANNA HI 9829 |
| TSS (mg /L) | 286 | Filtration method with suction pump |
| Temperature (°C) | 21 | Multiparameter HANNA HI 9829 |
| Electrical Conductivity (µs/cm) | 4600 | Multiparameter HANNA HI 9829 |
| Variables | Values | ||
|---|---|---|---|
| pH | 4 | 6.5 | 9 |
| Granulometry (mm) | 0.500 | 0.281 | 0.063 |
| Hauteur (cm) | 3 | 5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmansouri, I.; Lahkimi, A.; Kara, M.; Hmamou, A.; Mouhri, G.E.; Assouguem, A.; Chaouch, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; et al. A Continuous Fixed Bed Adsorption Process for Fez City Urban Wastewater Using Almond Shell Powder: Experimental and Optimization Study. Catalysts 2022, 12, 1535. https://doi.org/10.3390/catal12121535
Elmansouri I, Lahkimi A, Kara M, Hmamou A, Mouhri GE, Assouguem A, Chaouch M, Alrefaei AF, Kamel M, Aleya L, et al. A Continuous Fixed Bed Adsorption Process for Fez City Urban Wastewater Using Almond Shell Powder: Experimental and Optimization Study. Catalysts. 2022; 12(12):1535. https://doi.org/10.3390/catal12121535
Chicago/Turabian StyleElmansouri, Ibtissame, Amal Lahkimi, Mohammed Kara, Anouar Hmamou, Ghita El Mouhri, Amine Assouguem, Mehdi Chaouch, Abdulwahed Fahad Alrefaei, Mohamed Kamel, Lotfi Aleya, and et al. 2022. "A Continuous Fixed Bed Adsorption Process for Fez City Urban Wastewater Using Almond Shell Powder: Experimental and Optimization Study" Catalysts 12, no. 12: 1535. https://doi.org/10.3390/catal12121535
APA StyleElmansouri, I., Lahkimi, A., Kara, M., Hmamou, A., Mouhri, G. E., Assouguem, A., Chaouch, M., Alrefaei, A. F., Kamel, M., Aleya, L., Abdel-Daim, M. M., Eloutassi, N., Adachi, A., & Bekkari, H. (2022). A Continuous Fixed Bed Adsorption Process for Fez City Urban Wastewater Using Almond Shell Powder: Experimental and Optimization Study. Catalysts, 12(12), 1535. https://doi.org/10.3390/catal12121535













