Effects of Silicalite-1 Coating on the p-Xylene Selectivity and Catalytic Stability of HZSM-5 in Toluene Methylation with Methanol
Abstract
:1. Introduction
2. Results and Discussion
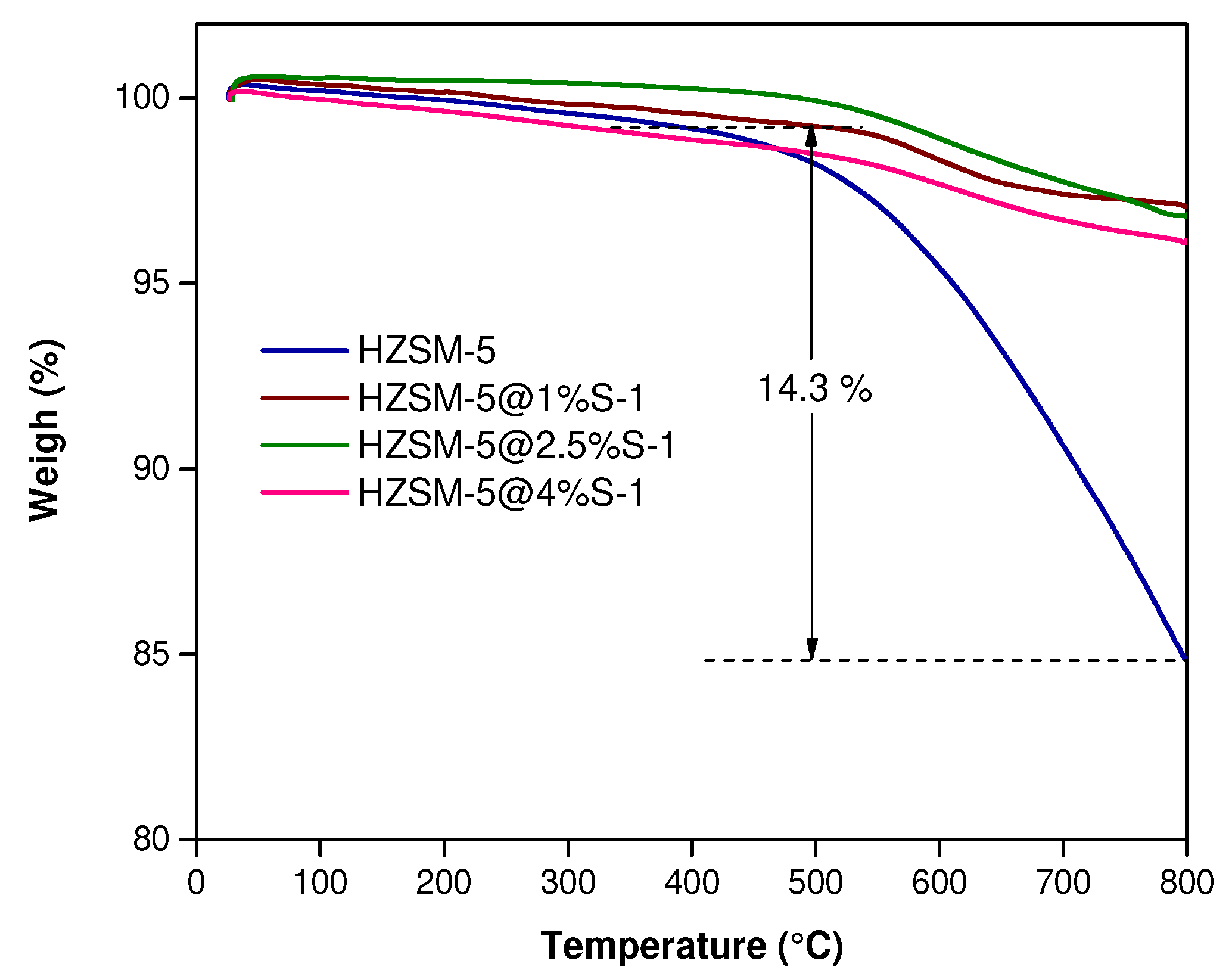
2.1. Catalysts Characterization
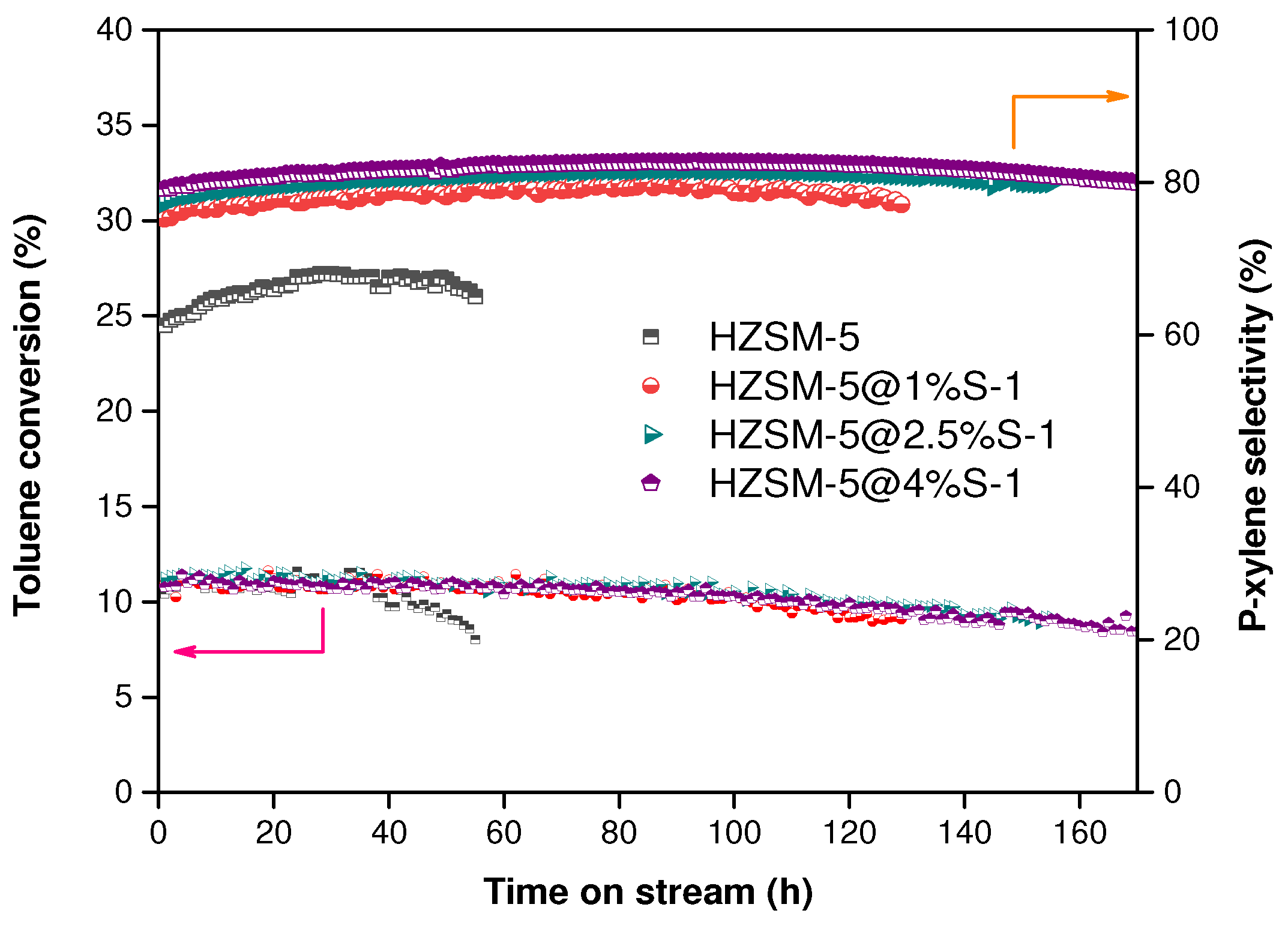
2.2. Catalytic Performance
3. Materials and Methods
3.1. Preparation of Parent HZSM-5 (Si/Al = 150)
3.2. Preparation of HZSM-5@Silicalite-1 Catalysts
3.3. Catalyst Characterization
3.4. Catalyst Evaluation Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Zhang, J.; Dong, P.; Li, G.; Fan, X.; Yang, Y. Novel Short Process for p-Xylene Production Based on the Selectivity Intensification of Toluene Methylation with Methanol. ACS Omega 2022, 7, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Chakinala, N.; Chakinala, A.G. Process Design Strategies to Produce P-Xylene via Toluene Methylation: A Review. Ind. Eng. Chem. Res. 2021, 60, 5331–5351. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, J.; Yang, B. Identifying the key steps determining the selectivity of toluene methylation with methanol over HZSM-5. Nat. Commun. 2021, 12, 3725. [Google Scholar] [CrossRef] [PubMed]
- Ulyev, L.M.; Kanischev, M.V.; Chibisov, R.E.; Vasilyev, M.A. Heat Integration of an Industrial Unit for the Ethylbenzene Production. Energies 2021, 14, 3839. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing Para-Xylene Selectivity in Making Aromatics from Methanol with a Surface-Modified Zn/P/ZSM-5 Catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Wang, G.; Wang, P.; Meng, Z.; Li, C. The origin of the activity and selectivity of silicalite-1 zeolite for toluene methylation to para-xylene. Chem. Eng. J. 2017, 327, 278–285. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Huang, X.; Zhu, Y.; Li, G.; Gu, Q.; Chen, J.; Ma, L.; Li, X.; He, Q.; et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene. Nat. Commun. 2019, 10, 4348. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wang, R.; Pan, X.; Wang, C.; Fan, C.; Zhu, Y.; Wang, Y.; Peng, Y. Catalyst design strategies towards highly shape-selective HZSM-5 for para-xylene through toluene alkylation. Green Energy. Environ. 2020, 5, 385–393. [Google Scholar] [CrossRef]
- Cuong, M.; Nguyen; Reyniers, M.; Marin, G.B. Adsorption thermodynamics of C1–C4 alcohols in H-FAU, H-MOR, H-ZSM-5, and H-ZSM-22. J. Catal. 2015, 322, 91–103. [Google Scholar]
- Runnebaum, R.C.; Ouyang, X.; Edsinga, J.A.; Rea, T.; Arslan, I.; Hwang, S.; Zones, S.I.; Katz, A. Role of Delamination in Zeolite-Catalyzed Aromatic Alkylation: UCB-3 versus 3-D Al-SSZ-70. ACS Catal. 2014, 4, 2364–2368. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Fei, Z.; Li, L.; Feng, X.; Ji, W.; Ding, W.; Chen, Y.; Yang, W.; Xie, Z. Effects of controlled SiO2 deposition and phosphorus and nickel doping on surface acidity and diffusivity of medium and small sized HZSM-5 for para-selective alkylation of toluene by methanol. Appl. Catal. A 2013, 453, 302–309. [Google Scholar] [CrossRef]
- Kaeding, W.W.; Chu, C.; Young, L.B.; Weinstein, B.; Butter, S.A. Selective alkylation of toluene with methanol to produce para-Xylene. J. Catal. 1981, 67, 159–174. [Google Scholar] [CrossRef]
- Li, G.; Wu, C.; Ji, D.; Dong, P.; Zhang, Y.; Yong, Y. Acidity and catalyst performance of two shape-selective HZSM-5 catalysts for alkylation of toluene with methanol. Reac. Kinet. Mech. Cat. 2020, 129, 963–974. [Google Scholar] [CrossRef]
- Tan, W.; Liu, M.; Zhao, Y.; Hou, K.; Wu, H.; Zhang, A.; Liu, H.; Wang, Y.; Song, C.; Guo, X. Para-selective methylation of toluene with methanol over nano-sized ZSM-5 catalysts: Synergistic effects of surface modifications with SiO2, P2O5 and MgO. Micropor. Mesopor. Mater. 2014, 196, 18–30. [Google Scholar] [CrossRef]
- Zheng, S.; Heydenrych, H.R.; Jentys, A.; Lercher, J.A. Influence of Surface Modification on the Acid Site Distribution of HZSM-5. J. Phys. Chem. B 2002, 106, 9552–9558. [Google Scholar] [CrossRef]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Ichikawa, S.; Egashira, Y.; Ueyama, K. Catalytic activities and structures of silicalite-1/H-ZSM-5 zeolite composites. Micropor. Mesopor. Mater. 2008, 115, 106–112. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kolvenbach, R.; Al-khattaf, S.S.; Jentys, A.; Lercher, J.A. Enhancing shape selectivity without loss of activity-novel mesostructured ZSM-5 catalysts for methylation of toluene to p-xylene. Chem. Commun. 2013, 49, 10584–10586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, Z.; Chen, Q.; Kong, D.; Li, W.; Wang, W.; Li, C. Chemical liquid deposition with polysiloxane of ZSM-5 and its effect on acidity and catalytic properties. Micropor. Mesopor. Mater. 2007, 101, 169–175. [Google Scholar] [CrossRef]
- Kim, J.; Ishida, A.; Okajima, M.; Niwa, M. Modification of HZSM-5 by CVD of Various Silicon Compounds and Generation of Para-Selectivity. J. Catal. 1996, 161, 387–392. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Zhu, Y.; Zhang, D.; Chen, J.; Chiang, F. P-Xylene selectivity enhancement in methanol toluene alkylation by separation of catalysis function and shape-selective function. Mol. Catal. 2017, 433, 242–249. [Google Scholar] [CrossRef]
- Nishiyama, N.; Miyamoto, M.; Egashira, Y.; Ueyama, K. Zeolite membrane on catalyst particles for selective formation of p-xylene in the disproportionation of toluene. Chem. Commun. 2001, 18, 1746–1747. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, A.; Gumidyala, A.; Grabow, L.C.; Crossley, S.P.; Rimer, J.D. Epitaxial Growth of ZSM-5@Silicalite-1: A Core-Shell Zeolite Designed with Passivated Surface Acidity. ACS Nano 2015, 9, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Long, Y.; Wang, Z. Optimization of conditions for preparation of ZSM-5@silicalite-1 core–shell catalysts via hydrothermal synthesis. Chin. J. Chem. Eng. 2018, 26, 2070–2076. [Google Scholar]
- Miyamoto, M.; Kamei, T.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Single Crystals of ZSM-5/Silicalite Composites. Adv. Mater. 2005, 17, 1985–1988. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, W.; Lv, H.; Zhang, Y.; Au, C.; Yin, S. Synthesis of HZSM-5@silicalite-1 core-shell composite and its catalytic application in the generation of p-xylene by methylation of toluene with methyl bromide. RSC Adv. 2014, 4, 37296–37301. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kolvenbach, R.; Neudeck, C.; Al-khattaf, S.S.; Jentys, A.; Lercher, J.A. Tailoring mesoscopically structured H-ZSM5 zeolites for toluene methylation. J. Catal. 2014, 311, 271–280. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Perez-Ramirez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Micropor. Mesopor. Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Althoff, R.; Schulz-Dobrick, B.; Schuth, F.; Unger, K. Controlling the spatial distribution of aluminum in ZSM-5 crystals. Micropor. Mater. 1993, 1, 207–218. [Google Scholar] [CrossRef]
- Luan, H.; Lei, C.; Ma, Y.; Wu, Q.; Zhu, L.; Xu, H.; Han, S.; Zhu, Q.; Liu, X.; Meng, X.; et al. Alcohol-assisted synthesis of high-silica zeolites in the absence of organic structure-directing agents. Chin. J. Catal. 2021, 42, 563–570. [Google Scholar] [CrossRef]
- Do, T.; Nossov, A.; Springuel-Huet, M.A.; Schneider, C.; Bretherton, J.L.; Fyfe, C.A.; Kaliaguine, S. Zeolite Nanoclusters Coated onto the Mesopore Walls of SBA-15. J. Am. Chem. Soc. 2004, 126, 14324–14325. [Google Scholar] [CrossRef]
- Zhao, S.; Collins, D.; Wang, L.; Huang, J. Influence of ZSM-5 porosity and binder introduction on the coke formation in the cracking of 1,3,5-triisopropylbenzene. Catal. Today 2021, 368, 211–216. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Xu, D.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation. Appl. Catal. B Env. 2021, 283, 119648. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ono, S.; Oumi, Y.; Uemiya, S.; Perre, S.V.; Virdis, T.; Baron, G.V.; Denayer, J.F.M. Nanoporous ZSM-5 Crystals Coated with Silicalite-1 for Enhanced p-Xylene Separation. ACS Appl. Nano Mater. 2019, 2, 2642–2650. [Google Scholar] [CrossRef]
- Breen, J.; Burch, R.; Kulkarni, M.; Collier, P.; Golunski, S. Enhanced Para-Xylene Selectivity in the Toluene Alkylation Reaction at Ultralow Contact Time. J. Am. Chem. Soc. 2005, 127, 5020–5021. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.-Y.; Liu, S.-B.; Wang, I. Enhanced para-selectivity by selective coking during toluene disproportionation over H–ZSM-5 zeolite. J. Catal. 1999, 185, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.H.; Chen, L.F.; Cao, Q.S.; Wen, Z.H.; Zhou, Z.; Xu, Y.R.; Zhu, X.D. Steamed Zn/ZSM-5 catalysts for improved methanol aromatization with high stability. Fuel Process. Technol. 2017, 162, 66–77. [Google Scholar] [CrossRef]

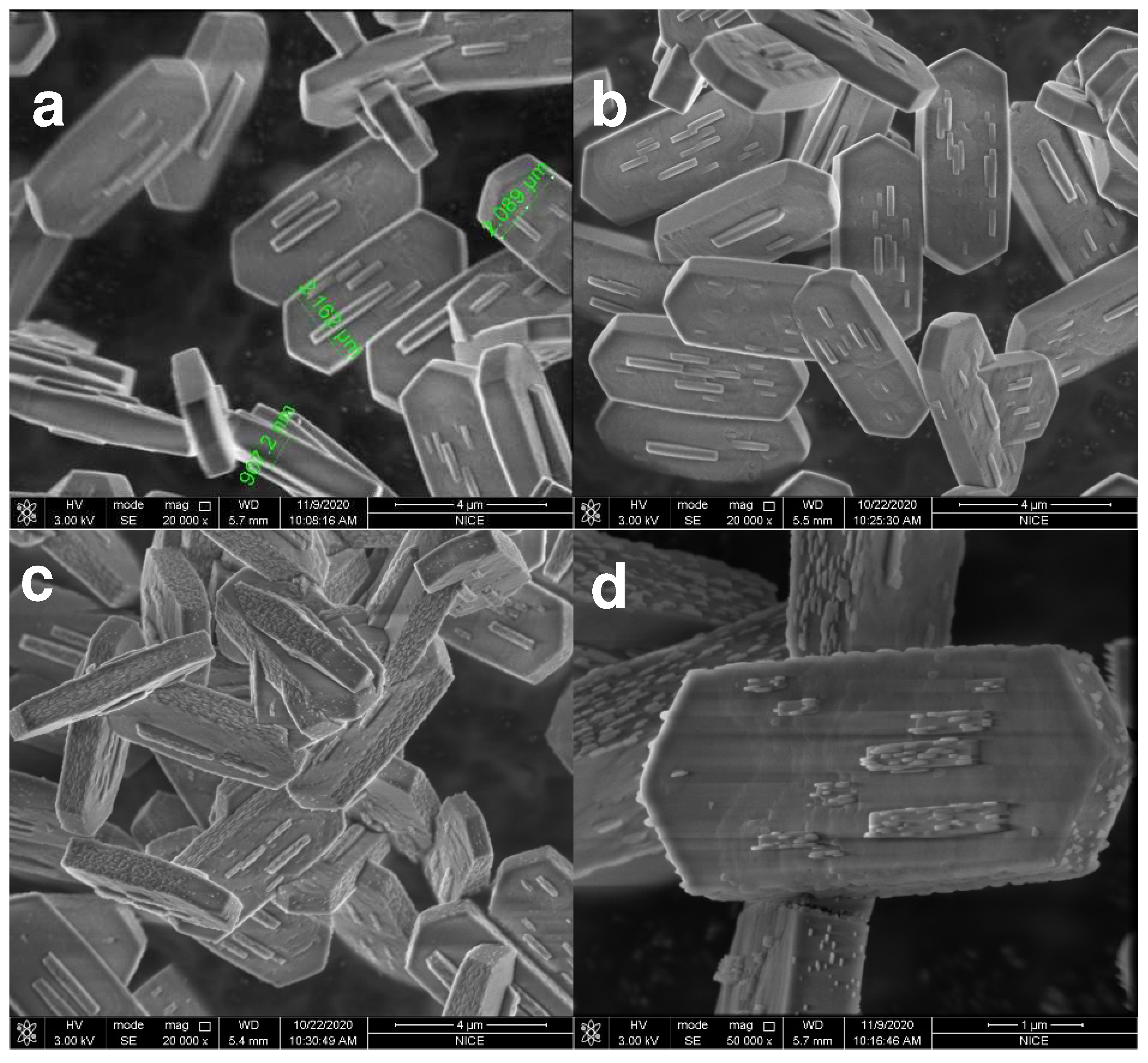

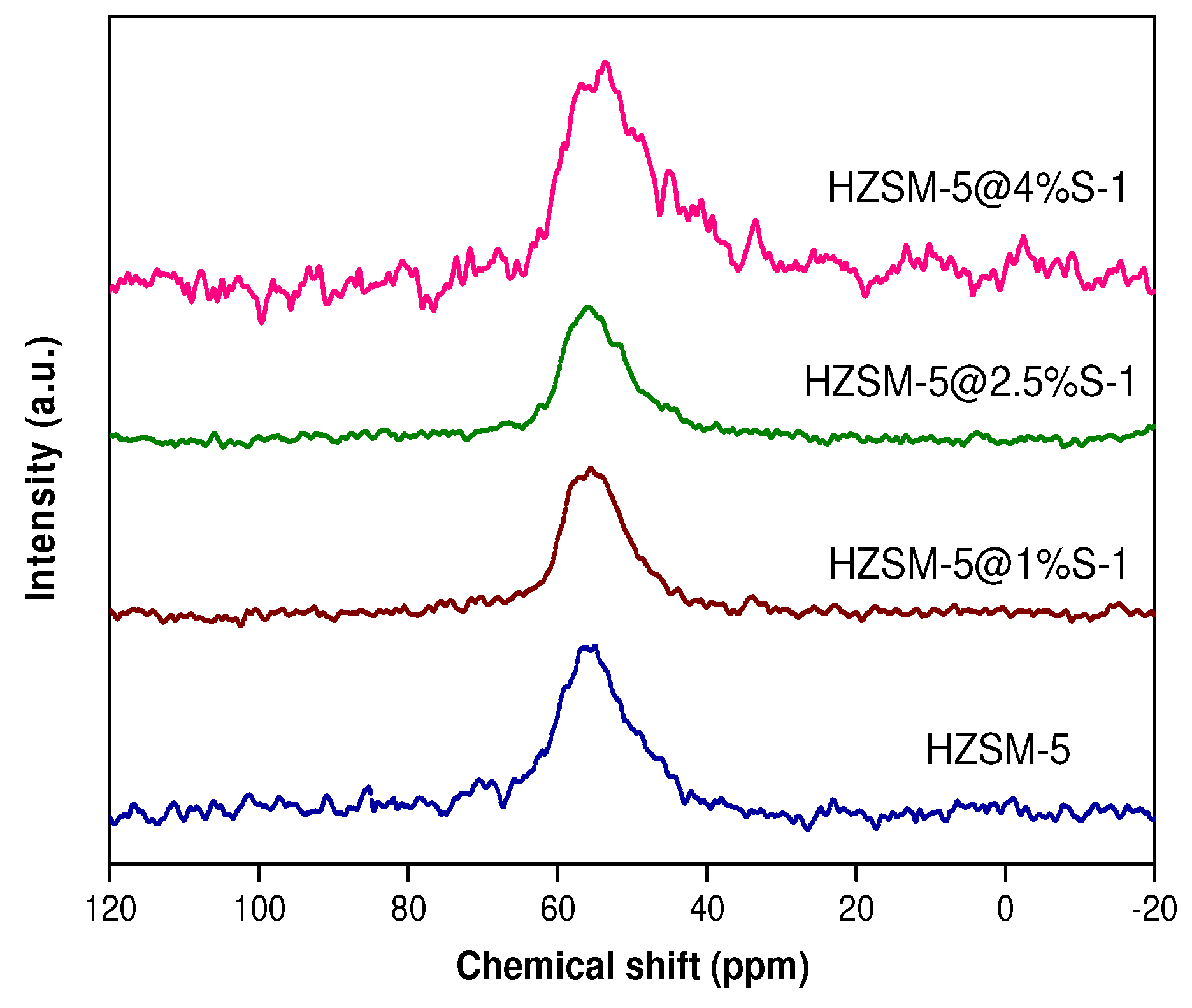

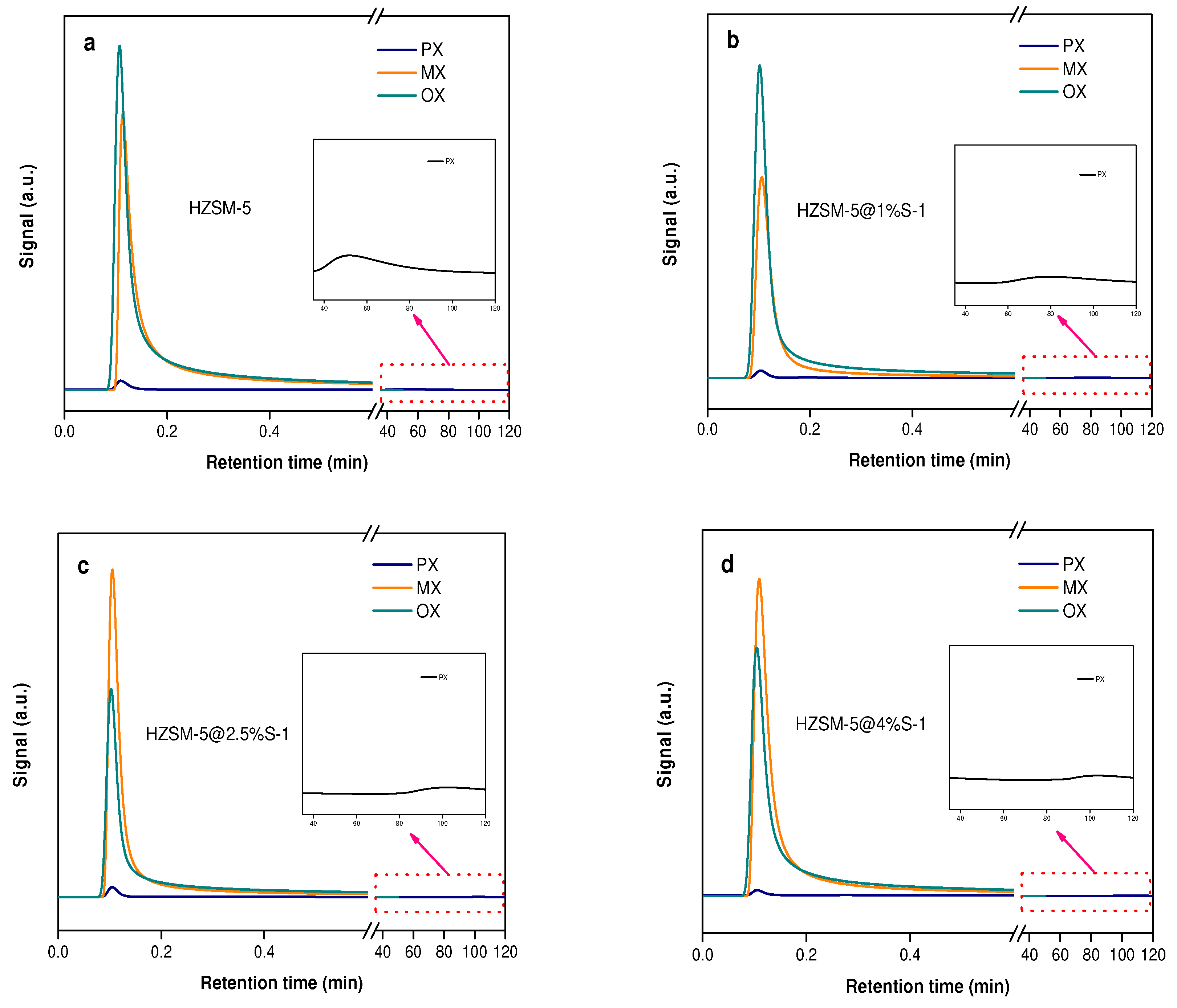
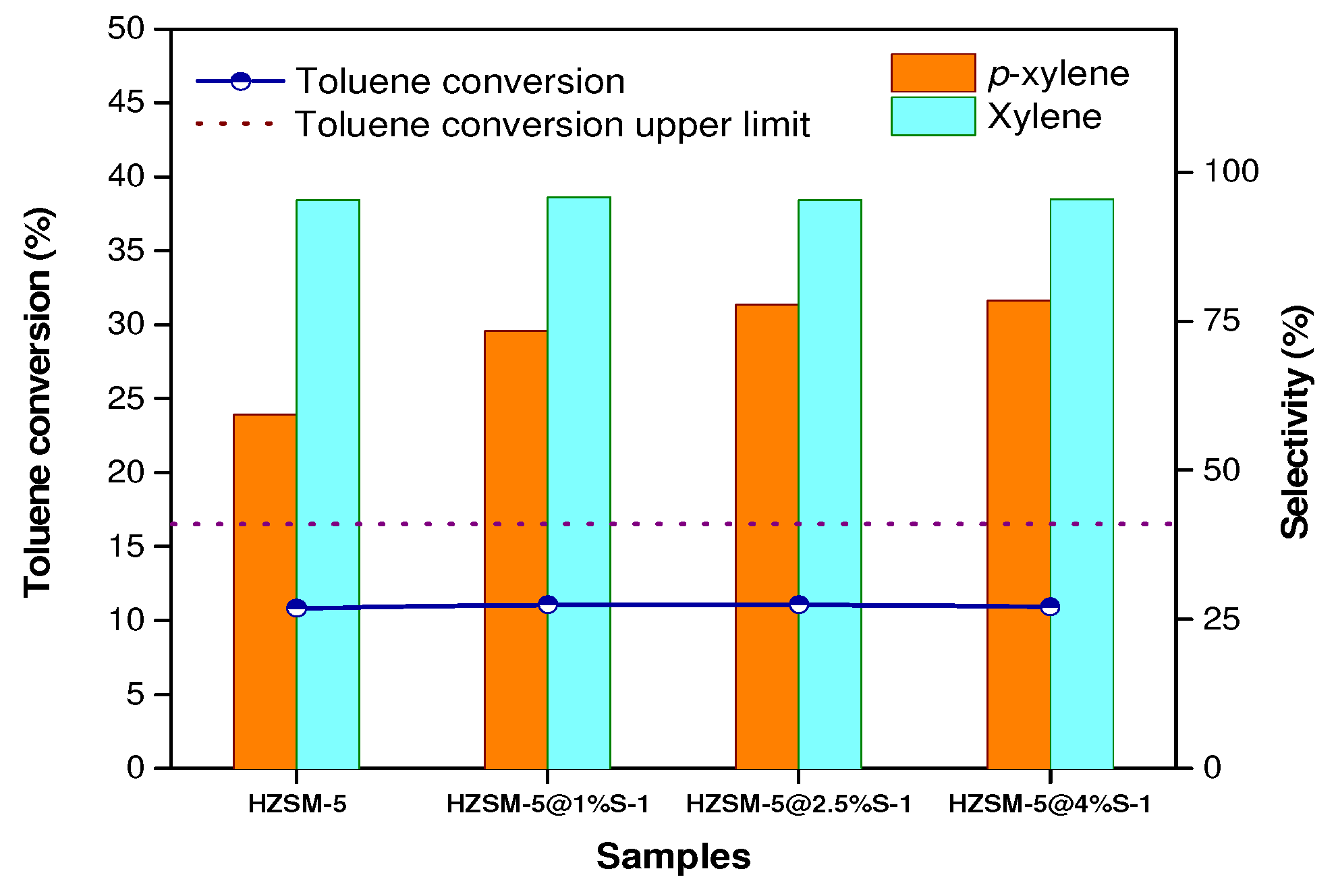
| Samples | SBET m2/g | Smicro m2/g | Sexternal m2/g | Vtotal cm3/g | Vmicro cm3/g | Vmeso cm3/g | Si/Al Ratio | |
|---|---|---|---|---|---|---|---|---|
| XRF | XPS | |||||||
| HZSM-5 | 352.6 | 269.3 | 83.3 | 0.19 | 0.11 | 0.08 | 186 | 72 |
| HZSM-5@1%S-1 | 297.2 | 198.2 | 99.0 | 0.16 | 0.07 | 0.09 | 195 | 118 |
| HZSM-5@2.5%S-1 | 348.7 | 253.5 | 95.2 | 0.19 | 0.10 | 0.09 | 196 | 128 |
| HZSM-5@4%S-1 | 347.1 | 255.0 | 92.1 | 0.20 | 0.11 | 0.09 | 195 | 152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Huang, X.; Wang, R.; Zhang, H.; Wei, H.; Chen, J.; Liu, S.; Sun, L.; Xu, D.; Liu, Y. Effects of Silicalite-1 Coating on the p-Xylene Selectivity and Catalytic Stability of HZSM-5 in Toluene Methylation with Methanol. Catalysts 2022, 12, 1538. https://doi.org/10.3390/catal12121538
Pan X, Huang X, Wang R, Zhang H, Wei H, Chen J, Liu S, Sun L, Xu D, Liu Y. Effects of Silicalite-1 Coating on the p-Xylene Selectivity and Catalytic Stability of HZSM-5 in Toluene Methylation with Methanol. Catalysts. 2022; 12(12):1538. https://doi.org/10.3390/catal12121538
Chicago/Turabian StylePan, Xu, Xin Huang, Ruizhuang Wang, Haiyong Zhang, Hui Wei, Jingyun Chen, Suyao Liu, Liping Sun, Deping Xu, and Yi Liu. 2022. "Effects of Silicalite-1 Coating on the p-Xylene Selectivity and Catalytic Stability of HZSM-5 in Toluene Methylation with Methanol" Catalysts 12, no. 12: 1538. https://doi.org/10.3390/catal12121538
APA StylePan, X., Huang, X., Wang, R., Zhang, H., Wei, H., Chen, J., Liu, S., Sun, L., Xu, D., & Liu, Y. (2022). Effects of Silicalite-1 Coating on the p-Xylene Selectivity and Catalytic Stability of HZSM-5 in Toluene Methylation with Methanol. Catalysts, 12(12), 1538. https://doi.org/10.3390/catal12121538







