SrMnO3/Functionalized h-BN Composite Modified Disposable Sensor for the Voltammetric Determination of Furaltadone Antibiotic Drug
Abstract
1. Introduction
2. Results and Discussion
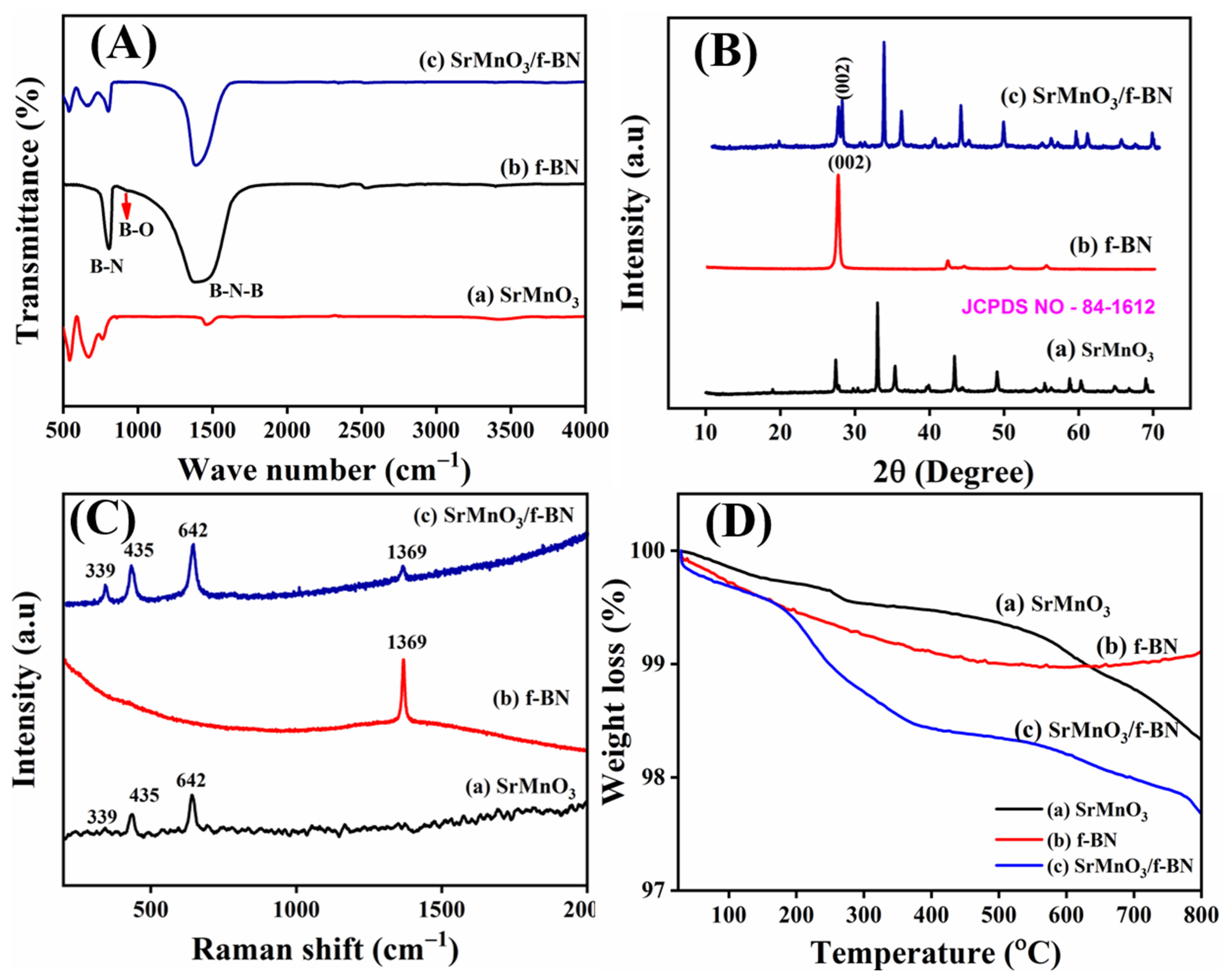
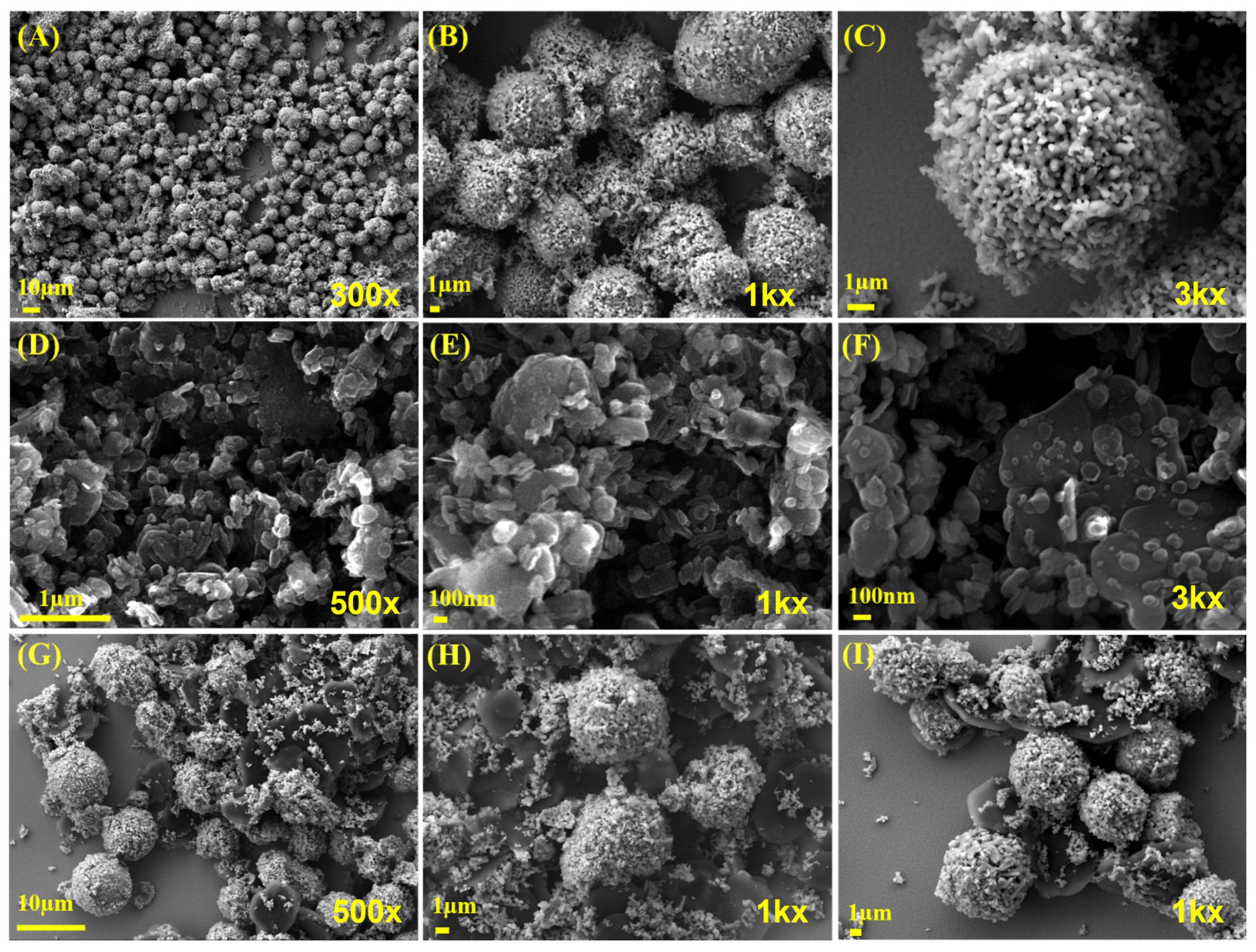
2.1. Materials Characterization
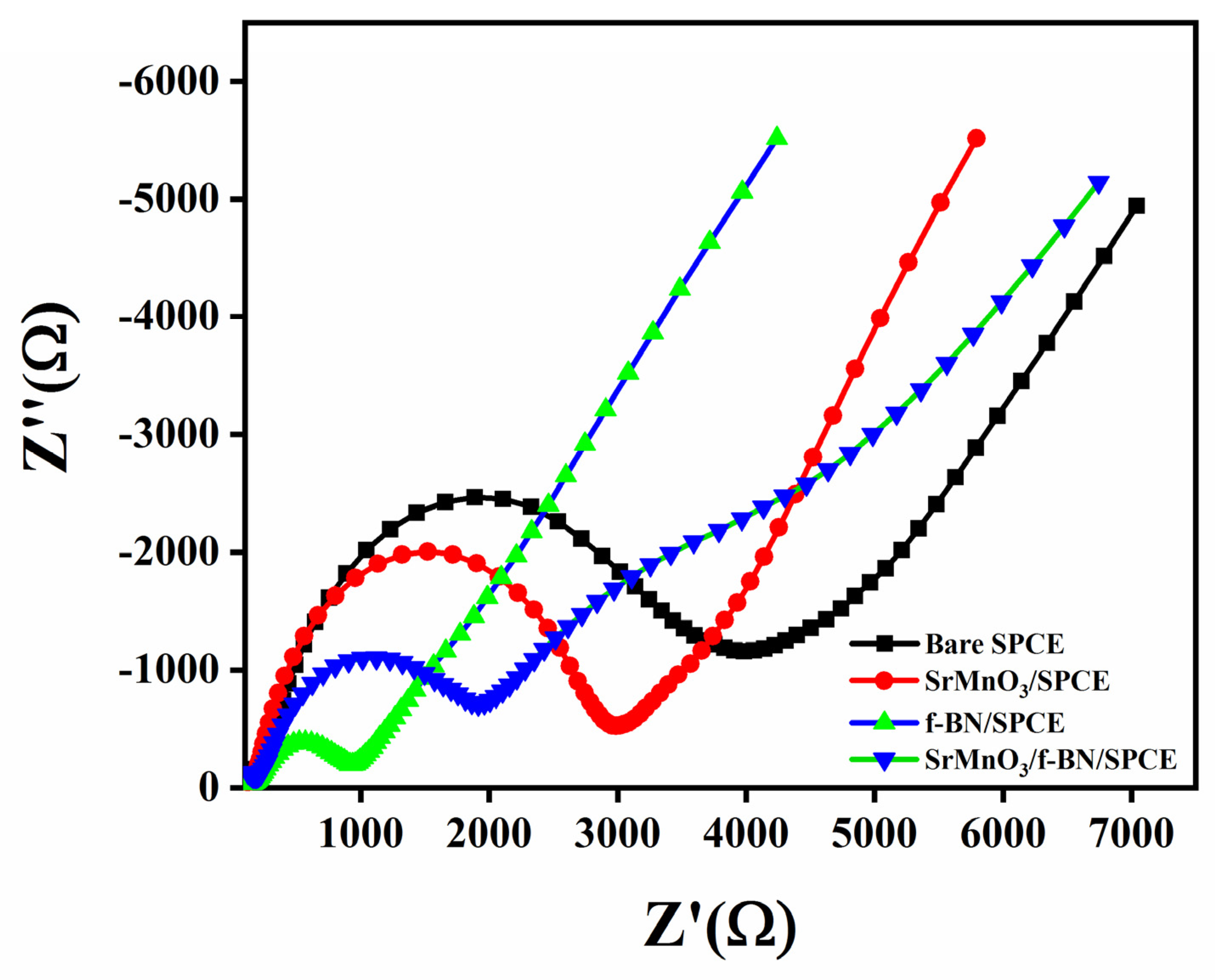
2.2. Electrochemical Properties
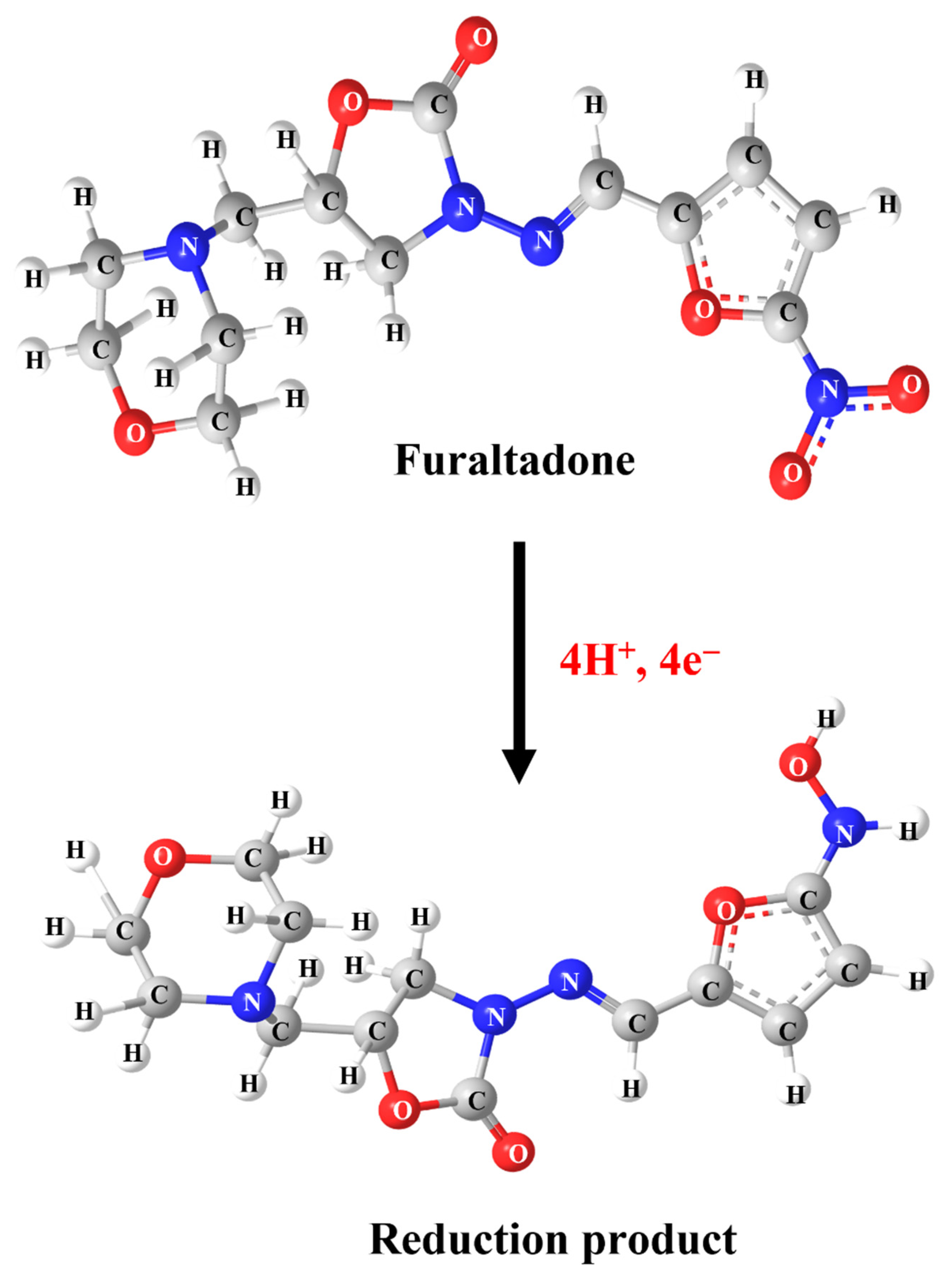
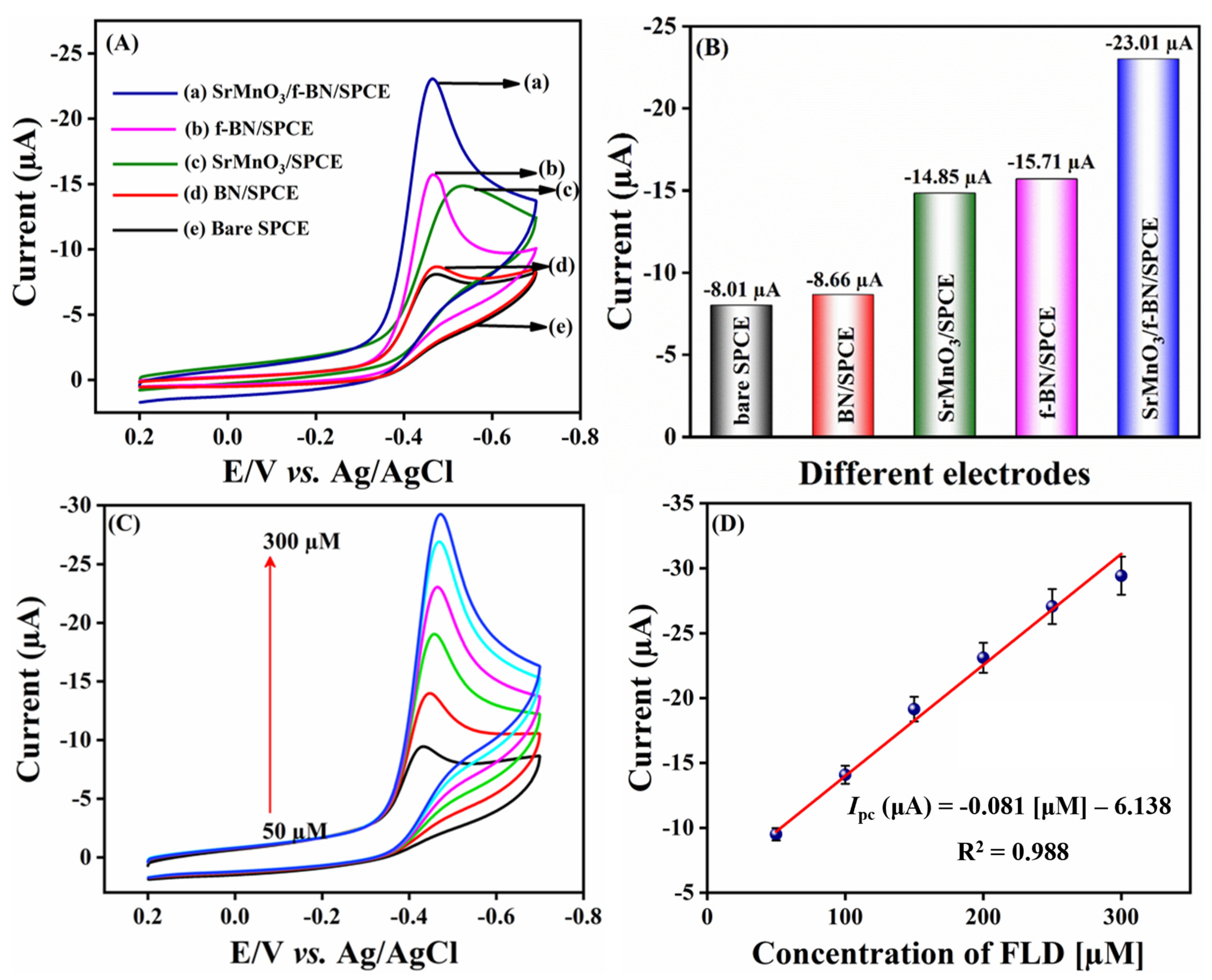
2.2.1. Electrochemical Behavior of FLD
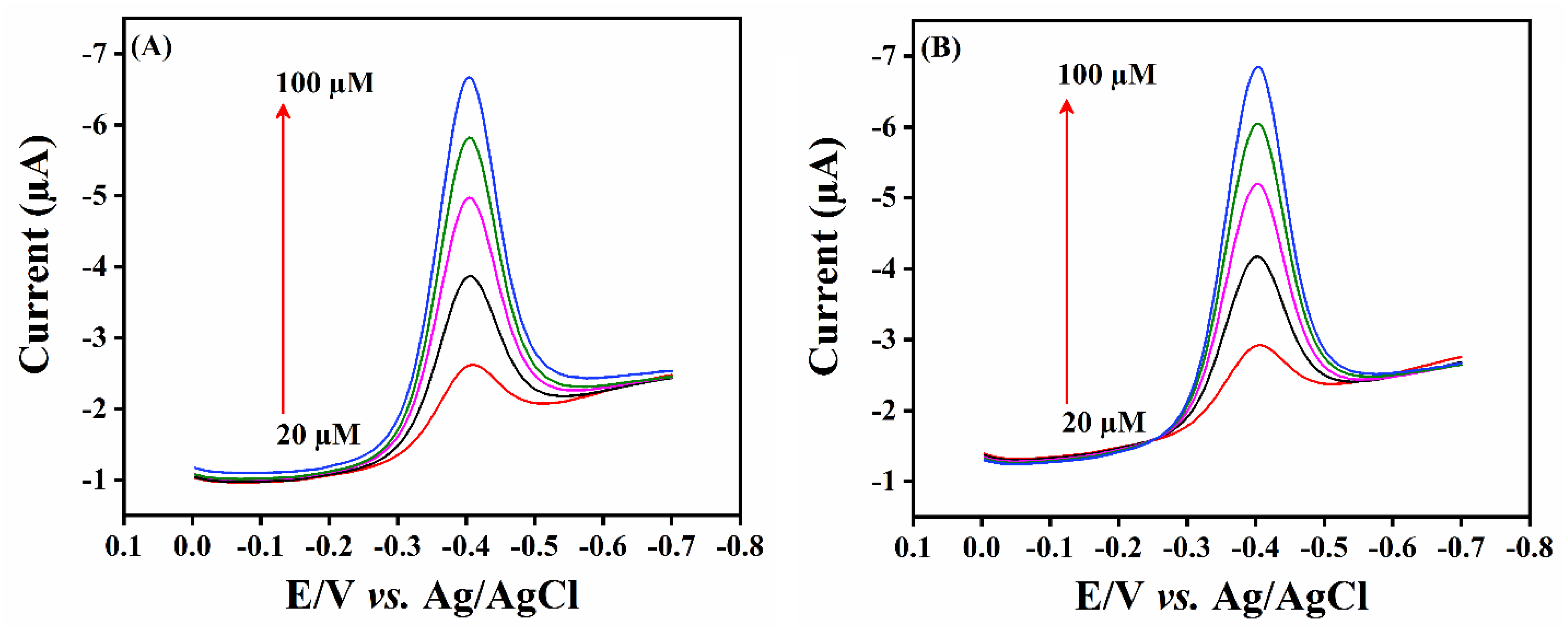
2.2.2. Effect of Concentration
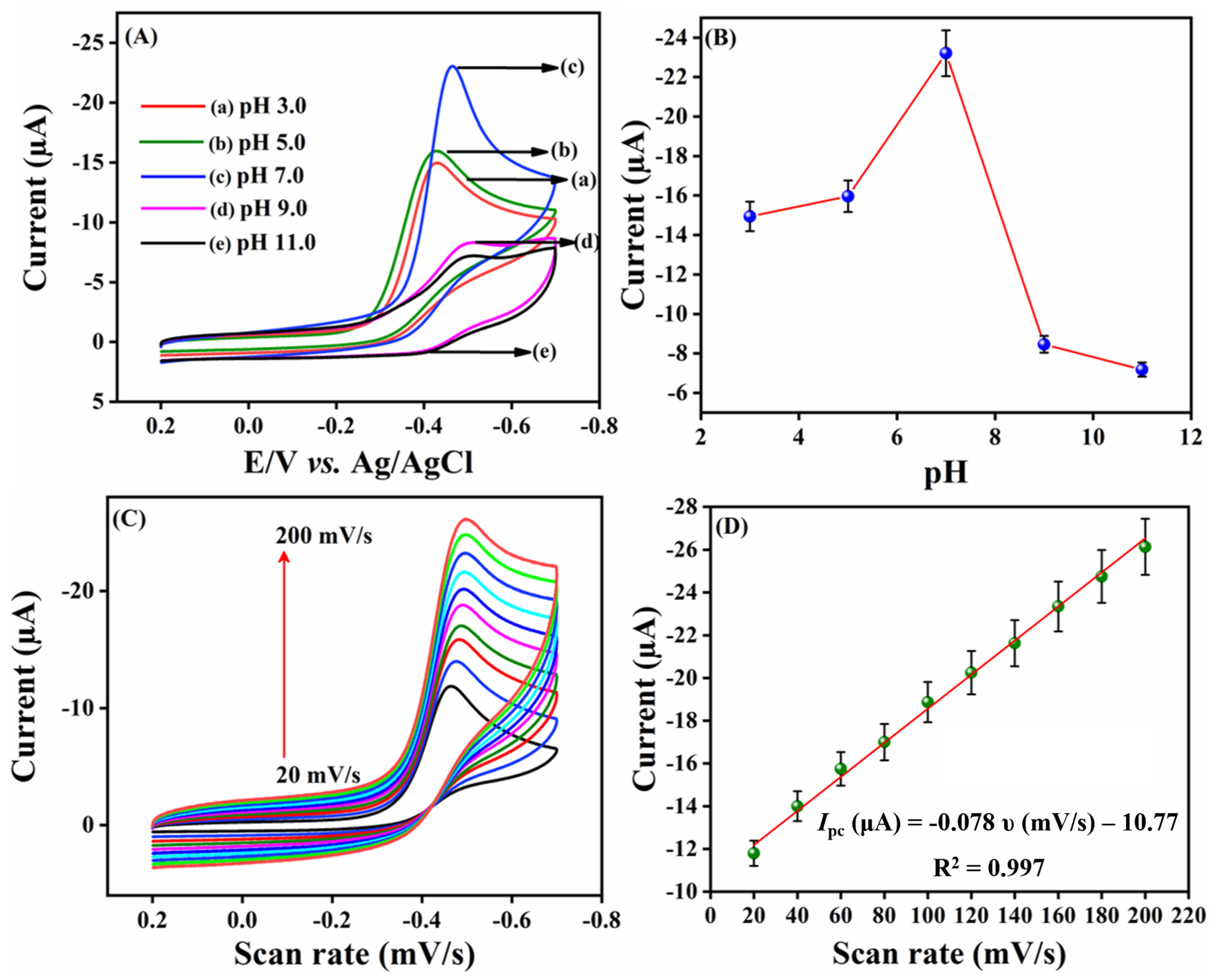
2.2.3. Effect of pH and Scan Rates
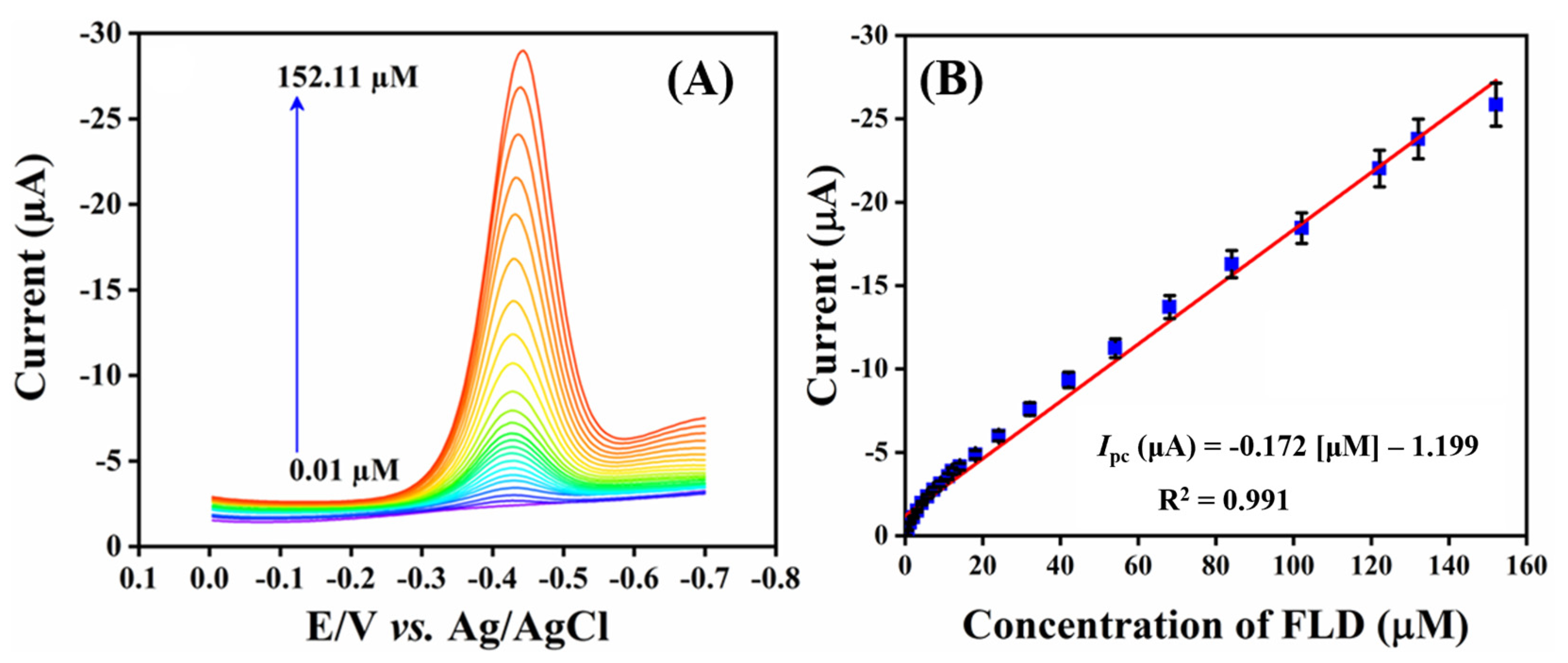
2.2.4. Differential Pulse Voltammetry (DPV) Analysis
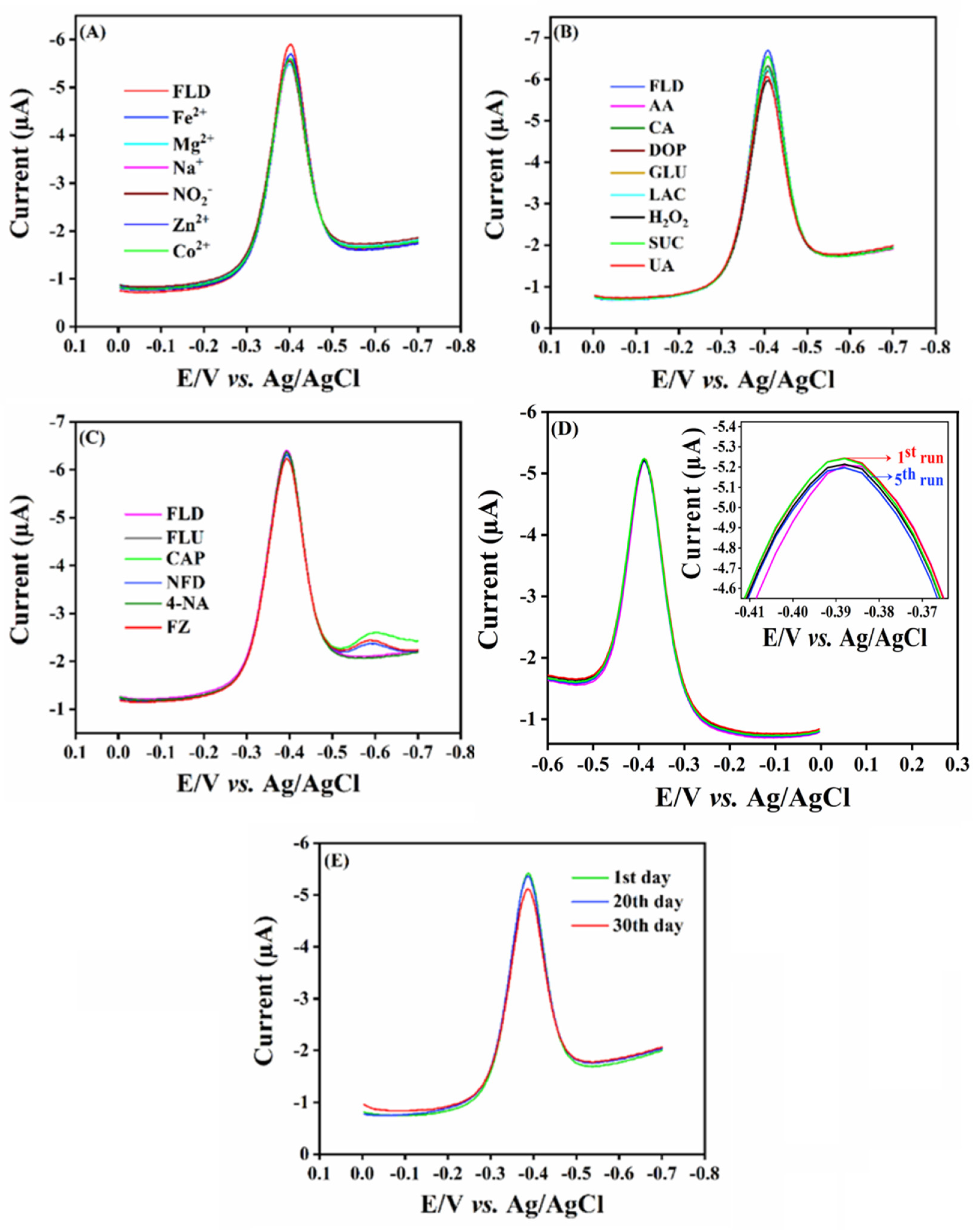
2.2.5. Selectivity, Repeatability, and Storage Stability Studies
2.2.6. Real Sample Analysis
3. Experimental
3.1. Chemicals and Reagents
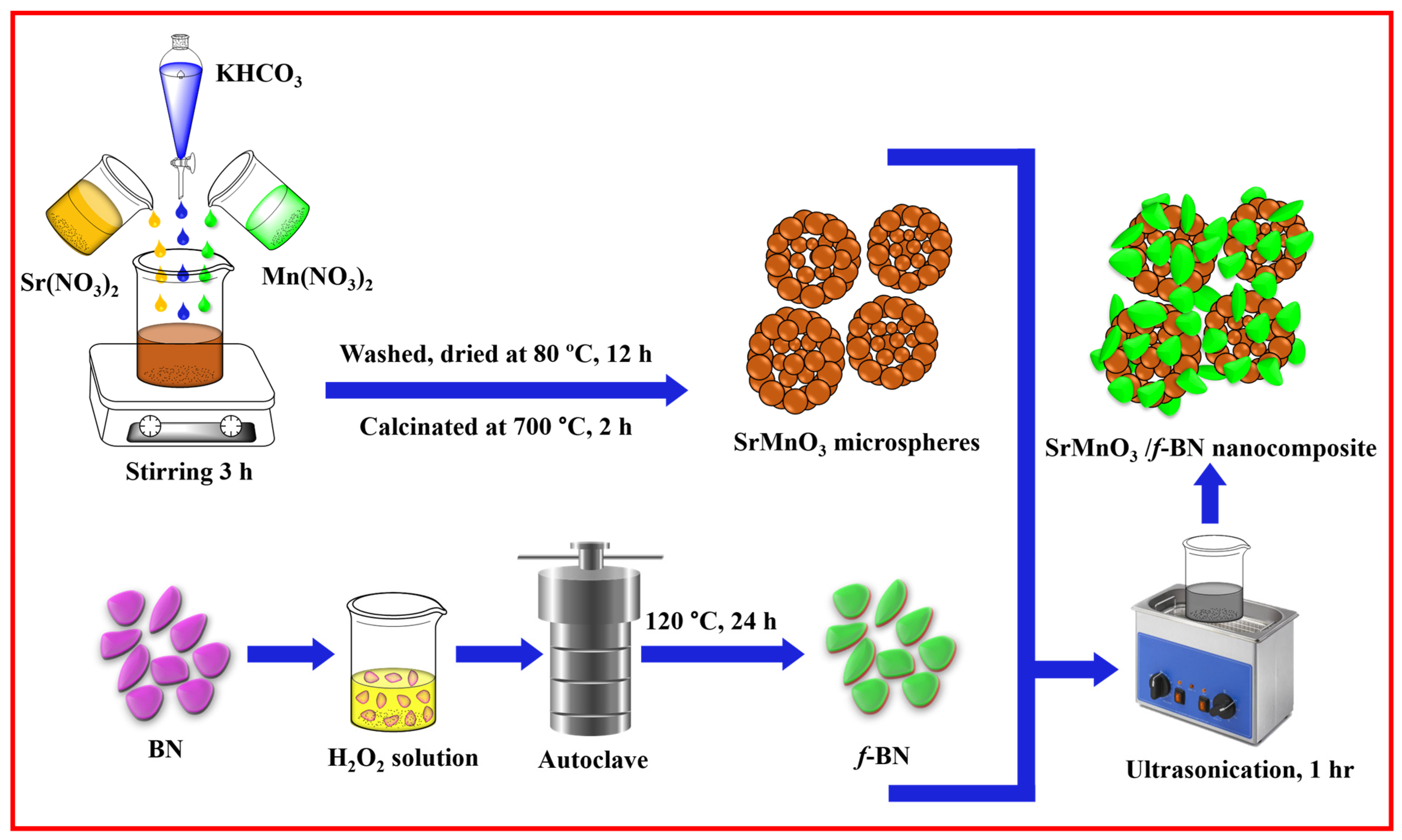
3.2. Synthesis of SrMnO3 Perovskite Microspheres
3.3. Functionalization of BN
3.4. Synthesis of SrMnO3/f-BN Composite
3.5. Instrumentation
3.6. Fabrication of the Sensor Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghian, E.; Marzi Khosrowshahi, E.; Sohouli, E.; Ahmadi, F.; Rahimi-Nasrabadi, M.; Safarifard, V. A new electrochemical sensor for the detection of fentanyl lethal drug by a screen-printed carbon electrode modified with the open-ended channels of Zn(ii)-MOF. New J. Chem. 2020, 44, 9271–9277. [Google Scholar] [CrossRef]
- Cooper, K.M.; Mulder, P.P.J.; van Rhijn, J.A.; Kovacsics, L.; McCracken, R.J.; Young, P.B.; Kennedy, D.G. Depletion of four nitrofuran antibiotics and their tissue-bound metabolites in porcine tissues and determination using LC-MS/MS and HPLC-UV. Food Addit. Contam. 2005, 22, 406–414. [Google Scholar] [CrossRef]
- Wu, W.; Yang, S.; Liu, J.; Mi, J.; Dou, L.; Pan, Y.; Mujtaba Mari, G.; Wang, Z. Progress in immunoassays for nitrofurans detection. Food Agric. Immunol. 2020, 31, 890–909. [Google Scholar] [CrossRef]
- Mahedero, M.C.; Díaz, T.G.; Pascual, S.G. Resolution of ternary mixtures of nitrofurantoin, furaltadone and furazolidone by partial least-square analysis to the spectrophotometric signals after photo-decomposition. J. Pharm. Biomed. Anal. 2002, 29, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Verdon, E.; Couedor, P.; Sanders, P. Multi-residue monitoring for the simultaneous determination of five nitrofurans (furazolidone, furaltadone, nitrofurazone, nitrofurantoine, nifursol) in poultry muscle tissue through the detection of their five major metabolites (AOZ, AMOZ, SEM, AHD, DNSA. Anal. Chim. Acta 2007, 586, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Hwa, K.Y.; Sharma, T.S.K. Nano assembly of NiFe spheres anchored on f-MWCNT for electrocatalytic reduction and sensing of nitrofurantoin in biological samples. Sci. Rep. 2020, 10, 12256. [Google Scholar] [CrossRef]
- Díaz, T.G.; Cabanillas, A.G.; Valenzuela, M.I.A.; Correa, C.A.; Salinas, F. Determination of nitrofurantoin, furazolidone and furaltadone in milk by high-performance liquid chromatograpby with electrochemical detection. J. Chromatogr. A 1997, 764, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Moura, S.; Barbosa, R.; Ramos, F.; da Silveira, M.I.N. Determination of nitrofurans in animal feeds by liquid chromatography-UV photodiode array detection and liquid chromatography-ionspray tandem mass spectrometry. Anal. Chim. Acta 2007, 586, 359–365. [Google Scholar] [CrossRef]
- Chiu, S.H.; Su, Y.L.; Le, A.V.T.; Cheng, S.H. Nanocarbon material-supported conducting poly(melamine) nanoparticle-modified screen-printed carbon electrodes for highly sensitive determination of nitrofuran drugs by adsorptive stripping voltammetry. Anal. Bioanal. Chem. 2018, 410, 6573–6583. [Google Scholar] [CrossRef]
- Khodari, M.; Salah El-Din, H.; Mersal, G.A.M. Electroreduction and quantification of furazolidone and furaltadone in different media. Mikrochim. Acta 2000, 135, 9–17. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Chen, Y.; Cheng, N.; Hui, Q. Hydrogen storage in perovskite-type oxides ABO3 for Ni/MH battery applications: A density functional investigation. Ind. Eng. Chem. Res. 2012, 51, 11821–11827. [Google Scholar] [CrossRef]
- Sugahara, K.; Kamata, K.; Muratsugu, S.; Hara, M. Amino Acid-Aided Synthesis of a Hexagonal SrMnO3 Nanoperovskite Catalyst for Aerobic Oxidation. ACS Omega 2017, 2, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, X.; Luo, B.; Li, L. BaTiO3-BiYbO3 perovskite materials for energy storage applications. J. Mater. Chem. A. 2015, 3, 18146–18153. [Google Scholar] [CrossRef]
- Björketun, M.E.; Castelli, I.E.; Rossmeisl, J.; Olsen, T.; Ukai, K.; Kato, M.; Dennler, G.; Jacobsen, K.W. Defect Chemistry and Electrical Conductivity of Sm-Doped La1-xSrxCoO3-δ for Solid Oxide Fuel Cells. J. Phys. Chem. C 2017, 121, 15017–15027. [Google Scholar] [CrossRef]
- Liu, X.; Gong, H.; Wang, T.; Guo, H.; Song, L.; Xia, W.; Gao, B.; Jiang, Z.; Feng, L.; He, J. Cobalt-Doped Perovskite-Type Oxide LaMnO3 as Bifunctional Oxygen Catalysts for Hybrid Lithium–Oxygen Batteries. Chem. Asian J. 2018 13, 528–535. [CrossRef]
- Atta, N.F.; El-Ads, E.H.; Galal, A.; Galal, A.E. Electrochemical Sensing Platform Based on Nano-Perovskite/Glycine/Carbon Composite for Amlodipine and Ascorbic Acid Drugs. Electroanalysis 2019, 31, 448–460. [Google Scholar] [CrossRef]
- Vidya Rajan, N.; Alexander, L.K. Electrochemical sensing of modified ABO3 perovskite: LaFe0.8R0.2O3 (R= Cr, Co, Al). AIP Conf. Proc. 2017, 1849, 020027. [Google Scholar] [CrossRef]
- Muthukutty, B.; Krishnapandi, A.; Chen, S.M.; Abinaya, M.; Elangovan, A. Innovation of Novel Stone-Like Perovskite Structured Calcium Stannate (CaSnO3): Synthesis, Characterization, and Application Headed for Sensing Photographic Developing Agent Metol. ACS Sustain. Chem. Eng. 2020, 8, 4419–4430. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- El-Ads, E.H.; Galal, A.; Atta, N.F. The effect of A-site doping in a strontium palladium perovskite and its applications for non-enzymatic glucose sensing. RSC Adv. 2016, 6, 16183–16196. [Google Scholar] [CrossRef]
- George, J.; Halali, V.V.; Sanjayan, C.G.; Suvina, V.; Sakar, M.; Balakrishna, R.G. Perovskite nanomaterials as optical and electrochemical sensors. Inorg. Chem. Front. 2020, 7, 2702–2725. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Das, N.; Kandimalla, S. Application of perovskites towards remediation of environmental pollutants: An overview: A review on remediation of environmental pollutants using perovskites. Int. J. Environ. Sci. Technol. 2017, 14, 1559–1572. [Google Scholar] [CrossRef]
- Gholamrezaei, S.; Amiri, M.; Amiri, O.; Salavati-Niasari, M.; Moayedi, H. Ultrasound-accelerated synthesis of uniform SrMnO3 nanoparticles as water-oxidizing catalysts for water splitting systems. Ultrason. Sonochem. 2020, 62, 104899. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavadivel, M.; Dhayabaran, V.V.; Subramanian, M. Nanosized BaMnO3 as high performance supercapacitor electrode material: Fabrication and characterization. Mater. Lett. 2016, 181, 335–339. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.; Chang, H.; Gan, J.; Yue, H.; Yang, Y. Hydrothermal Synthesis of Nanosized LiMnO2–Li2MnO3 Compounds and Their Electrochemical Performances. J. Electrochem. Soc. 2009, 156, A162. [Google Scholar] [CrossRef]
- Jin, Z.; Bard, A.J. Surface Interrogation of Electrodeposited MnOx and CaMnO3 Perovskites by Scanning Electrochemical Microscopy: Probing Active Sites and Kinetics for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Liu, M.; Shao, Z.; Ni, M. Recent Advances in Perovskite Oxides as Electrode Materials for Nonaqueous Lithium–Oxygen Batteries. Adv. Energy Mater. 2017, 7, 1602674. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, X.; Zhong, X. Fabrication and Characterization of Hexagonal SrMnO3 Nanofibers by Electrospinning. MATEC Web Conf. 2016, 67, 06093. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.M.; Xu, J.W.; Yuan, C.L.; Zhang, X.W. Synthesis and resistive switching behaviour of ZnMnO3 thin films with an Ag/ZnMnO3/ITO unsymmetrical structure. Bull. Mater. Sci. 2015, 38, 105–109. [Google Scholar] [CrossRef]
- Shafi, P.M.; Joseph, N.; Karthik, R.; Shim, J.; Bose, A.C.; Ganesh, V. Lemon juice-assisted synthesis of LaMnO3 perovskite nanoparticles for electrochemical detection of dopamine. Microchem. J. 2021, 164, 105945. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, C. Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis. Catalysts 2021, 11, 762021. [Google Scholar]
- Celorrio, V.; Calvillo, L.; Granozzi, G.; Russell, A.E.; Fermin, D.J. AMnO3 (A = Sr, La, Ca, Y) Perovskite Oxides as Oxygen Reduction Electrocatalysts. Top. Catal. 2018, 61, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jeerh, G.; Zou, P.; Lan, R.; Wang, M.; Wang, H.; Tao, S. Recent development of perovskite oxide-based electrocatalysts and their applications in low to intermediate temperature electrochemical devices. Mater. Today 2021, 49, 351–377. [Google Scholar] [CrossRef]
- George, G.; Jackson, S.L.; Luo, C.Q.; Fang, D.; Luo, D.; Hu, D.; Wen, J.; Luo, Z. Effect of doping on the performance of high-crystalline SrMnO3 perovskite nanofibers as a supercapacitor electrode. Ceram. Int. 2018, 44, 21982–21992. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L. Nanocrystalline SrMnO3 perovskite prepared by sol–gel self-combustion method for sensor applications. J. Sol-Gel Sci. Technol. 2021, 97, 146–154. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Brintakis, K.; Nasikas, N.K.; Stratakis, E. Perovskite nanocrystals for energy conversion and storage. Nanophotonics 2019, 8, 1607–1640. [Google Scholar] [CrossRef]
- Hong, J.; Aphale, A.N.; Heo, S.J.; Hu, B.; Reisert, M.; Belko, S.; Singh, P. Strontium Manganese Oxide Getter for Capturing Airborne Cr and S Contaminants in High-Temperature Electrochemical Systems. ACS Appl. Mater. Interfaces 2019, 11, 34878–34888. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharm. J. 2019, 27, 817–829. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Ceresoli, D.; Parisiades, P.; Prakapenka, V.B.; Yu, T.; Wang, Y.; Bremholm, M. Phase stability of the SrMnO3 hexagonal perovskite system at high pressure and temperature. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 90, 214101. [Google Scholar] [CrossRef]
- Sacchetti, A.; Baldini, M.; Postorino, P.; Martin, C.; Maignan, A. Raman spectroscopy on cubic and hexagonal SrMnO3. J. Raman Spectrosc. 2006, 37, 591–596. [Google Scholar] [CrossRef]
- Sacchetti, A.; Baldini, M.; Crispoldi, F.; Postorino, P.; Dore, P.; Martin, N.C.; Maignan, A. Temperature dependence of the optical phonons in SrMnO3 manganite: Evidence of a low-temperature structural transition in the hexagonal compound. Phys. Rev. BCondens. Matter Mater. Phys. 2005, 72, 172407. [Google Scholar] [CrossRef]
- dos Santos, O.A.L.; Sneha, M.; Devarani, T.; Bououdina, M.; Backx, B.P.; Vijaya, J.J.; Bellucci, S. Review—Perovskite/Spinel Based Graphene Derivatives Electrochemical and Biosensors. J. Electrochem. Soc. 2021, 168, 067506. [Google Scholar] [CrossRef]
- Chen, T.-W.; Ramachandran, R.; Chen, S.-M.; Anushya, G.; Divya Rani, S.; Mariyappan, V.; Elumalai, P.; Vasimalai, N. High-performance-based perovskite-supported nanocomposite for the development of green energy device applications: An overview. Nanomaterials 2021, 11, 1006. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mathur, P.; Mobin, S.M. Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics. Electrochim. Acta 2016, 215, 435–446. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, J.; Tian, Y.; Wu, Y.; Liu, X.; He, Q. Ultrasensitive electrochemical determination of tyrosine based on the α-Fe2O3@Co3O4-NRGO modified electrode. Microchem. J. 2021, 171, 106867. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Li, G.; Hu, J.; He, Q. Ultrasensitive detection of dopamine via electrochemical route on spindle-like α-Fe2O3 Mesocrystals/rGO modified GCE. Mater. Res. Bull. 2021, 133, 111050. [Google Scholar] [CrossRef]
- Govindasamy, M.; Wang, S.F.; Pan, W.C.; Subramanian, B.; Ramalingam, R.J.; Al-lohedan, H. Facile sonochemical synthesis of perovskite-type SrTiO3 nanocubes with reduced graphene oxide nanocatalyst for an enhanced electrochemical detection of α-amino acid (tryptophan). Ultrason. Sonochem. 2019, 56, 193–199. [Google Scholar] [CrossRef]
- Bi, Y.S.; Liu, B.; Liu, X.Y.; Qin, Y.; Zou, B.X. A h-BCN for electrochemical sensor of dopamine and uric acid. J. Nanomater. 2020, 2020, 4604820. [Google Scholar] [CrossRef]
- Maity, C.K.; Sahoo, S.; Verma, K.; Behera, A.K.; Nayak, G.C. Facile functionalization of boron nitride (BN) for the development of high-performance asymmetric supercapacitors. New J. Chem. 2020, 44, 8106–8119. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Kozuch, D.; Robinson, J.A. Large-scale synthesis and functionalization of hexagonal boron nitride nanosheets. Nanoscale 2014, 6, 11671–11675. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, H.F.; He, S.J.; Du, Y.H.; Yu, N.J.; Du, X.Z.; Lin, J.; Nazarenko, S. Thermal conductivity performance of polypropylene composites filled with polydopamine-functionalized hexagonal boron nitride. PLoS ONE 2017, 12, 0170523. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Bando, Y.; Huang, Y.; Zhi, C.; Golberg, D. Synthetic routes and formation mechanisms of spherical boron nitride nanoparticles. Adv. Funct. Mater. 2008, 18, 3653–3661. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; Ye, X.; Tian, K.; Huang, W.; He, J.; Guo, Y.; Tian, X. Synergistic enhancement of anisotropic thermal transport flexible polymer composites filled with multi-layer graphene (mG) and mussel-inspiring modified hexagonal boron nitride (h-BN). Compos. Part A Appl. Sci. Manuf. 2018, 111, 12–22. [Google Scholar] [CrossRef]
- Li, Q.; Huo, C.; Yi, K.; Zhou, L.; Su, L.; Hou, X. Preparation of flake hexagonal BN and its application in electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2018, 260, 346–356. [Google Scholar] [CrossRef]
- Jesu Amalraj, A.J.; Narasimha Murthy, U.; Sea-Fue, W. Ultrasensitive electrochemical detection of an antibiotic drug furaltadone in fish tissue with a ZnO-ZnCo2O4 self-assembled nano-heterostructure as an electrode material. Microchem. J. 2021, 169, 106566. [Google Scholar] [CrossRef]
- Vasu, D.; Karthi Keyan, A.; Sakthinathan, S.; Chiu, T.W. Investigation of electrocatalytic and photocatalytic ability of Cu/Ni/TiO2/MWCNTs Nanocomposites for detection and degradation of antibiotic drug Furaltadone. Sci. Rep. 2022, 12, 886. [Google Scholar] [CrossRef]
- Balamurugan, K.; Rajakumaran, R.; Chen, S.M.; Karthik, R.; Shim, J.J.; Shafi, P.M. Massive engineering of spinel cobalt tin oxide/tin oxide-based electrocatalyst for the selective voltammetric determination of antibiotic drug furaltadone in water samples. J. Alloys Compd. 2021, 882, 160750. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Musuvadhi Babulal, S.; Ming Chen, S.; Sukanya, R.; Karthik, R.; Muhammed Shafi, P.; Shim, J.-J.; Yo-Shiuana, C. Ingenious design of iron vanadate engulfed 3D porous reduced graphene oxide nanocomposites as a reliable electrocatalyst for the selective amperometric determination of furaltadone in aquatic environments. Appl. Surf. Sci. 2021, 569, 151046. [Google Scholar] [CrossRef]
| Working Electrode | Techniques | Linear Range (µM) | LOD (µM) | References |
|---|---|---|---|---|
| NC-Poly(melamine)/SPCE | ADSV | 0.012 | [9] | |
| ZnO-ZnCo2O4/GCE | DPV | 0.01–4.68; 15.0–100.0 | 0.034 | [56] |
| Cu/Ni/TiO2/MWCNTs/SPCE | A | 10.0–150.0 | 0.095 | [57] |
| Co2SnO4/SnO2/SPCE | DPV | 0.1–73.0; 91.0–1022.0 | 0.039 | [58] |
| Fe0.11V2O5.16/p-rGO/GCE | A | 0.5–84.0; 94.0–1319.0 | 0.138 | [59] |
| SrMnO3/f-BN/SPCE | DPV | 0.01–152.11 | 0.002 | This work |
| Sample | Added (µM) | Found (µM) | Recoveries (%) |
|---|---|---|---|
| FLD spiked urine | 20.0 | 19.8 | 99.0 |
| 40.0 | 39.8 | 99.5 | |
| 60.0 | 58.9 | 98.2 | |
| 80.0 | 78.5 | 98.1 | |
| 100.0 | 98.7 | 98.7 | |
| FLD spiked wastewater | 20.0 | 19.7 | 98.5 |
| 40.0 | 39.9 | 99.8 | |
| 60.0 | 59.8 | 99.7 | |
| 80.0 | 79.9 | 99.9 | |
| 100.0 | 99.2 | 99.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, K.; Rajakumaran, R.; Chen, S.-M.; Karuppasamy, P.; Banach, A.; Al-Onazi, W.A.; Sonadevi, S.; Krishnan, N.P.; Yang, C.-C.; Karuppiah, C.; et al. SrMnO3/Functionalized h-BN Composite Modified Disposable Sensor for the Voltammetric Determination of Furaltadone Antibiotic Drug. Catalysts 2022, 12, 1494. https://doi.org/10.3390/catal12121494
Venkatesh K, Rajakumaran R, Chen S-M, Karuppasamy P, Banach A, Al-Onazi WA, Sonadevi S, Krishnan NP, Yang C-C, Karuppiah C, et al. SrMnO3/Functionalized h-BN Composite Modified Disposable Sensor for the Voltammetric Determination of Furaltadone Antibiotic Drug. Catalysts. 2022; 12(12):1494. https://doi.org/10.3390/catal12121494
Chicago/Turabian StyleVenkatesh, Krishnan, Ramachandran Rajakumaran, Shen-Ming Chen, Periyakaruppan Karuppasamy, Artur Banach, Wedad A. Al-Onazi, Selvam Sonadevi, Nattamai Perumal Krishnan, Chun-Chen Yang, Chelladurai Karuppiah, and et al. 2022. "SrMnO3/Functionalized h-BN Composite Modified Disposable Sensor for the Voltammetric Determination of Furaltadone Antibiotic Drug" Catalysts 12, no. 12: 1494. https://doi.org/10.3390/catal12121494
APA StyleVenkatesh, K., Rajakumaran, R., Chen, S.-M., Karuppasamy, P., Banach, A., Al-Onazi, W. A., Sonadevi, S., Krishnan, N. P., Yang, C.-C., Karuppiah, C., & Ramaraj, S. K. (2022). SrMnO3/Functionalized h-BN Composite Modified Disposable Sensor for the Voltammetric Determination of Furaltadone Antibiotic Drug. Catalysts, 12(12), 1494. https://doi.org/10.3390/catal12121494











