Pseudomonas stutzeri Immobilized Sawdust Biochar for Nickel Ion Removal
Abstract
:1. Introduction
2. Results
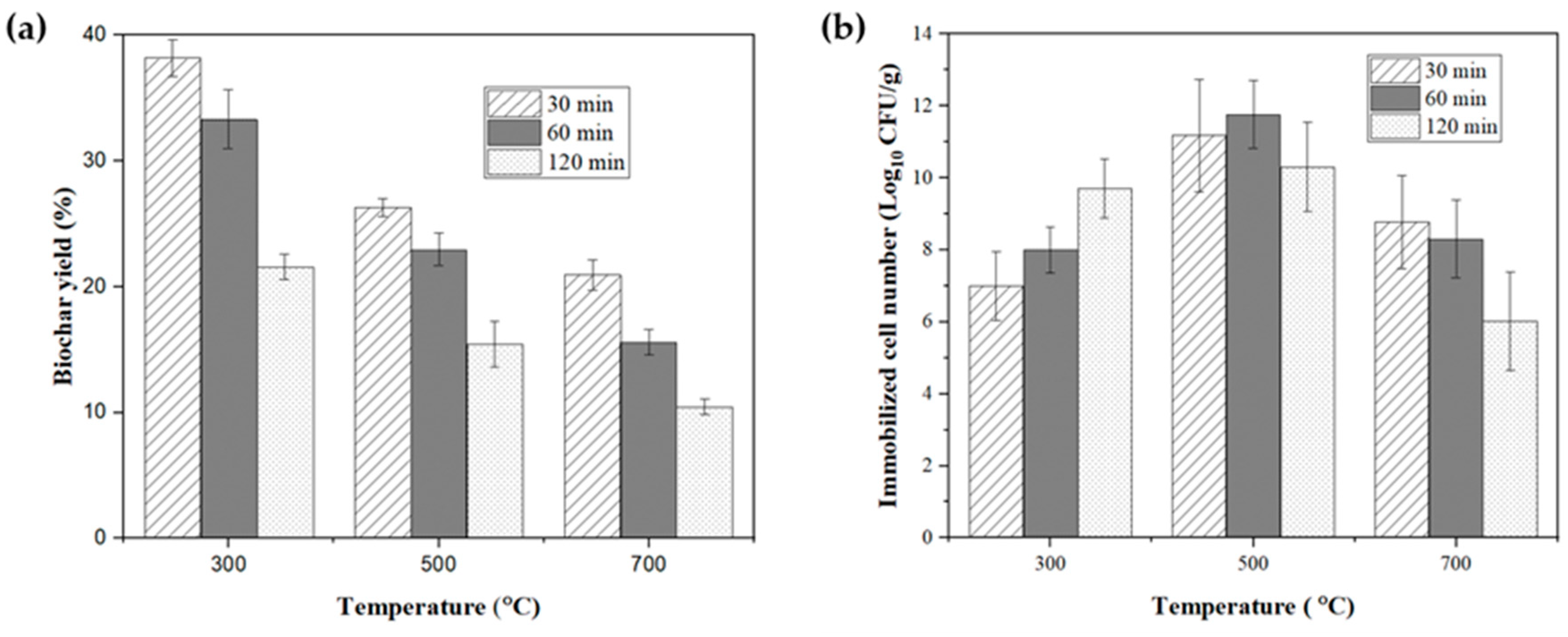
2.1. Biochar Yield
2.2. Immobilization and Selection of Biochar
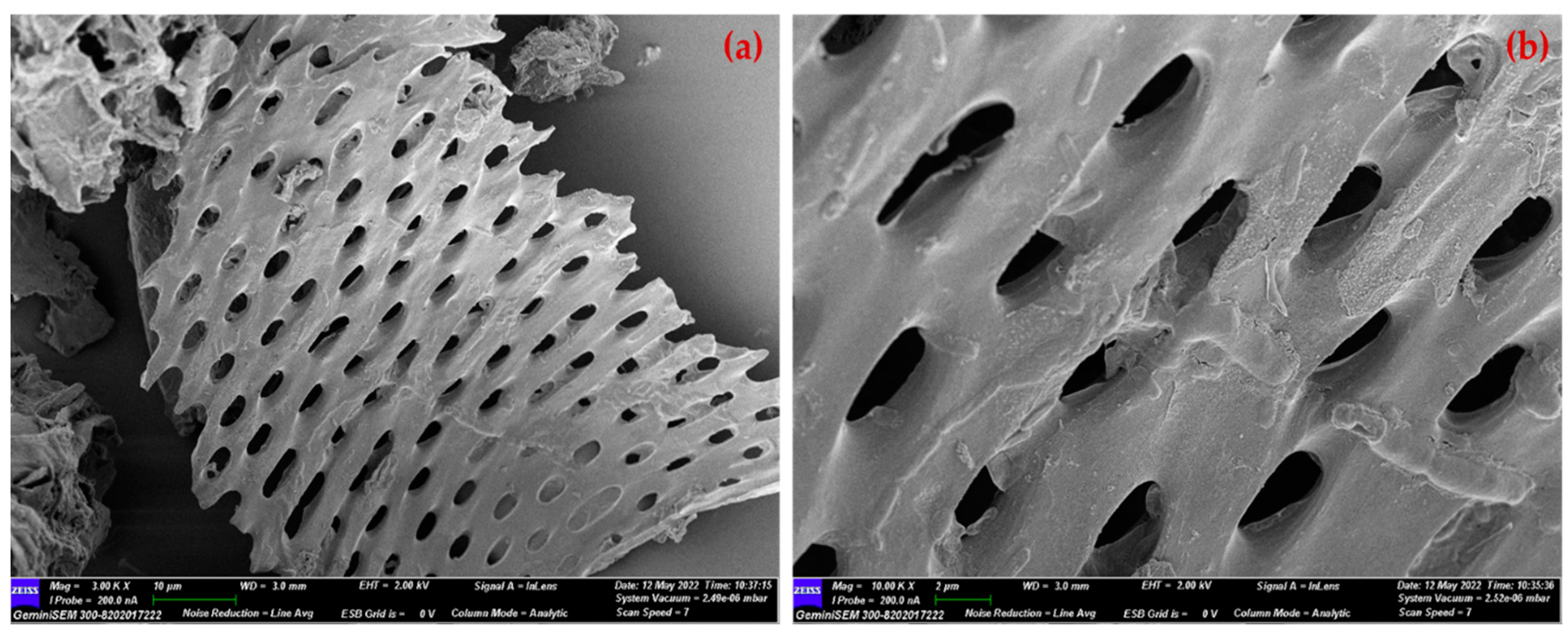
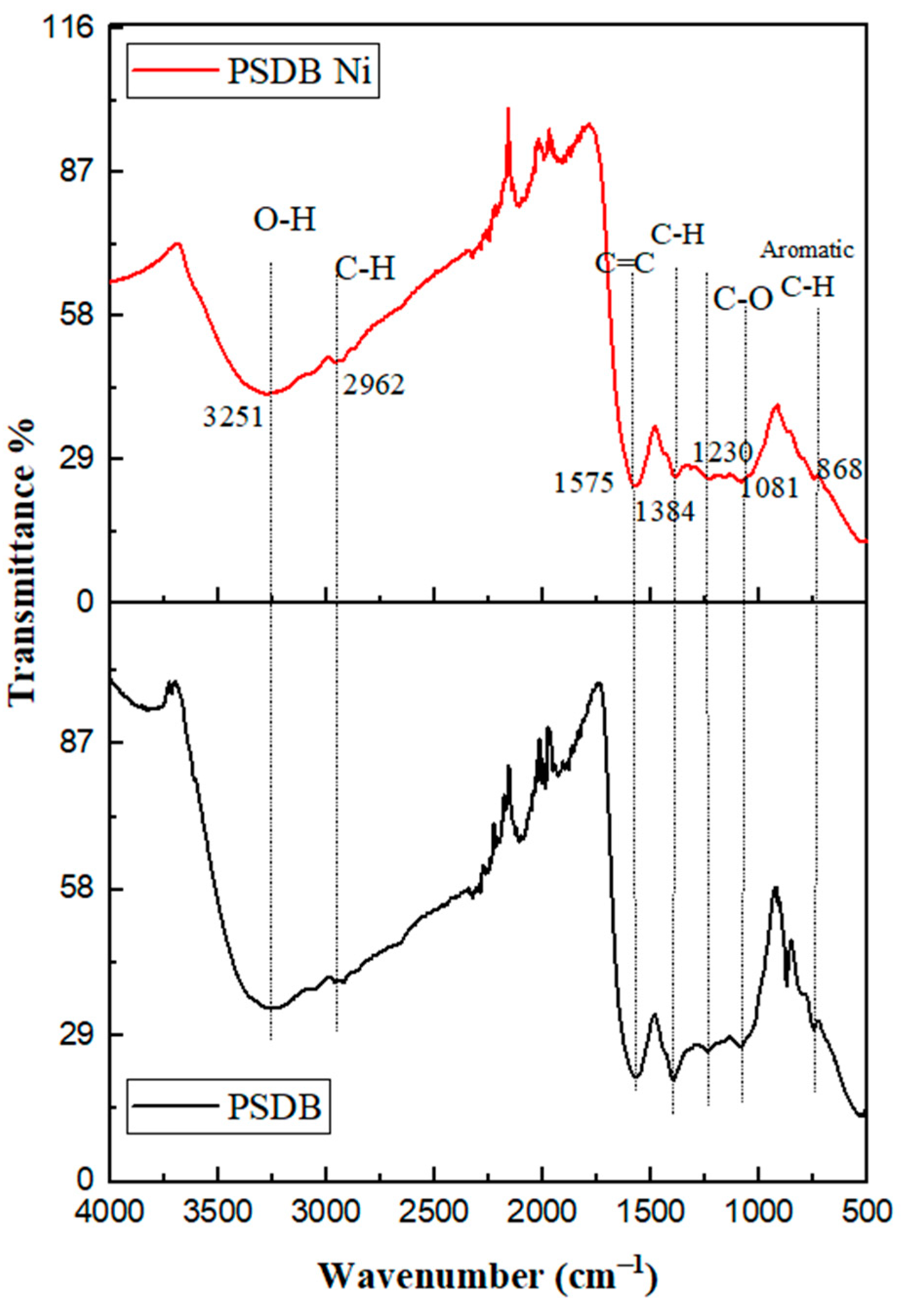
2.3. Characterization of SDB and PSDB
2.4. Effect of Operating Conditions on Ni2+ Removal
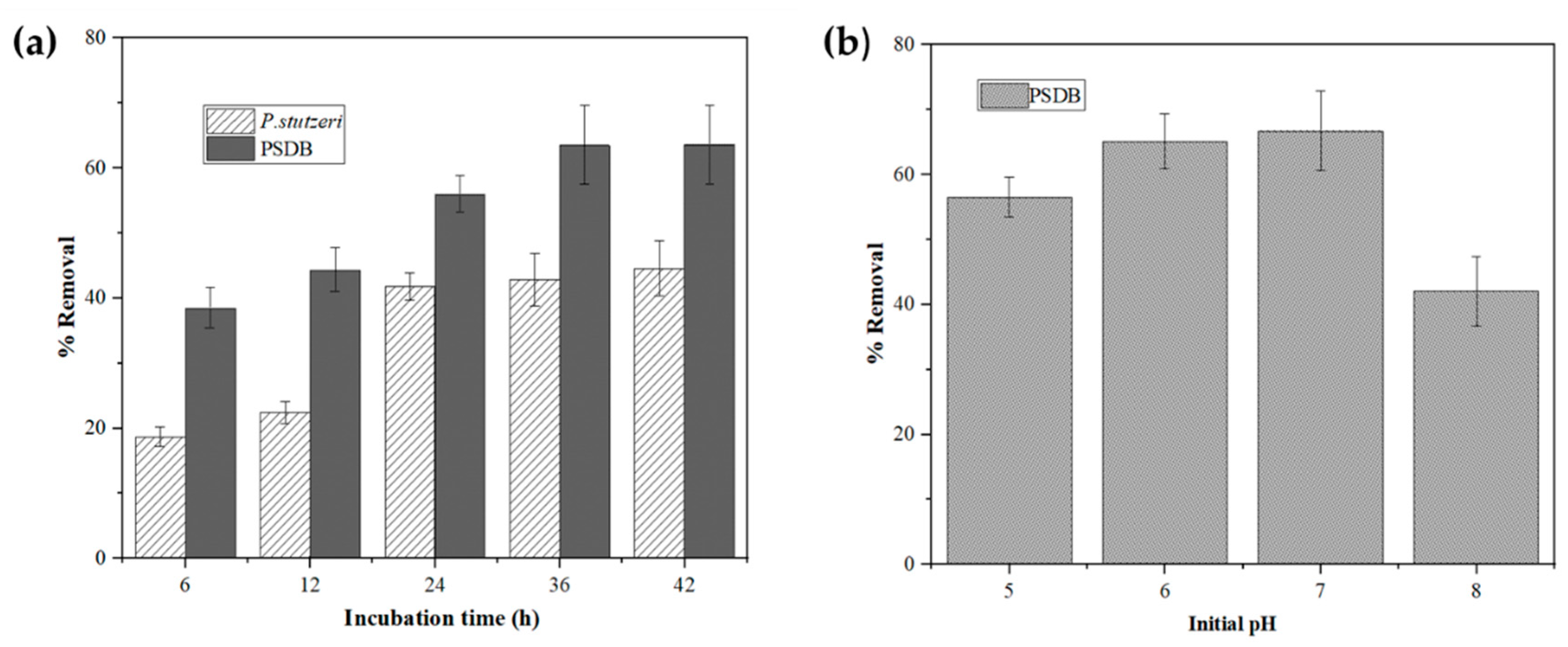
2.4.1. Effect of Incubation Time
2.4.2. Effect of Initial pH on Ni2+ Removal
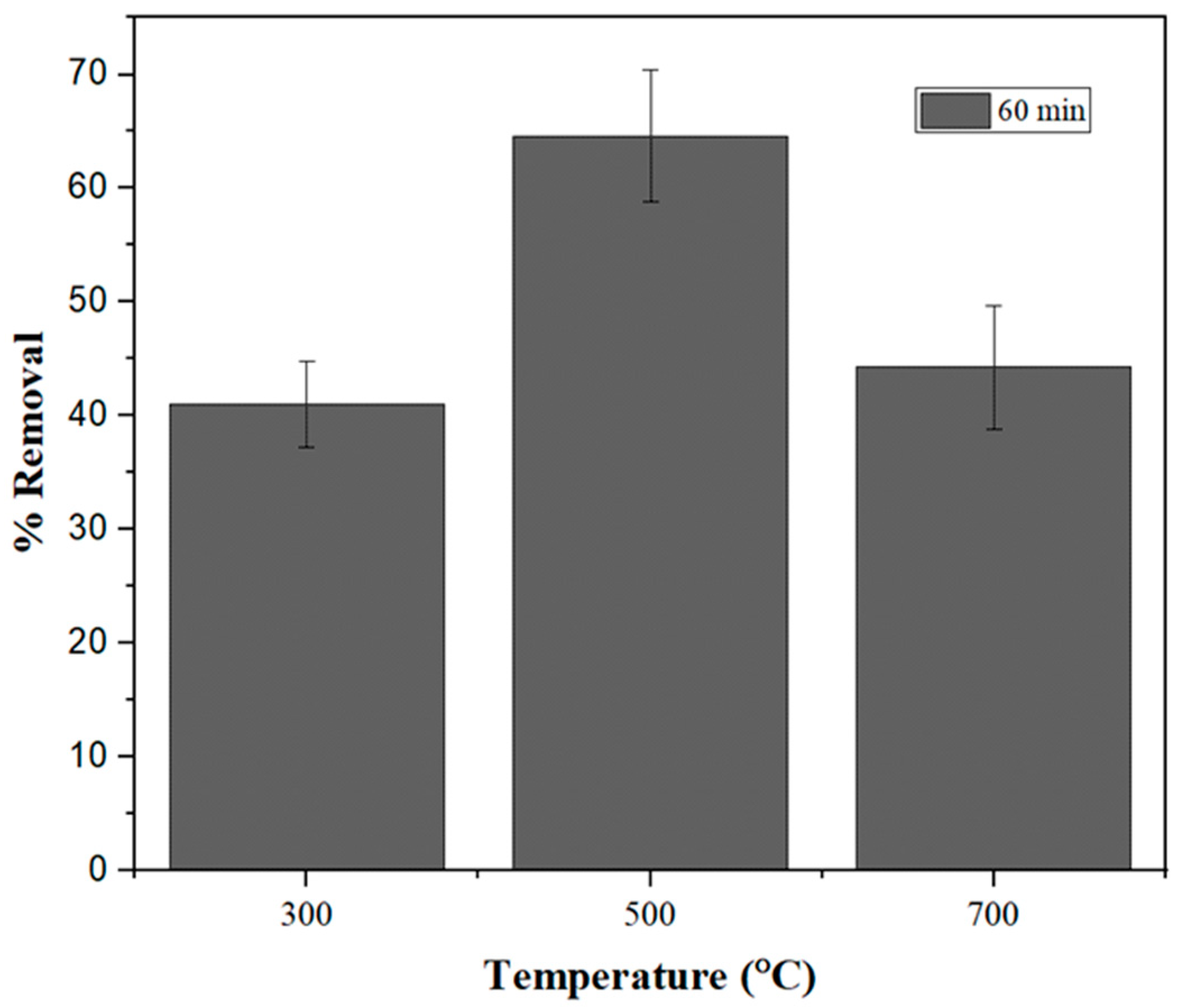
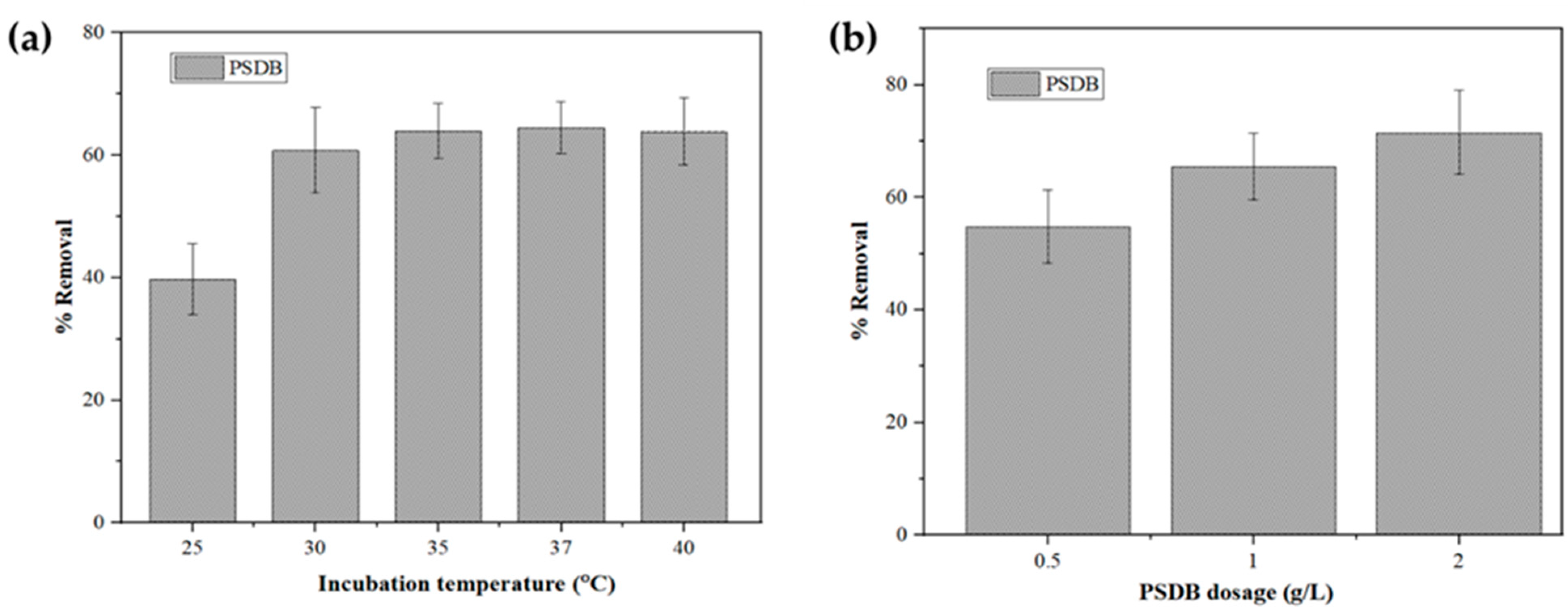
2.4.3. Effect of Temperature
2.4.4. Effect of PSDB Dosage
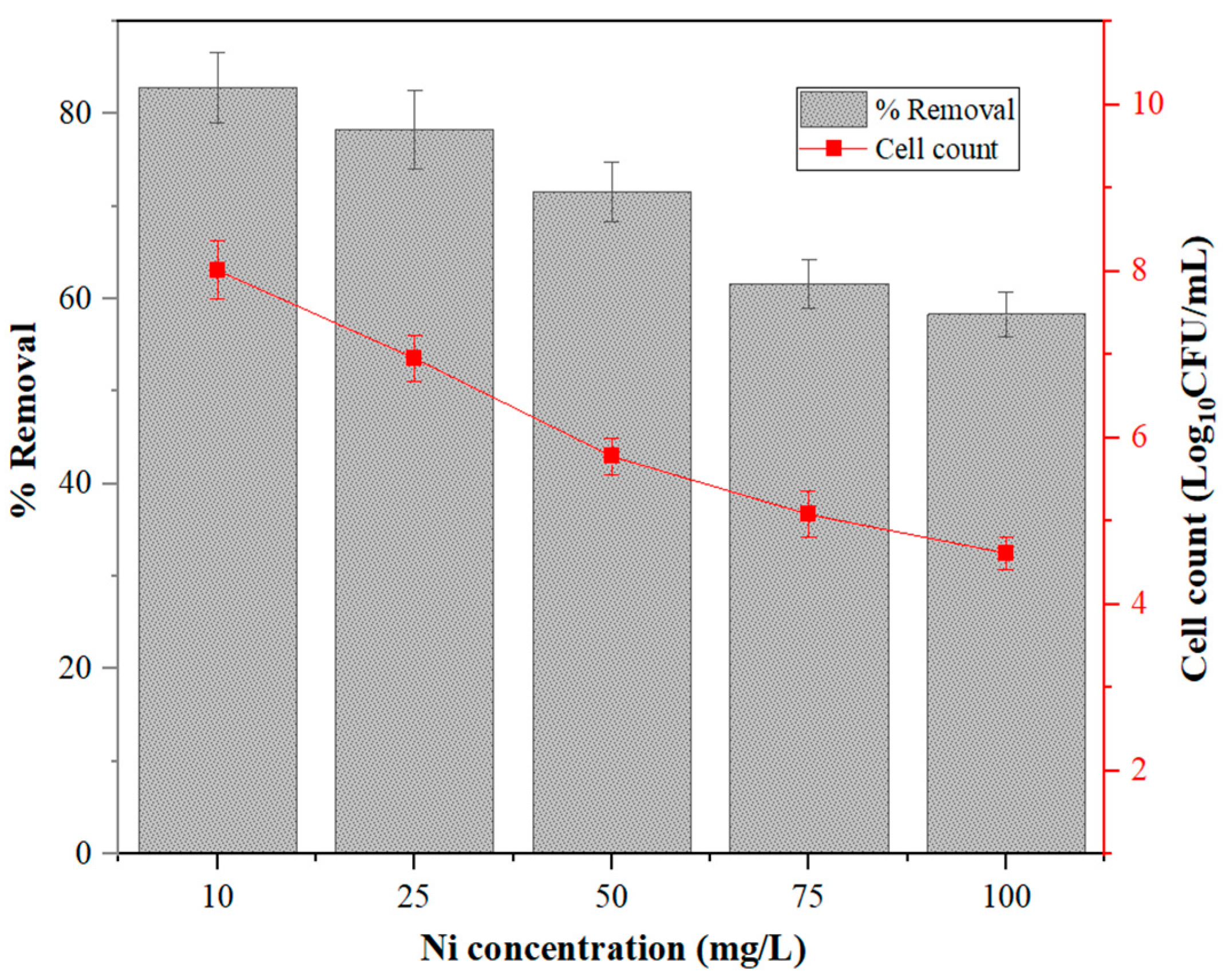
2.4.5. Effect of Initial Ni2+ Concentration
2.5. Adsorption Kinetics and Isotherm Study of PSDB
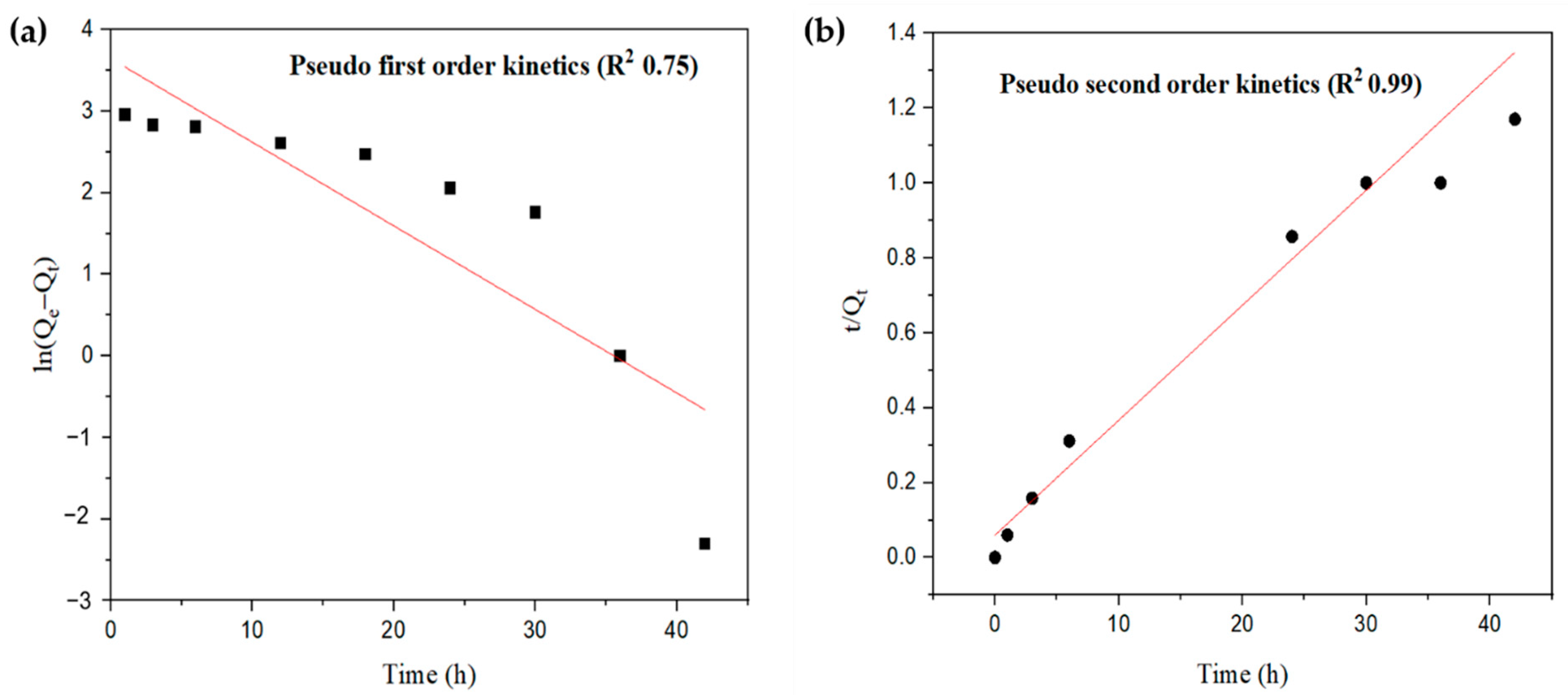
2.5.1. Adsorption Kinetics
2.5.2. Adsorption Isotherm
2.6. Ni2+ Removal by SDB, P. stutzeri, and PSDB at Optimized Operating Conditions
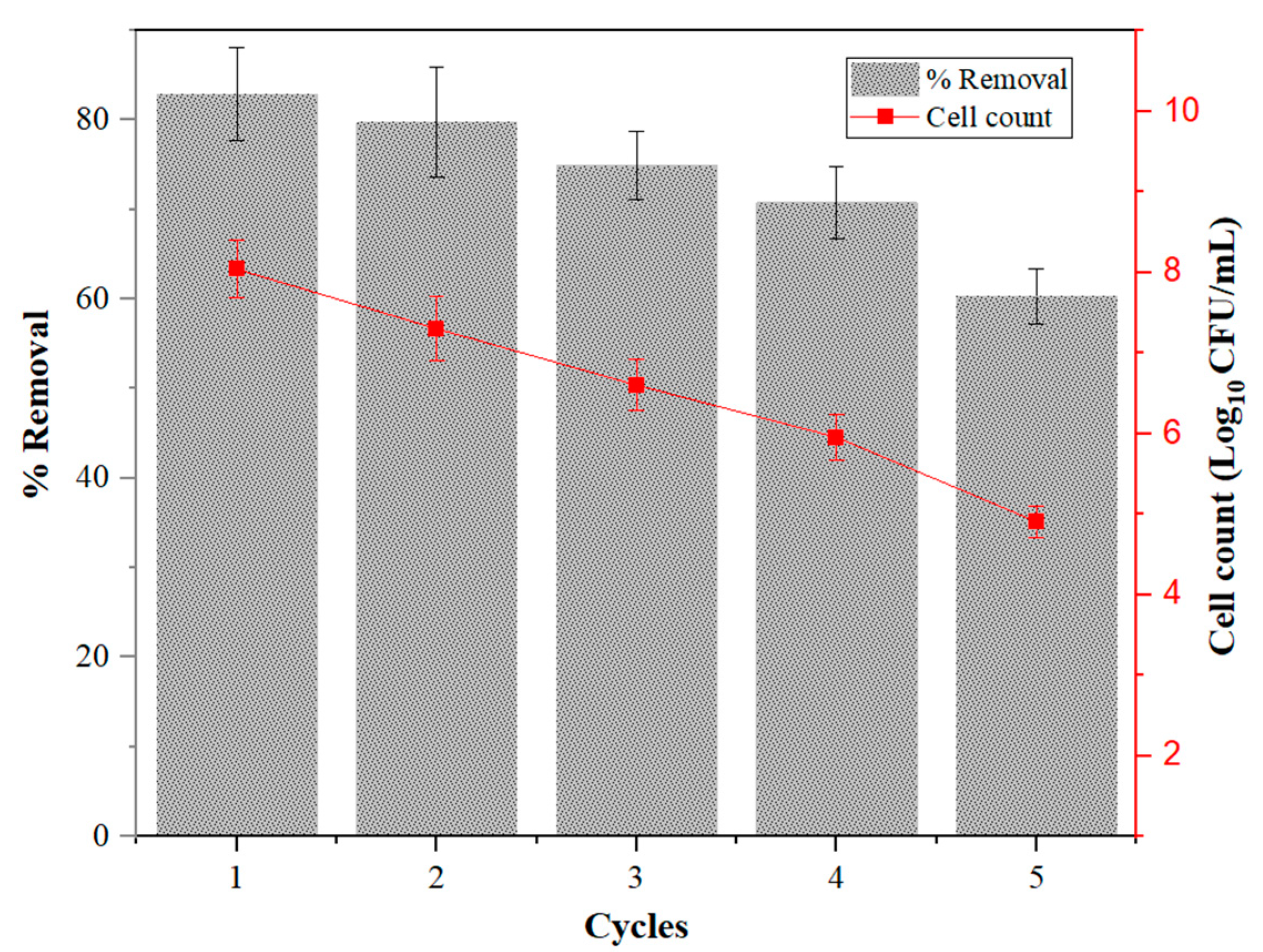
2.7. Reusability of Immobilized Cell
2.8. FTIR Analysis of PSDB after Ni Removal
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Biochar
3.3. Microbial Cell Immobilization
3.4. Selection of Biochar Based on Immobilization Potential
3.5. Characterization of SDB
3.6. Effect of Operating Conditions on Ni2+ Removal
3.7. Adsorption Kinetics and Isotherm Study
3.8. Ni2+ Removal by SDB, Pseudomonas stutzeri and PSDB at Optimized Operating Conditions
3.9. Experimental Design for the Reusability Study of Immobilized Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaumlöffel, D. Nickel species: Analysis and toxic effects. J. Trace Elem. Med. Biol. 2012, 26, 1–6. [Google Scholar] [CrossRef]
- Gillmore, M.L.; Gissi, F.; Golding, L.A.; Stauber, J.L.; Reichelt-Brushett, A.J.; Severati, A.; Humphrey, C.A.; Jolley, D.F. Effects of dissolved nickel and nickel-contaminated suspended sediment on the scleractinian coral. Acropora Muricata. Mar. Pollut. Bull. 2020, 152, 110886. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jasrotia, T.; Sharma, S.; Sharma, M.; Kumar, R.; Vats, R.; Kumar, R.; Umar, A.; Akhtar, M.S. Sustainable removal of Ni(II) from waste water by freshly isolated fungal strains. Chemosphere 2021, 282, 130871. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Liakopoulou-Kyriakides, M. Bioremoval of heavy metals by bacterial biomass. Environ. Monit. Assess. 2015, 187, 4173. [Google Scholar] [CrossRef] [PubMed]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef]
- Tyagi, S.; Rawtani, D.; Khatri, N.; Tharmavaram, M. Strategies for Nitrate removal from aqueous environment using Nanotechnology: A Review. J. Water Process Eng. 2018, 21, 84–95. [Google Scholar] [CrossRef]
- Sethu, V.S.; Aziz, A.R.; Aroua, M.K. Recovery and reutilisation of copper from metal hydroxide sludges. Clean Technol. Environ. Policy 2008, 10, 131–136. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Arabelo, A.; Banda, K.; Nyambe, I.; Chirwa, M.; Ito, M.; Sato, T.; Igarashi, T.; et al. Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Wu, X.; Huang, P.; Dong, C.; Deng, X. Nickel bioaccumulation by a marine bacterium Brevibacterium sp. (X6) isolated from Shenzhen Bay, China. Mar. Pollut. Bull. 2021, 170, 112656. [Google Scholar] [CrossRef]
- Aslam, F.; Yasmin, A.; Sohail, S. Bioaccumulation of lead, chromium, and nickel by bacteria from three different genera isolated from industrial effluent. Int. Microbiol. 2020, 23, 253–261. [Google Scholar] [CrossRef]
- Kathiravan, M.N.; Karthiga Rani, R.; Karthick, R.; Muthukumar, K. Mass transfer studies on the reduction of Cr(VI) using calcium alginate immobilized Bacillus sp. in packed bed reactor. Bioresour. Technol. 2010, 101, 853–858. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Giannakoudakis, D.A.; Prekodravac, J.R.; Carlos, J. Role of catalyst supports in biocatalysis. J. Chem. Technol. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z. Remoal of Heavy Metals from Industrial Effluent Using Saccharomyces Cerevisiae ( Baker ’ s Yeast ) Immobilised in Chitosan / lignosulphonate Matrix. J. Appl. Sci. Res. 2007, 3, 2091–2099. [Google Scholar]
- Noronha, F.R.; Manikandan, S.K.; Nair, V. Role of coconut shell biochar and earthworm (Eudrilus euginea) in bioremediation and palak spinach (Spinacia oleracea L.) growth in cadmium-contaminated soil. J. Environ. Manag. 2022, 302, 114057. [Google Scholar] [CrossRef]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Experimental validation of biochar based green Bronsted acid catalysts for simultaneous esterification and transesterification in biodiesel production. Bioresour. Technol. Rep. 2018, 2, 38–44. [Google Scholar] [CrossRef]
- Nair, V.; Vinu, R. Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresour. Technol. 2016, 216, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Ma, L.; Gao, G.; Wang, Y. Combination of biochar and immobilized bacteria in cypermethrin-contaminated soil remediation. Int. Biodeterior. Biodegrad. 2017, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Hu, T.; Liu, Y.; Liu, J.; Wang, Y.; Wang, P.; Zhou, J.; Chen, M.; Yang, B.; Li, L. Combination of biochar and immobilized bacteria accelerates polyacrylamide biodegradation in soil by both bio-augmentation and bio-stimulation strategies. J. Hazard. Mater. 2021, 405, 124086. [Google Scholar] [CrossRef]
- Higashikawa, F.S.; Conz, R.F.; Colzato, M.; Cerri, C.E.P.; Alleoni, L.R.F. Effects of feedstock type and slow pyrolysis temperature in the production of biochars on the removal of cadmium and nickel from water. J. Clean. Prod. 2016, 137, 965–972. [Google Scholar] [CrossRef]
- Manyuchi, M.M.; Sukdeo, N.; Stinner, W.; Mutusva, T.N. Influence of sawdust based biochar on gold tailings wastewater heavy metal contaminants removal. South Afr. J. Chem. Eng. 2021, 37, 81–91. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue–derived biochar. Water Air Soil Pollut. 2021, 232, 236. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, L.; Li, K.; Chen, C.; Lin, X.; Zhang, C.; Xie, Q. Enhanced bioremediation of diesel oil-contaminated seawater by a biochar-immobilized biosurfactant-producing bacteria Vibrio sp. LQ2 isolated from cold seep sediment. Sci. Total Environ. 2021, 793, 148529. [Google Scholar] [CrossRef] [PubMed]
- Shariff, A.; Mohamad Aziz, N.S.; Md Saleh, N.; Ruzali, N.S.I. The effect of feedstock type and slow pyrolysis temperature on biochar yield from coconut wastes. Int. J. Chem. Mol. Eng. 2016, 10, 1410–1414. [Google Scholar]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Ravindran, H.; Gupta, R.B.; Fasina, O.; Tu, M.; Fernando, S.D. Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour. Technol. 2010, 101, 8389–8395. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Wang, S.; Guo, S. Different biochars as microbial immobilization substrates for efficient copper II removal. Spectrosc. Lett. 2020, 53, 712–725. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J. Removal of chlortetracycline from water by Bacillus cereus immobilized on Chinese medicine residues biochar. Environ. Technol. Innov. 2021, 24, 101930. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Song, X.D.; Xue, X.Y.; Chen, D.Z.; He, P.J.; Dai, X.H. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Pua, F.L.; Sajab, M.S.; Chia, C.H.; Zakaria, S.; Rahman, I.A.; Salit, M.S. Alkaline-treated cocoa pod husk as adsorbent for removing methylene blue from aqueous solutions. J. Environ. Chem. Eng. 2013, 1, 460–465. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Yin, D.; Peng, B.; Tan, C.; Liu, Y.; Tan, X.; Wu, S. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manag. 2015, 153, 68–73. [Google Scholar] [CrossRef]
- Ye, J.; Joseph, S.D.; Ji, M.; Nielsen, S.; Mitchell, D.R.G.; Donne, S.; Horvat, J.; Wang, J.; Munroe, P.; Thomas, T. Chemolithotrophic processes in the bacterial communities on the surface of mineral-enriched biochars. ISME J. 2017, 11, 1087–1101. [Google Scholar] [CrossRef]
- Huang, F.; Guo, C.L.; Lu, G.N.; Yi, X.Y.; Zhu, L.D.; Dang, Z. Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere 2014, 109, 134–142. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, D.; Liu, Y.; Xu, H.; Nan, H.; Li, D.; Kan, Y.; Cao, X. Biochar as simultaneous shelter, adsorbent, pH buffer, and substrate of Pseudomonas citronellolis to promote biodegradation of high concentrations of phenol in wastewater. Water Res. 2020, 172, 115494. [Google Scholar] [CrossRef] [PubMed]
- Argun, M.E. Use of clinoptilolite for the removal of nickel ions from water: Kinetics and thermodynamics. J. Hazard. Mater. 2008, 150, 587–595. [Google Scholar] [CrossRef]
- Bogusz, A.; Nowak, K.; Stefaniuk, M.; Dobrowolski, R.; Oleszczuk, P. Synthesis of biochar from residues after biogas production with respect to cadmium and nickel removal from wastewater. J. Environ. Manag. 2017, 201, 268–276. [Google Scholar] [CrossRef]
- Saleem, M.; Pirzada, T.; Qadeer, R. Sorption of acid violet 17 and direct red 80 dyes on cotton fiber from aqueous solutions. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 292, 246–250. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, I.H.; Kim, Y.K.; Oh, B.K. Effective bioremediation of Cadmium (II), nickel (II), and chromium (VI) in a marine environment by using Desulfovibrio Desulfuricans. Biotechnol. Bioprocess Eng. 2015, 20, 937–941. [Google Scholar] [CrossRef]
- Lei, D.Y.; Liu, Z.; Peng, Y.H.; Liao, S.B.; Xu, H. Biosorption of copper, lead and nickel on immobilized Bacillus coagulans using experimental design methodologies. Ann. Microbiol. 2014, 64, 1371–1384. [Google Scholar] [CrossRef]
- An, Q.; Jin, N.; Deng, S.; Zhao, B.; Liu, M.; Ran, B.; Zhang, L. Ni (II), Cr (VI), Cu (II) and nitrate removal by the co-system of Pseudomonas hibiscicola strain L1 immobilized on peanut shell biochar. Sci. Total Environ. 2022, 814, 152635. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies. J. Hazard. Mater. 2004, 108, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Majumder, R.; Goswami, M.; Mazumder, S.; Maiti, S.; Ray, L. Implication of Greener Biocomposite Bead for Decontamination of Nickel(II): Column Dynamics Study. J. Polym. Environ. 2020, 28, 1985–1997. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; Silva, E.A. Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. J. Clean. Prod. 2017, 150, 58–64. [Google Scholar] [CrossRef]
- Gurel, L.; Senturk, I.; Bahadir, T.; Buyukgungor, H. Treatment of Nickel plating industrial wastewater by fungus immobilized onto rice Bran. J. Microb. Biochem. Technol. 2010, 2, 34–37. [Google Scholar] [CrossRef]
- Wong, P.K.; Kwok, S.C. Accumulation of nickel ion (Ni2+) by immobilized cells of Enterobacter species. Biotechnol. Lett. 1992, 7, 629–634. [Google Scholar] [CrossRef]
- Tsekova, K.; Todorova, D.; Ganeva, S. Removal of heavy metals from industrial wastewater by free and immobilized cells of Aspergillus niger. Int. Biodeterior. Biodegrad. 2010, 64, 447–451. [Google Scholar] [CrossRef]
- Huang, S.W.; Chen, X.; Wang, D.D.; Jia, H.L.; Wu, L. Bio-reduction and synchronous removal of hexavalent chromium from aqueous solutions using novel microbial cell/algal-derived biochar particles: Turning an environmental problem into an opportunity. Bioresour. Technol. 2020, 309, 123304. [Google Scholar] [CrossRef]
- López, A.; Lázaro, N.; Morales, S.; Marqués, A.M. Nickel biosorption by free and immobilized cells of Pseudomonas fluorescens 4F39: A comparative study. Water Air Soil Pollut. 2002, 135, 157–172. [Google Scholar] [CrossRef]
- Zeng, W.; Li, F.; Wu, C.; Yu, R.; Wu, X.; Shen, L.; Liu, Y.; Qiu, G.; Li, J. Role of extracellular polymeric substance (EPS) in toxicity response of soil bacteria Bacillus sp. S3 to multiple heavy metals. Bioprocess Biosyst. Eng. 2020, 43, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Jiang, Y.Q.; Nan, H.Y.; Yu, Y.; Jiang, J.N. Unraveling sorption of nickel from aqueous solution by KMnO4 and KOH-modified peanut shell biochar: Implicit mechanism. Chemosphere 2019, 214, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Shukla, P. A comparative analysis of heavy metal bioaccumulation and functional gene annotation towards multiple metal resistant potential by Ochrobactrum intermedium BPS-20 and Ochrobactrum ciceri BPS-26. Bioresour. Technol. 2021, 320, 124330. [Google Scholar] [CrossRef]
- Yakout, A.A.; Shaker, M.A.; Albishri, H.M. Application of bifunctional Mangifera indica L.-loaded Saccharomyces cerevisiae as efficacious biosorbent for bivalent cobalt and nickel cations from different wastewaters: Equilibrium and kinetic studies. Desalination Water Treat. 2016, 57, 8967–8980. [Google Scholar] [CrossRef]
- Shen, L.; Jin, Z.; Wang, D.; Wang, Y.; Lu, Y. Enhance wastewater biological treatment through the bacteria induced graphene oxide hydrogel. Chemosphere 2018, 190, 201–210. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, L.; Sun, F.L.; Luo, X.X.; Wang, H.F.; Liu, G.C.; Xu, Z.H.; Jiang, Z.X.; Pan, B.; Zheng, H. Effects of adding biochar on the properties and nitrogen bioavailability of an acidic soil. Eur. J. Soil Sci. 2017, 68, 559–572. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the effect of pyrolysis temperature on the Cd2+ adsorption characteristics of biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ali, A.; Su, J.; Zhang, S.; Fan, Y.; Sun, Y. Self-immobilized biochar fungal pellet combined with bacterial strain H29 enhanced the removal performance of cadmium and nitrate. Bioresour. Technol. 2021, 341, 125803. [Google Scholar] [CrossRef] [PubMed]




| Surface Area (m2 g−1) SDB | Total Pore Volume (cm3 g−1) | Average Pore Size (nm) | |
|---|---|---|---|
| SDB | 195.68 | 0.99 | 1.01 |
| PSDB | 3.19 | 0.14 | 0.5 |
| Ba | Fe | Mg | Mn | Zn | K | Cu | Pb |
|---|---|---|---|---|---|---|---|
| 2.21 | 673.97 | 61.41 | 8.69 | 11.57 | 12 | 40 | 2.7 |
| Models | Model Parameter | PSDB |
|---|---|---|
| Kinetic model | ||
| Pseudo-second order | Qe (mg g−1) | 32.59 |
| K2 (g mg−1 h−1) | 0.015 | |
| R2 | 0.99 | |
| Isotherm model | ||
| Langmuir | Qm (mg g−1) | 67.06 |
| KL (L mg−1) | 0.08 | |
| R2 | 0.99 | |
| Support Material | Microorganism | Initial Concentration of Ni2+ | Removal Efficiency | Time | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Loofa sponge | Chlorella sorokiniana | 200 mg L−1 | 60.38 mg g−1 | 15 min | Biosorption | [44] |
| Alginate | Bacillus cereus | 50 mg L−1 | 54 mg g−1 | - | Adsorption | [45] |
| Ca-alginate beads | Sargassum sp. | 7 mmol L−1 | 1.69 mmol g−1 | Adsorption | [46] | |
| Rice bran | Rhizopus arrhizus | 100 mg L−1 | 6.83 mg g−1 | 90 min | Biosorption | [47] |
| Polyacrylamide beads | Enterobacter species | 10 mg L−1 | 42% | 30 min | Accumulation | [48] |
| Polyvinyl alcohol hydrogel | Aspergillus niger, strain B 77 | 0.9 mg L−1 | 48.9% | 5 min | Biosorption | [49] |
| Ca-alginate | Aspergillus niger, strain | 0.9 mg L−1 | 54.4% | 5 min | Biosorption | [49] |
| Peanut shell biochar | Pseudomonas hibiscicola strain L1 | 20 mg L−1 | 77.34% | 120 h | - | [43] |
| Saw dust biochar | Pseudomonas stutzeri | 10 mg L−1 | 83% | 36 h | Adsorption Bioaccumulation | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manikandan, S.K.; Nair, V. Pseudomonas stutzeri Immobilized Sawdust Biochar for Nickel Ion Removal. Catalysts 2022, 12, 1495. https://doi.org/10.3390/catal12121495
Manikandan SK, Nair V. Pseudomonas stutzeri Immobilized Sawdust Biochar for Nickel Ion Removal. Catalysts. 2022; 12(12):1495. https://doi.org/10.3390/catal12121495
Chicago/Turabian StyleManikandan, Soumya Koippully, and Vaishakh Nair. 2022. "Pseudomonas stutzeri Immobilized Sawdust Biochar for Nickel Ion Removal" Catalysts 12, no. 12: 1495. https://doi.org/10.3390/catal12121495
APA StyleManikandan, S. K., & Nair, V. (2022). Pseudomonas stutzeri Immobilized Sawdust Biochar for Nickel Ion Removal. Catalysts, 12(12), 1495. https://doi.org/10.3390/catal12121495








