The Advanced Synthesis of MOFs-Based Materials in Photocatalytic HER in Recent Three Years
Abstract
1. Introduction
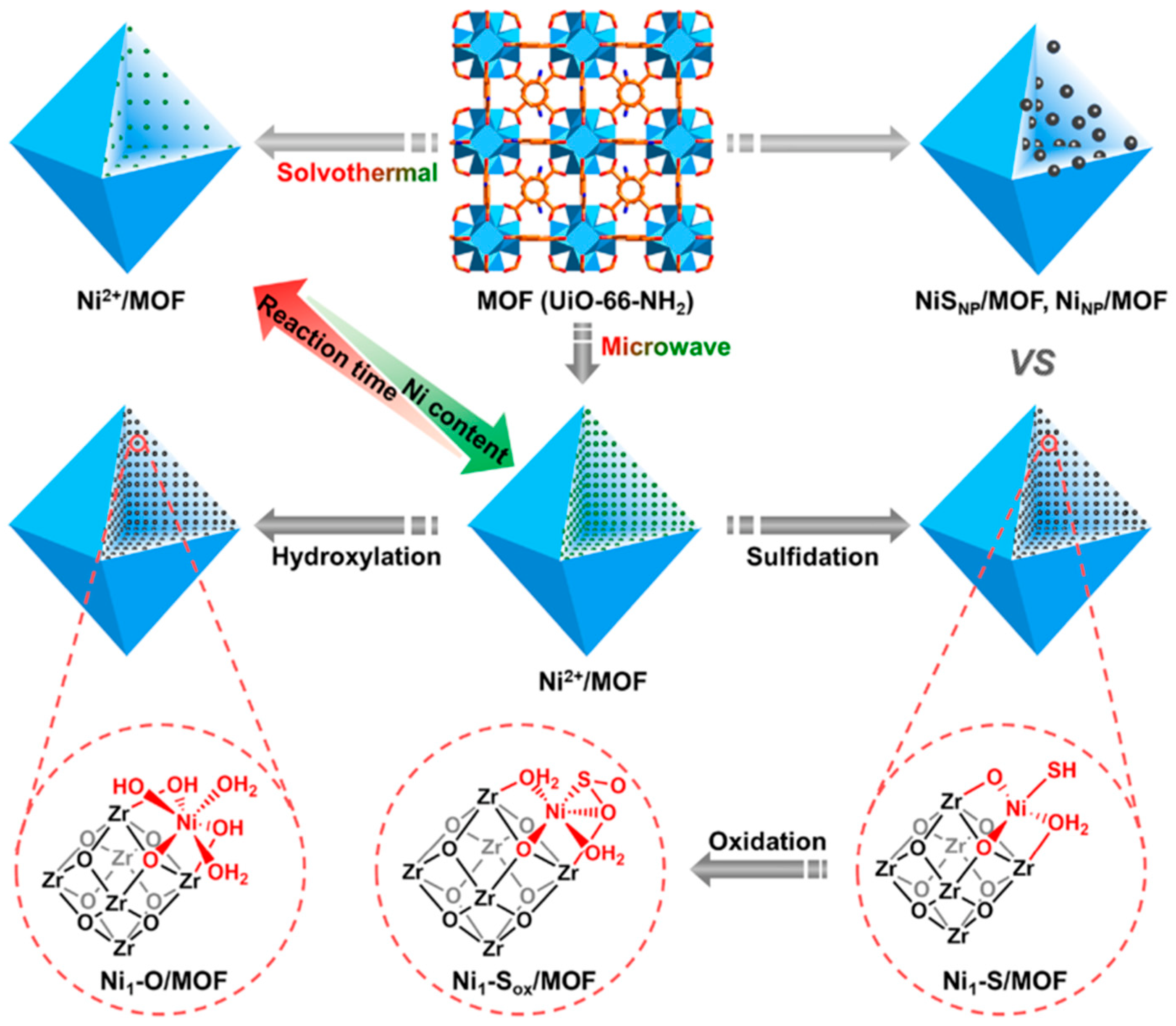
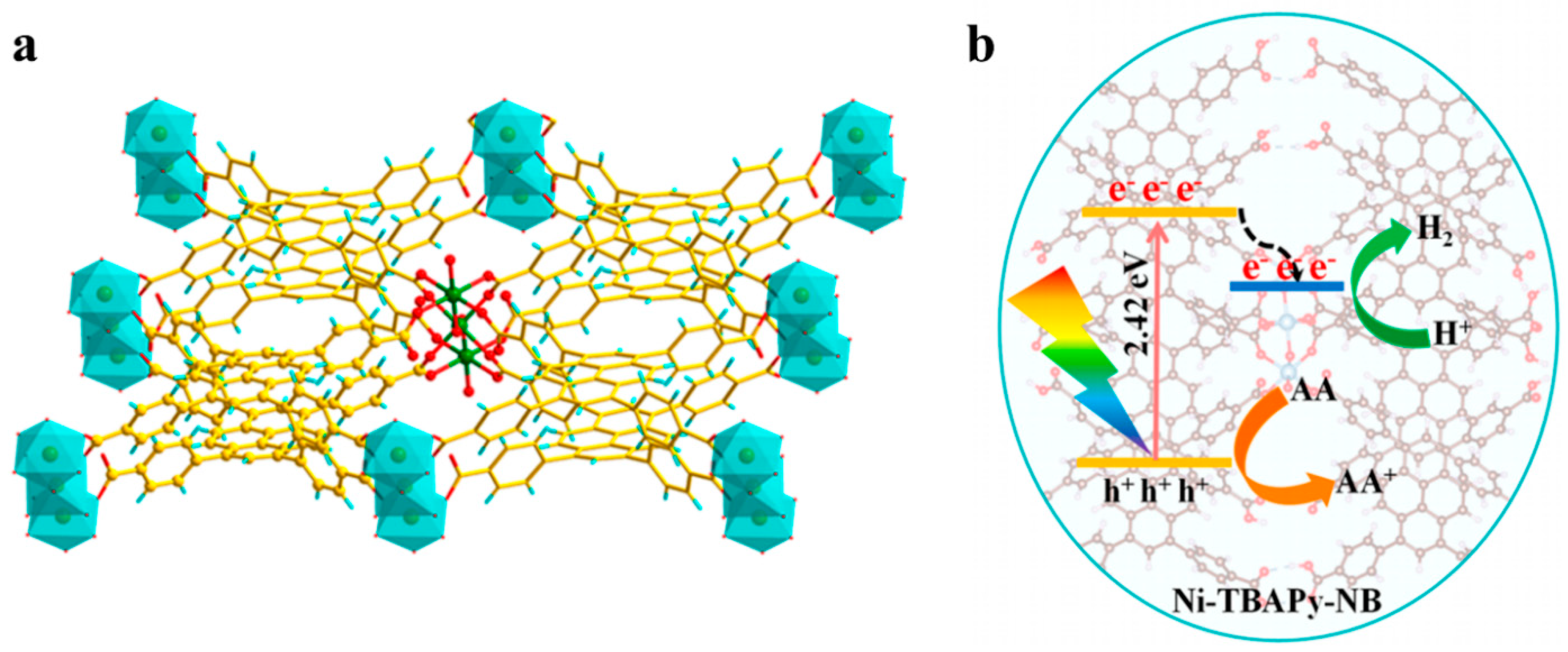
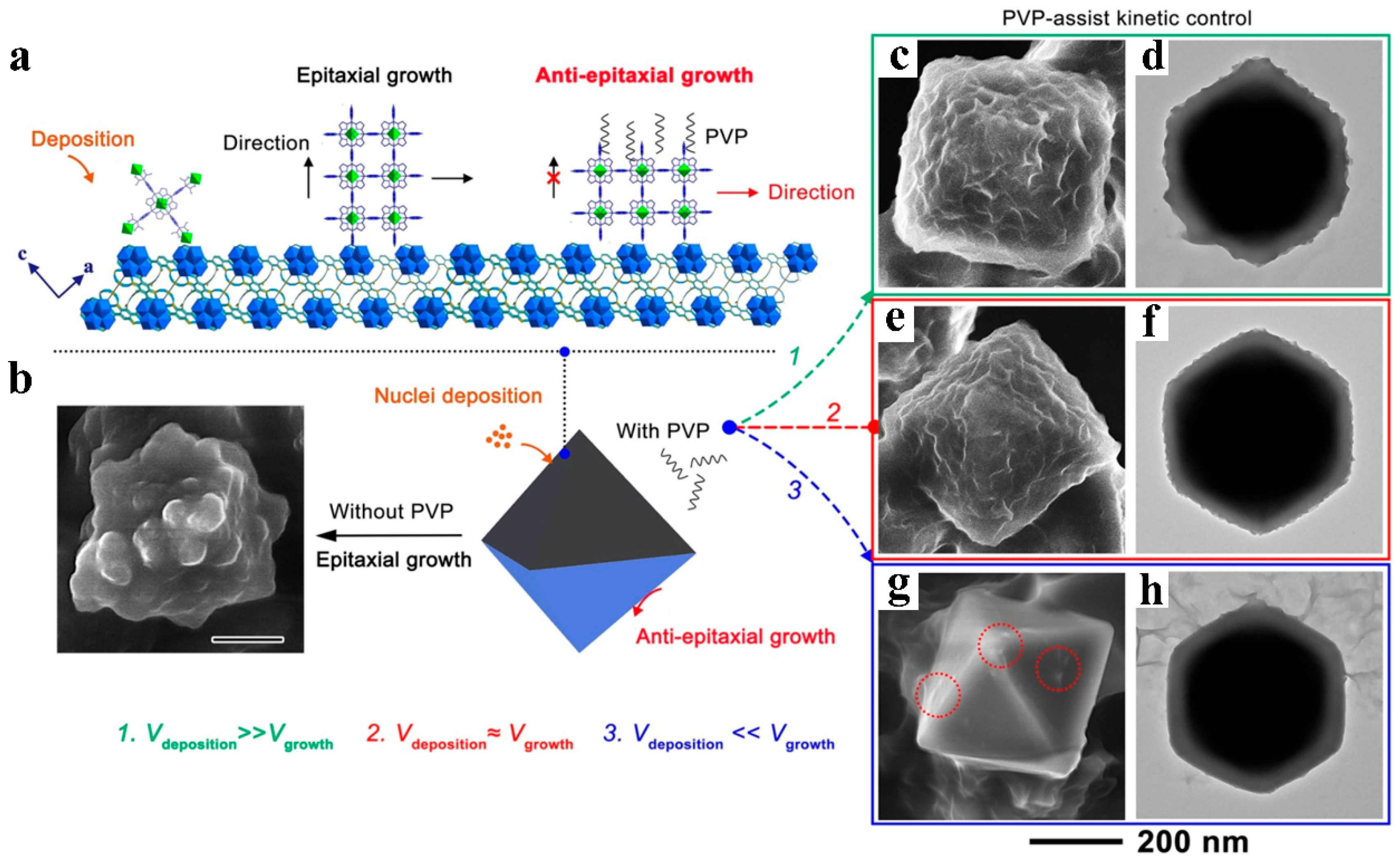
2. Internal Modification and Structure Optimization of MOFs
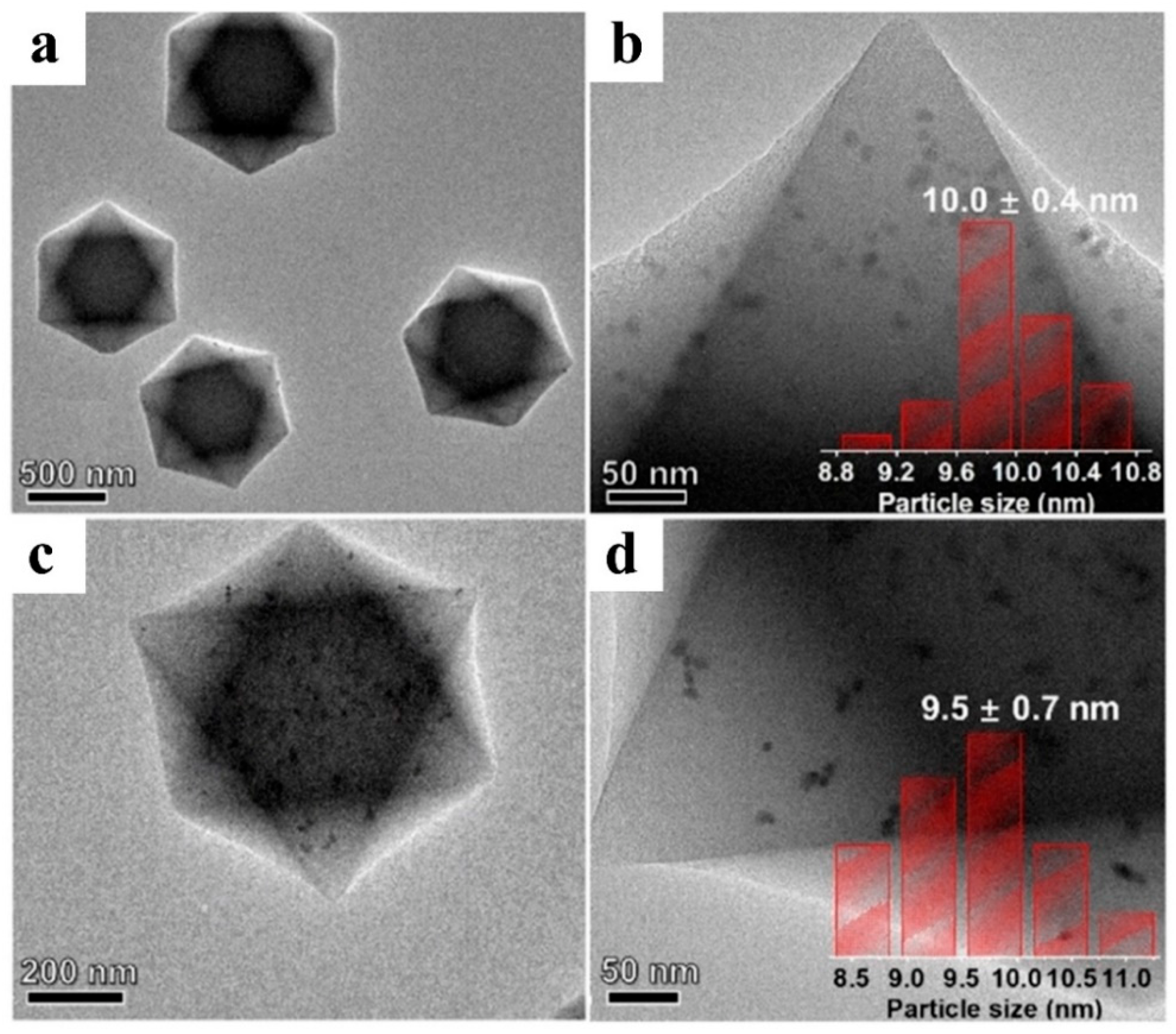
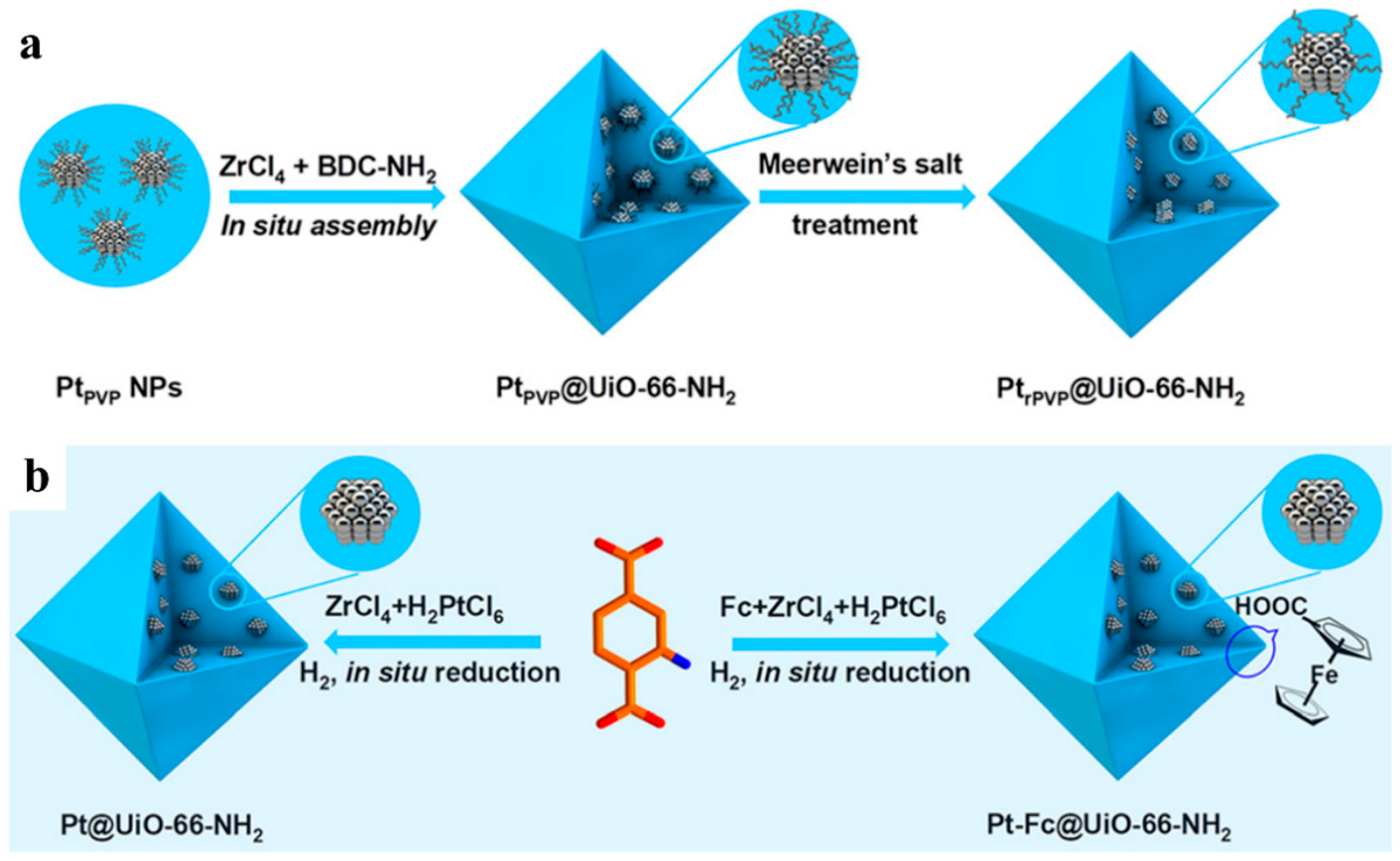
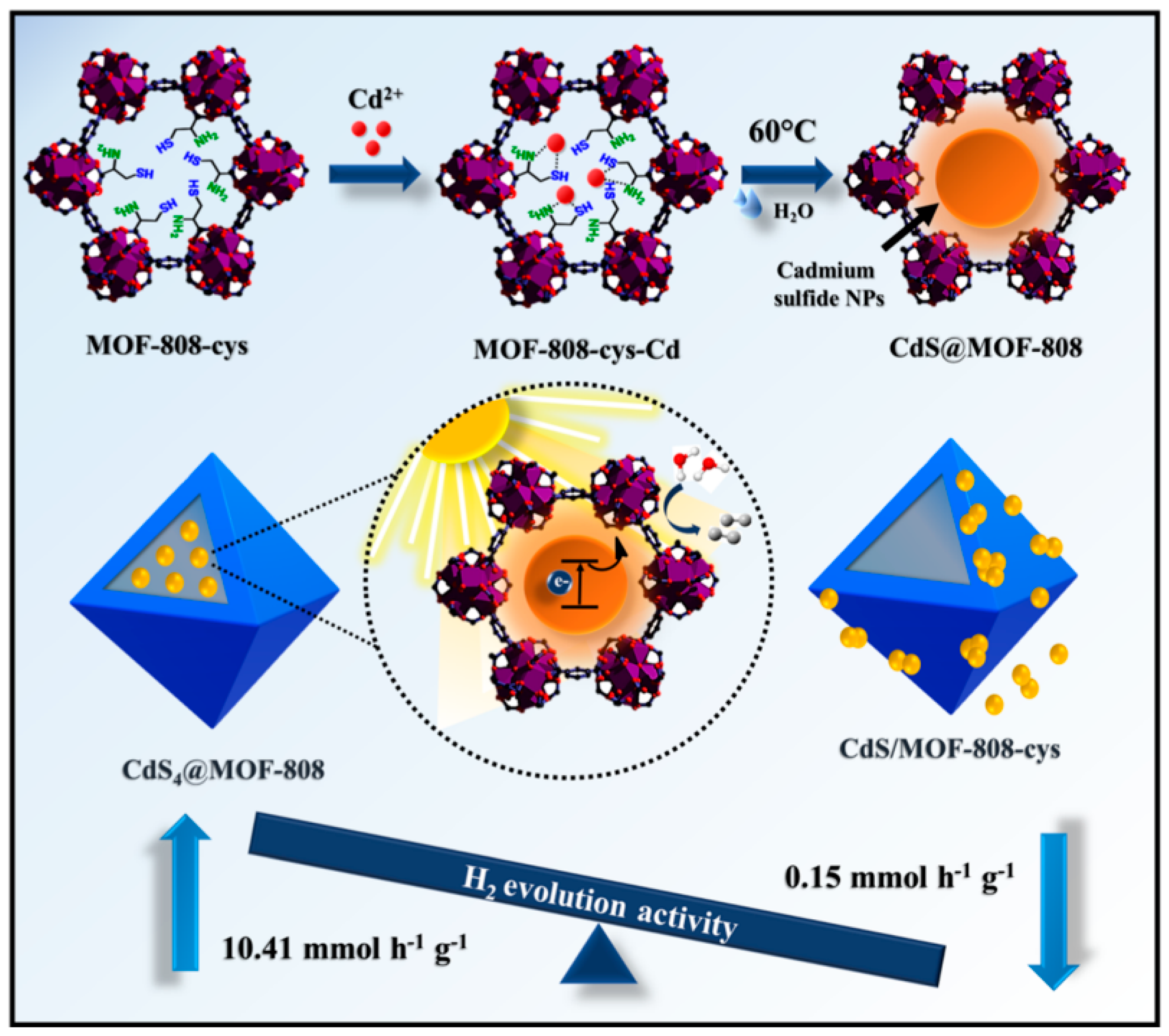
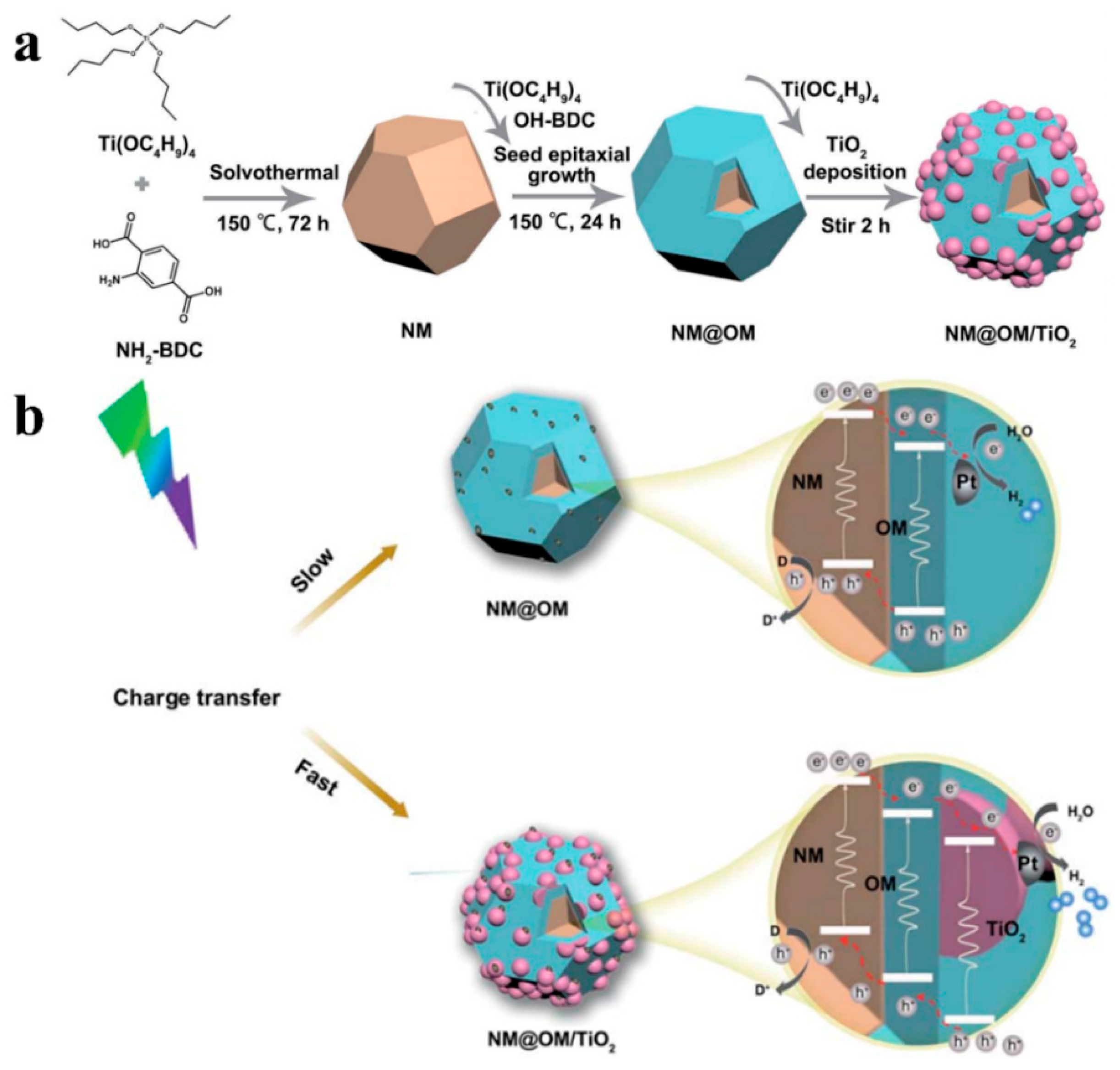
3. MOFs/Semiconductor Composites
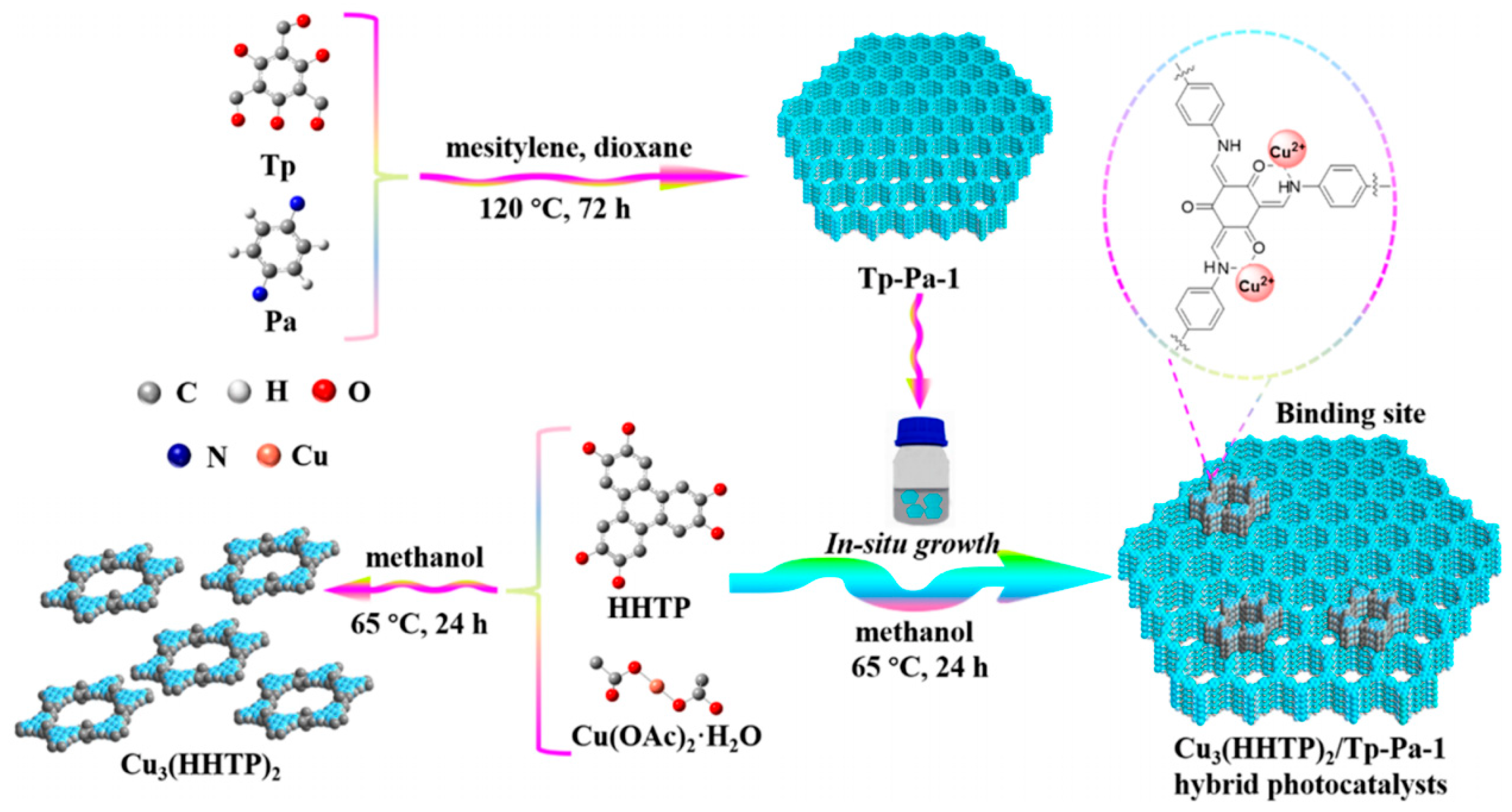
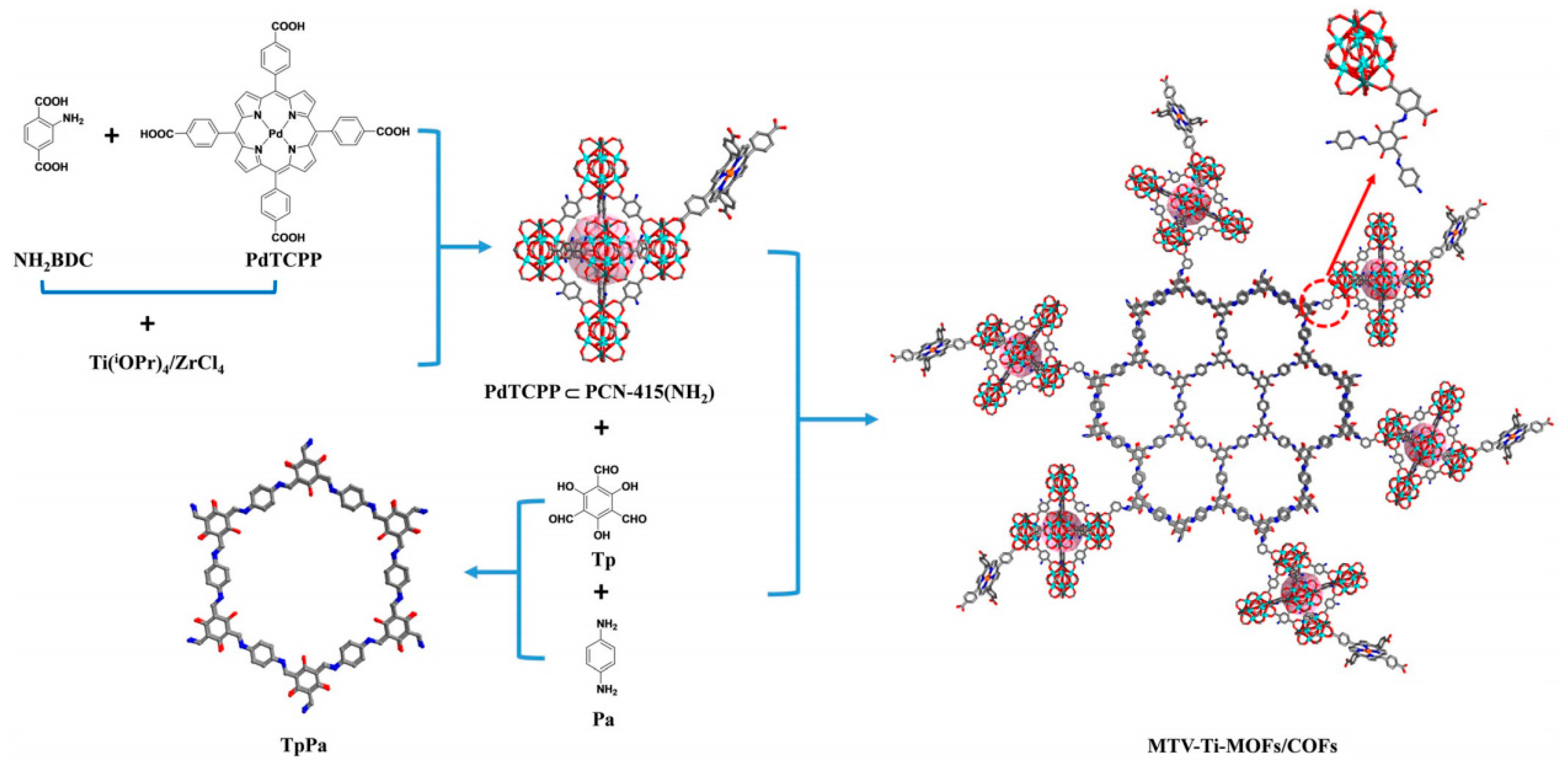
4. MOFs/COFs-Based Hybrids
5. MOF-Derived Materials
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Tian, D.; Denny, S.; Li, K.; Wang, H.; Kattel, S.; Chen, J. Density functional theory studies of transition metal carbides and nitrides as electrocatalysts. Chem. Soc. Rev. 2021, 50, 12338. [Google Scholar] [CrossRef] [PubMed]
- Guerra, O.; Eichman, J.; Denholm, P. Optimal energy storage portfolio for high and ultrahigh carbon-free and renewable power systems. Energy Environ. Sci. 2021, 14, 5132. [Google Scholar] [CrossRef]
- Lv, J.; Xie, J.; Mohamed, A.; Zhang, X.; Wang, Y. Photoelectrochemical energy storage materials: Design principles and functional devices towards direct solar to electrochemical energy storage. Chem. Soc. Rev. 2022, 51, 1511. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, P.; Fu, L.; Hu, Y.; Yang, L.; Wu, W.; Kong, X.; Jiang, L.; Wen, L. Engineered Cellulose Nanofiber Membranes with Ultrathin Low-Dimensional Carbon Material Layers for Photothermal-Enhanced Osmotic Energy Conversion. ACS Appl. Mater. Interfaces 2022, 14, 13223–13230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mian, M.R.; Lee, S.-J.; Chen, H.; Zhang, X.; Kirlikovali, K.O.; Shulda, S.; Melix, P.; Rosen, A.S.; Parilla, P.A.; et al. Fine-Tuning a Robust Metal-Organic Framework toward Enhanced Clean Energy Gas Storage. J. Am. Chem. Soc. 2021, 143, 18838–18843. [Google Scholar] [CrossRef]
- Lin, B.; Ma, B.; Chen, J.; Zhou, Y.; Zhou, J.; Yan, X.; Xue, C.; Luo, X.; Liu, Q.; Wang, J.; et al. Sea-urchin-like ReS2 nanosheets with charge edge-collection effect as a novel cocatalyst for high-efficiency photocatalytic H2 evolution. Chin. Chem. Lett. 2022, 33, 943–947. [Google Scholar] [CrossRef]
- Alves, H.; Frachoni, B.; Nunes, B.; Teixeira, P.; Paniago, R.; Bahnemann, D.; Paterno, L.; Patrocinio, A. Highly Stable Au/Hexaniobate Nanocomposite Prepared by a Green Intercalation Method for Photoinduced H2 Evolution Applications. ACS Appl. Energy Mater. 2022, 5, 8371–8380. [Google Scholar] [CrossRef]
- Bui, V.; Kumar, A.; Bui, H.; Lee, J.; Hwang, Y.; Le, H.; Kawazoe, Y.; Lee, H. Boosting Electrocatalytic HER Activity of 3D Interconnected CoSP via Metal Doping: Active and Stable Electrocatalysts for pH-Universal Hydrogen Generation. Chem. Mater. 2020, 32, 9591–9601. [Google Scholar] [CrossRef]
- Sun, Z.; Liang, Y.; Wu, Y.; Yu, Y.; Zhang, B. Boosting Electrocatalytic Hydrogen-Evolving Activity of Co/CoO Heterostructured Nanosheets via Coupling Photogenerated Carriers with Photothermy. ACS Sustain. Chem. Eng. 2018, 6, 11206–11210. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, Y.; Qi, S.; Zhang, K.; Chen, J.; Lou, Y.; Zhao, Y. Amorphous NiCoB-coupled MAPbI3 for efficient photocatalytic hydrogen evolution. Dalton Trans. 2021, 50, 17960. [Google Scholar] [CrossRef]
- Su, H.; Rao, C.; Zhou, L.; Pang, Y.; Lou, H.; Yang, D.; Qiu, X. Mo-Doped/Ni-supported ZnIn2S4-wrapped NiMoO4 S-scheme heterojunction photocatalytic reforming of lignin into hydrogen. Green Chem. 2022, 24, 2027. [Google Scholar] [CrossRef]
- Soltau, S.; Niklas, J.; Dahlberg, P.; Poluektov, O.; Tiede, D.; Mulfort, K.; Utschig, L. Aqueous light driven hydrogen production by a Ru–ferredoxin–Co biohybrid. Chem. Commun. 2015, 51, 10628–10631. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yu, Z.; Chen, D.; Zou, Z. Metal-complex chromophores for solar hydrogen generation. Chem. Soc. Rev. 2017, 46, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jacques, P.; Chavarot-Kerlidou, M.; Wang, M.; Sun, L.; Fontecave, M.; Artero, V. Phosphine Coordination to a Cobalt Diimine−Dioxime Catalyst Increases Stability during Light-Driven H2 Production. Inorg. Chem. 2012, 51, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Dai, H.; Li, Q.; Xu, F.; Mai, Y. A Hybrid Photocatalyst Composed of CdS Nanoparticles and Graphene Nanoribbons for Visible-Light-Driven Hydrogen Production. ACS Appl. Energy Mater. 2022, 5, 8621–8628. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, P.; Lin, J. Modulation of the Band Bending of CdS by Fluorination to Facilitate Photoinduced Electron Transfer for Efficient H2 Evolution over Pt/CdS. J. Phys. Chem. C 2022, 126, 7896–7902. [Google Scholar] [CrossRef]
- Xie, X.; Wang, R.; Ma, Y.; Chen, J.; Shi, Z.; Cui, Q.; Li, Z.; Xu, C. Sulfate-Functionalized Core−Shell ZnO/CdS/Ag2S Nanorod Arrays with Dual-Charge-Transfer Channels for Enhanced Photoelectrochemical Performance. ACS Appl. Energy Mater. 2022, 5, 6228–6237. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Cheng, B.; Zhang, L.; Al-Ghamdi, A.; Wageh, S.; Li, Y. Enhanced Photocatalytic H2-production Activity of CdS Nanoflower using Single Atom Pt and Graphene Quantum Dot as Dual Cocatalysts. Chin. J. Struct. Chem. 2022, 41, 2206006–2206014. [Google Scholar]
- Gao, R.; He, H.; Bai, J.; Hao, L.; Shen, R.; Zhang, P.; Li, Y.; Li, X. Pyrene-benzothiadiazole-based Polymer/CdS 2D/2D Organic/Inorganic Hybrid S-scheme Heterojunction for Efficient Photocatalytic H2 Evolution. Chin. J. Struct. Chem. 2022, 41, 2206031–2206038. [Google Scholar]
- Sahoo, S.; Mansingh, S.; Babu, P.; Parida, K. Black titania an emerging photocatalyst: Review highlighting the synthesis techniques and photocatalytic activity for hydrogen generation. Nanoscale Adv. 2021, 3, 5487. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Liu, X.; Zhou, J.; Zhao, H.; Wang, N.; Cui, Z.; Bai, J.; Zhao, Y. TiO2/g-C3N4 heterojunction hollow porous nanofibers as superior visible-light photocatalysts for H2 evolution and dye degradation. N. J. Chem. 2021, 45, 22123. [Google Scholar] [CrossRef]
- Pan, Y.; Xiong, B.; Li, Z.; Wu, Y.; Yan, C.; Song, H. In situ constructed oxygen-vacancy-rich MoO3-x/ porous g-C3N4 heterojunction for synergistically enhanced photocatalytic H2 evolution. RSC Adv. 2021, 11, 31219. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sun, X.; Liu, Y.; Ji, H.; Liu, W.; Wang, C.; Ma, W.; Cai, Z. A carbon-rich g-C3N4 with promoted charge separation for highly efficient photocatalytic degradation of amoxicillin. Chin. Chem. Lett. 2021, 32, 2787–2791. [Google Scholar]
- Hu, Y.; Li, X.; Wang, W.; Deng, F.; Han, L.; Gao, X.; Feng, Z.; Chen, Z.; Huang, J.; Zeng, F. Bi and S Co-doping g-C3N4 to Enhance Internal Electric Field for Robust Photocatalytic Degradation and H2 Production. Chin. J. Struct. Chem. 2022, 41, 2206069–2206078. [Google Scholar]
- Tao, S.; Wan, S.; Huang, Q.; Li, C.; Yu, J.; Cao, S. Molecular Engineering of g-C3N4 with Dibenzothiophene Groups as Electron Donor for Enhanced Photocatalytic H2-Production. Chin. J. Struct. Chem. 2022, 41, 2206048–2206054. [Google Scholar]
- Tang, Y.; Hu, X.; Liu, C. Perfect inhibition of CdS photocorrosion by graphene sheltering engineering on TiO2 nanotube array for highly stable photocatalytic activity. Phys. Chem. Chem. Phys. 2014, 16, 25321. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Zhao, H.; Dou, M.; Yin, X.; Yang, H.; Li, D.; Dou, J. A novel dinuclear cobalt-bis(thiosemicarbazone) complex as a cocatalyst to enhance visible-light-driven H2 evolution on CdS nanorods and a mechanism discussion. J. Photoch. Photobio. A 2022, 426, 113771. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Xu, P.; Xue, W.; Lei, L.; Cheng, M.; Wang, R.; Liu, X.; Deng, R. Semiconductor-based photocatalysts for photocatalytic and photoelectrochemical water splitting: Will we stop with photocorrosion? J. Mater. Chem. A 2020, 8, 2286. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, S.; Zhang, Q.; Luo, Y. Intermediate Complex-Mediated Interfacial Electron Transfer in a Radical Dianion/TiO2 Dye-Sensitized Photocatalytic System. J. Phys. Chem. Lett. 2022, 13, 8091–8096. [Google Scholar] [CrossRef]
- Yuan, C.; Shen, Y.; Zhu, C.; Zhu, P.; Yang, F.; Liu, J.; An, C. Ru Single-Atom Decorated Black TiO2 Nanosheets for Efficient Solar-Driven Hydrogen Production. ACS Sustain. Chem. Eng. 2022, 10, 10311–10317. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, C.; Jiang, N.; Zhao, Z.; Zhou, X.; Zhao, S.; Xu, A. g-C3N4 Hydrogen-Bonding Viologen for Significantly Enhanced Visible-Light Photocatalytic H2 Evolution. ACS Catal. 2017, 7, 8228–8234. [Google Scholar] [CrossRef]
- Hong, I.; Chen, Y.; Hsu, Y.; Yong, K. Triple-Channel Charge Transfer over W18O49/Au/g-C3N4 Z-Scheme Photocatalysts for Achieving Broad-Spectrum Solar Hydrogen Production. ACS Appl. Mater. Interfaces 2021, 13, 52670–52680. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lan, H.; Guan, G.; Yang, Q. Amino-Functionalized Microporous MOFs for Capturing Greenhouse Gases CF4 and NF3 with Record Selectivity. ACS Appl. Mater. Interfaces 2022, 14, 40072–40081. [Google Scholar] [CrossRef] [PubMed]
- Tarasi, S.; Ramazani, A.; Ramazani, A.; Hu, M.; Ghafghazi, S.; Tarasi, R.; Ahmadi, Y. Drug Delivery Using Hydrophilic Metal−Organic Frameworks (MOFs): Effect of Structure Properties of MOFs on Biological Behavior of Carriers. Inorg. Chem. 2022, 61, 13125–13132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Zong, M.; Xu, J.; Wang, D.; Bu, X. Energy Level Engineering: Ru Single Atom Anchored on Mo-MOF with a [Mo8O26(im)2]4− Structure Acts as a Biomimetic Photocatalyst. ACS Catal. 2022, 12, 7960–7974. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, G.; Kaur, J.; Singh, P. First transition series metal-organic frameworks: Synthesis, properties and applications. Mater. Adv. 2021, 2, 7308. [Google Scholar] [CrossRef]
- Li, K.; Yang, J.; Gu, J. Hierarchically Porous MOFs Synthesized by Soft-Template Strategies. Acc. Chem. Res. 2022, 55, 2235–2247. [Google Scholar] [CrossRef]
- Hao, J.; Geng, L.; Zheng, J.; Wei, J.; Zhang, L.; Feng, R.; Zhao, J.; Li, Q.; Pang, J.; Bu, X. Ligand Induced Double-Chair Conformation Ln12 Nanoclusters Showing Multifunctional Magnetic and Proton Conductive Properties. Inorg. Chem. 2022, 61, 3690–3696. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Yan, B. A multicolor-switchable fluorescent lanthanide MOFs triggered by anti-cancer drugs: Multifunctional platform for anti-cancer drug sensing and information anticounterfeiting. J. Mater. Chem. C 2022, 10, 3576. [Google Scholar] [CrossRef]
- Sharp, C.; Bukowski, B.; Li, H.; Johnson, E.; Ilic, S.; Morris, A.; Gersappe, D.; Snurr, R.; Morris, J. Nanoconfinement and mass transport in metal-organic frameworks. Chem. Soc. Rev. 2021, 50, 11530. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, Y.; Zhao, S.; Zhang, X.; Liu, Q.; Shi, X. Research progress in metal–organic frameworks (MOFs) in CO2 capture from post-combustion coalfired flue gas: Characteristics, preparation, modification and applications. J. Mater. Chem. A 2022, 10, 5174. [Google Scholar] [CrossRef]
- Zeeshan, M.; Shahid, M. State of the art developments and prospects of metal–organic frameworks for energy applications. Dalton Trans. 2022, 51, 1675. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jia, X.; Chen, H.; Chang, Z.; Li, L.; Wang, Y.; Li, J. Tuning the Pore Environment of MOFs toward Efficient CH4/N2 Separation under Humid Conditions. ACS Appl. Mater. Interfaces 2022, 14, 15830–15839. [Google Scholar] [CrossRef]
- Lisensky, G.; Yaghi, O. Visualizing Pore Packing and Topology in MOFs. J. Chem. Educ. 2022, 99, 1998–2004. [Google Scholar] [CrossRef]
- Ahmad, M.; Castro-Muñoz, R.; Budd, P. Boosting gas separation performance and suppressing the physical aging of polymers of intrinsic microporosity (PIM-1) by nanomaterial blending. Nanoscale 2020, 12, 23333. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, C.; Yu, M.; Bu, X. A unique 3D microporous MOF constructed by cross-linking 1D coordination polymer chains for effectively selective separation of CO2/CH4 and C2H2/CH4. Chin. Chem. Lett. 2021, 32, 1153–1156. [Google Scholar] [CrossRef]
- Qiao, Y.; Chang, X.; Zheng, J.; Yi, M.; Chang, Z.; Yu, M.; Bu, X. Self-Interpenetrated Water-Stable Microporous Metal−Organic Framework toward Storage and Purification of Light Hydrocarbons. Inorg. Chem. 2021, 60, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Liu, X.; Li, N.; Zhao, J.; Chang, Z.; Zheng, J.; Bu, X. Structural Transformation and Spatial Defect Formation of a Co(II) MOF Triggered by Varied Metal-Center Coordination Configuration. Inorg. Chem. 2020, 59, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chang, Z.; Li, N.; He, J.; Fu, Z.; Bu, X. Efficient Regulation of Energy Transfer in a Multicomponent Dye-Loaded MOF for White-Light Emission Tuning. ACS Appl. Mater. Interfaces 2020, 12, 51589–51597. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Zhu, Z.; Kottilil, D.; Rath, B.; Tian, W.; Tan, Z.; Liu, X.; Xu, Q.; Ji, W.; Vittal, J. Impact of the Structural Modification of Diamondoid Cd(II) MOFs on the Nonlinear Optical Properties. ACS Appl. Mater. Interfaces 2021, 13, 60163–60172. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Q.; Lv, J.; Li, Y.; Wang, K.; Li, J. Stable Metal-Organic Frameworks for Fluorescent Detection of Tetracycline Antibiotics. Inorg. Chem. 2022, 61, 8015–8021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, S.; Gan, Y.; Zhang, B.; Chen, L. Recent Progresses in Lanthanide Metal-organic Frameworks (Ln-MOFs) as Chemical Sensors for Ions, Antibiotics and Amino Acids. Chin. J. Struct. Chem. 2022, 41, 2211045–2211070. [Google Scholar] [CrossRef]
- Jin, S. How to Effectively Utilize MOFs for Electrocatalysis. ACS Energy Lett. 2019, 4, 1443–1445. [Google Scholar] [CrossRef]
- Dou, S.; Li, X.; Wang, X. Rational Design of Metal−Organic Frameworks towards Efficient Electrocatalysis. ACS Materials Lett. 2020, 2, 1251–1267. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, T.; Xu, X.; Wang, X.; Zhou, J.; Xie, H.; Liu, Z.; Chu, L.; Huang, M. Coupling of NiFe-Based Metal-Organic Framework Nanosheet Arrays with Embedded Fe-Ni3S2 Clusters as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Chin. J. Struct. Chem. 2022, 41, 2207074–2207080. [Google Scholar]
- Xia, T.; Lin, Y.; Li, W.; Ju, M. Photocatalytic degradation of organic pollutants by MOFs based materials: A review. Chin. Chem. Lett. 2021, 32, 1153–1156. [Google Scholar] [CrossRef]
- Kim, H.; Kim, N.; Ryu, J. Porous framework-based hybrid materials for solar-to-chemical energy conversion: From powder photocatalysts to photoelectrodes. Inorg. Chem. Front. 2021, 8, 4107. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, R.; Li, D.; Dou, J. A Review on Crystalline Porous MOFs Materials in Photocatalytic Transformations of Organic Compounds in Recent Three Years. Chin. J. Struct. Chem. 2022, 41, 2211071–2211083. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Zabihi, O.; Ahmadi, M.; Li, Q.; Blanchard, P.; Kiziltas, A.; Naebe, M. Metal-organic framework structure-property relationships for high-performance multifunctional polymer nanocomposite applications. J. Mater. Chem. A 2021, 9, 4348. [Google Scholar] [CrossRef]
- Li, X.; Rajasree, S.; Yu, J.; Deria, P. The role of photoinduced charge transfer for photocatalysis, photoelectrocatalysis and luminescence sensing in metal-organic frameworks. Dalton Trans. 2020, 49, 12892. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Yang, W.; Mao, G.; Zheng, L.; Jiang, H. Modulating Coordination Environment of Single-Atom Catalysts and Their Proximity to Photosensitive Units for Boosting MOF Photocatalysis. J. Am. Chem. Soc. 2021, 143, 12220–12229. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, J.; Chen, S.; Yang, W.; Ding, C.; Waseem, A.; Jiang, H. Linker engineering in metal-organic frameworks for dark photocatalysis. Chem. Sci. 2022, 13, 6696. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, S.; Guo, X.; Xiao, Y.; Yin, Z.; Yang, N.; Bao, Y.; Zhu, X.; Jin, S.; Feng, Z.; et al. Water-Stable Nickel Metal-Organic Framework Nanobelts for Cocatalyst-Free Photocatalytic Water Splitting to Produce Hydrogen. J. Am. Chem. Soc. 2022, 144, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Niu, B.; Liu, B.; Li, J.; Duan, W. Ultra-stable two-dimensional metal-organic frameworks for photocatalytic H2 production. Nanoscale 2022, 14, 7146. [Google Scholar] [CrossRef]
- Indra, A.; Acharjya, A.; Menezes, P.; Merschjann, C.; Hollmann, D.; Schwarze, M.; Aktas, M.; Friedrich, A.; Lochbrunner, S.; Thomas, A.; et al. Boosting Visible-Light-Driven Photocatalytic Hydrogen Evolution with an Integrated Nickel Phosphide-Carbon Nitride System. Angew. Chem. 2017, 129, 1675–1679. [Google Scholar] [CrossRef]
- Bi, W.; Zhang, L.; Sun, Z.; Li, X.; Jin, T.; Wu, X.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Insight into Electrocatalysts as Co-catalysts in Efficient Photocatalytic Hydrogen Evolution. ACS Catal. 2016, 6, 4253–4257. [Google Scholar] [CrossRef]
- Callejas, J.; McEnaney, J.; Read, C.; Crompton, J.; Biacchi, A.; Popczun, E.; Gordon, T.; Lewis, N.; Schaak, R. Electrocatalytic and Photocatalytic Hydrogen Production from Acidic and Neutral-pH Aqueous Solutions Using Iron Phosphide Nanoparticles. ACS Nano 2014, 8, 11101–11107. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Y.; Wang, C.; Lv, X.; Fu, W. Spectacular photocatalytic hydrogen evolution using metal-phosphide/CdS hybrid catalysts under sunlight irradiation. Chem. Commun. 2015, 51, 8708. [Google Scholar] [CrossRef]
- Sun, K.; Liu, M.; Pei, J.; Li, D.; Ding, C.; Wu, K.; Jiang, H. Incorporating Transition-Metal Phosphides into Metal-Organic Frameworks for Enhanced Photocatalysis. Angew. Chem. Int. Ed. 2020, 59, 22749–22755. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.; Sun, K.; Jiao, L.; Xie, C.; Ding, C.; Jiang, H. Interfacial Microenvironment Modulation Boosting Electron Transfer between Metal Nanoparticles and MOFs for Enhanced Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 16372–16376. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Li, J.; Yuan, Y.; Yang, J.; Xu, W.; An, P.; Xi, S.; Guo, J.; Liu, B.; et al. Two-Dimensional-on-Three-Dimensional Metal-Organic Frameworks for Photocatalytic H2 Production. Angew. Chem. 2022, 61, e202211031. [Google Scholar] [CrossRef]
- Ghosh, A.; Karmakar, S.; Rahimi, A.; Roy, R.; Nath, S.; Gautam, U.; Maji, T. Confinement Matters: Stabilization of CdS Nanoparticles inside a Postmodified MOF toward Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2022, 14, 25220–25231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, C.; Gao, Y.; Tao, X.; Ding, C.; Fan, F.; Jiang, H. Charge Separation by Creating Band Bending in Metal-Organic Frameworks for Improved Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202204108. [Google Scholar]
- Chen, Y.; Yang, D.; Xin, X.; Yang, Z.; Gao, Y.; Shi, Y.; Zhao, Z.; An, K.; Wang, W.; Tan, J.; et al. Multi-stepwise charge transfer via MOF@MOF/TiO2 dual-heterojunction photocatalysts towards hydrogen evolution. J. Mater. Chem. A 2022, 10, 9717. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Qi, H.; Chen, Y.; Guo, W.; Yu, H.; Chen, H.; Ying, Y. Humidity-Independent Artificial Olfactory Array Enabled by Hydrophobic Core-Shell Dye/MOFs@COFs Composites for Plant Disease Diagnosis. ACS Nano 2022, 16, 14297–14307. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jang, S.; Yim, S.; Ye, L.; Kim, D. Metal Doped Core–Shell Metal-Organic Frameworks@ Covalent Organic Frameworks (MOFs@COFs) Hybrids as a Novel Photocatalytic Platform. Adv. Funct. Mater. 2018, 28, 1707110. [Google Scholar] [CrossRef]
- Garzon-Tovar, L.; Perez-Carvajal, J.; Yazdi, A.; Hernandez-Munoz, J.; Tarazona, P.; Imaz, I.; Zamora, F.; Maspoch, D. A MOF@COF Composite with Enhanced Uptakethrough Interfacial Pore Generation. Angew. Chem. 2019, 131, 9612–9616. [Google Scholar] [CrossRef]
- Peng, H.; Raya, J.; Richard, F.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Samorì, P. Synthesis of Robust MOFs@COFs Porous Hybrid Materials via an Aza-Diels-Alder Reaction: Towards High-Performance Supercapacitor Materials. Angew. Chem. Int. Ed. 2020, 59, 19602–19609. [Google Scholar] [CrossRef]
- Xue, P.; Pan, X.; Huang, J.; Gao, Y.; Guo, W.; Li, J.; Tang, M.; Wang, Z. In Situ Fabrication of Porous MOF/COF Hybrid Photocatalysts for Visible-Light-Driven Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 59915–59924. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, Y.; Zhong, X.; Lan, P.; Wei, Z.; Pan, H.; Su, P.; Song, Y.; Chen, Y.; Nafady, A.; et al. Enhancing Photocatalytic Hydrogen Production via the Construction of Robust Multivariate Ti-MOF/COF Composites. Angew. Chem. Int. Ed. 2022, 61, e202114071. [Google Scholar]
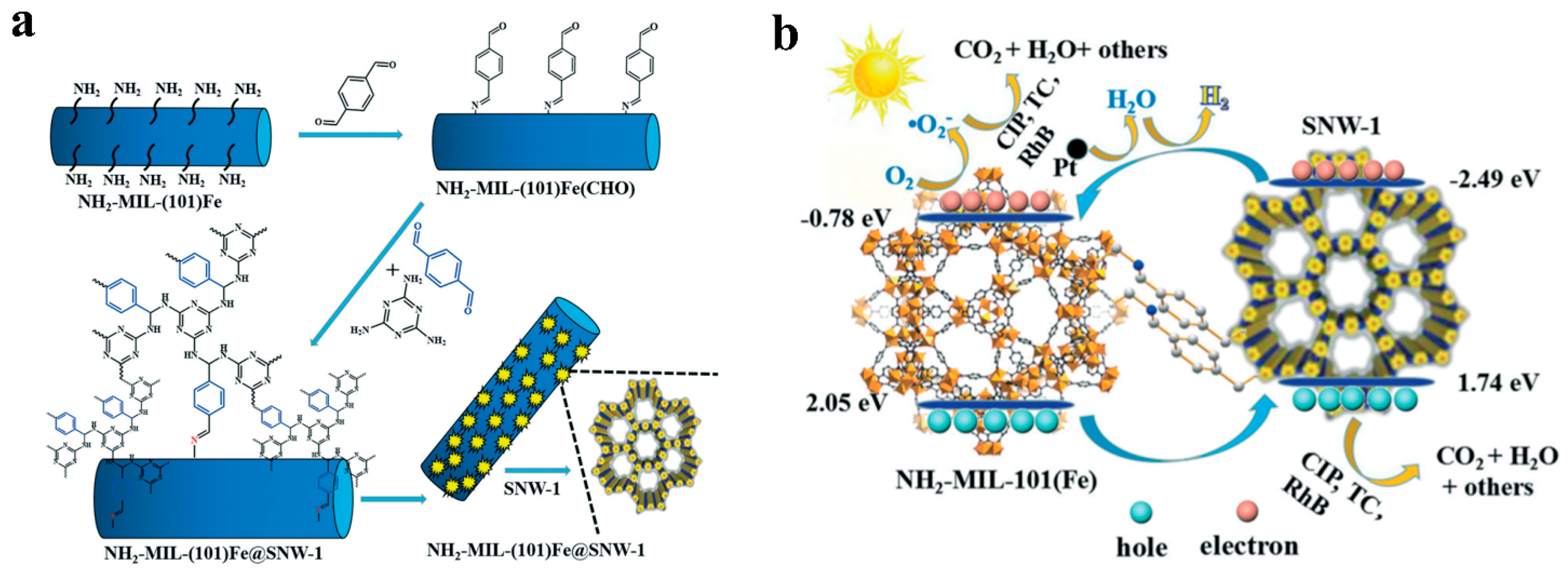
- Wang, Y.; Yang, H.; Shang, Q.; Cheng, Q.; Zhou, H.; Pan, Z. Integrating hollow spherical covalent organic frameworks on NH2-MIL-101(Fe) as high performance heterogeneous photocatalysts. Environ. Sci. Nano 2022, 9, 3081–3093. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Shi, B.; Dai, W.; Ren, H.; An, K.; Zhou, Z.; Zhao, Z.; Wang, W.; Jiang, Z. In situ construction of hydrazone-linked COF-based core-shell hetero-frameworks for enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2020, 8, 7724. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.; Lou, X. Construction of ZnIn2S4−In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Torad, N.; Hu, M.; Ishihara, S.; Sukegawa, H.; Belik, A.; Imura, M.; Ariga, K.; Sakka, Y.; Yamauchi, Y. Direct Synthesis of MOF-Derived Nanoporous Carbon with Magnetic Co Nanoparticles toward Efficient Water Treatment. Small 2014, 10, 2096–2107. [Google Scholar] [CrossRef]
- Qi, W.; Wang, C.; Yu, J.; Adimi, S.; Thomas, T.; Guo, H.; Liu, S.; Yang, M. MOF-Derived Porous Ternary Nickel Iron Nitride Nanocube as a Functional Catalyst toward Water Splitting Hydrogen Evolution for Solar to Chemical Energy Conversion. ACS Appl. Energy Mater. 2022, 5, 6155–6162. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, S.; Wu, W.; Qi, Y.; Lin, Z.; Li, Y.; Qin, Y. Hierarchical Hollow Zinc Oxide Nanocomposites Derived from Morphology-Tunable Coordination Polymers for Enhanced Solar Hydrogen Production. Angew. Chem. Int. Ed. 2022, 61, e202205312. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Jin, Z.; Tsubaki, N. Spatially separated catalytic sites supplied with the CdS-MoS2-In2O3 ternary dumbbell S-scheme heterojunction for enhanced photocatalytic hydrogen production. J. Mater. Chem. A 2022, 10, 10715. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Wang, X.; Li, L.; Li, Y.; Dai, W. In-N-In Sites Boosting Interfacial Charge Transfer in CarbonCoated Hollow Tubular In2O3/ZnIn2S4 Heterostructure Derived from In-MOF for Enhanced Photocatalytic Hydrogen Evolution. ACS Catal. 2021, 11, 6276–6289. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Li, X.; Wang, X.; Jin, Z. Design and synthesis of phosphating bimetallic CeCo-MOF for substantially improved photocatalytic hydrogen evolution. J. Mater. Chem. C 2022, 10, 8750. [Google Scholar] [CrossRef]
- Zhuang, G.; Fang, Q.; Wei, J.; Yang, C.; Chen, M.; Lyu, Z.; Zhuang, Z.; Yu, Y. Branched In2O3 Mesocrystal of Ordered Architecture Derived from the Oriented Alignment of a Metal-Organic Framework for Accelerated Hydrogen Evolution over In2O3-ZnIn2S4. ACS Appl. Mater. Interfaces 2021, 13, 9804–9813. [Google Scholar] [CrossRef]
- Hou, Q.; Li, X.; Pi, Y.; Xiao, J. Construction of In2S3@NH2-MIL-68(In)@In2S3 Sandwich Homologous Heterojunction for Efficient CO2 Photoreduction. Ind. Eng. Chem. Res. 2020, 59, 20711–20718. [Google Scholar] [CrossRef]
- Bariki, R.; Das, K.; Pradhan, S.; Prusti, B.; Mishra, B. MOF-Derived Hollow Tubular In2O3/MIIIn2S4 (MII: Ca, Mn, and Zn) Heterostructures: Synergetic Charge-Transfer Mechanism and Excellent Photocatalytic Performance to Boost Activation of Small Atmospheric Molecules. ACS Appl. Energy Mater. 2022, 5, 11002–11017. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.; Xu, H.; Su, J.; Rossi, D.; Chen, Y.; Zhang, L.; Lollar, C.; Wang, Q.; Jiang, H.; et al. [Ti8Zr2O12(COO)16] Cluster: An Ideal Inorganic Building Unit for Photoactive Metal-Organic Frameworks. ACS Cent. Sci. 2018, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Vu, T.; Le, D.; Doan, T.; Nguyen, V.; Phan, N. A Titanium-Organic Framework: Engineering of the Band-Gap Energy for Photocatalytic Property Enhancement. ACS Catal. 2017, 7, 338–342. [Google Scholar] [CrossRef]
- Gao, K.; Li, H.; Meng, Q.; Wu, J.; Hou, H. Efficient and Selective Visible-Light-Driven Oxidative Coupling of Amines to Imines in Air over CdS@Zr-MOFs. ACS Appl. Mater. Interfaces 2021, 13, 2779–2787. [Google Scholar] [CrossRef]
- Chen, P.; He, X.; Pang, M.; Dong, X.; Zhao, S.; Zhang, W. Iodine Capture Using Zr-Based Metal-Organic Frameworks (ZrMOFs): Adsorption Performance and Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 20429–20439. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, T.; Huang, H.; Yu, M.; Fan, X.; Chang, Z.; Bu, X. Metal-Organic-Framework-Based Photocatalysts Optimized by Spatially Separated Cocatalysts for Overall Water Splitting. Adv. Mater. 2020, 32, 2004747. [Google Scholar] [CrossRef]
- Mao, L.; Lu, B.; Shi, J.; Zhang, Y.; Kang, X.; Chen, Y.; Jin, H.; Guo, L. Rapid high-temperature hydrothermal post treatment on graphitic carbon nitride for enhanced photocatalytic H2 evolution. Catal. Today 2022, in press. [CrossRef]
- Soares, C.; Maurin, G.; Leitão, A. Computational Exploration of the Catalytic Degradation of Sarin and Its Simulants by a Titanium Metal-Organic Framework. J. Phys. Chem. C 2019, 123, 19077–19086. [Google Scholar] [CrossRef]







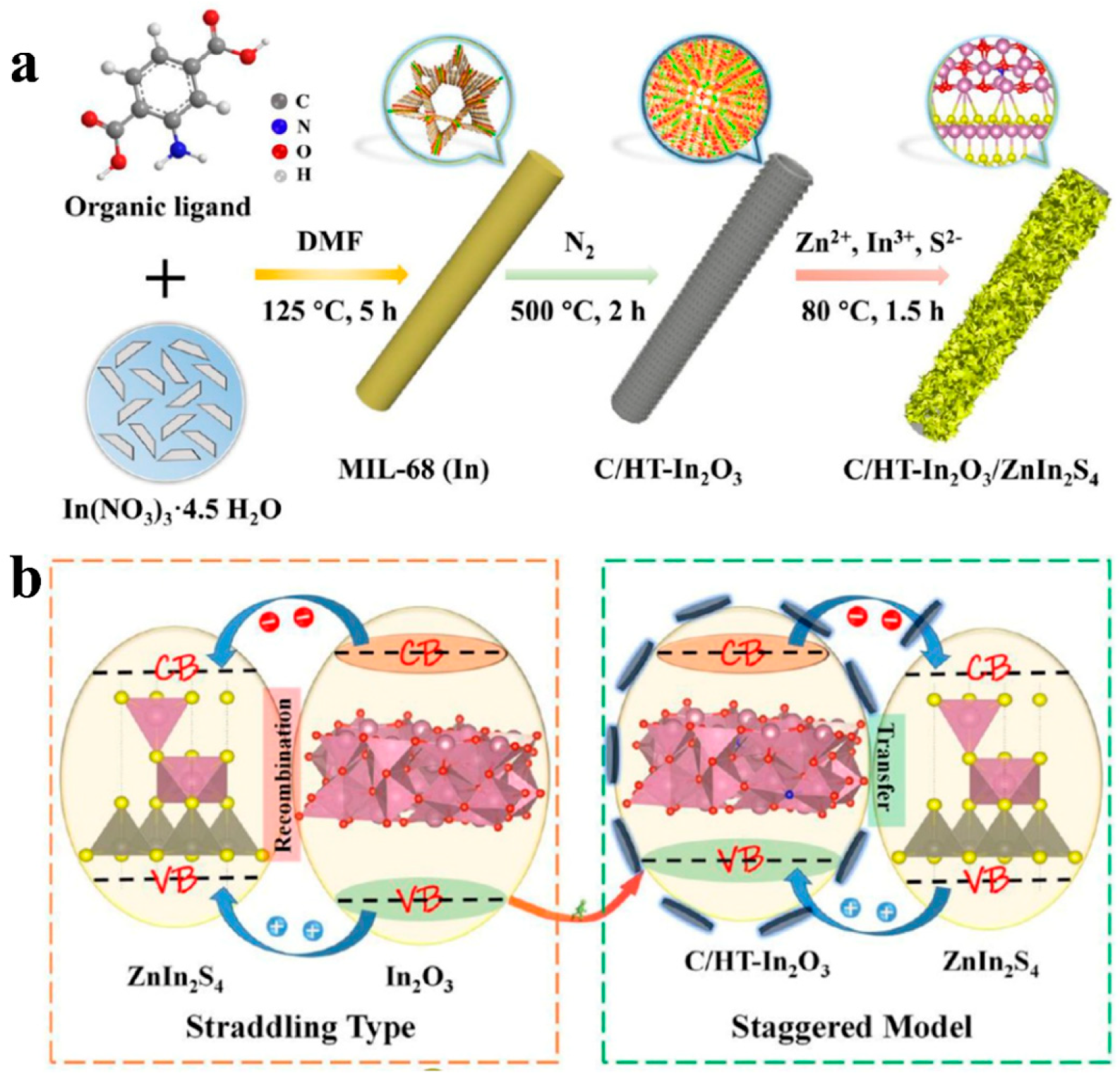
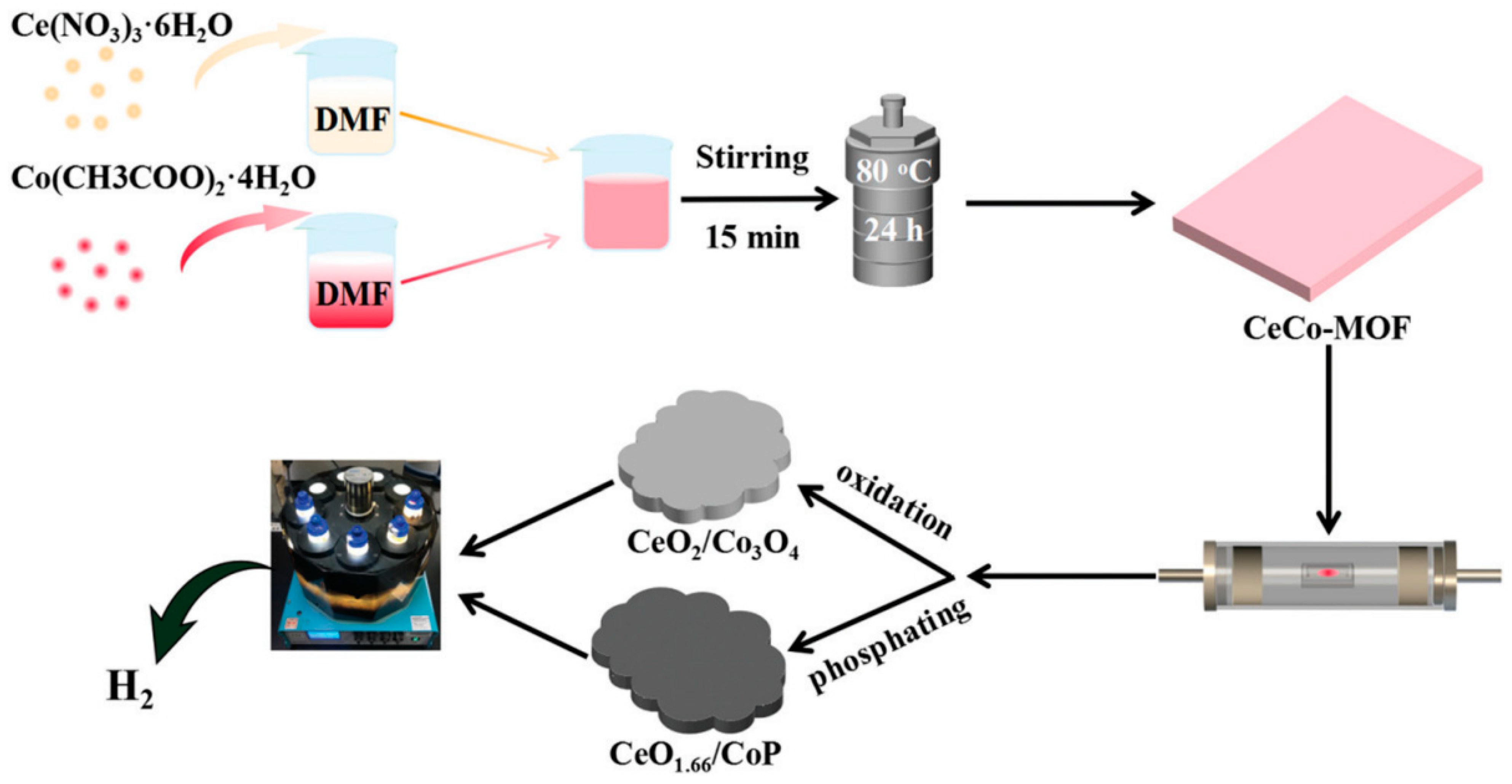
| MOFs | Cocatalysts/Derivatives | Light Source | Sacrificial Agents | H2 Evolution Rate (μmol∙g−1∙h−1) | Ref. |
|---|---|---|---|---|---|
| UiO-66-NH2 (5 mg) | Ni SACs | 300 W Xe lamp (λ > 380 nm) | TEA | 1360 | [61] |
| MIL-125-NH2 (10 mg) | Pt | 300 W Xe lamp (800 nm > λ > 200 nm) | TEOA | ~140 | [62] |
| Ni-TBAPy-NB (20 mg) | ― | 300 W Xe lamp (λ > 420 nm) | Ascorbic acid | 5000 | [63] |
| In-TCPP NS (5 mg) | Pt | 300 W Xe lamp | TEA | 539.07 | [64] |
| UiO-66-NH2 (5 mg) | Ni2P | 300 W Xe lamp (λ > 380 nm) | TEA | 409 | [69] |
| UiO-66-NH2 (5 mg) | Pt-Fc | 300 W Xe lamp (λ > 380 nm) | TEOA | 102 | [70] |
| UiO-66-NH2 (5 mg) | Cu-TCPP MOF | 300 W Xe lamp | TEOA | 25 | [71] |
| MOF-808 (5 mg) | CdS4 NPs | 300 W Xe lamp (800 nm > λ > 400 nm) | TEA | 10,410 | [72] |
| MIL-125-NH2 (10 mg) | MoO3 | 300 W Xe lamp (λ > 380 nm) | TEA | 399 | [73] |
| NH2-MIL-125@OH-MIL-125 (8 mg) | TiO2 | 300 W Xe lamp | TEOA | 7108 | [74] |
| Cu3(HHTP)2 (10 mg) | Tp-Pa-1-COF | 300 W Xe lamp (λ > 400 nm) | sodium ascorbate | 1760 | [79] |
| PdTCPP@PCN-415(NH2) (10 mg) | TpPa-COF | 300 W Xe lamp (λ > 400 nm) | Ascorbic acid | 13,980 | [80] |
| NH2-MIL-125(Fe) (50 mg) | SNW-1@Pt | 300 W Xe lamp | TEOA | 1949.56 | [81] |
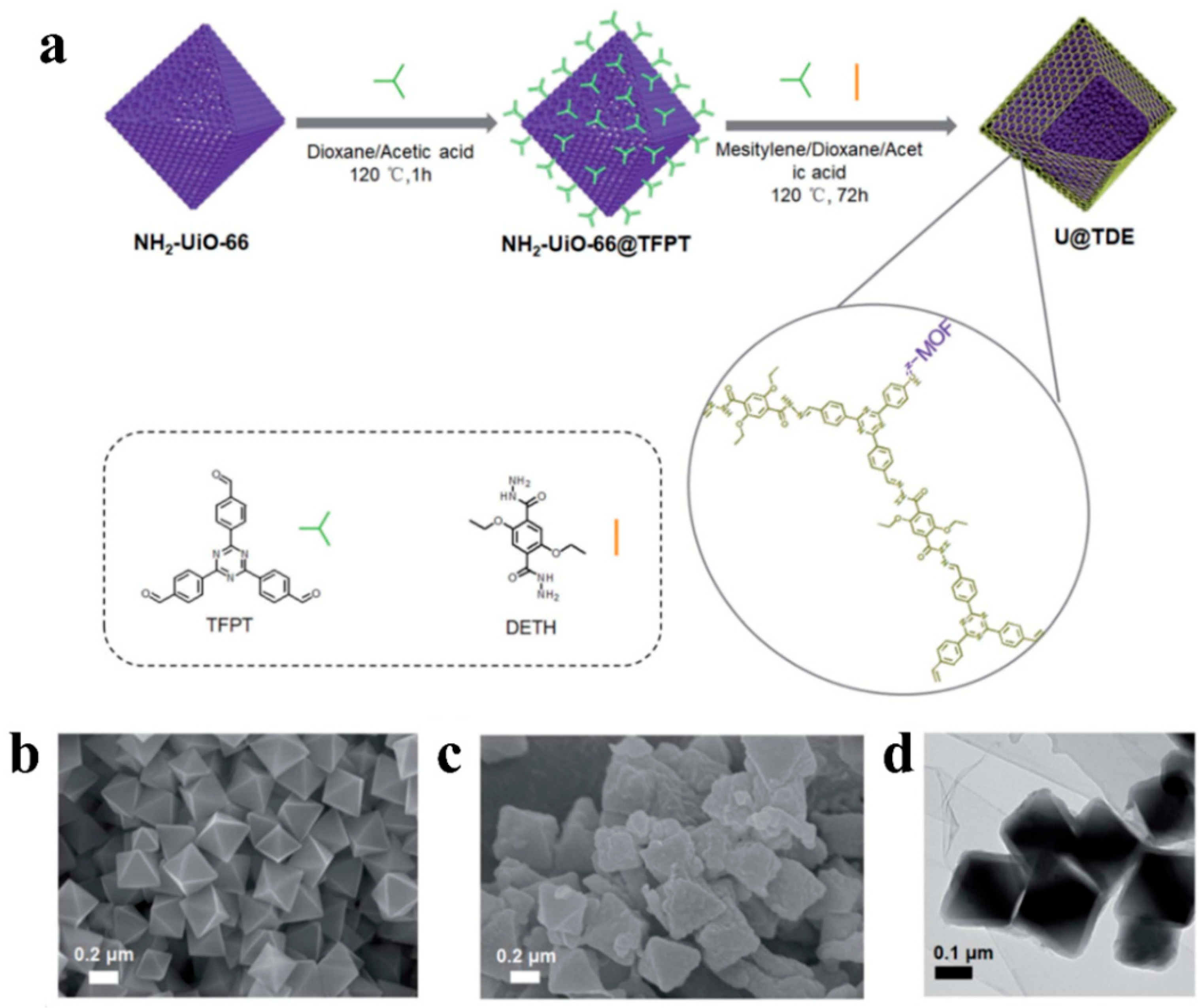
| NH2-UiO-66 (8 mg) | TFPT-DETH-COF | 300 W Xe lamp (λ > 420 nm) | Ascorbic acid | 7178 | [82] |
| Prussian blue analogues | Ni3FeN (derivative) | 300 W Xe lamp (λ > 400 nm) | TEOA | 16,960 | [85] |
| Zn-based CPP | O-ZnIn2S4/ZnO (derivative) | 300 W Xe lamp | Na2S and Na2SO3 | 4512.5 | [86] |
| In-CPP | CdS-In2O3(derivative) | 300 W Xe lamp | LA | 198,580 | [87] |
| MIL-68 | C/HT-In2O3 /Zn2InS4 | 300 W Xe lamp | Na2S and Na2SO3 | 230.1 μmol/m2/h | [88] |
| Ce/Co-MOF | CeO1.66/CoP(derivative) | 5 W LED (λ≥400 nm) | TEOA | 8040.9 | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, C.; Li, Y.; Pang, J.; Bu, X. The Advanced Synthesis of MOFs-Based Materials in Photocatalytic HER in Recent Three Years. Catalysts 2022, 12, 1350. https://doi.org/10.3390/catal12111350
Zhang H, Li C, Li Y, Pang J, Bu X. The Advanced Synthesis of MOFs-Based Materials in Photocatalytic HER in Recent Three Years. Catalysts. 2022; 12(11):1350. https://doi.org/10.3390/catal12111350
Chicago/Turabian StyleZhang, Hao, Cha Li, Yang Li, Jiandong Pang, and Xianhe Bu. 2022. "The Advanced Synthesis of MOFs-Based Materials in Photocatalytic HER in Recent Three Years" Catalysts 12, no. 11: 1350. https://doi.org/10.3390/catal12111350
APA StyleZhang, H., Li, C., Li, Y., Pang, J., & Bu, X. (2022). The Advanced Synthesis of MOFs-Based Materials in Photocatalytic HER in Recent Three Years. Catalysts, 12(11), 1350. https://doi.org/10.3390/catal12111350






