The Progress of Metal-Organic Framework for Boosting CO2 Conversion
Abstract
:1. Introduction
2. Strategies for CO2 Recycling
3. Electrochemical CO2 Reduction
3.1. Basic Issues of CO2ET
3.2. Construction of Electrolytic Cells
3.3. Type of Electrolyte
3.4. Metal–Organic Frameworks in Electrocatalytic CO2–RR
3.4.1. Fe–MOFs with Iron as the Active Centre
3.4.2. Co-MOFs with Cobalt as the Active Centre
3.4.3. Cu-MOFs with Copper as the Active Centre
3.4.4. Other Metal as the Active Centers
3.4.5. Application of Porphyrin-Based Metal–Organic Frameworks in Electrocatalytic CO2–RR
4. Photocatalytic CO2 Reduction
4.1. Metal Porphyrin Ligands for MOF Photocatalysts
4.2. Bipyridyl Metal Complex Ligands for MOF Photocatalysts
4.3. MOF Photocatalysts Containing the -NH2 Functional Group
4.4. Others
4.5. The Dynamic Role/Mechanism of MOFs Works as a Catalyst
5. Conclusions
- (1)
- design and construct MOF-based materials with high activity, high selectivity, high stability, low production cost, and low toxicity to achieve efficient and green conversion of CO2 under mild conditions;
- (2)
- further broaden the types of reactions for CO2 conversion by MOF-based materials, develop new ways of CO2 conversion, and convert CO2 into a variety of high value-added chemicals.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2. Catalysts 2020, 10, 1293. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, C.; Xiu, J.; Yang, L.; Duan, C. Metal-Organic Polymers Containing Discrete Single-Walled Nanotube as a Heterogeneous Catalyst for the Cycloaddition of Carbon Dioxide to Epoxides. J. Am. Chem. Soc. 2015, 137, 15066–15069. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Wang, B.; Hu, K.; Lyu, K.; Han, X.; Spencer, B.F.; Frogley, M.D.; Tuna, F.; McInnes, E.J.L.; Dryfe, R.A.W.; et al. Quantitative Electro-Reduction of CO2 to Liquid Fuel over Electro-Synthesized Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 17384–17392. [Google Scholar] [CrossRef]
- Agatemor, C.; Quintana, A.A.; Sztapka, L.M.; Ebinuma, V.C.S. Enabling Sustainable Chemistry with Ionic Liquids and Deep Eutectic Solvents: A Fad or the Future? Angew. Chem. Int. Ed. 2022, 134, e202205609. [Google Scholar]
- Wang, S.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Formation of Hierarchical In2S3-CdIn2S4 Heterostructured Nanotubes for Efficient and Stable Visible Light CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 17305–17308. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Whang, H.S.; Lim, J.; Choi, M.S.; Lee, J.; Lee, H. Heterogeneous catalysts for catalytic CO2 conversion into value-added chemicals. BMC Chem. Eng. 2019, 1, 9. [Google Scholar] [CrossRef]
- Liu, S.; Chen, F.; Li, S.; Peng, X.; Xiong, Y. Enhanced photocatalytic conversion of greenhouse gas CO2 into solar fuels over g-C3N4 nanotubes with decorated transparent ZIF-8 nanoclusters. Appl. Catal. B Environ. 2017, 211, 1–10. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Zhang, J. Enhanced photocatalytic CO2 reduction activity of MOF-derived ZnO/NiO porous hollow spheres. J. CO2 Util. 2018, 24, 548–554. [Google Scholar] [CrossRef]
- Dang, S.; Gao, P.; Liu, Z.; Chen, X.; Yang, C.; Wang, H.; Zhong, L.; Li, S.; Sun, Y. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]
- Fujiwara, M.; Ando, H.; Tanaka, M.; Souma, Y. Hydrogenation of carbon dioxide over Cu-Zn-chromate/zeolite composite catalyst: The effects of reaction behavior of alkenes on hydrocarbon synthesis. Appl. Catal. A Gen. 1995, 130, 105–116. [Google Scholar] [CrossRef]
- Di, Z.; Pang, J.; Hu, F.; Wu, M.; Hong, M. An ultra-stable microporous supramolecular framework with highly selective adsorption and separation of water over ethanol. Nano. Res. 2021, 14, 2584–2588. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Kafle, S.R.; Kim, B.-S. Surface Modification of Biochar for Dye Removal from Wastewater. Catalysts 2022, 12, 817. [Google Scholar] [CrossRef]
- Ren, F.; Ji, P. Recent Advances in the Application of Metal–Organic Frameworks for Polymerization and Oligomerization Reactions. Catalysts 2020, 10, 1141. [Google Scholar] [CrossRef]
- Fujlwara, M.; Kieffer, R.; Ando, H.; Xu, Q.; Souma, Y. Change of catalytic properties of Fe-ZnO/zeolite composite catalyst in the hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 154, 87–101. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Liu, B. Direct and selective hydrogenation of CO2 to ethylene and propene by bifunctional catalysts. Catal. Sci. Technol. 2017, 7, 5602–5607. [Google Scholar] [CrossRef]
- Gao, P.; Dang, S.; Li, S.; Bu, X.; Liu, Z.; Qiu, M.; Yang, C.; Wang, H.; Zhong, L.; Han, Y.; et al. Direct Production of Lower Olefins from CO2 Conversion via Bifunctional Catalysis. ACS Catal. 2017, 8, 571–578. [Google Scholar] [CrossRef]
- Goyal, S.; Shaharun, M.; Kait, C.; Abdullah, B.; Ameen, M. Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts 2018, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Ma, X.; Sun, X.; Han, B. Electrochemical Transformation of CO2 to Value-Added Chemicals and Fuels. CCS Chem. 2022, 4, 3213–3229. [Google Scholar] [CrossRef]
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Zheng, X.; Qi, Y.; Yuan, H.; Li, C.-P. Recent Advances in C2 Gases Separation and Purification by Metal-Organic Frameworks. Chin. J. Struct. Chem. 2022, 41, 2211031–2211044. [Google Scholar]
- Fang, Z.; Ren, R.; Wang, Y.; Hu, Y.; Dong, M.; Ye, Z.; He, Q.; Peng, X. Solar-driven all-in-one MOFs-based catalyst for highly efficient CO2 conversion. Appl. Catal. B Environ. 2022, 318, 121878. [Google Scholar] [CrossRef]
- Zhang, A.A.; Si, D.; Huang, H.; Xie, L.; Fang, Z.B.; Liu, T.F.; Cao, R. Partial Metalation of Porphyrin Moieties in Hydrogen-Bonded Organic Frameworks Provides Enhanced CO2 Photoreduction Activity. Angew. Chem. Int. Ed. 2022, 61, e202203955. [Google Scholar]
- Han, Y.; Xu, H.; Su, Y.; Xu, Z.L.; Wang, K.; Wang, W. Noble metal (Pt, Au@Pd) nanoparticles supported on metal organic framework (MOF-74) nanoshuttles as high-selectivity CO2 conversion catalysts. J. Catal. 2019, 370, 70–78. [Google Scholar] [CrossRef]
- Helal, A.; Cordova, K.E.; Arafat, M.E.; Usman, M.; Yamani, Z.H. Defect-engineering a metal–organic framework for CO2 fixation in the synthesis of bioactive oxazolidinones. Inorg. Chem. Front. 2020, 7, 3571–3577. [Google Scholar] [CrossRef]
- Luo, H.; Gu, Y.; Liu, D.; Sun, Y. Advances in Oxidative Desulfurization of Fuel Oils over MOFs-Based Heterogeneous Catalysts. Catalysts 2021, 11, 1557. [Google Scholar] [CrossRef]
- Qiu, X.F.; Huang, J.R.; Yu, C.; Zhao, Z.H.; Zhu, H.L.; Ke, Z.; Liao, P.Q.; Chen, X.M. A Stable and Conductive Covalent Organic Framework with Isolated Active Sites for Highly Selective Electroreduction of Carbon Dioxide to Acetate. Angew. Chem. Int. Ed. 2022, 61, e202206470. [Google Scholar]
- Wang, H.; Dong, J.; Allard, L.F.; Lee, S.; Oh, S.; Wang, J.; Li, W.; Shen, M.; Yang, M. Single-site Pt/La-Al2O3 stabilized by barium as an active and stable catalyst in purifying CO and C3H6 emissions. Appl. Catal. B Environ. 2019, 244, 327–339. [Google Scholar] [CrossRef]
- Overa, S.; Crandall, B.S.; Shrimant, B.; Tian, D.; Ko, B.H.; Shin, H.; Bae, C.; Jiao, F. Enhancing acetate selectivity by coupling anodic oxidation to carbon monoxide electroreduction. Nat. Catal. 2022, 5, 738–745. [Google Scholar] [CrossRef]
- Di, Z.; Liu, C.; Pang, J.; Zou, S.; Ji, Z.; Hu, F.; Chen, C.; Yuan, D.; Hong, M.; Wu, M. A Metal-Organic Framework with Nonpolar Pore Surfaces for the One-step Acquisition of C2H4 from a C2H4 and C2H6 Mixture. Angew. Chem. Int. Ed. 2022, 61, e202210343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Xu, Q. Metal–organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Ding, F.; Song, C.; Zhang, G.; Guo, X. Hydrothermally stable MOFs for CO2 hydrogenation over iron-based catalyst to light olefins. J. CO2 Util. 2016, 15, 89–95. [Google Scholar] [CrossRef]
- Valdebenito, G.; Gonzaléz-Carvajal, M.; Santibañez, L.; Cancino, P. Metal–Organic Frameworks (MOFs) and Materials Derived from MOFs as Catalysts for the Development of Green Processes. Catalysts 2022, 12, 136. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [Green Version]
- Maina, J.W.; Schutz, J.A.; Grundy, L.; Des Ligneris, E.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumee, L.F. Inorganic Nanoparticles/Metal Organic Framework Hybrid Membrane Reactors for Efficient Photocatalytic Conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Ziemba, M.; Radtke, M.; Schumacher, L.; Hess, C. Elucidating CO2 Hydrogenation over In2O3 Nanoparticles using Operando UV-vis and Impedance Spectroscopies. Angew. Chem. Int. Ed. 2022, 61, e202209388. [Google Scholar] [CrossRef]
- Zang, Y.; Liu, T.; Wei, P.; Li, H.; Wang, Q.; Wang, G.; Bao, X. Selective CO2 Electroreduction to Ethanol over Carbon-Coated CuOx Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202209629. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Wang, T.; Wang, D.; Mao, J. P and Cu Dual Sites on Graphitic Carbon Nitride for Photocatalytic CO2 Reduction to Hydrocarbon Fuels with High C2H6 Evolution. Angew. Chem. Int. Ed. 2022, 61, e202210789. [Google Scholar]
- Santibáñez, L.; Escalona, N.; Torres, J.; Kremer, C.; Cancino, P.; Spodine, E. CuII- and CoII-Based MOFs: {[La2Cu3(µ-H2O)(ODA)6(H2O)3]∙3H2O}n and {[La2Co3(ODA)6(H2O)6]∙12H2O}n. The Relevance of Physicochemical Properties on the Catalytic Aerobic Oxidation of Cyclohexene. Catalysts 2020, 10, 589. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Isaeva, V.I.; Tkachenko, O.P.; Chernyshev, V.V.; Kustov, L.M. Conversion of CO2 into liquid hydrocarbons in the presence of a Co-containing catalyst based on the microporous metal-organic framework MIL-53(Al). Fuel. Proc. Tech. 2018, 176, 101–106. [Google Scholar] [CrossRef]
- Sun, X.; Sun, L.; Li, G.; Tuo, Y.; Ye, C.; Yang, J.; Low, J.; Yu, X.; Bitter, J.H.; Lei, Y.; et al. Phosphorus Tailors the d-Band Center of Copper Atomic Sites for Efficient CO2 Photoreduction under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2022, 61, e202207677. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Oliveira, E.P.; Reinares-Fisac, D.; Aguirre-Diaz, L.M.; Esteban-Betegon, F.; Pintado-Sierra, M.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, A.; Gandara, F. Framework Adaptability and Concerted Structural Response in a Bismuth Metal-Organic Framework Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202209335. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnO Nanoparticles in Metal-Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef]
- Becerra, J.; Nguyen, D.-T.; Gopalakrishnan, V.-N.; Do, T.-O. Plasmonic Au Nanoparticles Incorporated in the Zeolitic Imidazolate Framework (ZIF-67) for the Efficient Sunlight-Driven Photoreduction of CO2. ACS Appl. Energy Mater. 2020, 3, 7659–7665. [Google Scholar] [CrossRef]
- Cancino, P.; Santibañez, L.; Stevens, C.; Fuentealba, P.; Audebrand, N.; Aravena, D.; Torres, J.; Martinez, S.; Kremer, C.; Spodine, E. Influence of the channel size of isostructural 3d–4f MOFs on the catalytic aerobic oxidation of cycloalkenes. New J. Chem. 2019, 43, 11057–11064. [Google Scholar] [CrossRef]
- Lu, S.; Lou, F.; Yu, Z. Recent Progress in Two-Dimensional Materials for Electrocatalytic CO2 Reduction. Catalysts 2022, 12, 228. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Hintsho-Mbita, N.C. Green Derived Zinc Oxide (ZnO) for the Degradation of Dyes from Wastewater and Their Antimicrobial Activity: A Review. Catalysts 2022, 12, 833. [Google Scholar] [CrossRef]
- Lv, J.J.; Yin, R.; Zhou, L.; Li, J.; Kikas, R.; Xu, T.; Wang, Z.J.; Jin, H.; Wang, X.; Wang, S. Microenvironment Engineering for the Electrocatalytic CO2 Reduction Reaction. Angew. Chem. Int. Ed. 2022, 61, e202207252. [Google Scholar] [CrossRef] [PubMed]
- Grammatico, D.; Bagnall, A.J.; Riccardi, L.; Fontecave, M.; Su, B.L.; Billlon, L. Heterogenised molecular catalysts for sustainable electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2022, 61, e202206399. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, L.; Tang, J.; Malgras, V.; Asahina, S.; Liu, G.; Zhang, H.; Meng, X.; Chang, K.; He, J.; et al. A Co3O4− embedded porous ZnO rhombic dodecahedron prepared using zeolitic imidazolate frameworks as precursors for CO2 photoreduction. Nanoscale 2016, 8, 6712–6720. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, Z.; Wu, J. Construction of a Z-scheme heterojunction for high-efficiency visible-light-driven photocatalytic CO2 reduction. Nanoscale 2021, 13, 4359–4389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, T.; Wang, J.; Liu, H.; Dao, T.D.; Li, M.; Liu, G.; Meng, X.; Chang, K.; Shi, L.; et al. Surface-Plasmon-Enhanced Photodriven CO2 Reduction Catalyzed by Metal-Organic-Framework-Derived Iron Nanoparticles Encapsulated by Ultrathin Carbon Layers. Adv. Mater. 2016, 28, 3703–3710. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Zhou, X.; Liu, Y.-N.; Shen, C.-C.; Yuan, C.-Z.; Jiang, Y.-F.; Zhao, S.-J.; Ma, L.-B.; Cheang, T.-Y.; Xu, A.-W. Ultrasmall Ni nanoparticles embedded in Zr-based MOFs provide high selectivity for CO2 hydrogenation to methane at low temperatures. Catal. Sci. Technol. 2018, 8, 3160–3165. [Google Scholar] [CrossRef]
- Yi, J.-D.; Xie, R.; Xie, Z.-L.; Chai, G.-L.; Liu, T.-F.; Chen, R.-P.; Huang, Y.-B.; Cao, R. Highly Selective CO2 Electroreduction to CH4 by In Situ Generated Cu2O Single-Type Sites on a Conductive MOF: Stabilizing Key Intermediates with Hydrogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 23641–23648. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, Q.; Wu, W.; Ye, Y.; Yao, Z.; Ma, X.; Xiang, S.; Zhang, Z. Isostructural MOFs with Higher Proton Conductivity for Improved Oxygen Evolution Reaction Performance. ACS Appl. Mater. Interfaces 2020, 12, 16367–16375. [Google Scholar] [CrossRef]
- Zhou, Z.; He, C.; Yang, L.; Wang, Y.; Liu, T.; Duan, C. Alkyne Activation by a Porous Silver Coordination Polymer for Heterogeneous Catalysis of Carbon Dioxide Cycloaddition. ACS Catal. 2017, 7, 2248–2256. [Google Scholar] [CrossRef]
- Gong, Y.N.; Jiao, L.; Qian, Y.; Pan, C.Y.; Zheng, L.; Cai, X.; Liu, B.; Yu, S.H.; Jiang, H.L. Regulating the Coordination Environment of MOF-Templated Single-Atom Nickel Electrocatalysts for Boosting CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Q.-L. MOF-based materials for photo- and electrocatalytic CO2 reduction. EnergyChem 2020, 2, 100033. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Dong, L.-Z.; Liu, J.; Li, S.-L.; Lan, Y.-Q. From molecular metal complex to metal-organic framework: The CO2 reduction photocatalysts with clear and tunable structure. Coord. Chem. Rev. 2019, 390, 86–126. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Liu, B.; Qiao, J.; Lv, L.; Gao, X.; Zhang, X.; Xu, D.; Liu, W.; Liu, J.; et al. Recent Advances in MOF-based Nanocatalysts for Photo-Promoted CO2 Reduction Applications. Catalysts 2019, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Bhugun, I.; Lexa, D.; Saveant, J.-M. Ultraefficient Selective Homogeneous Catalysis of the Electrochemical Reduction of Carbon Dioxide by an Iron(0) Porphyrin Associated with a Weak Bronsted Acid Cocatalyst. J. Am. Chem. Soc. 1994, 116, 5015–5016. [Google Scholar] [CrossRef]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. Fe-Porphyrin-Based Metal–Organic Framework Films as High-Surface Concentration, Heterogeneous Catalysts for Electrochemical Reduction of CO2. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, R.-K.; Mao, M.-J.; Chai, G.-L.; Yi, J.-D.; Zhao, S.-S.; Huang, Y.-B.; Cao, R. Integration of Strong Electron Transporter Tetrathiafulvalene into Metalloporphyrin-Based Covalent Organic Framework for Highly Efficient Electroreduction of CO2. ACS Energy Lett. 2020, 5, 1005–1012. [Google Scholar] [CrossRef]
- Ejsmont, A.; Jankowska, A.; Goscianska, J. Insight into the Photocatalytic Activity of Cobalt-Based Metal–Organic Frameworks and Their Composites. Catalysts 2022, 12, 110. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal-organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.L.; Wang, Y.R.; He, W.W.; Zhong, R.L.; Liu, Q.Z.; Xu, S.; Xu, J.M.; Han, X.L.; Ge, X.; Li, S.L.; et al. Efficient Electron Transfer from Electron-Sponge Polyoxometalate to Single-Metal Site Metal-Organic Frameworks for Highly Selective Electroreduction of Carbon Dioxide. Small 2021, 17, e2100762. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Zhou, D.; Sun, M.; Wang, S.; Chen, W. Factors Influencing the Performance of Copper-Bearing Catalysts in the CO2 Reduction System. ACS Energy Lett. 2021, 6, 3992–4022. [Google Scholar] [CrossRef]
- Hinogami, R.; Yotsuhashi, S.; Deguchi, M.; Zenitani, Y.; Hashiba, H.; Yamada, Y. Electrochemical Reduction of Carbon Dioxide Using a Copper Rubeanate Metal Organic Framework. ECS Electrochem. Lett. 2012, 1, H17–H19. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Kang, X.; Li, L.; Sheveleva, A.; Han, X.; Li, J.; Liu, L.; Tuna, F.; McInnes, E.J.L.; Han, B.; Yang, S.; et al. Electro-reduction of carbon dioxide at low over-potential at a metal-organic framework decorated cathode. Nat. Commun. 2020, 11, 5464. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, L.; Yang, W.; Xie, C.; Jiang, H.L. Rational Fabrication of Low-Coordinate Single-Atom Ni Electrocatalysts by MOFs for Highly Selective CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 7607–7611. [Google Scholar] [CrossRef]
- Zhang, M.-D.; Si, D.-H.; Yi, J.-D.; Yin, Q.; Huang, Y.-B.; Cao, R. Conductive phthalocyanine-based metal-organic framework as a highly efficient electrocatalyst for carbon dioxide reduction reaction. Sci China Chem. 2021, 64, 1332–1339. [Google Scholar] [CrossRef]
- Jiao, L.; Yang, W.; Wan, G.; Zhang, R.; Zheng, X.; Zhou, H.; Yu, S.H.; Jiang, H.L. Single-Atom Electrocatalysts from Multivariate Metal-Organic Frameworks for Highly Selective Reduction of CO2 at Low Pressures. Angew. Chem. Int. Ed. 2020, 59, 20589–20595. [Google Scholar] [CrossRef]
- Wang, Y.R.; Huang, Q.; He, C.T.; Chen, Y.; Liu, J.; Shen, F.C.; Lan, Y.Q. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018, 9, 4466. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal-organic frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-Based MOFs for Photocatalytic CO2 Reduction: Role of Coordination Unsaturated Sites and Dual Excitation Pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, J.; Feng, L.; Wang, Q.; Guan, W.; Dong, L.Z.; Zhang, L.; Yan, L.K.; Lan, Y.Q.; Zhou, H.C. Multielectron transportation of polyoxometalate-grafted metalloporphyrin coordination frameworks for selective CO2-to-CH4 photoconversion. Natl. Sci. Rev. 2020, 7, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yao, W.; Lin, J.; Ding, Z.; Wang, X. Cobalt imidazolate metal-organic frameworks photosplit CO2 under mild reaction conditions. Angew. Chem. Int. Ed. 2014, 53, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-K.; Liu, J.; Zhang, L.; Dong, L.-Z.; Li, S.-L.; Kan, Y.-H.; Li, D.-S.; Lan, Y.-Q. Monometallic Catalytic Models Hosted in Stable Metal–Organic Frameworks for Tunable CO2 Photoreduction. ACS Catal. 2019, 9, 1726–1732. [Google Scholar] [CrossRef]
- Zhang, H.X.; Hong, Q.L.; Li, J.; Wang, F.; Huang, X.; Chen, S.; Tu, W.; Yu, D.; Xu, R.; Zhou, T.; et al. Isolated Square-Planar Copper Center in Boron Imidazolate Nanocages for Photocatalytic Reduction of CO2 to CO. Angew. Chem. Int. Ed. 2019, 58, 11752–11756. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.J.; Lin, Z. Nickel Metal-Organic Framework Monolayers for Photoreduction of Diluted CO2: Metal-Node-Dependent Activity and Selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef]
- Shinde, G.Y.; Mote, A.S.; Gawande, M.B. Recent Advances of Photocatalytic Hydrogenation of CO2 to Methanol. Catalysts 2022, 12, 94. [Google Scholar] [CrossRef]
- He, H.; Sun, Q.; Gao, W.; Perman, J.A.; Sun, F.; Zhu, G.; Aguila, B.; Forrest, K.; Space, B.; Ma, S. A Stable Metal-Organic Framework Featuring a Local Buffer Environment for Carbon Dioxide Fixation. Angew. Chem. Int. Ed. 2018, 57, 4657–4662. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Li, J.; Luo, D.; Yang, C.; He, S.; Chen, S.; Lin, J.; Zhu, L.; Li, X. Copper(II) imidazolate frameworks as highly efficient photocatalysts for reduction of CO2 into methanol under visible light irradiation. J. Solid State Chem. 2013, 203, 154–159. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Zhou, J.; Chen, W.; Liu, M. Encapsulating CuO quantum dots in MIL-125(Ti) coupled with g-C3N4 for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2020, 399, 125782. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, X.; Chen, D.; Li, C.; Zhang, M. Porous Copper/Zinc Bimetallic Oxides Derived from MOFs for Efficient Photocatalytic Reduction of CO2 to Methanol. Catalysts 2020, 10, 1127. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Stulp, S.; de Brito, J.F.; Flor, J.B.S.; Frem, R.C.G.; Zanoni, M.V.B. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Helal, A.; Usman, M.; Arafat, M.E.; Abdelnaby, M.M. Allyl functionalized UiO-66 metal-organic framework as a catalyst for the synthesis of cyclic carbonates by CO2 cycloaddition. J. Ind. Eng. Chem. 2020, 89, 104–110. [Google Scholar] [CrossRef]
- Di, Z.; Liu, C.; Pang, J.; Chen, C.; Hu, F.; Yuan, D.; Wu, M.; Hong, M. Cage-like Porous Materials with Simultaneous High C2H2 Storage and Excellent C2H2/CO2 Separation Performance. Angew. Chem. Int. Ed. 2021, 60, 10828–10832. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chen, Q.; Lu, J.; Liu, H. Z-Scheme Photocatalytic CO2 Reduction on a Heterostructure of Oxygen-Defective ZnO/Reduced Graphene Oxide/UiO-66-NH2 under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 550–562. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Wang, G.; He, C.T.; Huang, R.; Mao, J.; Wang, D.; Li, Y. Photoinduction of Cu Single Atoms Decorated on UiO-66-NH2 for Enhanced Photocatalytic Reduction of CO2 to Liquid Fuels. J. Am. Chem. Soc. 2020, 142, 19339–19345. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Stewart, D.J.; Raja, R. Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal–Organic Frameworks. Catalysts 2020, 10, 1176. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, C.; Li, Q.; Xiong, L.; Chen, R.; Wan, X.; Wang, Z.; Chen, W.; Deng, Z.; Peng, Y. Selective reduction of CO2 by conductive MOF nanosheets as an efficient co-catalyst under visible light illumination. Appl. Catal. B Environ. 2018, 238, 339–345. [Google Scholar] [CrossRef]
- Garrido, M.; Volland, M.K.; Munich, P.W.; Rodriguez-Perez, L.; Calbo, J.; Orti, E.; Herranz, M.A.; Martin, N.; Guldi, D.M. Mono- and Tripodal Porphyrins: Investigation on the Influence of the Number of Pyrene Anchors in Carbon Nanotube and Graphene Hybrids. J. Am. Chem. Soc. 2020, 142, 1895–1903. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal-Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-Enhanced Photocatalytic CO Conversion within Metal-Organic Frameworks under Visible Light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Li, Z.; Veroneau, S.S.; Zhu, Y.Y.; Xu, Z.; Wang, C.; Lin, W. Photosensitizing Metal-Organic Layers for Efficient Sunlight-Driven Carbon Dioxide Reduction. J. Am. Chem. Soc. 2018, 140, 12369–12373. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO2 reduction over NH2 -Uio-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal-organic frameworks. Chem. Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.; Liu, J.J.; Dong, L.Z.; Xin, Z.F.; Teng, Y.L.; Lan, Y.Q. Adenine Components in Biomimetic Metal-Organic Frameworks for Efficient CO2 Photoconversion. Angew. Chem. Int. Ed. 2019, 58, 5226–5231. [Google Scholar] [CrossRef] [PubMed]

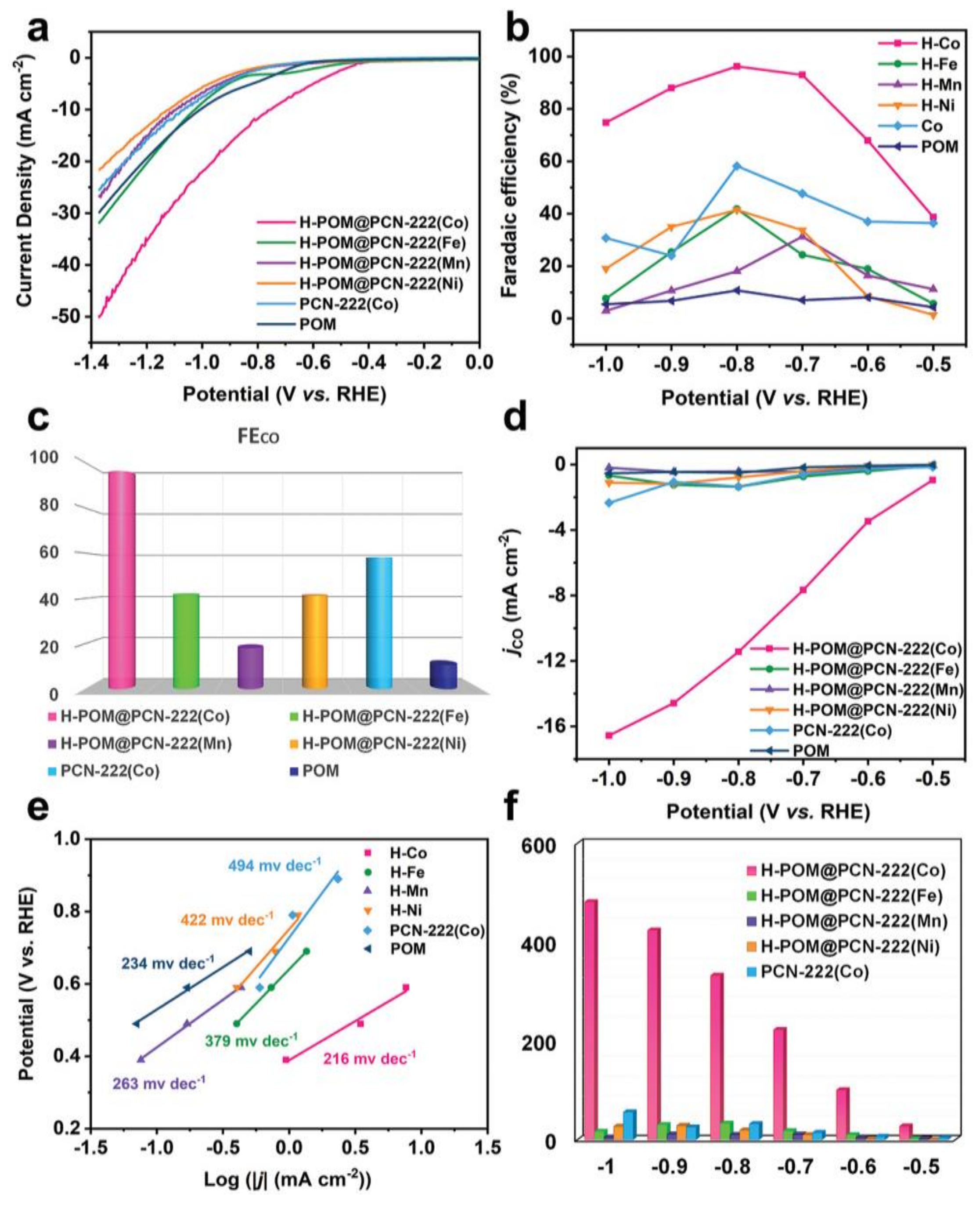

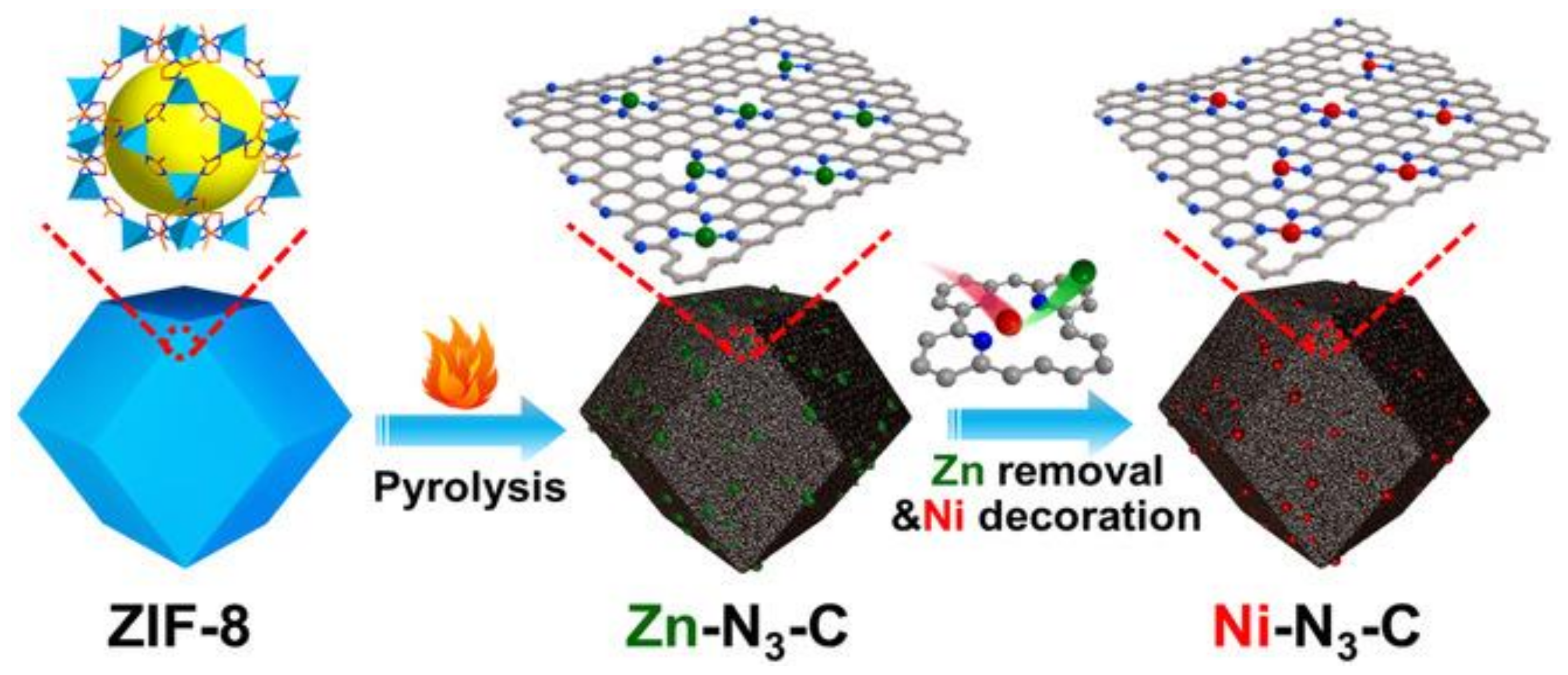

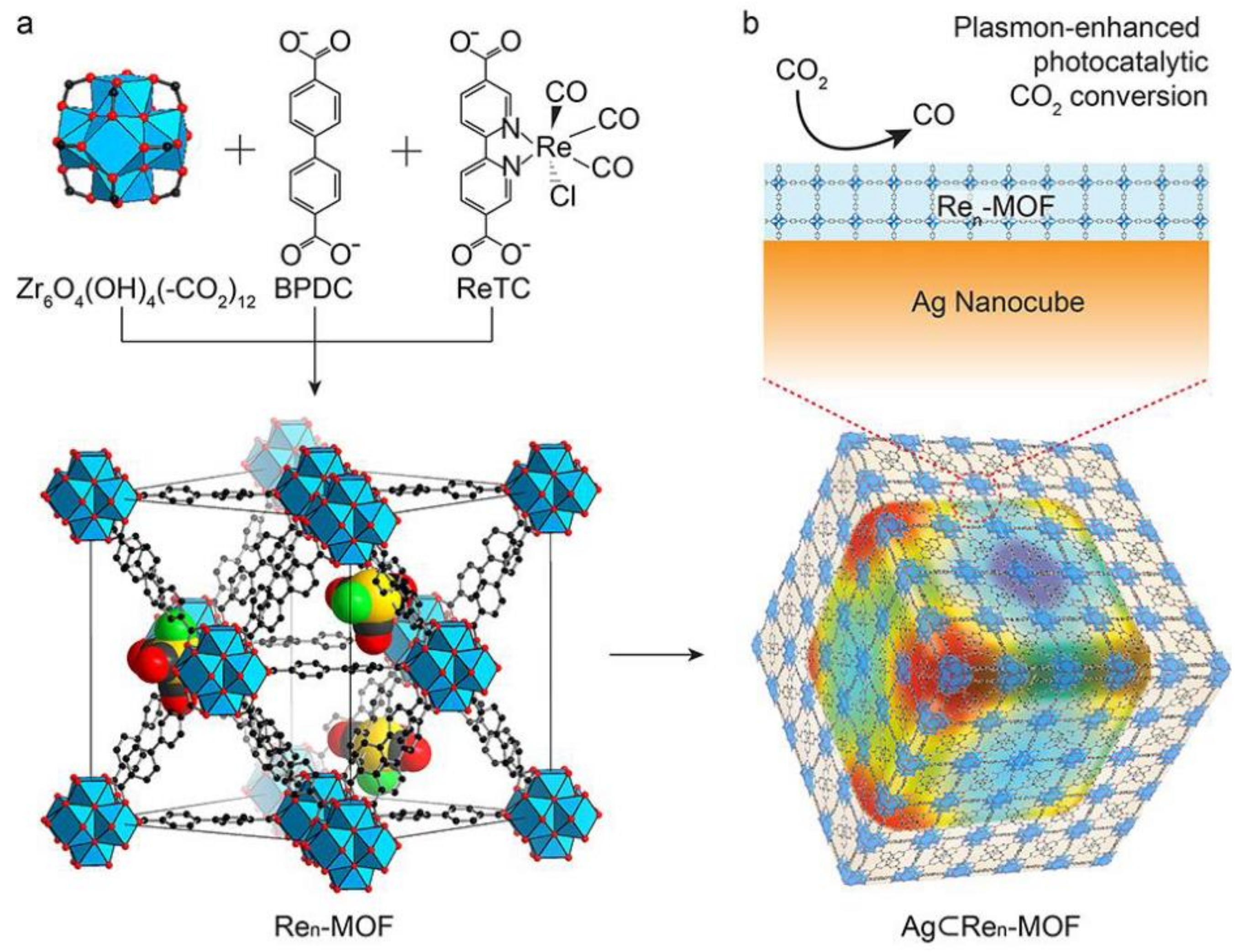
| Reaction | E0/[V vs RHE (Reversible Hydrogen Electrode)] |
|---|---|
| 2H2O → O2 + 4H+ + 4e− | 1.23 |
| xCO2 + nH+ + ne− → product + yH2O | - |
| CO2 + 2H+ + 2e− → HCOOH(aq) | −0.12 |
| CO2 + 2H+ + 2e− → CO(g) + H2O | −0.10 |
| CO2 + 4H+ + 4e− → C(s) + 2H2O | 0.21 |
| CO2 + 6H+ + 6e− → CH3OH(aq) + H2O | 0.03 |
| CO2 + 8H+ + 8e− → CH4(g) + 2H2O | 0.17 |
| 2CO2 + 12H+ + 12e− → C2H4(g) + 4H2O | 0.08 |
| 2CO2 + 12H+ + 12e− → C2H5OH(aq) + 3H2O | 0.09 |
| 2CO2 + 14H+ + 14e− → C2H6(g) + 4H2O | 0.14 |
| 3CO2 + 18H+ + 18e− → C3H7OH(aq) + 5H2O | 0.10 |
| 2CO2 + 2H+ + 2e− → (COOH)2(s) | −0.47 |
| 2CO2 + 8H+ + 8e− → CH3COOH(aq) + 2H2O | 0.11 |
| 2CO2 + 10H+ + 10e− → CH3CHO(aq) + 3H2O | 0.06 |
| 3CO2 + 16H+ + 16e− → C2H5CHO(aq) + 5H2O | 0.09 |
| xCO + nH+ + ne− → product + yH2O | - |
| CO + 6H+ + 6e− → CH4(g) + H2O | 0.26 |
| 2CO + 8H+ + 8e− → CH3CH2OH(aq) + H2O | 0.19 |
| 2CO + 8H+ + 8e− → C2H4(g) + 2H2O | 0.17 |
| 2H+ + 2e− → H2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Z.; Qi, Y.; Yu, X.; Hu, F. The Progress of Metal-Organic Framework for Boosting CO2 Conversion. Catalysts 2022, 12, 1582. https://doi.org/10.3390/catal12121582
Di Z, Qi Y, Yu X, Hu F. The Progress of Metal-Organic Framework for Boosting CO2 Conversion. Catalysts. 2022; 12(12):1582. https://doi.org/10.3390/catal12121582
Chicago/Turabian StyleDi, Zhengyi, Yu Qi, Xinxin Yu, and Falu Hu. 2022. "The Progress of Metal-Organic Framework for Boosting CO2 Conversion" Catalysts 12, no. 12: 1582. https://doi.org/10.3390/catal12121582
APA StyleDi, Z., Qi, Y., Yu, X., & Hu, F. (2022). The Progress of Metal-Organic Framework for Boosting CO2 Conversion. Catalysts, 12(12), 1582. https://doi.org/10.3390/catal12121582






