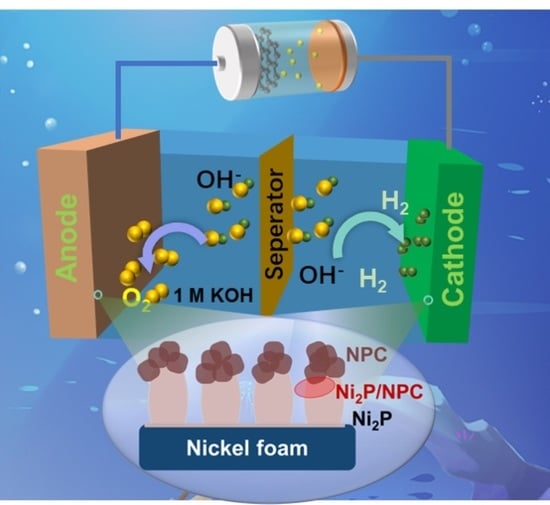
In Situ Growth of Self-Supporting MOFs-Derived Ni2P on Hierarchical Doped Carbon for Efficient Overall Water Splitting
Abstract
1. Introduction
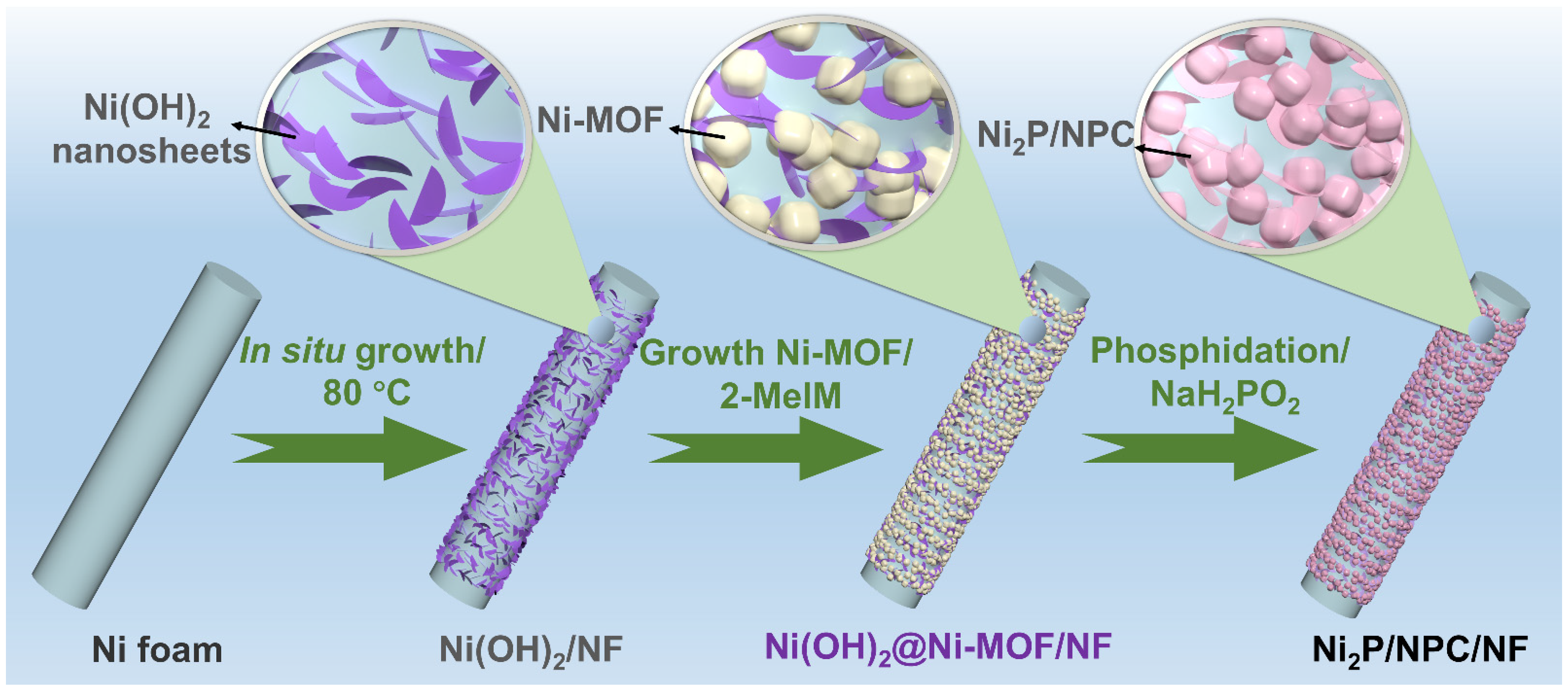
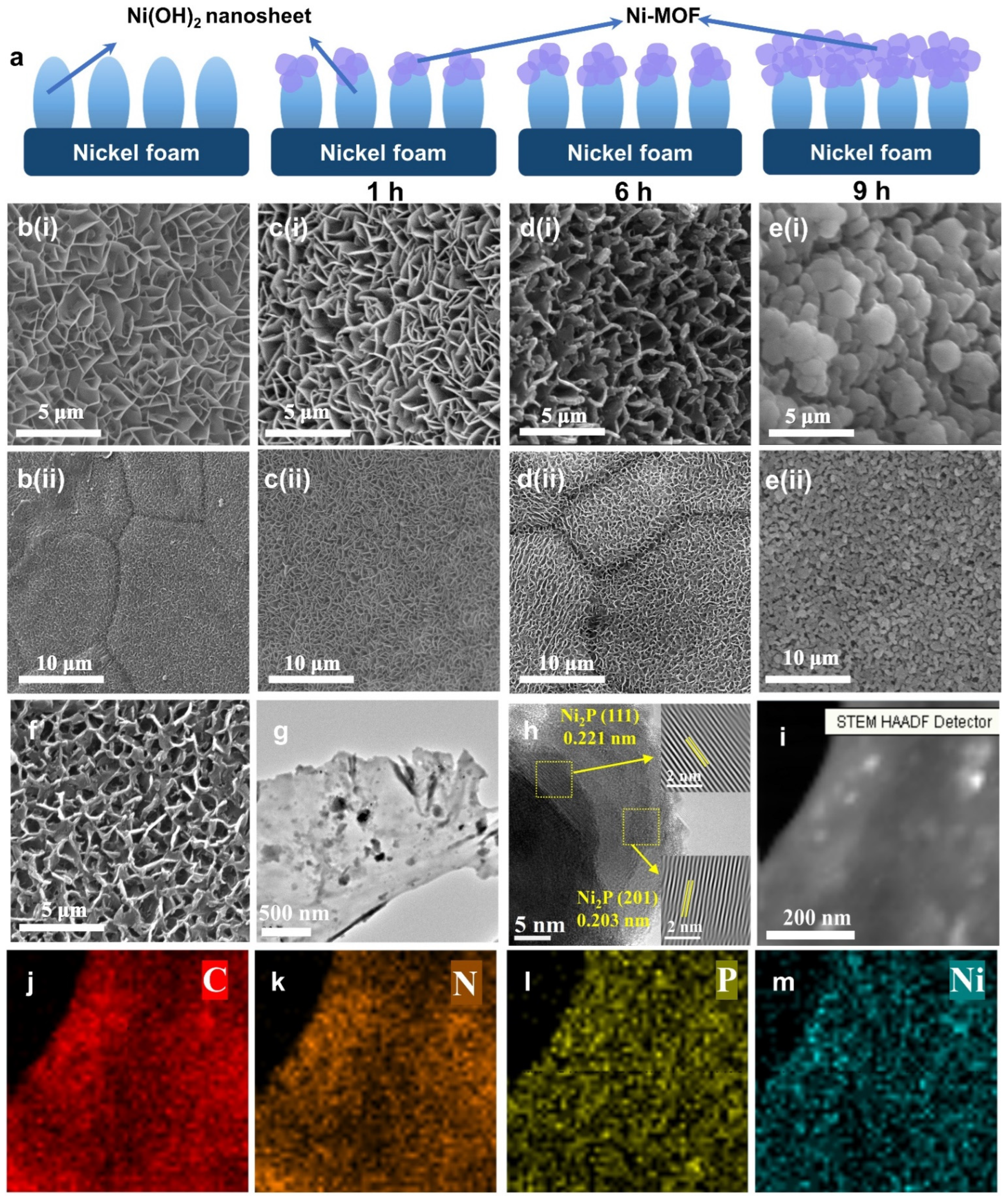
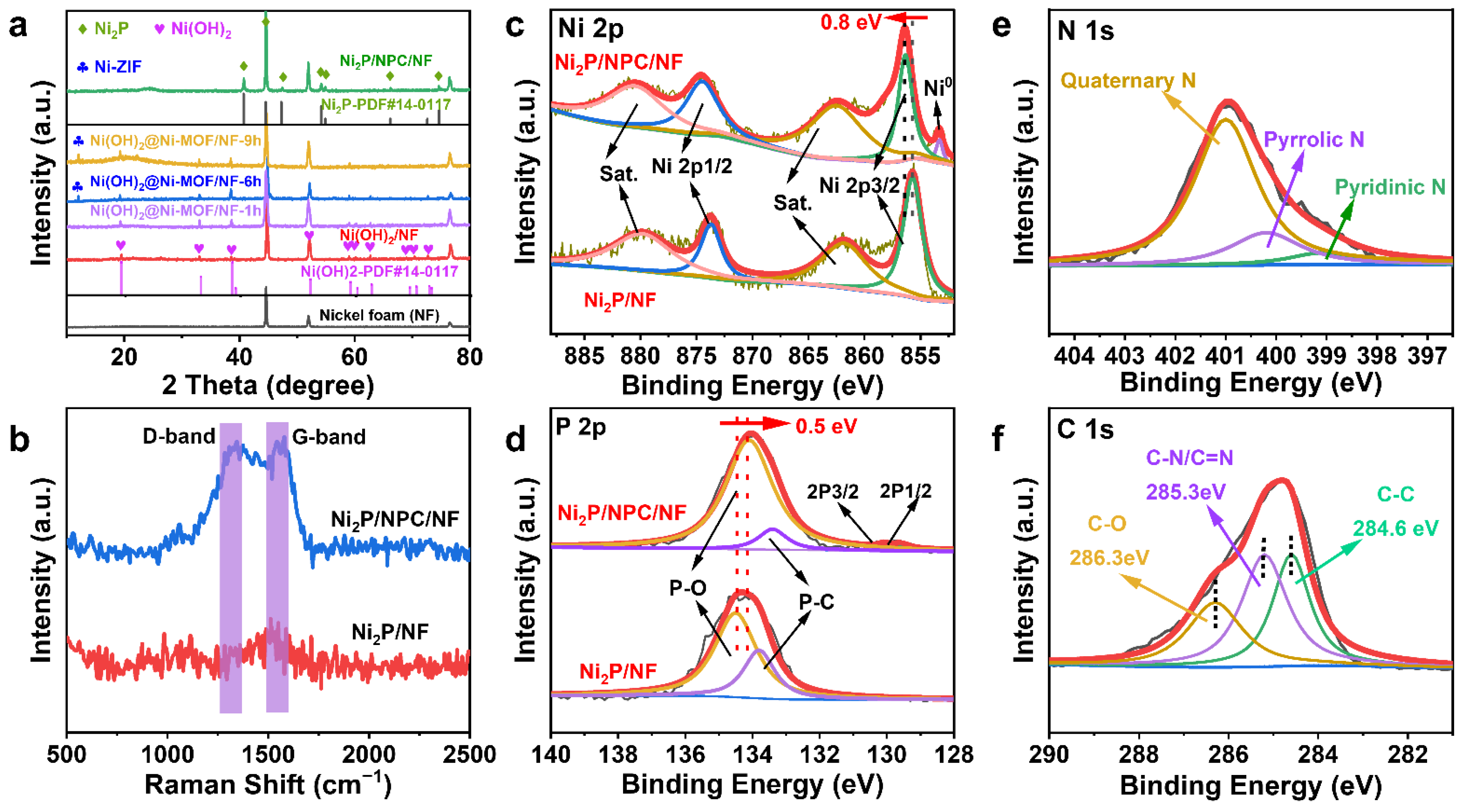
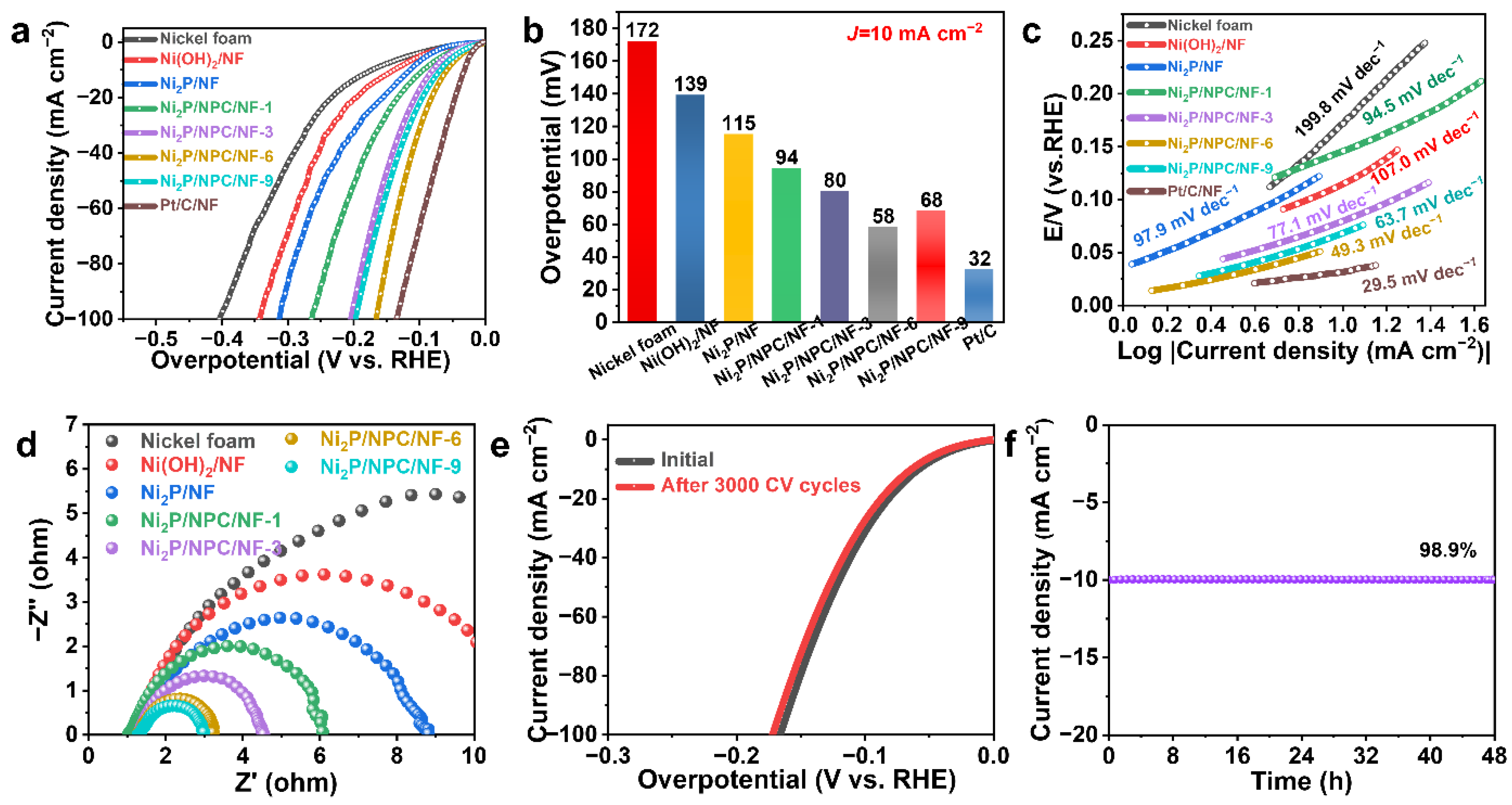
2. Results
3. Experimental Section
3.1. Chemicals
3.2. In Situ Growth of Ni(OH)2 Nanosheets on Nickel Foam
3.3. Synthesis of Ni-MOF/NF
3.4. Synthesis of Ni2P/NPC/NF Nanosheets
3.5. Synthesis of Ni2P/NF
3.6. Characterization of Electrode Materials
3.7. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Weng, G.M.; Li CY, V.; Chan, K.Y. Hydrogen battery using neutralization energy. Nano Energy 2018, 53, 240–244. [Google Scholar] [CrossRef]
- Li, Z.; Feng, Y.; Liang, Y.L.; Cheng, C.Q.; Dong, C.K.; Liu, H.; Du, X.W. Stable Rhodium (IV) Oxide for Alkaline Hydrogen Evolution Reaction. Adv. Mater. 2020, 32, e1908521. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, H.Q.; Cheng, J.; Zhang, K.; Chen, D.; Shen, D.; Wu, S.; Jiao, T.; Kong, X.; Gao, Q.; et al. Hydrogen Evolution Reaction: Nitrogen-Doped Graphene-Encapsulated Nickel–Copper Alloy Nanoflower for Highly Efficient Electrochemical Hydrogen Evolution Reaction (Small 48/2019). Small 2019, 15, 1970260. [Google Scholar] [CrossRef]
- Li, Z.X.; Hu, M.L.; Wang, P.; Liu, J.H.; Yao, J.S.; Li, C.Y. Heterojunction catalyst in electrocatalytic water splitting. Coord. Chem. Rev. 2021, 439, 213953. [Google Scholar] [CrossRef]
- Bae, S.-Y.; Mahmood, J.; Jeon, I.-Y.; Baek, J.-B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020, 5, 43–56. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Feng, X. Support and Interface Effects in Water-Splitting Electrocatalysts. Adv. Mater. 2019, 31, e1808167. [Google Scholar] [CrossRef]
- Ai, L.H.; Li, N.; Chen, M.; Jiang, H.L.; Jiang, J. Photothermally boosted water splitting electrocatalysis by broadband solar harvesting nickel phosphide within a quasi-MOF. J. Mater. Chem. A 2021, 9, 16479–16488. [Google Scholar] [CrossRef]
- Geva, R.; Levy, N.R.; Tzadikov, J.; Cohen, R.; Weitman, M.; Xing, L.D.; Abisdris, L.; Barrio, J.; Xia, J.W.; Volokh, M.; et al. Molten state synthesis of nickel phosphides: Mechanism and composition-activity correlation for electrochemical applications. J. Mater. Chem. A 2021, 9, 27629–27638. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Zhu, B.; Li, G.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C. Boosting Electrochemical Hydrogen Evolution of Porous Metal Phosphides Nanosheets by Coating Defective TiO2 Overlayers. Small 2018, 14, e1802755. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Gupta, K.; Jang, J.H.; Park, H.S.; Jang, I.; Jang, J.H.; Lee, Y.K.; Lee, S.C.; Yoo, S.J. Rationalization of electrocatalysis of nickel phosphide nanowires for efficient hydrogen production. Nano Energy 2016, 26, 496–503. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.G.; Yang, S.; Wu, C.Q.; Tan, Y.W. Anion-modulated nickel-based nanoheterostructures as high performance electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 12013–12027. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.A.; Klysubun, W.; Wang, G.G.; Sattayaporn, S.; Li, F.; Cai, Y.W.; Zhang, F.; Yu, J.; Yang, Y. Interfacial electronic structure engineering on molybdenum sulfide for robust dual-pH hydrogen evolution. Nat. Commun. 2021, 12, 5260. [Google Scholar] [CrossRef] [PubMed]
- Kucernak, A.R.J.; Naranammalpuram Sundaram, V.N. Nickel phosphide: The effect of phosphorus content on hydrogen evolution activity and corrosion resistance in acidic medium. J. Mater. Chem. A 2014, 2, 17435–17445. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Tian, W.; Zhang, H.; Li, J.; Wang, S.; Wang, Y. Non-Metal Single-Phosphorus-Atom Catalysis of Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 23791–23799. [Google Scholar] [CrossRef]
- Chen, N.; Meng, Z.H.; Wang, R.; Cai, S.C.; Guo, W.B.; Tang, H.L. Bimetal-organic framework-derived carbon nanocubes with 3D hierarchical pores as highly efficient oxygen reduction reaction electrocatalysts for microbial fuel cells. Sci. China Mater. 2021, 64, 2926–2937. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, N.; Cai, S.; Wang, R.; Wu, J.; Tang, H. Recent advances of hierarchically porous bifunctional oxygen electrocatalysts derived from metal–organic frameworks for Zn–air batteries. Mater. Chem. Front. 2021, 5, 2649–2667. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, Y.; Ma, T.; Yang, C.; Peng, Y.; Zhou, Z.; Zhou, M.; Li, S.; Wang, Y.; Cheng, C. Designing MOF Nanoarchitectures for Electrochemical Water Splitting. Adv. Mater. 2021, 33, e2006042. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Qing, H.L.; Zhou, K.; Sun, D.L.; Wu, R.B. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
- Cao, C.S.; Ma, D.D.; Xu, Q.; Wu, X.T.; Zhu, Q.L. Semisacrificial Template Growth of Self-Supporting MOF Nanocomposite Electrode for Efficient Electrocatalytic Water Oxidation. Adv. Funct. Mater. 2019, 29, 1807418. [Google Scholar] [CrossRef]
- Hou, C.C.; Zou, L.; Wang, Y.; Xu, Q. MOF-Mediated Fabrication of a Porous 3D Superstructure of Carbon Nanosheets Decorated with Ultrafine Cobalt Phosphide Nanoparticles for Efficient Electrocatalysis and Zinc-Air Batteries. Angew. Chem. Int. Ed. 2020, 59, 21360–21366. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.D.; Guan, J.Q. Recent progress on MOF-derived electrocatalysts for hydrogen evolution reaction. Appl. Mater. Today 2019, 16, 146–168. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Jin, Z. Phosphorus modified Ni-MOF–74/BiVO4 S-scheme heterojunction for enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2022, 307, 121166. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ye, C.; Liu, H.; Xu, M.; Bao, S.J. Nanosized Metal Phosphides Embedded in Nitrogen-Doped Porous Carbon Nanofibers for Enhanced Hydrogen Evolution at All pH Values. Angew. Chem. Int. Ed. 2018, 57, 1963–1967. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Zhao, H.; Huang, H.; Jiao, H.; Du, Y. MOF-derived porous Ni2P nanosheets as novel bifunctional electrocatalysts for the hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2018, 6, 18720–18727. [Google Scholar] [CrossRef]
- Guo, C.; Liu, X.; Gao, L.; Kuang, X.; Ren, X.; Ma, X.; Zhao, M.; Yang, H.; Sun, X.; Wei, Q. Fe-doped Ni2P nanosheets with porous structure for electroreduction of nitrogen to ammonia under ambient conditions. Appl. Catal. B 2020, 263, 118296. [Google Scholar] [CrossRef]
- Cao, R.; Yang, H.; Zhang, S.; Xu, X. Engineering of Z-scheme 2D/3D architectures with Ni(OH)2 on 3D porous g-C3N4 for efficiently photocatalytic H2 evolution. Appl. Catal. B 2019, 258, 117997. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Z.N.; Li, L.; Yin, P.; Liu, Z.; Zhang, H. Ultrathin Ni(0)-Embedded Ni(OH)2 Heterostructured Nanosheets with Enhanced Electrochemical Overall Water Splitting. Adv. Mater. 2020, 32, e1906915. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, A.; Wang, C.; Zhou, W.; Liu, S.; Guo, L. Interfacial Electron Transfer of Ni2P-NiP2 Polymorphs Inducing Enhanced Electrochemical Properties. Adv. Mater. 2018, 30, e1803590. [Google Scholar] [CrossRef]
- Wang, X.D.; Hu, Q.; Li, G.D.; Wei, S.M.; Yang, H.P.; He, C.X. Regulation of the adsorption sites of Ni2P by Ru and S co-doping for ultra-efficient alkaline hydrogen evolution. J. Mater. Chem. A 2021, 9, 15648–15653. [Google Scholar] [CrossRef]
- Zhao, T.W.; Shen, X.J.; Wang, Y.; Hocking, R.K.; Li, Y.B.; Rong, C.L.; Dastafkan, K.; Su, Z.; Zhao, C. In Situ Reconstruction of V-Doped Ni2P Pre-Catalysts with Tunable Electronic Structures for Water Oxidation. Adv. Funct. Mater. 2021, 31, 2100614. [Google Scholar] [CrossRef]
- Wang, X.; Kolen’ko, Y.V.; Bao, X.Q.; Kovnir, K.; Liu, L. One-Step Synthesis of Self-Supported Nickel Phosphide Nanosheet Array Cathodes for Efficient Electrocatalytic Hydrogen Generation. Angew. Chem. Int. Ed. 2015, 54, 8188–8192. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Su, F.; Li, H.; Sun, Y.; Liang, Z.; Liang, W.; Zhang, J.; Qin, W.; Geyer, S.M.; Qiu, Y.; et al. A Ni2P nanocrystal cocatalyst enhanced TiO2 photoanode towards highly efficient photoelectrochemical water splitting. Chem. Eng. J. 2020, 385, 123878. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Cai, C.; Wang, M.; Zhao, Z.L.; Li, M.; Huang, X.; Han, S.; Zhou, H.; Feng, Z.; et al. Single Iridium Atom Doped Ni2P Catalyst for Optimal Oxygen Evolution. J. Am. Chem. Soc 2021, 143, 13605–13615. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, e1806326. [Google Scholar] [CrossRef]
- Chen, G.; Ma, T.; Liu, Z.; Li, N.; Su, Y.; Davey, K.; Qiao, S. Efficient and Stable Bifunctional Electrocatalysts Ni/NixMy (M = P, S) for Overall Water Splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]
- Chang, K.; Tran, D.T.; Wang, J.; Kim, N.H.; Lee, J. A 3D hierarchical network derived from 2D Fe-doped NiSe nanosheets/carbon nanotubes with enhanced OER performance for overall water splitting. J. Mater. Chem. A 2022, 10, 3102–3111. [Google Scholar] [CrossRef]
- Singh, T.I.; Maibam, A.; Cha, D.C.; Yoo, S.; Babarao, R.; Lee, S.U.; Lee, S. High-Alkaline Water-Splitting Activity of Mesoporous 3D Heterostructures: An Amorphous-Shell@Crystalline-Core Nano-Assembly of Co-Ni-Phosphate Ultrathin-Nanosheets and V- Doped Cobalt-Nitride Nanowires. Adv. Sci. 2022, 9, 2201311. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.C.; Dong, P.; Craig, S.R.; Ajayan, P.M.; Ye, M.X.; Shen, J.F. Transforming Nickel Hydroxide into 3D Prussian Blue Analogue Array to Obtain Ni2P/Fe2P for Efficient Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800484. [Google Scholar] [CrossRef]
- Yu, S.H.; Chen, W.; Wang, H.; Pan, H.; Chua DH, C. Highly stable tungsten disulfide supported on a self-standing nickel phosphide foam as a hybrid electrocatalyst for efficient electrolytic hydrogen evolution. Nano Energy 2019, 55, 193–202. [Google Scholar] [CrossRef]
- Li, J.Z.; Wei, G.D.; Zhu, Y.K.; Xi, Y.L.; Pan, X.X.; Ji, Y.; Zatovsky, I.V.; Han, W. Hierarchical NiCoP nanocone arrays supported on Ni foam as an efficient and stable bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2017, 5, 14828–14837. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.P.; Hahnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.C.; Chen, Y.T.; Yuan, Z.H. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380. [Google Scholar] [CrossRef]
- Luo, X.; Ji, P.X.; Wang, P.Y.; Cheng, R.L.; Chen, D.; Lin, C.; Zhang, J.A.; He, J.W.; Shi, Z.H.; Li, N.; et al. Interface Engineering of Hierarchical Branched Mo-Doped Ni3S2/NixPy Hollow Heterostructure Nanorods for Efficient Overall Water Splitting. Adv. Energy Mater. 2020, 10, 1903891. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhang, Z.; Ji, Y.F.; Yang, J.D.; Fan, K.; Ma, X.Z.; Wang, C.; Shu, R.Y.; Chen, Y. Surface engineering induced hierarchical porous Ni12P5-Ni2P polymorphs catalyst for efficient wide pH hydrogen production. Appl. Catal. B 2021, 282, 119609. [Google Scholar] [CrossRef]
- Gong, Y.; Xu, Z.; Pan, H.; Lin, Y.; Yang, Z.; Wang, J. A 3D well-matched electrode pair of Ni–Co–S//Ni–Co–P nanoarrays grown on nickel foam as a high-performance electrocatalyst for water splitting. J. Mater. Chem. A 2018, 6, 12506–12514. [Google Scholar] [CrossRef]
- Arunkumar, P.; Gayathri, S.; Han, J.H. A Complementary Co− Ni Phosphide/Bimetallic Alloy-Interspersed N-Doped Graphene Electrocatalyst for Overall Alkaline Water Splitting. ChemSusChem 2021, 14, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Khiarak, B.N.; Golmohammad, M.; Shahraki, M.M.; Simchi, A. Facile synthesis and self-assembling of transition metal phosphide nanosheets to microspheres as a high-performance electrocatalyst for full water splitting. J. Alloys Compd. 2021, 875, 160049. [Google Scholar] [CrossRef]
- Wei, X.J.; Zhang, Y.H.; He, H.C.; Peng, L.; Xiao, S.H.; Yao, S.R.; Xiao, P. Carbon-incorporated porous honeycomb NiCoFe phosphide nanospheres derived from a MOF precursor for overall water splitting. Chem. Commun. 2019, 55, 10896–10899. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, F.; Xu, Y.J.; Chang, M.J.; Jin, X.J.; Zhou, Y.L.; Piao, J.H.; Lei, J.F. CoP/Ni2P heteronanoparticles integrated with atomic Co/Ni dual sites for enhanced electrocatalytic performance toward hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 8431–8443. [Google Scholar] [CrossRef]
- Bu, F.X.; Chen, W.S.; Aboud MF, A.; Shakir, I.; Gu, J.J.; Xu, Y.X. Microwave-assisted ultrafast synthesis of adjustable bimetal phosphide/graphene heterostructures from MOFs for efficient electrochemical water splitting. J. Mater. Chem. A 2019, 7, 14526–14535. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, Y.; Shen, X.C.; Tao, J.Y.; Yang, X.W.; Xiong, Y.J.; Peng, Z.M. Porous amorphous NiFeOx/NiFeP framework with dual electrocatalytic functions for water electrolysis. J. Power Source 2019, 428, 76–81. [Google Scholar] [CrossRef]
- Saad, A.; Shen, H.; Cheng, Z.; Ju, Q.; Guo, H.; Munir, M.; Turak, A.; Wang, J.; Yang, M. Three-dimensional mesoporous phosphide–spinel oxide heterojunctions with dual function as catalysts for overall water splitting. ACS Appl. Energy Mater. 2020, 3, 1684–1693. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Xue, Q.; Xue, Y.Y.; Ding, Y.; Li, F.M.; Jin, P.J.; Chen, P.; Chen, Y. Iridium Nanotubes as Bifunctional Electrocatalysts for Oxygen Evolution and Nitrate Reduction Reactions. ACS Appl. Mater. Interfaces 2020, 12, 14064–14070. [Google Scholar] [CrossRef]
- Quan, Q.; Lai, Z.X.; Bao, Y.; Bu, X.M.; Meng, Y.; Wang, W.; Takahashi, T.; Hosomi, T.; Nagashima, K.; Yanagida, T.; et al. Self-Anti-Stacking 2D Metal Phosphide Loop-Sheet Heterostructures by Edge-Topological Regulation for Highly Efficient Water Oxidation. Small 2021, 17, 2006860. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Xie, Y.; Sun, B.; Jiang, J.; Jiang, W.J.; Xi, S.; Yang, H.Y.; Yan, K.; Wang, S.; et al. Constructing Atomic Heterometallic Sites in Ultrathin Nickel-Incorporated Cobalt Phosphide Nanosheets via a Boron-Assisted Strategy for Highly Efficient Water Splitting. Nano Lett. 2021, 21, 823–832. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W.D. Construction of hierarchical Ni–Co–P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Chai, L.; Liu, S.L.; Pei, S.T.; Wang, C. Electrodeposited amorphous cobalt-nickel-phosphide-derived films as catalysts for electrochemical overall water splitting. Chem. Eng. J. 2021, 420, 129686. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, X.T.; Xiao, L.G. Prussian blue analogues-derived bimetallic phosphide hollow nanocubes grown on Ni foam as water splitting electrocatalyst. J. Mater. Sci. 2019, 54, 7087–7095. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-organic frameworks derived nanotube of nickel–cobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Shuai, C.; Mo, Z.L.; Niu, X.H.; Zhao, P.; Dong, Q.B.; Chen, Y.; Liu, N.J.; Guo, R.B. Nickel/cobalt bimetallic phosphides derived metal-organic frameworks as bifunctional electrocatalyst for oxygen and hydrogen evolution reaction. J. Alloys Compd. 2020, 847, 156514. [Google Scholar] [CrossRef]
- Xu, H.; Wei, J.J.; Zhang, K.; Shiraish, Y.; Du, Y.K. Hierarchical NiMo Phosphide Nanosheets Strongly Anchored on Carbon Nanotubes as Robust Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 29647–29655. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Wang, J.; Xia, W.; Peng, Z.; Wu, Z.; Lei, W.; Xia, K.; Xin, H.L.; Wang, D. Porous structured Ni–Fe–P nanocubes derived from a prussian blue analogue as an electrocatalyst for efficient overall water splitting. ACS Appl. Mater. Interfaces 2017, 9, 26134–26142. [Google Scholar] [CrossRef]
- Guo, M.; Song, S.; Zhang, S.; Yan, Y.; Zhan, K.; Yang, J.; Zhao, B. Fe-Doped Ni–Co phosphide nanoplates with planar defects as an efficient bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 2020, 8, 7436–7444. [Google Scholar] [CrossRef]
- Ledendecker, M.; Calderon, S.K.; Papp, C.; Steinruck, H.P.; Antonietti, M.; Shalom, M. The Synthesis of Nanostructured Ni5P4 Films and their Use as a Non-Noble Bifunctional Electrocatalyst for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Che, S.; Liu, H.; Ta, N.; Li, G.; Chen, F.; Ma, G.; Yang, F.; Li, Y. In Situ Growth of Self-Supporting MOFs-Derived Ni2P on Hierarchical Doped Carbon for Efficient Overall Water Splitting. Catalysts 2022, 12, 1319. https://doi.org/10.3390/catal12111319
Chen N, Che S, Liu H, Ta N, Li G, Chen F, Ma G, Yang F, Li Y. In Situ Growth of Self-Supporting MOFs-Derived Ni2P on Hierarchical Doped Carbon for Efficient Overall Water Splitting. Catalysts. 2022; 12(11):1319. https://doi.org/10.3390/catal12111319
Chicago/Turabian StyleChen, Neng, Sai Che, Hongchen Liu, Na Ta, Guohua Li, Fengjiang Chen, Guang Ma, Fan Yang, and Yongfeng Li. 2022. "In Situ Growth of Self-Supporting MOFs-Derived Ni2P on Hierarchical Doped Carbon for Efficient Overall Water Splitting" Catalysts 12, no. 11: 1319. https://doi.org/10.3390/catal12111319
APA StyleChen, N., Che, S., Liu, H., Ta, N., Li, G., Chen, F., Ma, G., Yang, F., & Li, Y. (2022). In Situ Growth of Self-Supporting MOFs-Derived Ni2P on Hierarchical Doped Carbon for Efficient Overall Water Splitting. Catalysts, 12(11), 1319. https://doi.org/10.3390/catal12111319