PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Au/rGO and PtAu/rGO
2.1.1. SEM/EDS Characterization
2.1.2. XPS Characterization
2.2. Hydrogen Evolution on Au/rGO and PtAu/rGO
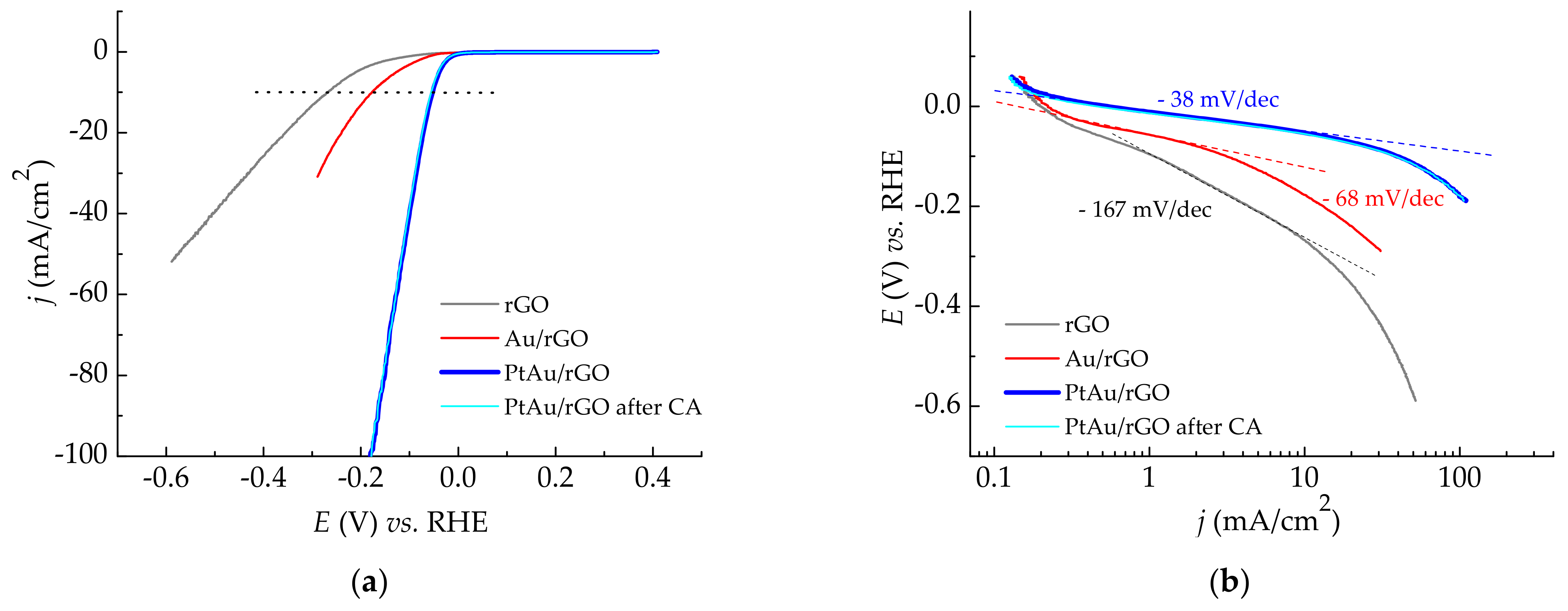
2.2.1. Cyclic Voltammetry Characterization of Au/rGO and PtAu/rGO
2.2.2. HER Catalytic Activity of rGO, Au/rGO, and PtAu/rGO
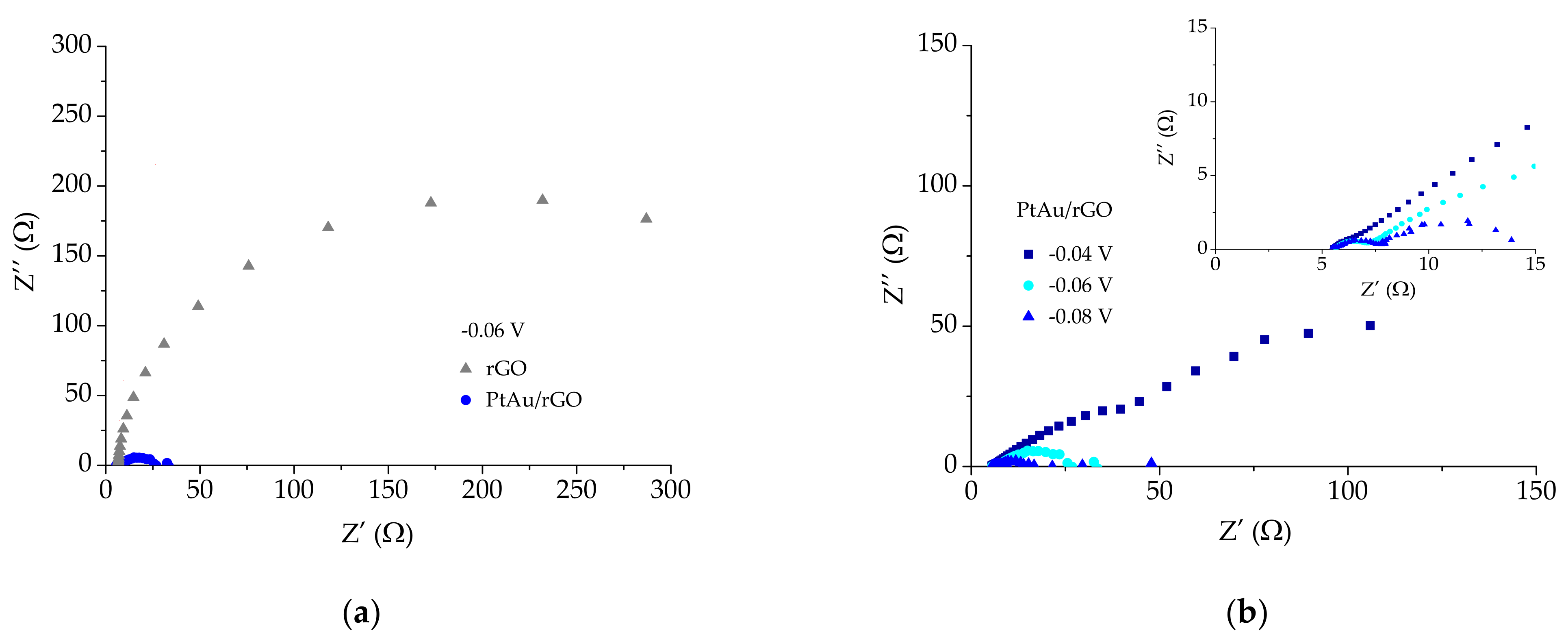
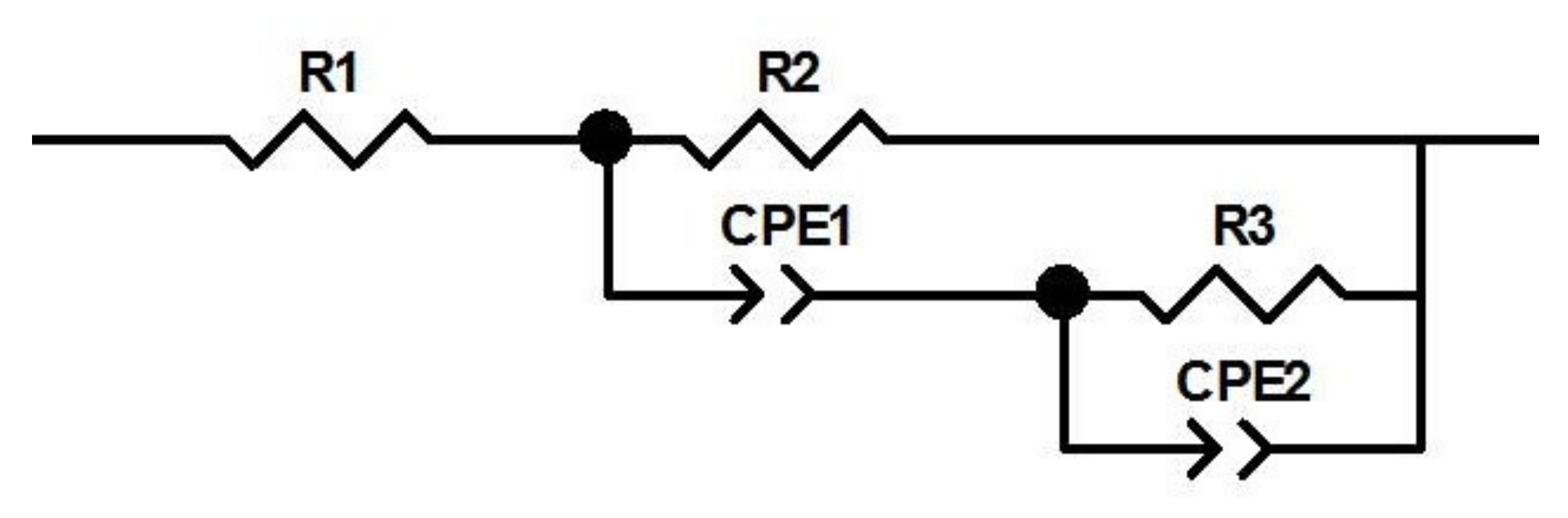
2.2.3. EIS Measurements
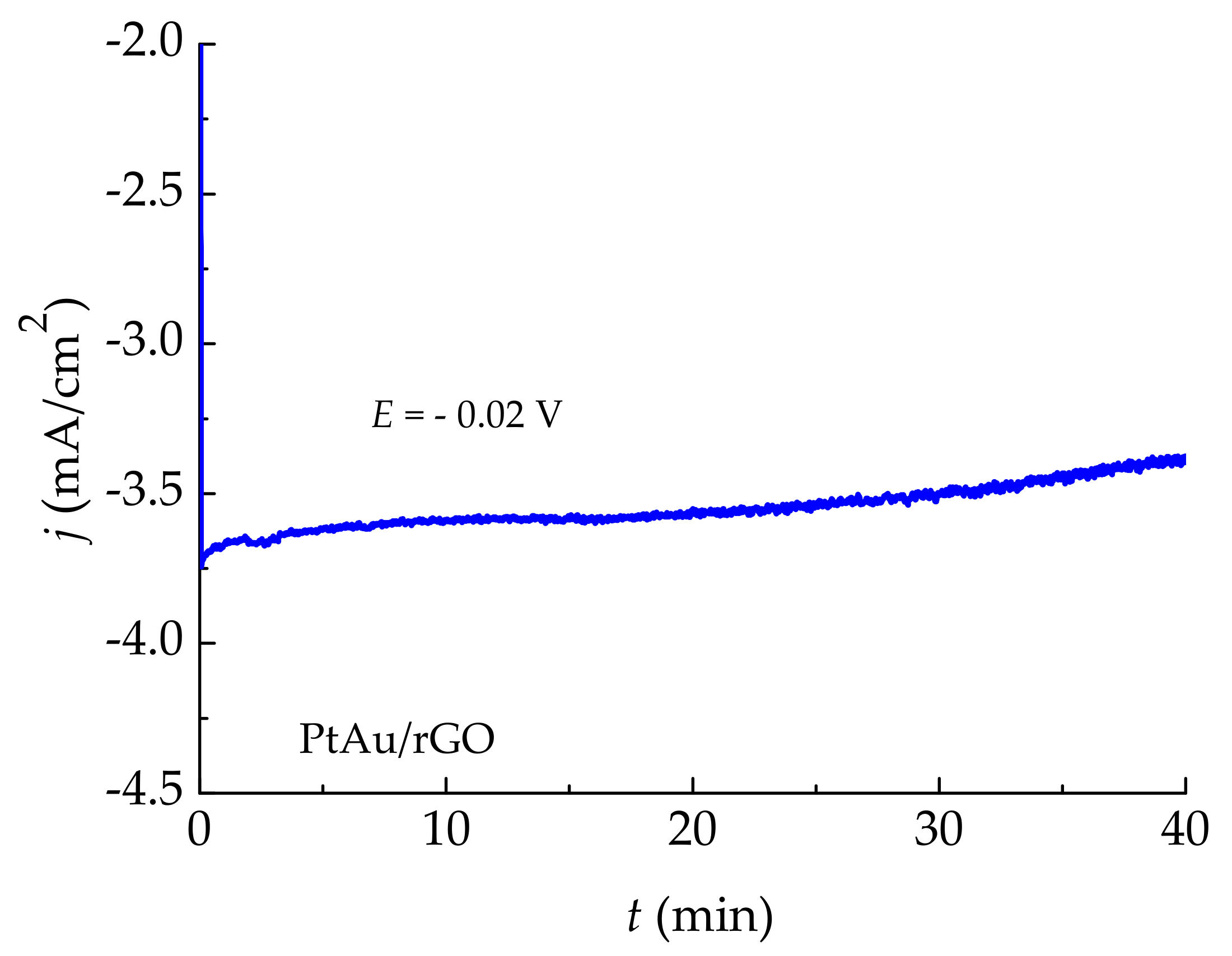
2.2.4. Stability Test for Hydrogen Evolution on PtAu/rGO
3. Materials and Methods
3.1. Materials Preparation
3.2. Materials Characterization
3.3. Electrochemical Measurements
3.4. Chemicals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shinagawa, T.; Takanabe, K. Towards versatile and sustainable hydrogen production through electrocatalytic water splitting: Electrolyte engineering. ChemSusChem 2017, 10, 1318–1336. [Google Scholar] [CrossRef] [Green Version]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrog. Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef]
- Bhalothia, D.; Krishnia, L.; Yang, S.-S.; Yan, C.; Hsiung, W.-H.; Wang, K.-W.; Chen, T.-Y. Recent advancements and future prospects of noble metal-based heterogeneous nanocatalysts for oxygen reduction and hydrogen evolution reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.-B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S.; et al. Single-Atom Catalysts for Electrochemical Hydrogen Evolution Reaction: Recent Advances and Future Perspectives. Nano-Micro Lett. 2020, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xianyun Peng, X.; Liu, X. Single-Atom Catalysts for the Hydrogen Evolution Reaction. ChemElectroChem 2018, 5, 2963–2974. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, L.; Zhuang, C.; Zhang, G.; Sun, S. Fast synthesis of Pt single-atom catalyst with high intrinsic activity for hydrogen evolution reaction by plasma sputtering. Mater. Today Energy 2021, 22, 100877. [Google Scholar] [CrossRef]
- Li, J.; Banis, M.N.; Ren, Z.; Adair, K.R.; Doyle-Davis, K.; Meira, D.M.; Finfrock, Y.Z.; Zhang, L.; Kong, F.; Sham, T.-K.; et al. Unveiling the Nature of Pt Single-Atom Catalyst during Electrocatalytic Hydrogen Evolution and Oxygen Reduction Reactions. Small 2021, 17, 2007245. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Xing, Z.; Yang, X. Single-atom ruthenium based catalyst for enhanced hydrogen evolution. Appl. Catal. B Environ. 2019, 249, 91–97. [Google Scholar] [CrossRef]
- Hossain, M.D.; Liu, Z.; Zhuang, M.; Yan, X.; Xu, G.L.; Gadre, C.A.; Tyagi, A.; Abidi, I.H.; Sun, C.-J.; Wong, H.; et al. Rational design of graphene-supported single atom catalysts for hydrogen evolution reaction. Adv. Energy Maert. 2019, 9, 1803689. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Zhong, H.; Liu, D.; Sarnello, E.; Fang, L.; Xu, M.; Engelhard, M.H.; Tian, H.; Li, T.; et al. An Ion-Imprinting Derived Strategy to Synthesize Single-Atom Iron Electrocatalysts for Oxygen Reduction. Small 2021, 17, 2004454. [Google Scholar] [CrossRef]
- Jing, H.; Zhu, P.; Zheng, X.; Zhang, Z.; Wang, D.; Li, Y. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Ceram. Int. 2021, in press. [Google Scholar] [CrossRef]
- Murthya, A.P.; Madhavana, J.; Murugan, K. Recent advances in hydrogen evolution reaction catalysts on carbon/carbon-based supports in acid media. J. Power Sources 2018, 398, 9–26. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-based electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrog. Energy 2021, 46, 21401–21418. [Google Scholar] [CrossRef]
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y.; et al. Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction. Adv. Mater. 2019, 31, 1903415. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, C.; Shi, G. Graphene based catalysts. Energy Environ. Sci. 2012, 5, 8848–8868. [Google Scholar] [CrossRef]
- Rakočević, L.; Srejić, I.; Maksić, A.; Golubović, J.; Štrbac, S. Hydrogen evolution on reduced graphene oxide-supported PdAu nanoparticles. Catalysts 2021, 11, 481. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Chen, S.-S.; Feng, J.-J.; Lin, X.-X.; Wang, W.; Wang, A.J. Dicationic ionic liquid mediated fabrication of Au@Pt nanoparticles supported on reduced graphene oxide with highly catalytic activity for oxygen reduction and hydrogen evolution. Appl. Surf. Sci. 2018, 441, 438–447. [Google Scholar] [CrossRef]
- Shi, Y.; Zhai, T.T.; Zhou, Y.; Xu, W.-X.; Yang, D.-R.; Wang, F.-B.; Xia, X.-H. Atomic level tailoring of the electrocatalytic activity of Au-Pt core-shell nanoparticles with controllable Pt layers toward hydrogen evolution reaction. J. Electroanal. Chem. 2018, 819, 442–446. [Google Scholar] [CrossRef]
- Shao, F.-Q.; Lin, X.-X.; Feng, J.-J.; Yuan, J.; Chen, J.-R.; Wang, A.-J. Simple fabrication of core-shell AuPt@Pt nanocrystals supported on reduced graphene oxide for ethylene glycol oxidation and hydrogen evolution reactions. Electrochim. Acta 2016, 219, 321–329. [Google Scholar] [CrossRef]
- Feng, J.-J.; Chen, L.-X.; Ma, X.; Yuan, J.; Chen, J.-R.; Wang, A.-J.; Xu, Q.-Q. Bimetallic AuPt alloy nanodendrites/reduced graphene oxide: One-pot ionic liquid-assisted synthesis and excellent electrocatalysis towards hydrogen evolution and methanol oxidation reactions. Int. J. Hydrog. Energy 2017, 42, 1120–1129. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Lai, C.-H.; Lin, C.-C.; Yang, C.-P. Insertion of a graphene oxide layer into a Cu/SiO2/Pt structure to overcome performance degradation in a vaporless environment. Appl. Sci. 2019, 9, 1432. [Google Scholar] [CrossRef] [Green Version]
- Eng, A.Y.S.; Sofer, Z.; Sedmidubský, D.; Pumera, M. Synthesis of Carboxylated-graphenes by the Kolbe-Schmitt process. ACS Nano 2017, 11, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Peuckert, M.; Coenen, F.P.; Bonzel, H.P. XPS study of the electrochemical surface oxidation of Platinum in N H2SO4 acid electrolyte. Electrochim. Acta 1984, 29, 1305–1314. [Google Scholar] [CrossRef]
- Heimann, P.; Van der Veen, J.F.; Eastman, D.E. Structure-dependent surface core level shifts for the Au(111), Au(100), and Au(110) surfaces. Solid State Commun. 1981, 38, 595–598. [Google Scholar] [CrossRef]
- Štrbac, S.; Smiljanić, M.; Rakočević, Z. Spontaneously deposited Rh on Au(111) observed by AFM and XPS: Electrocatalysis of hydrogen evolution. J. Electrochem. Soc. 2016, 163, D3027–D3033. [Google Scholar] [CrossRef]
- Romeo, M.; Majerus, J.; Legare, P.; Castellani, N.; Leroy, D. Photoemission study of Pt adlayers on Ni(111). Surf. Sci. 1990, 238, 163–168. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Wang, Z.-B.; Li, C.; Zhao, L.; Liu, J.; Zhang, L.-M.; Gu, D.-M. Multiwall-carbon nanotube modified by N-doped carbon quantum dots as Pt catalyst support for methanol electrooxidation. J. Power Sources 2015, 289, 63–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Z.; Shen, Q.; Li, M.; Xu, L.; Luo, Z. Aqueous Preparation of platinum nanoflowers on three-dimensional graphene for efficient methanol oxidation. Catalysts 2018, 8, 519. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.G.M.; Brownson, D.A.C.; Banks, C.E. Investigating the integrity of graphene towards the electrochemical hydrogen evolution reaction (HER). Sci. Rep. 2019, 9, 15961. [Google Scholar] [CrossRef] [PubMed]
- Smiljanić, M.; Srejić, I.; Grgur, B.; Rakočević, Z.; Štrbac, S. Catalysis of hydrogen evolution on Au(111) modified by spontaneously deposited Pd islands. Electrocatalysis 2012, 3, 369–375. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Liao, H.; Sun, S.; Li, S.; Ager, J.W., III; Xu, Z.J. Activation effect of electrochemical cycling on gold nanoparticles towards the hydrogen evolution reaction in sulfuric acid. Electrochim. Acta 2016, 209, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Shinagava, T.; Garcia-Espanza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.D.; Henderson, M. Impedance plane display of a reaction with an adsorbed intermediate. J. Electroanal. Chem. 1972, 39, 81–90. [Google Scholar] [CrossRef]
- Amaral, L.; Cardoso, D.S.P.; Šljukić, B.; Santos, D.M.F.; Sequeira, C.A.C. Electrochemistry of hydrogen evolution in ionic aqueous mixtures. Mater. Res. Bull. 2019, 112, 407–412. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Lacconi, G.I.; Corti, H.R. Kinetics of the hydrogen evolution on nickel in alkaline solution: New insight from rotating disk electrode and impedance spectroscopy analysis. Electrochim. Acta 2015, 159, 210–218. [Google Scholar] [CrossRef]
- Krstajić, N.; Popović, M.; Grgur, B.; Vojnović, M.; Šepa, D. On the kinetics of the hydrogen evolution reaction on nickel in alkaline solution—Part I. The mechanism. J. Electroanal. Chem. 2001, 512, 16–26. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Zhang, Z.; Qi, H.; Li, H.; Zhong, J. 3D binder-free MoSe2 nanosheets/carbon cloth electrodes for efficient and stable hydrogen evolution prepared by simple electrophoresis deposition strategy. Sci. Rep. 2016, 6, 22516. [Google Scholar] [CrossRef]
- Mirdamadi-Esfahani, M.; Mostafavi, M.; Keita, B.; Nadjo, L.; Kooyman, P.; Remita, H. Bimetallic Au-Pt nanoparticles synthesized by radiolysis: Application in electro-catalysis. Gold Bull. 2010, 43, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Xiahou, Y.; Xing, L.; Zhang, X.; Li, H.; Wu, C.S.; Xia, H. Fe(II)-assisted one-pot synthesis of ultra-small core-shell Au-Pt nanoparticles as superior catalysts towards HER and ORR. Nanoscale 2020, 12, 20456–20466. [Google Scholar] [CrossRef]

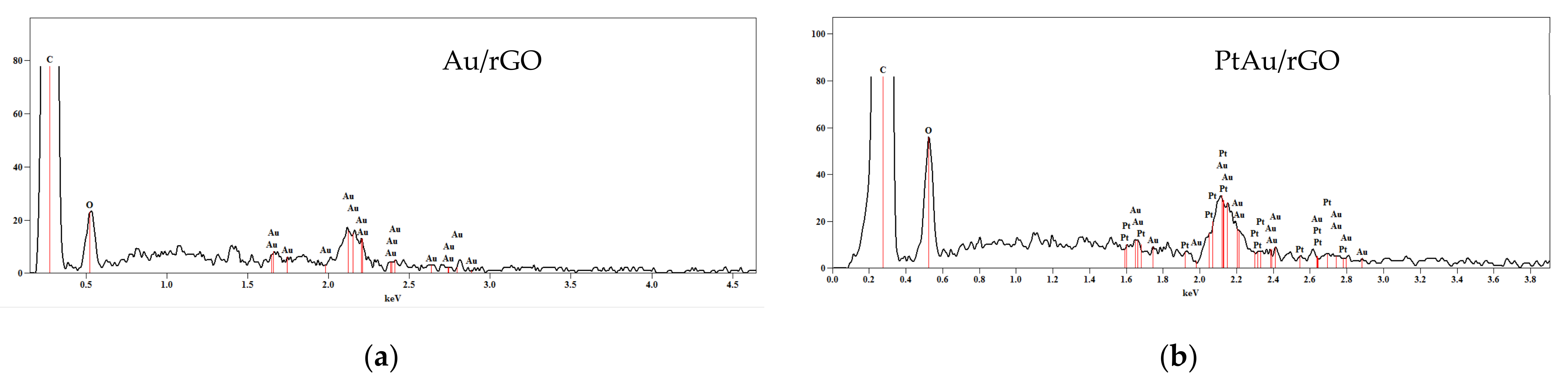
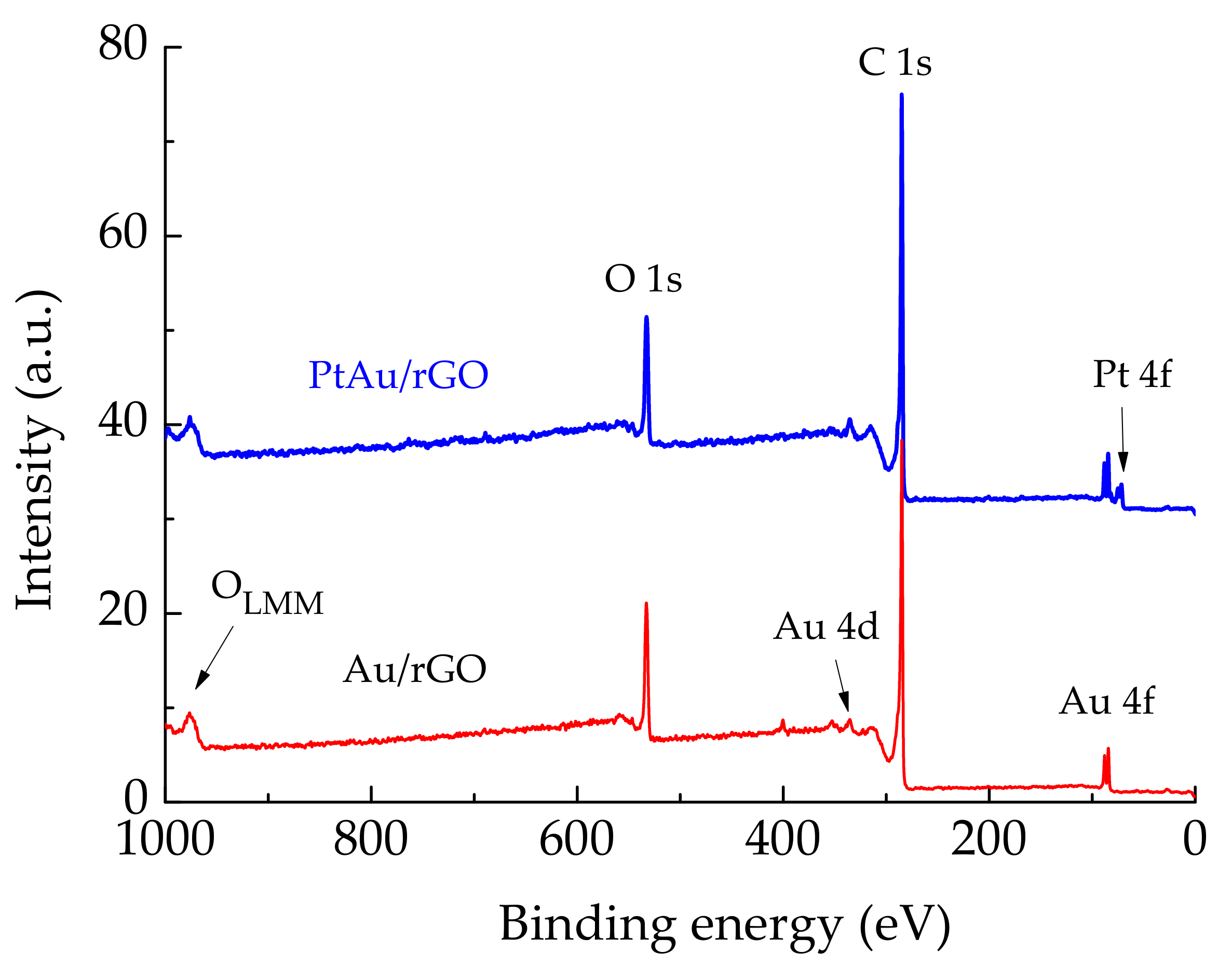
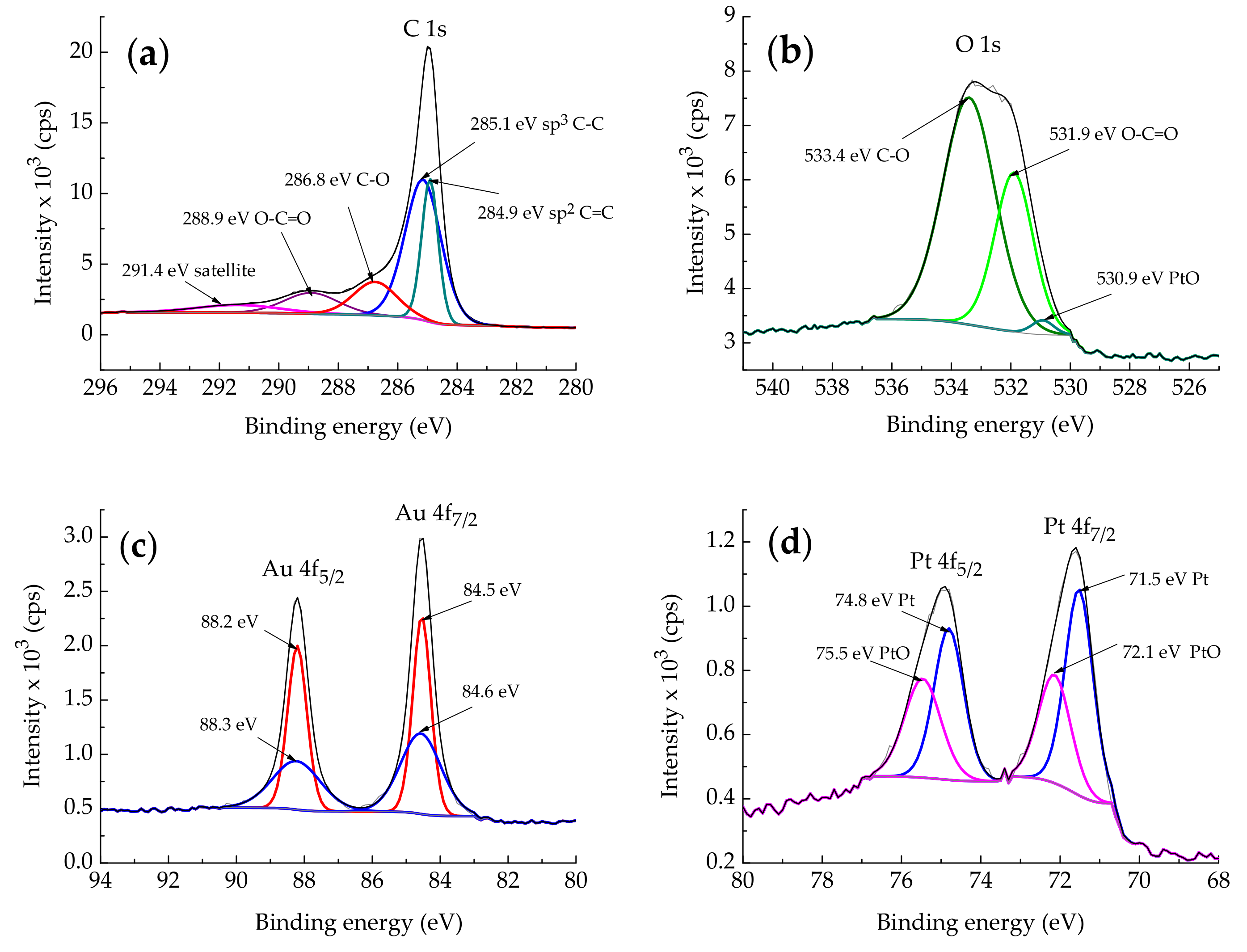

| Element/Line Type | Au/rGO | PtAu/rGO | ||
|---|---|---|---|---|
| Weight % | Atom % | Weight % | Atom % | |
| C K | 85.63 | 94.76 | 81.32 | 93.80 |
| O K | 5.60 | 4.65 | 6.14 | 5.31 |
| Au M | 8.77 | 0.59 | 4.14 | 0.29 |
| Pt M | 8.40 | 0.60 | ||
| Line | Au/rGO Atom % | PtAu/rGO Atom % |
|---|---|---|
| C 1s | 83.3 | 82.8 |
| O 1s | 16.0 | 15.0 |
| Au 4f7/2 | 0.7 | 0.6 |
| Pt 4f7/2 | 0.3 |
| E (V) vs. RHE | −0.04 | −0.06 | −0.08 |
|---|---|---|---|
| Rs (Ω) | 5.33 | 5.55 | 5.78 |
| R2 (Ω) | 11.8 | 2.28 | 2.4 |
| R3 (Ω) | 262.6 | 20.8 | 11.8 |
| CPE1 (F) | 27.8 × 10−3 | 4.98 × 10−3 | 1.958 × 10−3 |
| CPE2 (F) | 3.075 × 10−3 | 72.04 × 10−3 | 0.127 |
| Catalyst | Onset Potential E (mV) vs. RHE | Tafel Slope (mV/dec) | Ref. |
|---|---|---|---|
| Au@Pt NPs/rGO | −20 | 42 | [22] |
| Au-monolayer Pt/rGO | ≈0 | 27 | [23] |
| AuPt@Pt nanocrystals/rGO | −25 | 33 | [24] |
| AuPt alloy nanodendrites/rGO | −37 | 34 | [25] |
| Au-Pt (54:47) alloy NPs/GC | −6 | 34 | [44] |
| Au38.4@Au9.3Pt52.3 -NP/C | −4 | 14 | [45] |
| Pt/C | −5 | 31 | [45] |
| PtAu/rGO | −5 | 38 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakočević, L.; Simatović, I.S.; Maksić, A.; Rajić, V.; Štrbac, S.; Srejić, I. PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution. Catalysts 2022, 12, 43. https://doi.org/10.3390/catal12010043
Rakočević L, Simatović IS, Maksić A, Rajić V, Štrbac S, Srejić I. PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution. Catalysts. 2022; 12(1):43. https://doi.org/10.3390/catal12010043
Chicago/Turabian StyleRakočević, Lazar, Ivana Stojković Simatović, Aleksandar Maksić, Vladimir Rajić, Svetlana Štrbac, and Irina Srejić. 2022. "PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution" Catalysts 12, no. 1: 43. https://doi.org/10.3390/catal12010043
APA StyleRakočević, L., Simatović, I. S., Maksić, A., Rajić, V., Štrbac, S., & Srejić, I. (2022). PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution. Catalysts, 12(1), 43. https://doi.org/10.3390/catal12010043






