Catalytic Synthesis of Methacrolein via the Condensation of Formaldehyde and Propionaldehyde with L-Proline Intercalated Layered Double Hydroxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Re-MgxAl–LDHs and Re-CaxAl–LDHs
2.1.1. Powder X-ray Diffractometry (XRD)
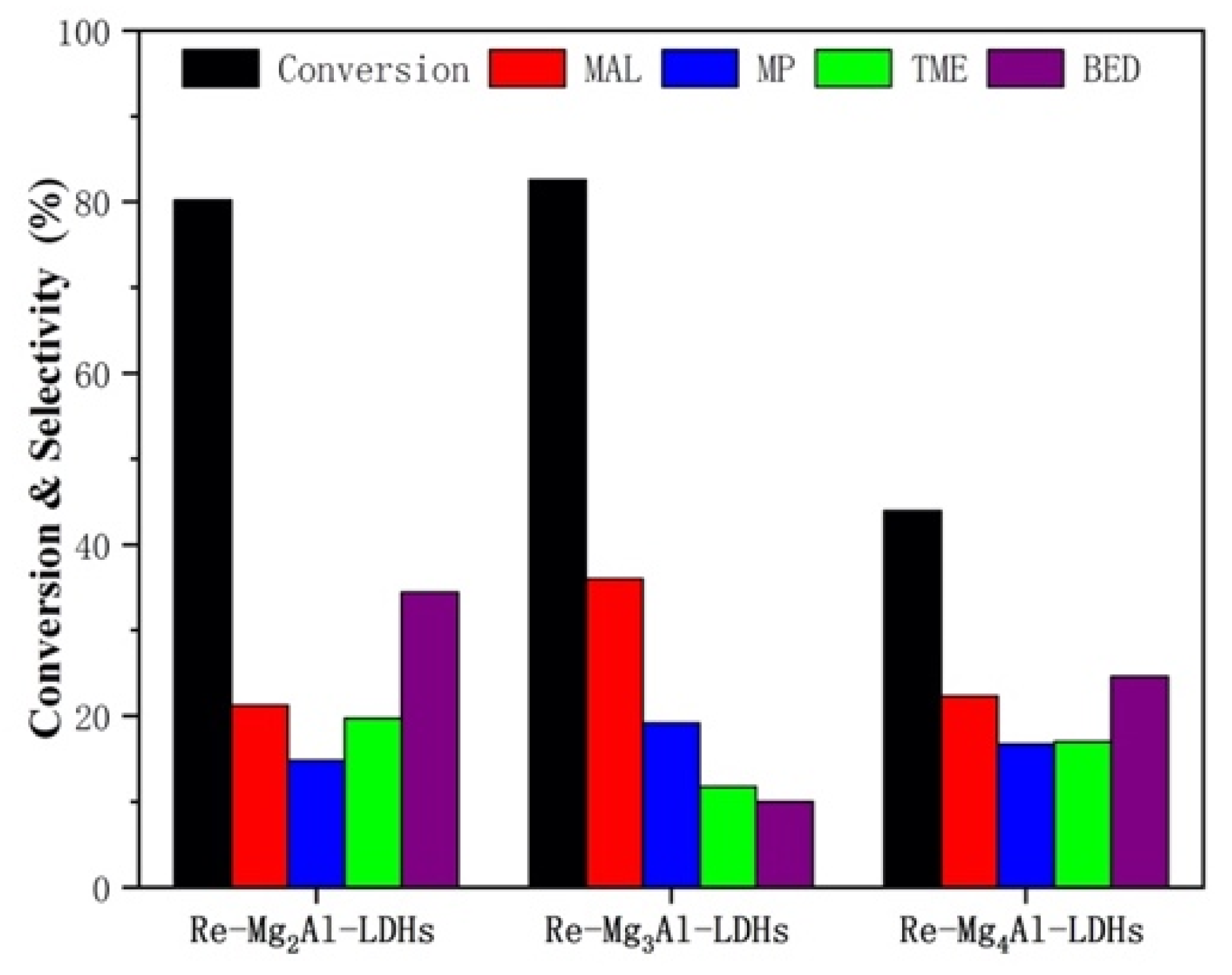
2.1.2. Evaluation of the Catalytic Performance
2.2. Re-Mg3Al–Pro-LDHs and Mg3Al–Pro-LDHs
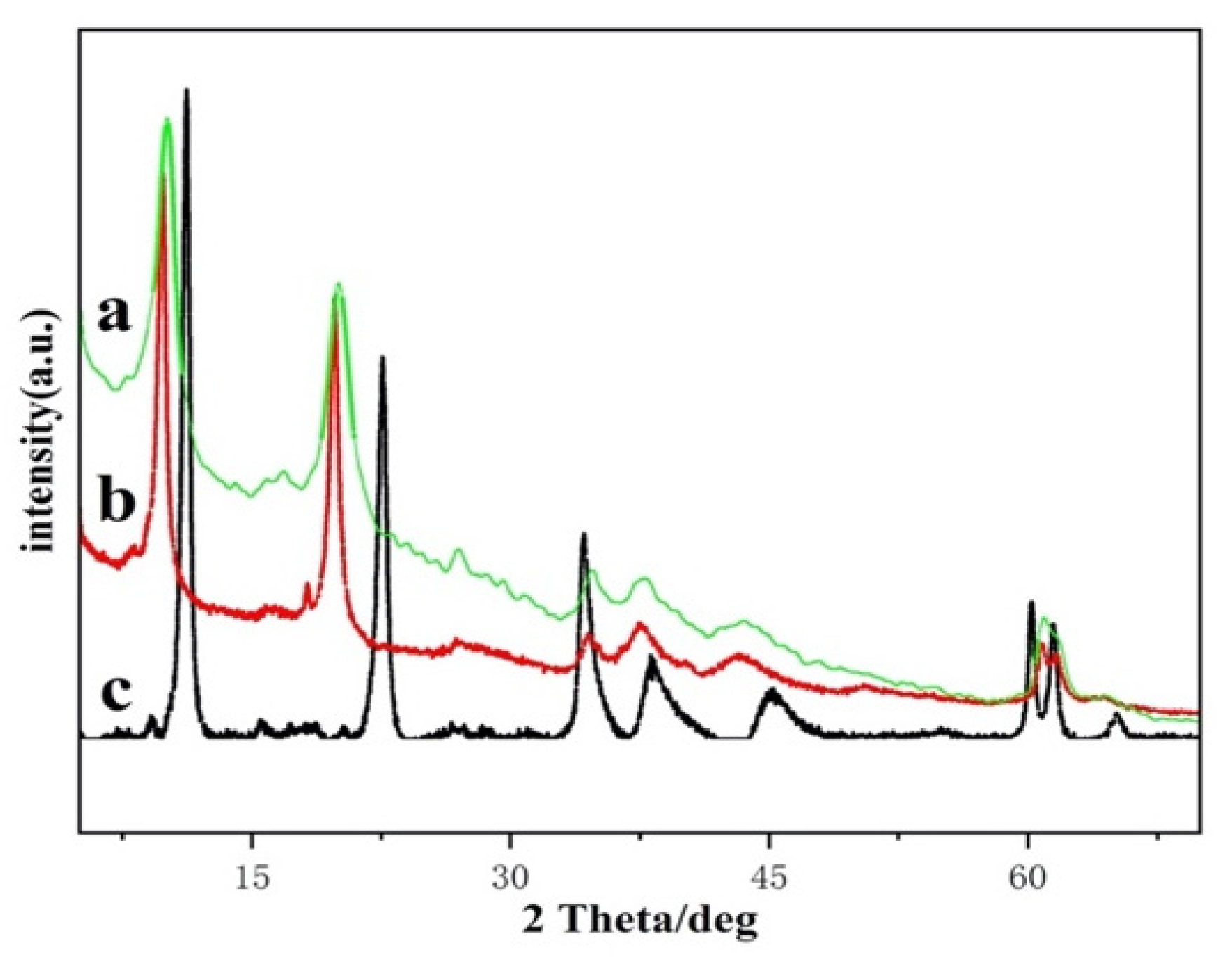
2.2.1. XRD
2.2.2. FTIR
2.2.3. Evaluation of the Catalytic Performance
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Catalysts Preparation
3.3. Synthesis of MAL from FA and PA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diao, Y.; Yang, P.; Yan, R.; Jiang, L.; Wang, L.; Zhang, H.; Li, C.; Li, Z.; Zhang, S. Deactivation and regeneration of the supported bimetallic Pd-Pb catalyst in direct oxidative esterification of methacrolein with methanol. Appl. Catal. B Environ. 2013, 142, 329–336. [Google Scholar] [CrossRef]
- Jaber, D.; Jean-Luc, D.; Fabrizio, C.; Rostamizadeh, M.; Patience, G.S. Catalysis for the synthesis of methacrylic acid and methyl methacrylate. Chem. Soc. Rev. 2018, 47, 7703–7738. [Google Scholar]
- Fan, L.; Xu, B.; Li, J.; Yan, R.; Diao, Y.; Li, C. Kinetic studies on both synthesis of methacrolein catalyzed by an ionic liquid and catalyst deactivation. Ind. Eng. Chem. Res. 2021, 60, 5411–5420. [Google Scholar] [CrossRef]
- Yan, R.; Li, Z.; Diao, Y.; Fu, C.; Wang, H.; Li, C.; Chen, Q.; Zhang, X.; Zhang, S. Green process for methacrolein separation with ionic liquids in the production of methyl methacrylate. Aiche J. 2011, 57, 2388–2396. [Google Scholar] [CrossRef]
- Li, Y.C.; Yan, R.Y.; Wang, L.; Liu, L.; Liu, D.; Zhang, H.; Diao, Y.Y.; Li, Z.X.; Zhang, S.J. Synthesis of methacrolein by condensation of propionaldehyde with formaldehyde. Adv. Mater. Res. 2012, 396–398, 1094–1097. [Google Scholar] [CrossRef]
- West, R.M.; Liu, Z.Y.; Peter, M.; Gärtner, C.A.; Dumesic, J.A. Carbon–Carbon bond formation for biomass-derived furfurals and ketones by aldol condensation in a biphasic system. Mol. Catal. 2008, 296, 18–27. [Google Scholar] [CrossRef]
- Vashishtha, M.; Mishra, M.; Shah, D.O. A novel approach for selective cross aldol condensation using reusable NaOH-cationic micellar systems. Appl. Catal. A Gen. 2013, 466, 38–44. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Wang, L.; Fu, Z. Oxidative esterification of methacrolein to methyl methacrylate over supported gold catalysts prepared by colloid deposition. Chemcatchem 2017, 11, 1960–1968. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Yin, D.; Li, D. Latest progress and application of Mannich reaction. Chin. J. Org. Chem. 2016, 36, 927–938. [Google Scholar] [CrossRef]
- de María, P.D.; Bracco, P.; Castelhano, L.F.; Bargeman, G. Influence of the organocatalyst in the Aldol/Mannich-Type product selectivities in C−C bond forming reactions. ACS Catal. 2011, 1, 70–75. [Google Scholar] [CrossRef]
- Lei, L.; Tao, R.; Shi, J.; Jing, X.; Ma, H. Rapid and continuous synthesis of methacrolein with high selectivity by condensation of propanal with formaldehyde in laboratory. Can. J. Chem. Eng. 2017, 95, 1985–1992. [Google Scholar] [CrossRef]
- Yu, J.; Jensen, A.D.; Wang, L.; Li, C.; Zhang, S. Catalytic synthesis of methacrolein via the condensation of formaldehyde and propionaldehyde with L-proline. Green Chem. 2020, 22, 4222–4230. [Google Scholar] [CrossRef]
- Elmekawy, A.A.; Sweeney, J.B.; Brown, D.R. Efficient synthesis of supported proline catalysts for asymmetric aldol reactions. Catal. Sci. Technol. 2015, 5, 690–696. [Google Scholar] [CrossRef]
- Feng, X.; Jena, H.S.; Leus, K.; Wang, G.; Ouwehand, J.; Van Der Voort, P. L-proline modulated zirconium metal organic frameworks: Simple chiral catalysts for the aldol addition reaction. J. Catal. 2018, 365, 36–42. [Google Scholar] [CrossRef]
- An, Z.; Zhang, W.; Shi, H.; He, J. An effective heterogeneous L-proline catalyst for the asymmetric aldol reaction using anionic clays as intercalated support. J. Catal. 2006, 241, 319–327. [Google Scholar] [CrossRef]
- Heravi, M.M.; Mohammadi, P. Layered double hydroxides as heterogeneous catalyst systems in the cross-coupling reactions: An overview. Mol. Divers. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Li, X.; Bai, P.; Yan, W.; Yu, J. Layered inorganic cationic frameworks beyond layered double hydroxides: Structures and applications. Eur. J. Inorg. Chem. 2020, 43, 4055–4063. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Wang, N.; Huang, Z.; Li, X.; Li, J.; Ji, S.; An, Q.-F. Tuning molecular sieving channels of layered double hydroxides membrane with direct intercalation of amino acids. J. Mater. Chem. A 2018, 6, 17148–17155. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, L.; Li, J.; Yu, H. Adsorption of heavy metals by L-cysteine intercalated layered double hydroxide: Kinetic, isothermal and mechanistic studies. J. Colloid Interface Sci. 2020, 562, 149–158. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M. An effective, low-cost and recyclable bio-adsorbent having amino acid intercalated LDH@Fe3O4/PVA magnetic nanocomposites for removal of methyl orange from aqueous solution. Appl. Clay Sci. 2019, 15, 127–137. [Google Scholar] [CrossRef]
- Rives, V.; Arco, M.D.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2013, 88, 239–269. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Gaskell, E.E.; Ha, T.; Hamilton, A.R. Ibuprofen intercalation and release from different layered double hydroxides. Ther. Deliv. 2018, 9, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Co(II)-amino acid-CaAl-layered double hydroxide composites construction and characterization. J. Mol. Struct. 2019, 1179, 263–268. [Google Scholar] [CrossRef]
- Deng, X.; Huang, J.; Wan, H.; Chen, F.; Lin, Y.; Xu, X.; Ma, R.; Sasaki, T. Application progress of functionalized layered double hydroxides. J. Mater. Sci. Eng. 2019, 37, 509–516. [Google Scholar]
- Huo, Y.S.; Zhu, L.H.; Yang, J.; Sun, Y.L. Structure, Properties and preparation of layered double hydroxides and its application in field of catalysis. Bull. Chin. Ceram. Soc. 2013, 21, 429–433. [Google Scholar]
- Deng, X.; Huang, J.; Wan, H.; Chen, F.; Lin, Y.; Xu, X.; Ma, R.; Sasaki, T. Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation. J. Energy Chem. 2018, 32, 93–104. [Google Scholar] [CrossRef]
- Prevot, V.; Bourgeat-Lami, E. Recent advances in layered double hydroxide/polymer latex nanocomposites: From assembly to in situ formation. Layer. Double Hydroxide Polym. Nanocompos. 2020, 461–495. [Google Scholar] [CrossRef]
- Vijaikumar, S.; Dhakshinamoorthy, A.; Pitchumani, K. L-proline anchored hydrotalcite clays: An efficient catalyst for asymmetric Michael addition. Appl. Catal. A Gen. 2008, 340, 25–32. [Google Scholar] [CrossRef]
- Chen, S.; Ma, J.; Zhang, T.; Zhang, S.; Zhang, C.; Cheng, H.; Ge, Y.; Liu, L.; Tong, Z.; Zhang, B. Intercalated cobalt porphyrin between layered double hydroxide nanosheets as an efficient and recyclable catalyst for aerobic epoxidation of alkenes. Appl. Clay Sci. 2020, 187, 105478. [Google Scholar]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Demir, F.; Demir, B.; Yalcinkaya, E.E.; Cevik, S.; Demirkol, D.O.; Anik, U.; Timur, S. Amino acid intercalated montmorillonite: Electrochemical biosensing applications. RSC Adv. 2014, 4, 50107–50113. [Google Scholar] [CrossRef]
- Stamate, A.E.; Pavel, O.D.; Zavoianu, R.; Marcu, I.C. Highlights on the catalytic properties of polyoxometalate-intercalated layered double hydroxides: A review. Catalysts 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- He, S.; An, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide-based catalysts: Nanostructure design and catalytic performance. Chem. Commun. 2013, 49, 5912–5920. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Pang, H. Intercalation of Mg-Al layered double hydroxides by L-proline: Synthesis and characterization. Res. Chem. Intermed. 2012, 38, 629–638. [Google Scholar] [CrossRef]
- Fudala, Á.; Pálinkó, I.; Kiricsi, I. Preparation and characterization of hybrid organic-inorganic composite materials using the amphoteric property of amino acids: Amino acid intercalated layered double hydroxide and montmorillonite. Inorg. Chem. 1999, 38, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, T.; Dhakshinamoorthy, A.; Pitchumani, K. Amino acid intercalated layered double hydroxide catalyzed chemoselective methylation of phenols and thiophenols with dimethyl carbonate. Cheminform 2013, 54, 7167–7170. [Google Scholar]
- Wang, G.; Li, Z.; Fan, L.; Li, C.; Zhang, S. Sec-amine grafted D301 resin catalyzed fixed-bed process for continuous preparation of methacrolein via Mannich reaction. Chem. Eng. J. 2019, 370, 625–636. [Google Scholar] [CrossRef]
- Bing, W.; Zheng, L.; He, S.; Rao, D.; Xu, M.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. Insights on active sites of CaAl-hydrotalcite as a high-performance solid base catalyst toward aldol condensation. ACS Catal. 2017, 8, 656–664. [Google Scholar] [CrossRef]
- Bing, W.; Wang, H.; Zheng, L.; Rao, D.; Yang, Y.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. CaMnAl-hydrotalcite solid basic catalyst toward aldol condensation reaction with a comparable level to liquid alkali catalysts. Green Chem. 2018, 20, 3071–3080. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Weng, B.; Atrens, A.; Ma, S.; Liu, L.; Pan, F. Sealing of anodized magnesium alloy AZ31 with MgAl layered double hydroxides layers. RSC Adv. 2018, 8, 2248–2259. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M.; Hung, C.I.; Gnanamani, E. Tuning the reactivity of ketones through unsaturation: Construction of cyclic and acyclic quaternary stereocenters via Zn-ProPhenol catalyzed mannich reactions. ACS Catal. 2019, 9, 1549–1557. [Google Scholar] [CrossRef]
- An, Y.-J.; Wang, C.-C.; Liu, Z.-P.; Tao, J.-C. Isosteviol proline conjugates as highly efficient amphiphilic organocatalysts for asymmetric three-component Mannich reactions in the presence of water. Helv. Chim. Acta 2012, 95, 43–51. [Google Scholar] [CrossRef]
- Arya, K.; Rajesh, U.C.; Rawat, D.S. Proline confined FAU zeolite: Novel hybrid catalyst for synthesis of spiro heterocycles via mannich type reaction. Green Chem. 2012, 14, 3344–3351. [Google Scholar] [CrossRef]
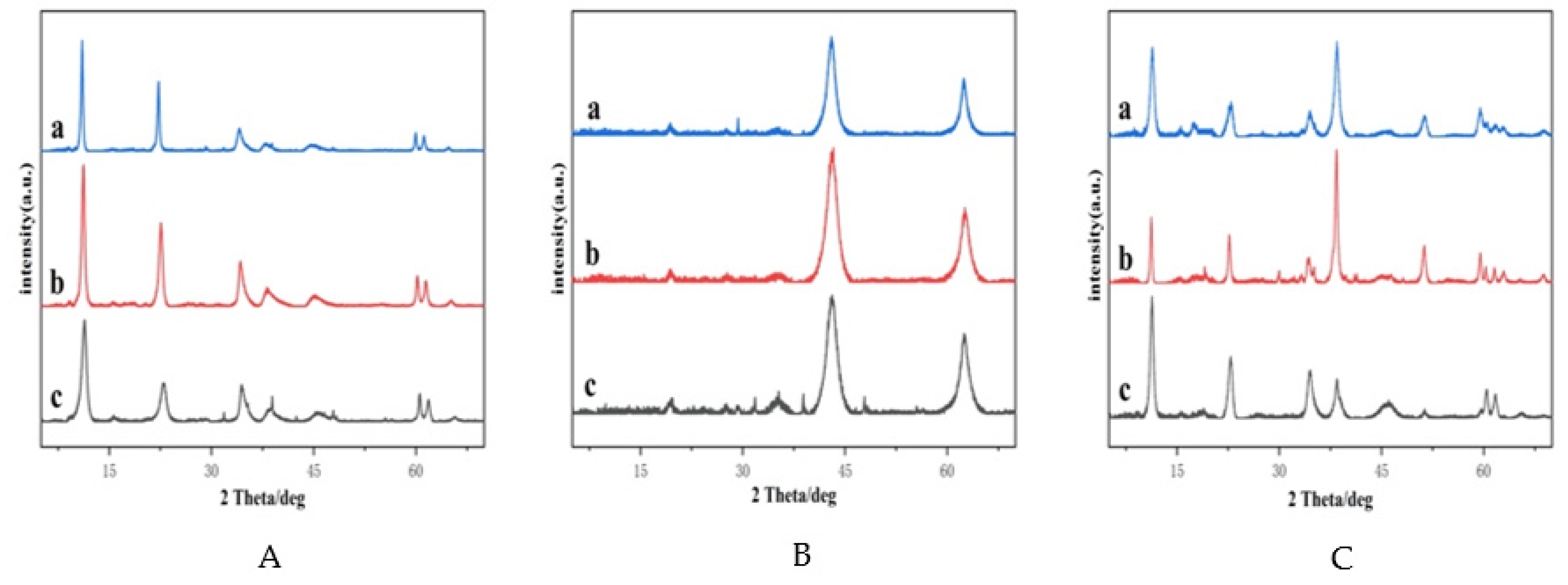
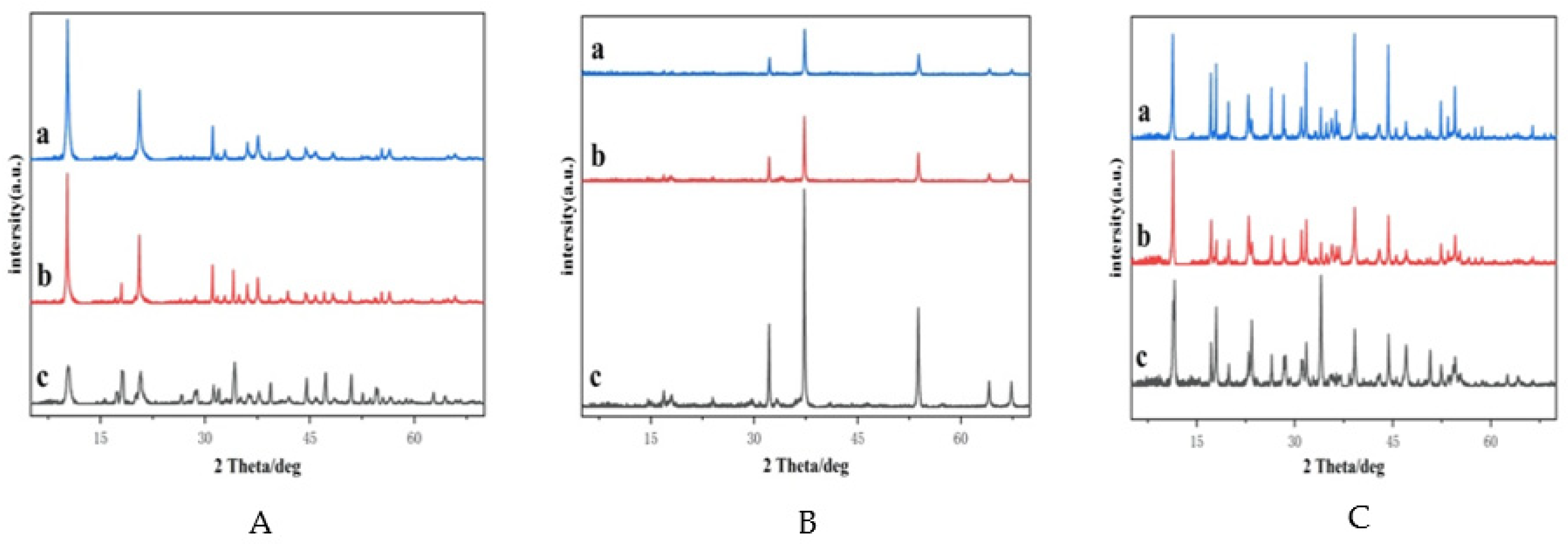
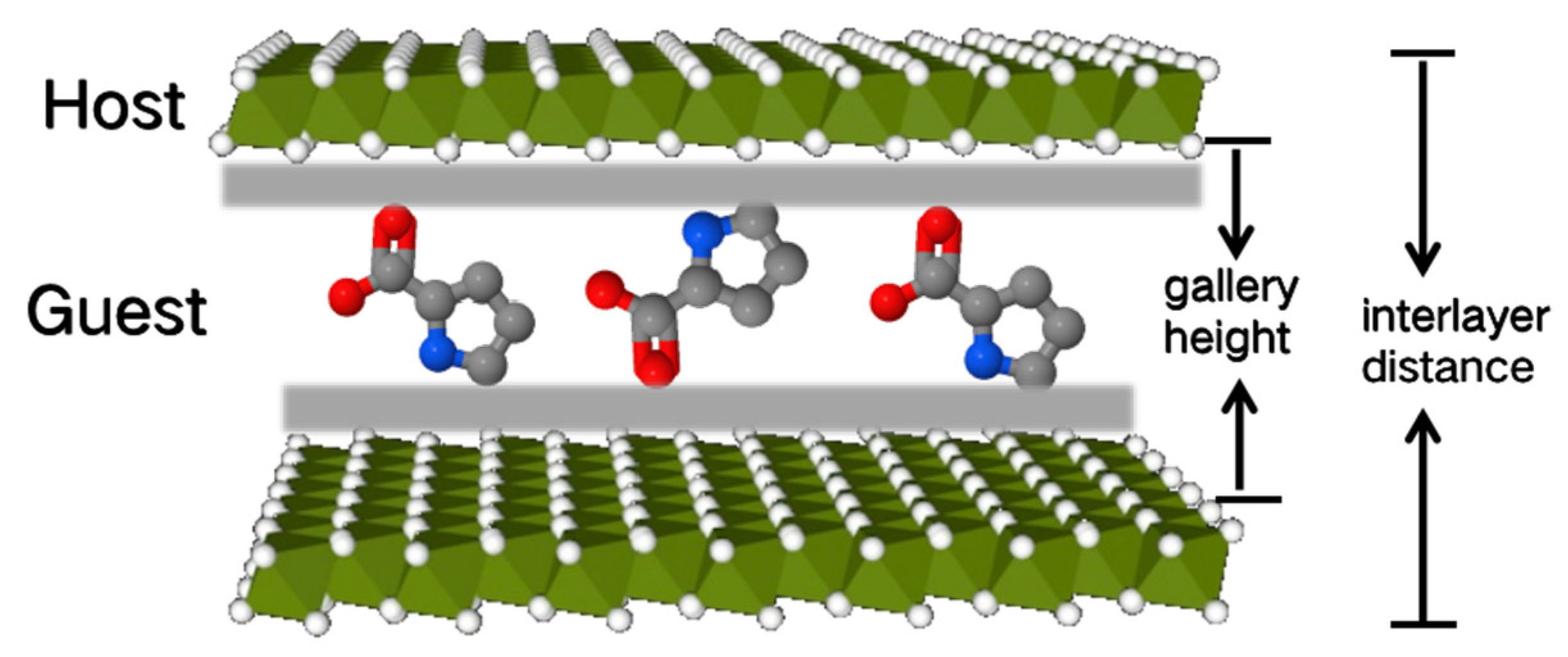

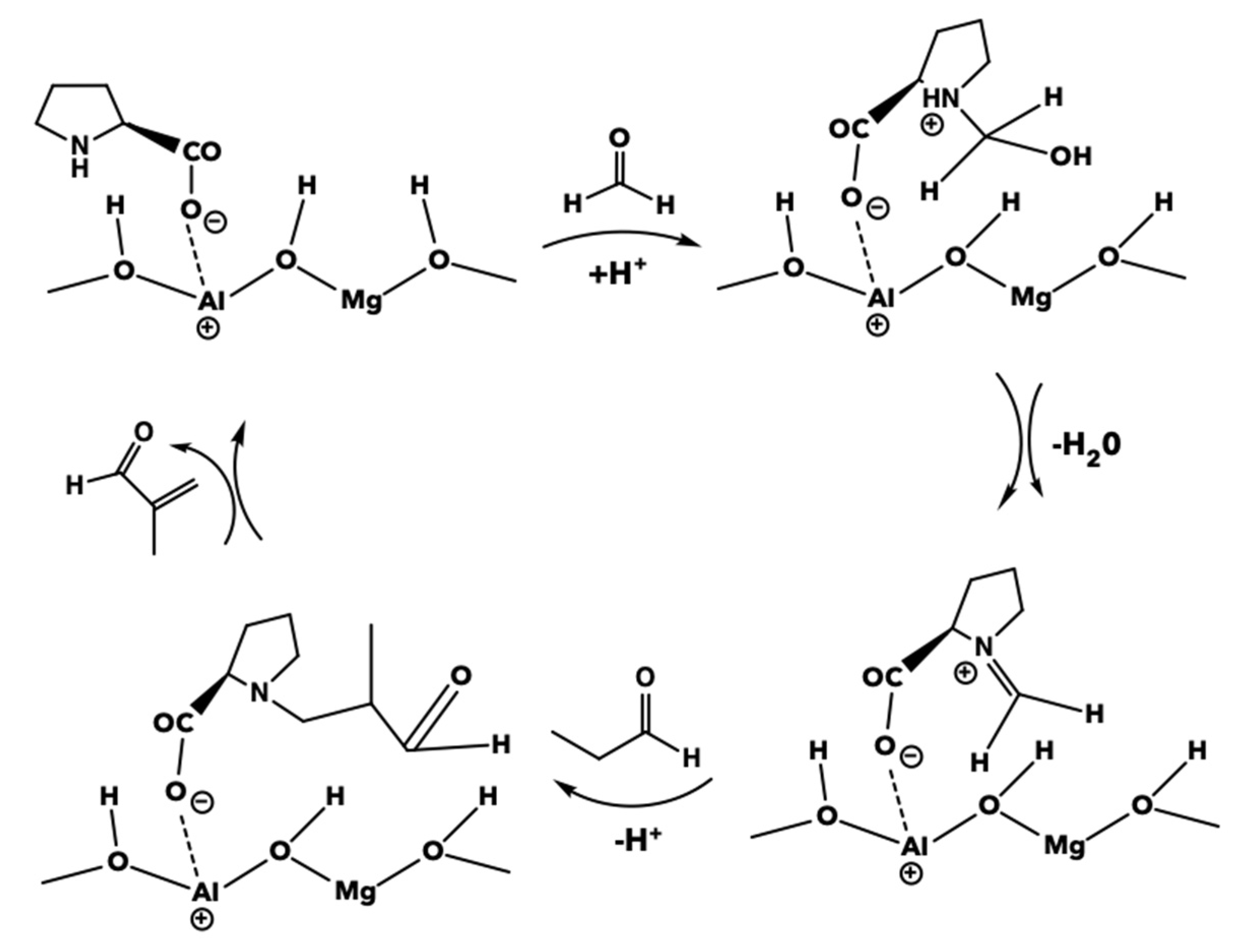
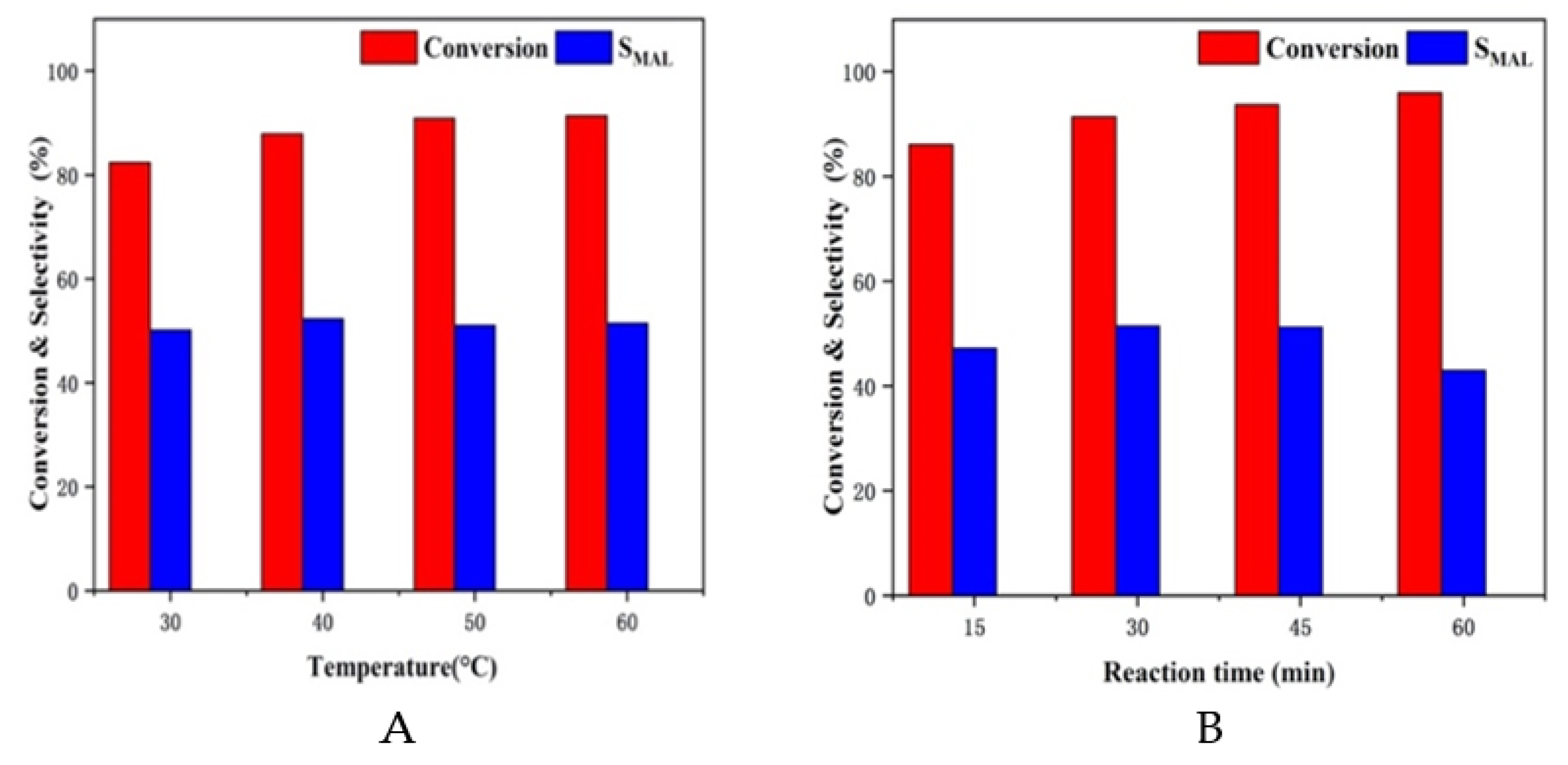

| Catalyst | PA Conversion (%) | MAL Selectivity (%) |
|---|---|---|
| re-Ca2Al–LDHs | 73.61 | 17.93 |
| re-Ca3Al–LDHs | 79.30 | 18.22 |
| re-Ca4Al–LDHs | 84.56 | 24.85 |
| re-Mg2Al–LDHs | 80.18 | 21.25 |
| re-Mg3Al–LDHs | 82.59 | 36.01 |
| re-Mg4Al–LDHs | 43.95 | 22.31 |
| Mg3Al–LDHs | Mg3Al–Pro-LDHs | re-Mg3Al–Pro-LDHs | ||||
|---|---|---|---|---|---|---|
| Diffraction Peaks | 2θ (°) | d (nm) | 2θ (°) | d (nm) | 2θ (°) | d (nm) |
| 003 | 11.20 | 0.78 | 9.80 | 0.90 | 10.20 | 0.86 |
| 006 | 22.56 | 0.39 | 19.80 | 0.45 | 20.02 | 0.44 |
| 009 | 34.28 | 0.26 | 34.50 | 0.26 | 34.82 | 0.26 |
| 110 | 60.22 | 0.15 | 60.78 | 0.15 | 60.94 | 0.15 |
| Catalyst | PA Conversion (%) | MAL Selectivity (%) |
|---|---|---|
| Mg3Al–Pro-LDHs | 21.09 | 94.58 |
| re-Mg3Al–Pro-LDHs | 19.50 | 64.72 |
| Substrate Amount (mmol) | PA Conversion (%) | MAL Selectivity (%) |
|---|---|---|
| 5 | 97.67 | 32.97 |
| 25 | 41.72 | 45.25 |
| 50 | 20.89 | 96.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.; Li, G.; Luo, H. Catalytic Synthesis of Methacrolein via the Condensation of Formaldehyde and Propionaldehyde with L-Proline Intercalated Layered Double Hydroxides. Catalysts 2022, 12, 42. https://doi.org/10.3390/catal12010042
Ju L, Li G, Luo H. Catalytic Synthesis of Methacrolein via the Condensation of Formaldehyde and Propionaldehyde with L-Proline Intercalated Layered Double Hydroxides. Catalysts. 2022; 12(1):42. https://doi.org/10.3390/catal12010042
Chicago/Turabian StyleJu, Longxin, Gang Li, and Hongxian Luo. 2022. "Catalytic Synthesis of Methacrolein via the Condensation of Formaldehyde and Propionaldehyde with L-Proline Intercalated Layered Double Hydroxides" Catalysts 12, no. 1: 42. https://doi.org/10.3390/catal12010042
APA StyleJu, L., Li, G., & Luo, H. (2022). Catalytic Synthesis of Methacrolein via the Condensation of Formaldehyde and Propionaldehyde with L-Proline Intercalated Layered Double Hydroxides. Catalysts, 12(1), 42. https://doi.org/10.3390/catal12010042







