Effect of Heating Rate on the Photocatalytic Activity of Ag–TiO2 Nanocomposites by One-Step Process via Aerosol Routes
Abstract
1. Introduction
2. Results and Discussions
2.1. Heating Rate Effect on the Surface Morphology and Thickness of the Ag–TiO2 Nanocomposite Film
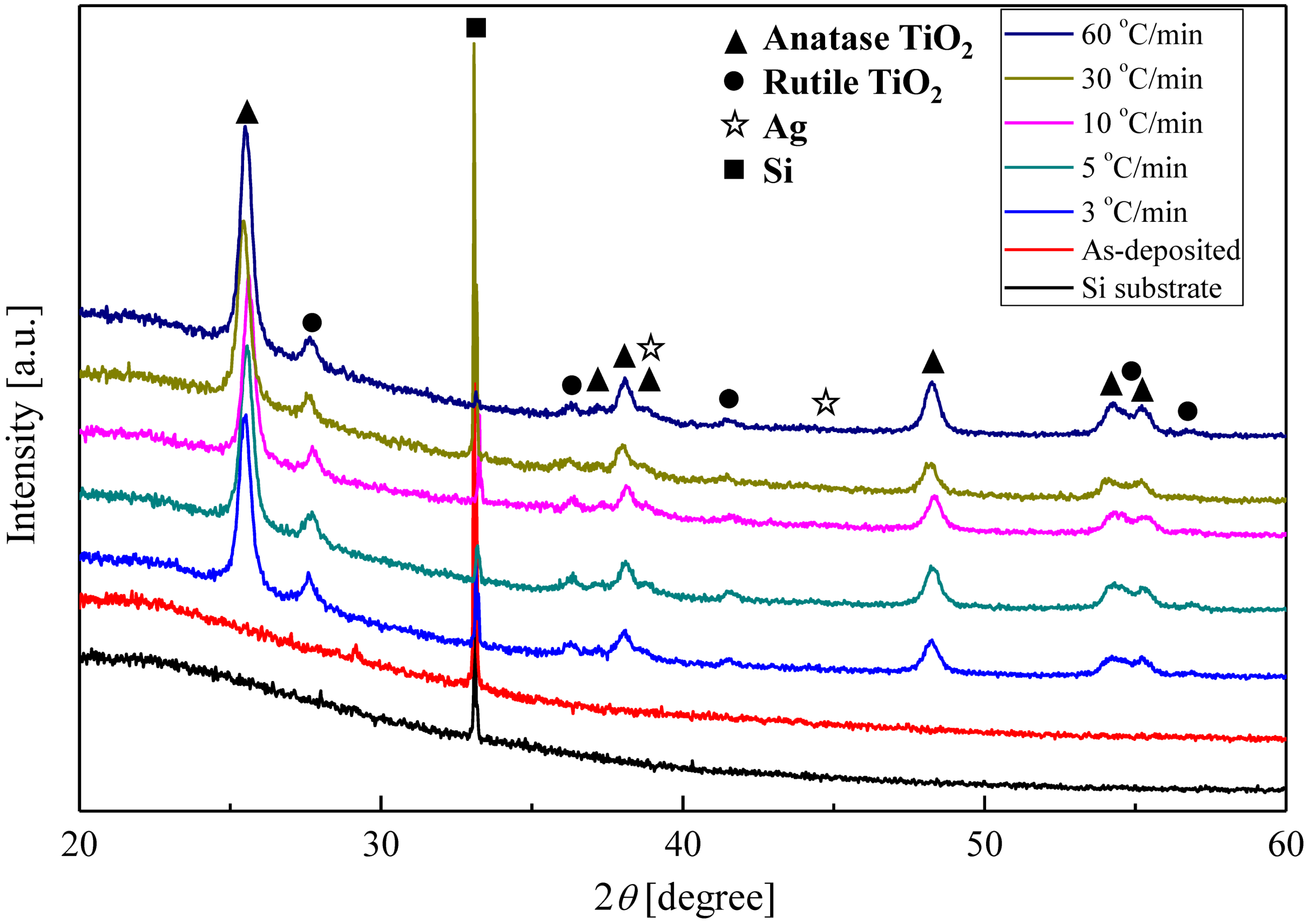
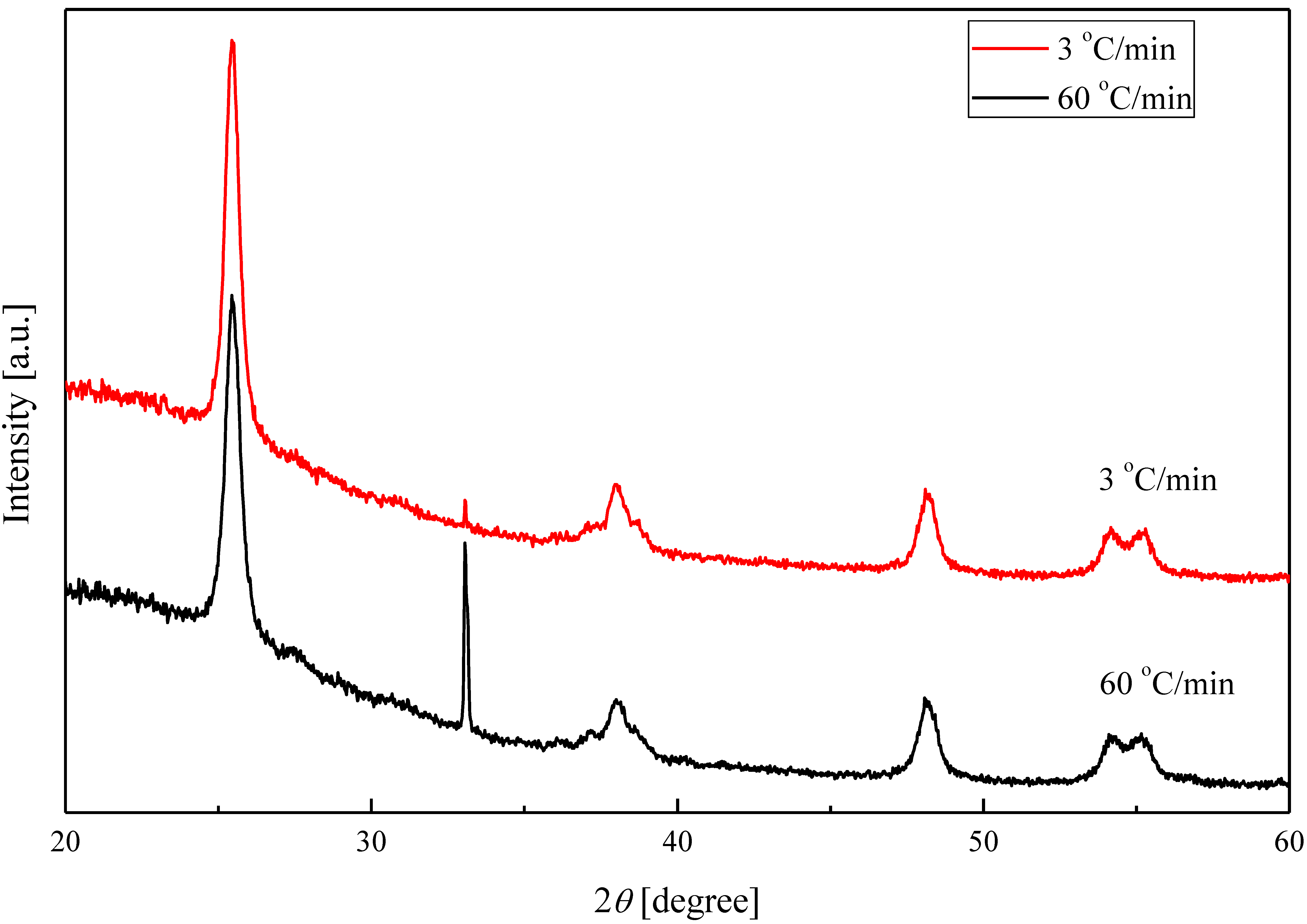
2.2. Crystalline Phase of Ag–TiO2 Nanocomposite Film
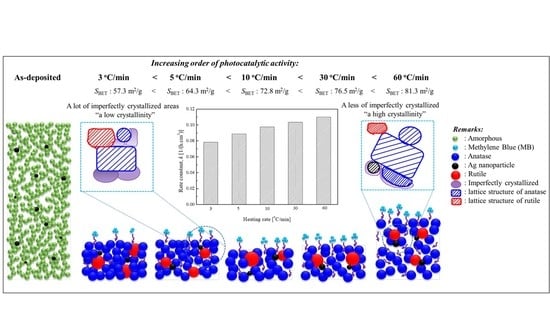
2.3. Photocatalytic Activity Evaluation of Ag–TiO2 Nanocomposite Films
3. Materials and Method
3.1. Materials and Experimental Setup
3.2. Characterization
3.3. Photocatalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrogen Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Luo, Z.; Poyraz, A.S.; Kuo, C.-H.; Miao, R.; Meng, Y.; Chen, S.-Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline Mixed Phase (Anatase/Rutile) Mesoporous Titanium Dioxides for Visible Light Photocatalytic Activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef] [PubMed]
- Galizia, P.; Maizza, G.; Galassi, C. Heating rate dependence of anatase to rutile transformation. Process. Appl. Ceram. 2016, 10, 235–241. [Google Scholar] [CrossRef]
- Dikici, T.; Demirci, S.; Tünçay, M.M.; Yildirim, B.K.; Kaya, N. Effect of heating rate on structure, morphology and photocatalytic properties of TiO2 particles: Thermal kinetic and thermodynamic studies. J. Sol-Gel Sci. Technol. 2021, 97, 622–637. [Google Scholar] [CrossRef]
- Santos, L.M.; Machado, W.A.; França, M.D.; Borges, K.A.; Paniago, R.M.; Patrocinio, A.O.T.; Machado, A.E.H. Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity. RSC Adv. 2015, 5, 103752–103759. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Airinei, A.; Olaru, N.; Rosca, I.; Koudoumas, E.; Suchea, M.P. Innovative Ag–TiO2 Nanofibers with Excellent Photocatalytic and Antibacterial Actions. Catalysts 2021, 11, 1234. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Kusdianto, K.; Jiang, D.; Kubo, M.; Shimada, M. Effect of annealing temperature on the photocatalytic activity of Ag-TiO2 nanocomposite films by one-step gas-phase deposition. Mater. Res. Bull. 2018, 97, 497–505. [Google Scholar] [CrossRef]
- Mansour, S.A. Non-isothermal crystallization kinetics of nano-sized amorphous TiO2 prepared by facile sonochemical hydrolysis route. Ceram. Int. 2019, 45, 2893–2898. [Google Scholar] [CrossRef]
- Gunnewiek, R.F.; Kiminami, R.H. Effect of heating rate on microwave sintering of nanocrystalline zinc oxide. Ceram. Int. 2014, 40, 10667–10675. [Google Scholar] [CrossRef]
- Sierra-Uribe, H.; Tuta, E.M.C.; Acevedo-Peña, P. The Effect of the Heating Rate on Anatase Crystal Orientation and Its Impact on the Photoelectrocatalytic Performance of TiO2Nanotube Arrays. J. Electrochem. Soc. 2017, 164, H279–H285. [Google Scholar] [CrossRef]
- Bouhdjer, A.; Attaf, A.; Saidi, H.; Benkhetta, Y.; Aida, M.S.; Bouhaf, I.; Rhil, A. Influence of annealing temperature on In2O3 properties grown by an ultrasonic spray CVD process. Optik 2016, 127, 6329–6333. [Google Scholar] [CrossRef]
- Guhel, Y.; Toloshniak, T.; Bernard, J.; Besq, A.; Germanicus, R.C.; El Fallah, J.; Pesant, J.; Descamps, P.; Boudart, B. Rapid thermal annealing of cerium dioxide thin films sputtered onto silicon (111) substrates: Influence of heating rate on microstructure and electrical properties. Mater. Sci. Semicond. Process. 2015, 30, 352–360. [Google Scholar] [CrossRef]
- Kubo, M.; Mantani, Y.; Shimada, M. Effects of Annealing on the Morphology and Porosity of Porous TiO2 Films Fabricated by Deposition of Aerosol Nanoparticles. J. Chem. Eng. Jpn. 2015, 48, 292–299. [Google Scholar] [CrossRef][Green Version]
- Ozaki, K.; Hanatani, T.; Nakamura, T. Analysis of crystalline phases in airborne particulates by grazing incidence X-ray diffractometry. Analyst 2005, 130, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.M.; Wong, W.M.P.; Chai, S.-P.; Mohamed, A.R. One-pot synthesis of Ag-MWCNT@TiO2 core–shell nanocomposites for photocatalytic reduction of CO2 with water under visible light irradiation. Chem. Eng. J. 2015, 278, 272–278. [Google Scholar] [CrossRef]
- Masuda, Y.; Kato, K. Synthesis and phase transformation of TiO2 nano-crystals in aqueous solutions. J. Ceram. Soc. Jpn. 2009, 117, 373–376. [Google Scholar] [CrossRef]
- Kusdianto, K.; Jiang, D.; Kubo, M.; Shimada, M. Fabrication of TiO2–Ag nanocomposite thin films via one-step gas-phase deposition. Ceram. Int. 2017, 43, 5351–5355. [Google Scholar] [CrossRef]
- Tao, C.; Xu, L.; Guan, J. Well-dispersed mesoporous Ta2O5 submicrospheres: Enhanced photocatalytic activity by tuning heating rate at calcination. Chem. Eng. J. 2013, 229, 371–377. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Cheng, B.; Ong, H.C. Microwave-hydrothermal preparation and visible-light photoactivity of plasmonic photocatalyst Ag-TiO2 nanocomposite hollow spheres. Chem. Asian J. 2010, 5, 1466–1674. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B: Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology, and Applications 2014, 2nd ed.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Sing, K.S.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kubo, M.; Ishihara, Y.; Mantani, Y.; Shimada, M. Evaluation of the factors that influence the fabrication of porous thin films by deposition of aerosol nanoparticles. Chem. Eng. J. 2013, 232, 221–227. [Google Scholar] [CrossRef]



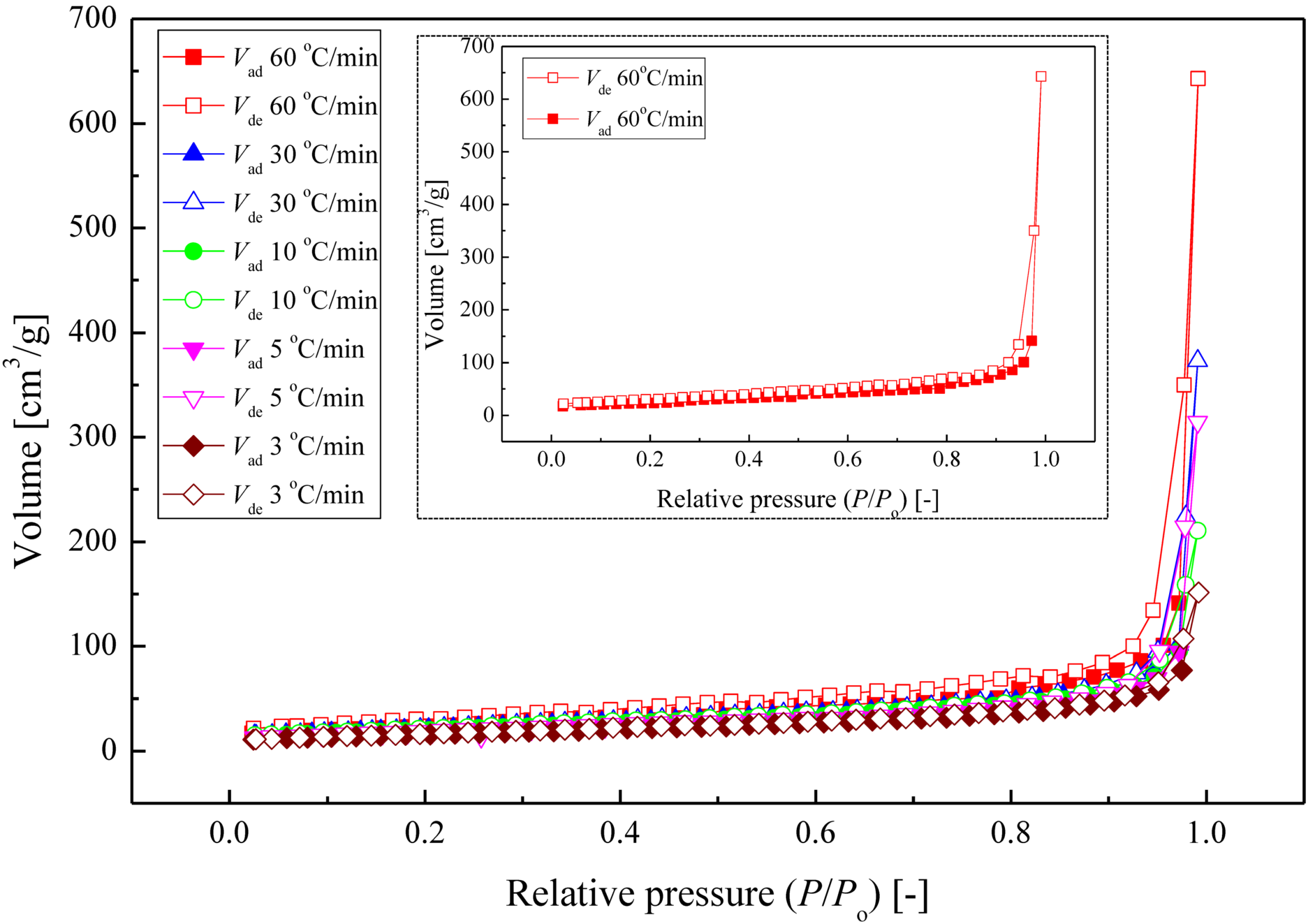

| Annealing | Heating | Crystalline | GMD | Phase Content | Crystallite Size | Relative | Rate | ||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | Rate | Phase | Anatase | Rutile | Anatase | Rutile | Thickness | Constant (k) | |
| [°C] | [°C/min] | - | [nm] | [%] | [%] | [nm] | [nm] | - | [1/(h·cm2)] |
| As-deposited | - | amorphous | 13 | - | - | - | - | 1.00 | - |
| 600 | 3 | anatase/rutile | 22 | 85 | 15 | 25.9 | 41.4 | 0.24 | 0.079 |
| 600 | 5 | anatase/rutile | 25 | 84 | 16 | 25.2 | 30.8 | 0.25 | 0.089 |
| 600 | 10 | anatase/rutile | 24 | 85 | 15 | 29.2 | 44.8 | 0.19 | 0.098 |
| 600 | 30 | anatase/rutile | 20 | 87 | 13 | 27.3 | 39.1 | 0.22 | 0.104 |
| 600 | 60 | anatase/rutile | 22 | 88 | 12 | 27.6 | 32.3 | 0.38 | 0.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusdianto, K.; Hudandini, M.; Jiang, D.; Kubo, M.; Shimada, M. Effect of Heating Rate on the Photocatalytic Activity of Ag–TiO2 Nanocomposites by One-Step Process via Aerosol Routes. Catalysts 2022, 12, 17. https://doi.org/10.3390/catal12010017
Kusdianto K, Hudandini M, Jiang D, Kubo M, Shimada M. Effect of Heating Rate on the Photocatalytic Activity of Ag–TiO2 Nanocomposites by One-Step Process via Aerosol Routes. Catalysts. 2022; 12(1):17. https://doi.org/10.3390/catal12010017
Chicago/Turabian StyleKusdianto, Kusdianto, Meditha Hudandini, Dianping Jiang, Masaru Kubo, and Manabu Shimada. 2022. "Effect of Heating Rate on the Photocatalytic Activity of Ag–TiO2 Nanocomposites by One-Step Process via Aerosol Routes" Catalysts 12, no. 1: 17. https://doi.org/10.3390/catal12010017
APA StyleKusdianto, K., Hudandini, M., Jiang, D., Kubo, M., & Shimada, M. (2022). Effect of Heating Rate on the Photocatalytic Activity of Ag–TiO2 Nanocomposites by One-Step Process via Aerosol Routes. Catalysts, 12(1), 17. https://doi.org/10.3390/catal12010017