Abstract
The valorization of carbon dioxide by diverting it into useful chemicals through reduction has recently attracted much interest due to the pertinent need to curb increasing global warming, which is mainly due to the huge increase of CO2 emissions from domestic and industrial activities. This approach would have a double benefit when using the green hydrogen generated from the electrolysis of water with renewable electricity (solar and wind energy). Strategies for the chemical storage of green hydrogen involve the reduction of carbon dioxide to value-added products such as methane, syngas, methanol, and their derivatives. The reduction of CO2 at ambient pressure to methane or carbon monoxide are rather facile processes that can be easily used to store renewable energy or generate an important starting material for chemical industry. While the methanation pathway can benefit from existing infrastructure of natural gas grids, the production of syngas could be also very essential to produce liquid fuels and olefins, which will also be in great demand in the future. In this review, we focus on the recent advances in the thermocatalytic reduction of CO2 at ambient pressure to basically methane and syngas on the surface of supported metal nanoparticles, single-atom catalyst (SACs), and supported bimetallic alloys. Basically, we will concentrate on activity, selectivity, stability during reaction, support effects, metal-support interactions (MSIs), and on some recent approaches to control and switch the CO2 reduction selectivity between methane and syngas. Finally, we will discuss challenges and requirements for the successful introduction of these processes in the cycle of renewable energies. All these aspects are discussed in the frame of sustainable use of renewable energies.
1. Introduction—CO2 Reduction and Energy Perspectives
The catalytic reduction of carbon dioxide to useful chemical products is gaining exponentially increasing interest as it is considered one of the most important targets for the energy sector and at the same time is of high importance for obvious ecological reasons. CO2 is related to global warming and resulting climate changes, which may lead to serious environmental and economic problems in the near future [1]. The symptoms of this climate problem, which have recently appeared in many areas across the world, have led to the quest to push global warming well below 2 °C, a level still higher than the average earth temperature of the pre-industrial era [2]. This aspiration needs either industrial activities to cease, or efficient technologies to be developed to capture CO2 and then use it in a closed energy cycle [3,4,5]. Considering the enormous economic damage that would be caused by the suppression of industrial activities and the expected terrible socio-political consequences all over the world, the second option, which aims at strict mitigation of carbon emissions and their cyclic utilization is the only possible way forward.
The anticipated technical approach to solve this problem is based on: (i) harvesting carbon dioxide from air or at the outlet of emitters, which would need the development of suitable materials and technologies, (ii) the production of green hydrogen by water electrolysis using electricity generated from renewable energy resources, basically solar and wind energy, and finally, (iii) the reaction of green hydrogen with harvested CO2 to produce either energy carriers (e.g., methanol, formic acid or methane) or other chemicals (see proposed energy cycle illustrated in Figure 1). This approach is not only ecologically favorable, but also central for new scientific concepts related to the sustainable use of renewable energy resources. Given the seasonality of electricity generation from renewable energy resources (i.e., fluctuations in energy supply due to change in weather, solar radiation, wind speed, etc.), the chemical storage of excess electricity, which is produced during periods of maximum electric power generation, in a stable and easily convertible energy carrier (e.g., as methanol, formic acid, or methane), would help to buffer the expected fluctuations in renewable energy supply.
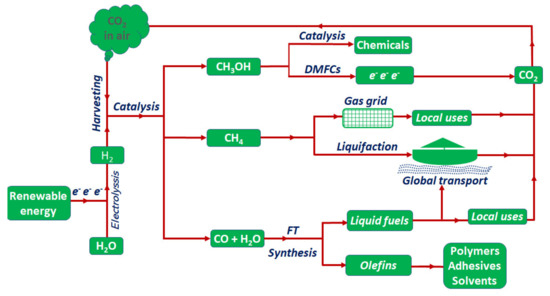
Figure 1.
Schematic representation of the pathways for the conversion of carbon dioxide harvested from air using green hydrogen produced form renewable energy (DMFCs: direct methanol fuel cells; gas grid: the natural gas grid; FT: Fischer Tropsch process).
For this purpose, different strategies were proposed to react hydrogen with carbon dioxide, either to produce energy carriers with higher energy density, or otherwise convert both molecules into a value-added compound. One of the top targets is the direct conversion of CO2 to methanol, which serves as energy carrier in the first place (power-to-liquid). Methanol can be stored and transferred globally in a much easier way than hydrogen, which needs a huge amount of energy for liquefaction and more importantly requires extreme safety measures [6]. In this way, the liquid and easily transportable and storable methanol can be used to release stored hydrogen, or to produce other useful chemicals [7], or it can be directly used to generate electricity in the direct methanol fuel cells (DMFCs) [8]. The development of efficient catalysts for the synthesis of methanol from CO2 reduction is still in the beginning and a lot of progress is still needed to achieve scalable, highly active, methanol selective, and commercially feasibly catalytic materials [9]. The other simple approach is the transformation of CO2 to methane or syngas (i.e., power-to-gas concept).
Direct methanation of carbon dioxide, known as Sabatier reaction, is considered as a well-developed process that is in the course of being introduced into the renewable energy sector [10]. In this approach, the resulting methane, referred to as synthetic natural gas, can be stored and distributed and even transferred (exported/imported) globally using the already existing infrastructures (natural gas grid, transfer pipelines, liquefaction stations, and carrier ships) (see scheme in Figure 1). The methanation process is also very preferable from the technical point of view, as the operation conditions and safety measures are less complicated than the methanol production process. On the one hand it has been studied for over one hundred years, since it was first introduced by Paul Sabatier on a wide range of catalytic materials [11,12], which makes it close to a well-established technology for large scale production [13]. On the other hand, methanation of CO2 can be operated at ambient pressure or slightly increased pressure with high activity and reasonable selectivity compared to methanol synthesis, which needs the use of much higher pressures to achieve enough selectivity and activity toward methanol yield. At this point, it is essential to mention the industrial implementation of the power-to-gas concept, originally in Germany. Preliminary studies, which focused on the development of so-called alpha plants, were carried out in Stuttgart. These findings then led to the possibility of building the Audi e-gas plant in Werlte. The electrical input from electrolysis here is 6 MW [14]. The hydrogen obtained from this step is compressed to 10 bar and brought to the Sabatier reaction with biogas-CO2 at temperatures between 200 and 350 °C via staged dosage. A nickel catalyst is used, which promotes the conversion of carbon dioxide to over 90%. This development has been the seed of many other similar power-to-gas projects in several countries all over the world [15,16].
The reduction of CO2 to syngas is considered as another important route with increasing importance over the recent years. This is related to the need for sustainable production of petrochemical commodities from renewable resources and anthropogenic carbon dioxide, especially with the continuous decrease of proven world reserves of crude oil. Here, it should be noted that syngas is typically produced from the reforming of natural gas or crude oil. In the not so far future, the syngas produced from CO2 reduction will be able to be converted to a wide range of platform chemicals used in chemical industry, basically we refer to (i) the production of light olefins used in the polymer industries (ethylene, propylene and butene) [17], (ii) the commercial production of methanol [18], and (iii) liquid hydrocarbon fuels via Fischer Tropsch synthesis [19]. These products will continue to be indispensable for the chemical industry in the future, but also for air and maritime transport (see schematic in Figure 1). Based on these aspects, it seems that both reaction pathways, CO2 methanation and RWGS reactions, are complementary for the sustainable use of renewable energy (i.e., green hydrogen) in the near future, even after the optimization of CO2 reduction to methanol. It is thus highly desirable to find strategies for switching the selectivity of CO2 reduction between these two processes to allow alternatives for both applications.
2. Thermodynamic and Kinetic Considerations
The product of CO2 reduction depends decisively on the reaction conditions (temperature, pressure, and stoichiometry of the reactants) that affect the thermodynamic equilibrium. Considering a fixed pressure (i.e., here ambient pressure) and constant gas composition of CO2/H2 = 1:1, we can see in Figure 2 the effect of variation of the reaction temperature on the thermodynamic equilibrium composition. The direct pathways of CO2 reduction at ambient pressure can proceed either via reverse water gas shift (RWGS: CO2 + H2 => CO + H2O; ΔH = −41.1 kJ mol−1) or via the Sabatier reaction/CO2 methanation (CO2 + 4 H2 => CH4 + 2H2O + ΔH = −165 kJ mol−1) [20,21]. Given the much higher exothermicity for CO2 methanation compared to RWGS reaction, one can expect in the first glance, that RWGS would be more promoted at high temperatures and at the same time CO2 methanation would be less pronounced. Looking at the equilibrium conversion of CO2 to different products, it can be observed that the start of the decline of CO2 methanation pathway commences at a temperature of 350 °C and continues to decrease with the increase of the temperature, which goes along with the decline in methane yield (T > 600 °C; methane yield < 30% (see Figure 2)).
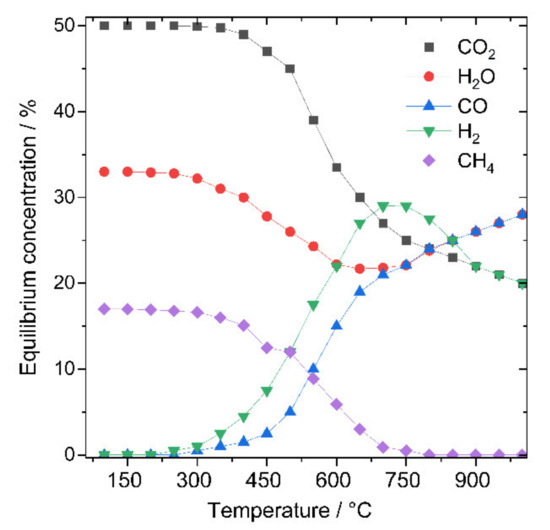
Figure 2.
The thermodynamic equilibrium composition of different products of the conversion of CO2/H2 (1:1) at ambient pressure in the temperature range from 100 to 1000 °C (reprinted with permission from Ref. [22] © 2020, Frontiers, Lausanne, Switzerland).
The onset of CO formation via RWGS can be observed at a temperature of about 350 °C and continues to increase with temperature. In general, we see that methanation is thermodynamically more favorable at lower temperatures than CO formation. This primarily determines the working temperatures of the desired catalytic reaction, in which methanation (Sabatier pathway) or CO formation via RWGS should be operated [22]. These thermodynamic conditions should always precede a reaction design, as the catalyst can only change the chemical kinetics. However, considering the high importance of syngas production from CO2 as outlined above, it is highly desirable to overcome kinetic limitations of the RWGS pathway to achieve high productivity at the lowest possible temperature. This can be achieved by the development of catalytic materials able to significantly lower the energy barrier for CO formation, prevent CO-poisoning, and at the same time lifting the energy barrier for CH4 formation. This would mean that the RWGS pathway will prevail over the CO2 reduction pathway. This issue will be discussed in more detail in Section 5.
3. Catalysts for CO2 Reduction—Activity, Stability, and Selectivity of Different Supported Metals
3.1. Historical Background
The direct hydrogenation of CO2 was first introduced by Paul Sabatier during his work in the beginning of the twentieth century on the development of a direct hydrogenation method on metal surfaces [23]. These early trials included the reduction/hydrogenation of a wide range of compounds including, nitriles, aromatics, unsaturated hydrocarbons, and carbon oxides, where gaseous hydrogen was basically used as reducing agent [12]. Focusing on the methanation of carbon oxides, Sabatier and Senderens found out that the contact of gaseous hydrogen with CO or CO2 on the surface of nickel powder results in the formation of methane [11]. Different metals have been studied over time such as Ru, Rh, Ir, Co, Ni, Fe, and Pd, and were found active for the methanation of both CO and CO2 [13]. In their early work on the synthesis of higher hydrocarbons from carbon oxides, Fischer and Tropsch compared in a systematic way the activity of different metals toward methanation activity and for the synthesis of higher hydrocarbons, known later as FT synthesis [24]. As a general consensus, it could be concluded that the metals active for methanation of CO are active for CO2 methanation but are expected to be less active toward the FT synthesis of higher hydrocarbons [25]. For instance, the behavior of Ru and Ni catalysts on the one side and Fe and Co catalysts on the other side are clearly proving the correctness of this concept. Ru and Ni are by far the most active metals for methanation of CO and CO2 at ambient pressure, but once transferred to the FT reaction regime they show poor selectivity in FT synthesis while Fe and Co shows the opposite behavior, i.e., promoting FT pathway and suppressing the CO2 reduction to methane and/or CO [13,19]. Ru and Ni are, however, studied and used as promoters for FT catalysts.
Focusing on the widely studied CO2 reduction under ambient pressure conditions, we will discuss and compare the behavior of different metals that are more active for the transformation of CO2 to methane and syngas at a wide range of temperatures depending on the nature of catalyst and its support materials. In the following, we will contrast the advantages and disadvantages in possible applications for the most studied metals (Ni, Ru, and Rh) in addition to the recently emerging bimetallic catalysts based on different combinations of these metals together with other cheaper elements.
3.2. Nickel-Based Catalysts
Nickel as catalytically active element received an enormous amount of interest since the methanation process was developed by Sabatier and Senderens [11], not only because of its relatively high activity in CO2 reduction at ambient pressure, but also because to its much lower price compared to other more active metals such as Ru, Rh, and Pt. Nickel has also been combined in different compositions with different oxide supports including the famous catalyst known as Raney nickel (nickel-aluminum alloy) [13], and nickel nanoparticles supported on a wide range of oxides such as Ni/Al2O3 [26,27], Ni/SiO2 [28], Ni/TiO2 [29,30], Ni/ZrO2 [31], Ni/CeO2 [32,33,34,35]. In this way, the use of reducible oxide supports was found to significantly improve the activity of nickel. In particular, compared to the standard Ni/Al2O3 catalysts, Ni/CeO2 showed higher activity at low temperature, which allowed for higher selectivity toward methane (this parameter will be discussed in detail in Section 4). Recent trials to improve the performance of nickel catalysts in methanation and methane reforming involved the use of mixed oxide or ternary composites (Al2O3-ZrO2 [36], WO3–ZrO2 [37], Ni-Mg-Al [38]) and also doping with transition metals such as Fe, Co, Mn [39].
The major challenge in the use of conventional nickel-based catalysts is basically their strong and continuous deactivation with time-on-stream. As an example, Ewald et al., demonstrated systematically that Ni/Al2O3 prepared with different loadings using co-precipitation and impregnation methods show continuous loss of activity at temperatures in the range from 250 to 350 °C [40]. Figure 3a shows the relative activity loss of such a catalyst in a narrow temperature range (250 to 350 °C). The deactivation rate of these catalysts was found to depend on the method of catalyst preparation. However, in general the loss of catalyst surface area along with the sintering of Ni nanoparticles during CO2 reduction with time-on-stream were considered as the major reasons for the process. In connection with these findings, Munnik et al. [41], observed similar deactivation behavior for Ni/SiO2, but during CO methanation in CO/H2 (1:2) at 1 bar and at 230 °C (Figure 3b). These authors explained the continuous deactivation over time by the formation of large Ni nanoparticles. This is driven by the formation of Ni(CO)4 species in the presence of CO and their decomposition on the support to form adatomic Ni or tiny Nix clusters, which migrate across the surface merging to the larger particles (ripening effect) and therefore leading to continuous loss of active sites (i.e., sintering via Ostwald ripening).
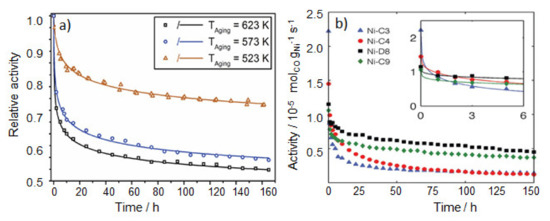
Figure 3.
(a) Time-on-stream change of relative activity of NiAlOx catalyst (Ni:Al ratio of 3:1) during CO2 reduction (H2/CO2/Ar/N2 = 8/2/9/1) gas mixture at ambient pressure at three different temperatures. Catalytic activity measurements were done using 50 mg of the catalyst diluted by 450 mg of SiC using a total gas flow of 500 mL min−1. Relative activity is defined as the activity on stream normalized to the initial activity (reprinted with permission from Ref [40] © 2019, Elsevier Inc, Amsterdam, The Netherlands). (b) Time-on-stream change of the Ni-mass normalized activity of CO reduction (H2/CO = 2:1) at 230 °C on Ni/SiO2 catalysts with different initial particle sizes of NiO/Ni (Ni-D8: 7.7/7.5 nm; Ni-C9:8.7/9 nm; Ni-C4: 3.9/4.3; Ni-C3: 3.2/3.2 nm). Measurements were carried out on a powder mixture of 10 mg of Ni/SiO2 catalyst and 200 mg of SiC and a total gas flow of 6 Nml (STP) min−1 resulting in GHSV of 18,000 h−1 (reprinted from Ref [41] © 2014, Wiley Inc (Hoboken, NJ, USA)).
Considering this explanation, the following criteria should be taken into account for the efficient use of these catalysts, and hence prevent their continuous deactivation. First, the use of Ni catalysts at low temperatures <350 °C is not desirable if the catalyst is partially active for RWGS due to the formation of Ni(CO)4, which would lead to catalyst deactivation in addition to its poisonous nature, which raises major safety issues. Second, Ni catalysts would be more desired for use at high temperature for the reduction of CO2 to CO, where methane is also thermodynamically less preferred compared to CO formation, and at the same time Ni(CO)4 formation is avoided. Some of these supported nickel catalysts such as Ni/SiO2 [28], Ni/MgO [42], Ni/MgAl2O4 [43], Ni/Al2O3-CeO2 catalysts are reported as excellent candidates for the RWGS over methanation and has been widely studied for this aspect [44]. Furthermore, nickel is known for its Janus-like catalytic behavior in the reductive conversion of CO2 with hydrogen. The reaction preferably follows one of two possible mechanisms: (i) the redox mechanism forming COad species on the catalyst surface, and (ii) an H2 mediated cycle via HCOOad and COOHad to COad adsorbates [45,46,47]. After these elementary steps, either desorption of CO (RWGS) or further hydrogenation to CH4 (Sabatier reaction) takes place, which is dependent on the activation barriers of both the steps. Probably, the origin of this ambivalent behavior lies in the variation of the adsorption strength of the species formed on the catalyst surface with changes in the structure of the catalysts. In this regards, it is also known that the selectivity for methane increases with increasing the Ni particle size [48]. These mechanistic aspects are not yet fully understood and further studies are indispensable to tailor more stable and selective Ni catalysts for CO2 reduction.
3.3. Ru-Based Catalysts
Supported Ru catalysts have been also one of the most studied catalysts for the reduction of CO2 for over four decades [49,50,51,52,53,54]. In this regard, these catalysts have recently attracted intensive investigations in the recent few years by several research groups [55,56,57,58,59,60,61,62,63] as one of the promising options for power-to-gas applications. This high interest is supported by their impressively high activity at rather low temperatures [64,65]. In comparison with supported Ni catalysts prepared using similar methods and with comparable structural characteristics (support material, metal loading, treatment), Garbarino et al. showed that Ru/Al2O3 outperforms Ni/Al2O3 catalyst in activity and stability [26]. In terms of metal-based catalytic activity, supported Ru catalysts were found to be at least one order of magnitude more active [66]. The unsurpassed high activity in particular at low temperature of supported Ru catalysts is preferable not only for rationalizing the overall costs of the catalytic process, but also it gives preference for the methanation pathway when catalyst preparation and activation steps are well controlled.
Different from supported Ni catalysts, Ru nanoparticles (1–3 nm) supported on different metal oxides such as Al2O3 [67,68,69], TiO2 [66,70], ZrO2 [68,71], were found to be highly stable during time-on-stream measurements in CO2 methanation. As an example, the comparison of the Ru mass normalized activity for CO2 reduction at 190 °C/1 bar on different oxide supports (Al2O3, TiO2, and ZrO2) shows almost no/or very limited deactivation with time-on-stream (see Figure 4).
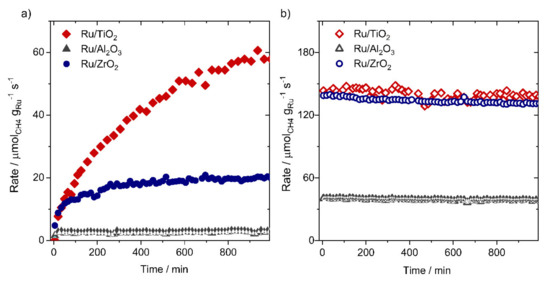
Figure 4.
(a) Time-on-stream CO2 methanation reaction rate recorded on Ru/TiO2 [66], Ru/Al2O3 [67], and Ru/ZrO2 [71] during CO2 methanation after oxidative treatment in 10% O2/N2 at 150 °C for 30 min (O150), and (b) after high temperature reaction treatment (TPR:190 to 350 °C). All catalytic measurements on these catalysts were done on powder samples using 200 mg of Ru/MOx catalyst diluted by thermally stable and catalytically inactive α–Al2O3 (1:10–Ru/MOx: α–Al2O3) using a flow of 41.6 Nml min−1 at constant GHSV. Data in this figure are produced from Refs [66,67].
Note that the high stability of these catalysts during CO2 methanation is independent of the initial catalyst pretreatment step, whether a reductive, or an oxidative gas atmosphere is applied for this purpose. We see that in both cases, the catalysts are highly stable (oxidative pretreatment -Figure 4a; reductive pretreatment-Figure 4b), although the activity is much more pronounced after reductive pretreatment. It is also clear that there is an initial activation phase for reducible oxides after the oxidative treatment (Figure 4a), which is related to the slow reduction of the support and the formation of O-vacancy defects, which are needed for the activation of CO2 on the catalyst surface. Here, we need to underline that different from the behavior of supported Ru catalysts in CO2 reduction, these catalysts (Ru/MOx) show different behavior in the methanation of CO in CO/H2 gas mixtures or in CO/CO2/H2 mixtures. In the presence of CO, modest deactivation can be observed, in particular for the reducible oxides such as Ru/TiO2 catalysts [70,72,73,74]. This effect is also observable on Ru NPs (1–3 nm) supported on non-reducible oxides in the presence of water vapor in CO/CO2/H2 feed gases with limited CO partial pressures (0.01%CO) [75,76,77]. The reason for the deactivation of these catalysts in the presence of CO could be attributed to a combination of effects evolving simultaneously with time including (i) the change of the particle shape of Ru NPs from flat to hemispherical or spherical [72], (ii) sintering of small Ru NPs [72], and (iii) encapsulation of Ru NPs by reduced TiOx species at high temperatures [66]. These effects lead individually or together to the loss of active Ru surface area during CO/CO2 methanation with time on stream. More importantly, it was demonstrated that supported Ru catalysts (Ru/Al2O3) are also better catalysts than the corresponding Ni-based catalyst (Ni/Al2O3) under dynamic operation conditions involving the variation of reactor load [78,79]. This property is one of the prerequisites for the future decentralized use of CO2 methanation reactors/stations. Therefore, a suitable stability of the catalyst under dynamic changes is mandatory to enable real applications.
Referring to the selectivity between methanation and reverse water gas shift reaction, except for supported Ru catalysts on TiO2 with large specific surface area (> 200 m2g−1), which show pronounced CO formation, most of these Ru/TiO2 catalysts (SSA ≤ 125 m2g−1) are active for methanation in the temperature range from 50 up to 350 °C [65,66,67,71,80], with selectivity almost of 100%. This aspect will be discussed in more detail in Section 5.2.
3.4. Rh-Based Catalysts
Like supported Ru catalysts, the thermo-catalytic reduction of CO2 on oxide supported rhodium catalysts attracted significant attention due to their high activity at moderate to low reaction temperatures (<200 °C). These catalysts were intensively investigated by Solymosi and coworkers and were reported in several papers [81,82,83,84]. These authors showed that Rh is also active for the photocatalytic reduction of CO2 in the presence of water vapor to different products other than CO and CH4, such as formaldehyde, formic acid, methanol, which are viewed as candidates for hydrogen fixation in liquid carriers [85]. The thermal reduction of CO2 by reaction with methane to CO + H2, commonly known as dry reforming of methane, on supported Rh catalysts has been studied by several groups on different supports including Rh supported on TiO2 [86], SiO2 [86,87], Al2O3 [86,88,89]. This reaction can serve as a possible pathway for the sustainable production of syngas and at the same time for valorizing the flaring methane at the natural gas wells. The approach can be useful in the periods of shortage of hydrogen production from renewable resources, where methane stored can be used for hydrogen or syngas by direct reaction with CO2. Although supported Rh catalysts are known for their high methanation selectivity at low to moderate temperatures (<250 °C), recent results by Matsubu et al. demonstrated that the selectivity of CO2 reduction can be steered toward CO by either atomic dispersion of Rh on metal oxide [90], and also through the modification of metal support interaction [91]. These results shall be discussed in more details in Section 5.
3.5. Bimetallic Alloy Catalysts
The combination of two or more metals in one alloy is intended on the one hand to create better electronic/structural properties, which are not reachable on supported single metals, and on the other hand is an approach to rationalize the use of one of two metals, which is usually a precious rare metal such as Ru, Pt, or Rh, by combination with less expensive metal such as Ni, Co, or Fe, e.g., the core-shell materials where an expensive metal is prepared as outer shell of a cheaper metal. The first example, which is widely studied, and is considered highly promising as cheap and stable option for CO2 methanation, is NiFe alloys, which were first described by Andersson et al. [92].
In their study, these authors found out that the activity for CO hydrogenation depends decisively on the ratio of Ni:Fe in the alloy, with the maximum reaction rate achieved for an alloy composition of 1:1 (see Figure 5) [92]. These catalysts received recently increasing attention in experimental studies for CO2 reduction at ambient pressure [93,94,95,96,97,98,99]. The amount of Fe in the NiFe alloy was found to affect the activity decisively and an optimum amount in the range from 20 to 25 mol% results in the highest catalytic activity at lower temperatures (200–400 °C) when compared to supported Ni catalysts [99,100]. In this regard, Meshkini et al. demonstrated that an alloy based on 80% Ni and 20% Fe supported on Al2O3 achieves about 100% CO2 conversion to methane at 270 °C, which is not feasible on supported Ni catalysts [96]. The quantitative comparison of the methanation activity of the Ni alloy with different metals including Fe (Fe, Co, Zr, La, and Cu) indicated that FeNi alloy outperforms the catalytic activity and selectivity of the Ni alloys with other elements, with the highest CO2 conversion rate and methanation selectivity close to 100% at 350 °C [101].
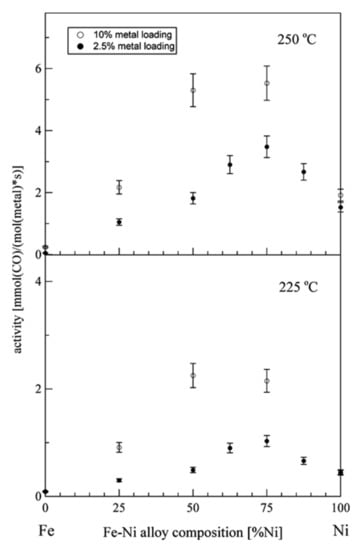
Figure 5.
Measured rate of CO removal for a gas containing 2% CO in 1 bar of H2 on FeNi catalyst loaded on spinel MgAl2O4 support, as a function of the Fe content in FeNi alloy catalysts. Results are shown for two different temperatures and two metal concentrations of powder catalysts. Measurements were done using 150 mg of the FeNi/MgAl2O4 catalyst confined by two layers of quartz wool at GHSV of 40,000 h−1 (reprinted from Ref [92] © 2006, Elsevier Inc).
Possible explanation for the role of Fe was discussed by Huong et al., who used FTIR spectroscopy and DFT calculations for this purpose [102]. These results led to the conclusion that the overall activation barrier of CH4 formation is significantly lowered on the alloy surface. In another study, Sherr et al. demonstrated that FeOx clusters can be observed during reaction on top of the surface of FeNi nanoparticles, which play a vital role in the activation of CO2 during reaction [103]. In addition to the higher activity of these catalysts, they are advantageously preferred during reaction on stream due to their enhanced stability. In a comparative study of the behavior of commercial Ni catalysts and the supported NiFe alloy (Ni3Fe/Al2O3), Mutz et al. [104], reported much higher activity and time-on-stream stability, with much limited deactivation of the Ni3Fe alloy (<5% loss of activity in 40 h at 358 °C). This highlights the vital role of alloying in solving one of the severe drawbacks of pure or supported nickel catalysts.
The second example is bimetallic RuNi alloy, which has been recently studied as a cheaper alternative for expensive Ru, offering a combination of characteristics of Ru and Ni catalysts [105,106]. As an example, Lange et al., showed that low Ru concentration-based RuNi alloy supported on ZrO2 supports can achieve comparable activity to the pure Ru catalysts supported on ZrO2, which are more active and stable during the CO2 methanation than Ni/ZrO2 catalysts [105]. The addition of Ru as promoter to the mesoporous NiAlOx oxides promoted by CaO was also found to increase the methanation activity for CO2 at lower temperatures with higher sintering resistance of Ni nanoparticles compared to the pure Ru free catalysts [105]. Although there is no significant enhancement of the performance of the resulting bimetallic catalysts, they are, however, much cheaper than the pure Ru counterparts [105]. These catalysts have been studied recently also for selective CO methanation [107,108]. Nevertheless, it is clear that these RuNi alloys have not received so far, enough attention for CO2 reduction/methanation compared to the NiFe system. In general, the addition of small amounts of Ru to Ni in an alloy or as promoter in mesoporous NiAlOx oxides results in the enhancement of the reducibility of Ni and therefore the adsorption and spillover of hydrogen, which increase their methanation activity and on-stream stability [107]. The alloying of Ru with Fe was also reported to yield an active catalyst for CO2 reduction/hydrogenation [109,110], in addition to earlier reports about its use in CO methanation [111].
Proano et al. also studied the effect of alloying ruthenium or platinum with Ni to improve the catalytic properties of nickel catalysts for CO2 methanation [112]. These alloys were combined with alkaline adsorbent for the capture of carbon dioxide and its subsequent methanation. Both metals, Ru and Pt, were found to promote the reduction of NiO formed during catalyst calcination. Ru resulted, however, in a more facile reduction of NiO than Pt and therefore alloying with Ru resulted in a higher activity to CO2 methanation than with Pt. Note that the PtNi bimetallic alloys are proposed as active catalysts for the dry reforming of methane using CO2 as oxidant [113], which may be considered for extensive studies for the direct reduction of CO2. Overall, it can be concluded that RuNi and PtNi in general did not receive enough investigations and insofar are not the first choice for possible applications.
In general, we conclude that the alloying approach is very essential to efficiently enhance the catalytic properties of both Ni and Ru metals for the reduction of CO2. The NiFe alloys in particular, which have been intensively investigated in recent years, seem very promising as a more active and stable alternative for commercial supported Ni catalysts for potential application. Nevertheless, a lot of progress is still needed to further lower the onset methanation temperature of these catalysts, which is a pertinent issue for future power-to-gas applications.
4. Support Effects on Different Metals
To discuss the influence of catalyst supports, we decided to focus on some selected examples both for the supported Ni and Ru catalysts, which attracted so far, the greatest attention for CO2 reduction applications. It should be noted that the elucidation of the effect of this parameter (i.e., support effect) require the strict disentanglement from other influencing parameters like differences in metal particle size, specific surface area, metal loading, pretreatment, reaction conditions. In other words, the catalysts studied should be iso-structural. In this way, the differences in activity can be attributed solely to the chemical nature of catalyst support. We have summarized the TOF values in several studies for CO2 reduction on different metals on different oxide supports together with their corresponding reaction conditions and structural aspects in Table 1. Thus, we want to help the reader to draw conclusions on this influencing parameter. For supported Ru catalysts, Kowalczyk et al. showed that Ru nanoparticles of similar average sizes/metal dispersion show significant variation of the CO2 methanation activity in a 0.4% CO2/H2 gas mixture with the change of the nature of support [114]. Although Al2O3, MgO, Al2MgO4, and activated carbon (AC) are all non-reducible supports, there is clear differences in the Ru mass normalized reaction rate; activity measured at 240 °C increases in the order Ru/AC < Ru/MgO < Ru/MgAl2O4 < Ru/Al2O3 (see TOF value sin Table 1). This observation highlights that the chemical nature of the support, which does not differ much in reducibility, is enough to affect the catalytic activity.

Table 1.
Comparison of structural parameters of supported metal catalysts and their catalytic activity.
Panagiotopolou et al. also studied the impact of the support material for Ru NPs on the activity for CO and CO2 reduction (1% CO, 15% CO2, 50%H2, balance He) in the presence and in the absence of varying amounts of water vapor in the feed gases. By comparing similar metal dispersions (17–21%) for 5.0 wt.% Ru loading on different oxides, the activity for CO2 reduction followed the order <Ru/CeO2 < Ru/SiO2 < Ru/TiO2 < Ru/Al2O3 (see Table 1). These results show a higher activity of the Ru/Al2O3 catalyst in comparison with the reducible supports [115]. However, in this case, the catalysts were prepared with oxide supports having significant differences in the specific surface areas (<Ru/CeO2: 3.3 m2 g−1; Ru/TiO2 42 m2 g−1; Ru/Al2O3: 83 m2 g−1; Ru/SiO2: 144 m2 g−1). This parameter was recently shown to strongly affect the MSIs and as a consequence the activity of these catalysts for the methanation of carbon oxides [70,72].
Based on these findings, Behm and coworkers studied the effect of catalyst support by using iso-structural Ru/MOx catalysts that were prepared by keeping the following parameters nearly similar for all catalysts: (i) specific surface area (110–120 m2g−1), (ii) Ru loading (2.1 wt.% Ru loading), (iii) Ru NP size/Ru dispersion (1.5–1.8 nm/50–60% dispersion, (iv) catalyst pretreatment, and (v) reaction conditions [68]. For CO2 methanation in 15% CO2/H2 gas mixture, the authors showed that the activity of Ru NPs supported on reducible oxides (TiO2) [121] outperforms that for catalysts based on non-reducible (γ–Al2O3) [67] or weakly reducible supports (ZrO2) [71]. The activity of these catalysts measured at 190 °C after temperature programmed reduction (TPR) in reaction gas between 190 and 350 °C followed the order Ru/TiO2 (244 μmol gRu−1 s−1) [121] > Ru/ZrO2 (140 μmol gRu−1 s−1) [71] > Ru/γ–Al2O3 (42 μmol gRu−1 s−1) [67]. In general, the reason for the enhancement of the activity of these catalysts after the reductive treatment is the modification of metal support interaction for ZrO2 and TiO2, and the change in the support basicity for γ–Al2O3, which helped to (i) enhance the electronic metal support interaction (EMSI) and charger transfer at Ru-TiO2/Ru-ZrO2 interface and (ii) increase the fraction of flatter Ru NPs on γ–Al2O3 support. These findings underline the pivotal role of the support material on the activity of Ru NPs for CO2 reduction.
For supported nickel catalysts, the situation was about similar to those findings reported for supported Ru catalysts. Vance and Bartholomew showed that the activity for supported Ni catalysts, both in CO and CO2 reduction, increases in the order of Ni/SiO2 < Ni/Al2O3 < Ni/TiO2 [116], i.e., with the increase of support reducibility (see Table 1). The selectivity of these catalysts for methane formation from CO2 increased also in the same order. The authors attributed the observed increase in catalytic activity to an increase of the metal-support interactions (MSIs), which is highest for the Ni/TiO2 catalysts. The origin of the enhancement of selectivity on these catalysts remains, however, an open question for future mechanistic studies.
In another study, Zawadzki and coworkers showed similar results for supported Ni catalysts, where activity decreased in the order Ni/CeO2 > Ni/ZrO2 > Ni/Al2O3 [122]. The authors employed H2-temperature programmed reduction (H2-TPR) measurements to evaluate the impact of support on the nature/reducibility of nickel nanoparticles, where a strong dependence on the support reducibility was correlated.
For detailed discussion about the parameters evolving with the variation of catalyst support and their impact on the CO2 methanation on supported Ni catalysts, we refer to the review published by Shen et al. [123].
In contrast to findings reported on supported Ni catalysts, Pandey and Deo showed that the activity for CO2 methanation on NiFe supported alloys is more pronounced for the non-reducible Al2O3 support compared to reducible oxides such as TiO2 and Nb2O5. The activity toward CO2 methanation at 250 °C follows the order NiFe/Al2O3 > NiFe/ZrO2 > NiFe/TiO2 > NiFe/SiO2 > NiF/Nb2O5 (see Figure 6) [124]. Based on their results, all prepared catalysts showed comparable (i) specific surface area, (ii) degree of reduction, and (iii) crystallite sizes of the support or NiFe alloys. This led to the conclusion that the difference in activity is merely related to the nature of the support. The authors explained the enhancement of activity by improved CO2 adsorption on NiFe/Al2O3 catalysts compared to other catalysts (NiFe/TiO2, NiFe/SiO2, NiF/Nb2O5). In comparison with previous observations on supported Ni and also supported Ru nanoparticles, it seems that there is special synergism between these NiFe alloys and the Al2O3 support leading to unexpected CO2 methanation activity. The authors related this behavior to the improvement of CO2 adsorption. We believe that other reasons should also be studied to explain this behavior such as the support basicity, concentration of OH groups, and adsorption strength of reaction products.
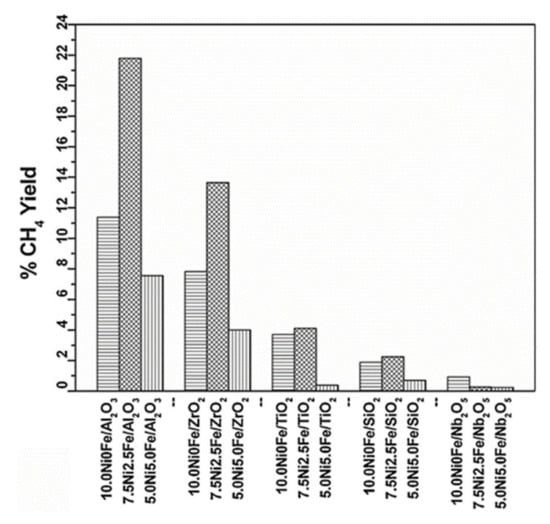
Figure 6.
CH4 yield for Al2O3, ZrO2, TiO2, SiO2, and Nb2O5 supported Ni and series of Ni–Fe bimetallic alloy catalysts at 250 °C. Catalytic measurements were done in fixed bed flow reactor at 250 °C on 150 mg under continuous flow of 4% v/v CO2 in H2 (80 mL min−1) after reductive treatment (4 h in a flow of H2 at 500 °C) of the calcined samples (reprinted from Ref [124] © 2006, Elsevier Inc).
5. Nonconventional Approaches to Switch CO2 Reduction Selectivity
In this section, we will discuss in detail some of the new approaches that have been recently reported by some groups for switching the selectivity of CO2 reduction from methanation pathway to the CO formation via RWGS reaction. These involve the development of single atom catalysts (SCAs) and the modulation of the metal support interactions by recently emerging approaches.
5.1. Heterogeneous Single Atom Catalysts (SACs) Supported on Metal Oxides for CO2 Reduction
The miniaturization of the size of active metal nanoparticles (NPs) in heterogeneous catalysts to their atomic limits, ultimately to the so-called single-atom catalysts (SACs), is emerging as an attractive approach for engineering new catalytic materials [125,126]. This is motivated on the one hand by the need to maximize the fraction of active sites on the surface of catalyst supports and therefore reduce the price of expensive metals used in catalytic applications. On the other hand, this approach is adopted to enhance the selectivity of metal active sites in specific reaction pathways. The latter aspect, in particular, is emerging recently as an approach to bridge heterogeneous catalysis with molecular homogeneous catalysis and is anticipated to help engineering unprecedented properties of heterogeneous catalysts (for further discussion on this aspect we refer to Ref. [127]). The use of SACs in the reduction of CO2 emerged also recently as an attractive approach to engineer the unique properties of different metals such as Rh [90,91], Ru [59], and Pt [128], which by default are known as the most active catalysts for CO2 hydrogenation/reduction reactions. Isolated nickel single atom catalysts were also prepared in stable forms and were active for the photo- and electrocatalytic reduction of CO2 [129,130]. The stability of nickel single sites under thermo-catalytic reaction in CO/CO2/H2 gases is quite challenging due to the much higher tendency of these isolated sites to form Ni(CO)4, at low temperatures. Therefore, much progress in the design of catalyst support is still needed for the architecture of such catalysts. Hence, we will focus on some key examples for Ru, Rh, and Pt where the atomic dispersion of the metals, i.e., as SACs result in the change of catalytic performance.
5.1.1. Rh-Single-Atom Catalysts
Recent work of Matsubu et al. on supported Rh/TiO2 catalysts has shown that the dispersion of Rh nanoparticles/clusters to their atomic limits results in a drastic change of the selectivity of this element for the catalytic CO2 reduction at ambient pressure and 200 °C from CH4 to CO formation [90]. By leaching the Rh nanoparticles on a series of Rh/TiO2 catalysts using H2O2/HCl mixture and correlating the activity of resulting catalysts with the fraction of Rh single sites on TiO2, these authors found out a strong correlation between the activity toward CO formation with the fraction of isolated Rh single sites. In this study, the authors showed that the temperature onset of CO formation (CH4 formation) is strongly affected by the ratio of CO2:H2 in the reaction gas mixture, where methane formation is promoted with the increase of the H2/CO2 ratio [90]. Further studies of Rh single sites on TiO2 showed that the activity and selectivity of these atomic species are strongly dependent on the local surface environment, which is strongly dependent on the pretreatment (gas atmosphere/temperature) and the reaction gas mixture used in CO2 reduction [131]. Moreover, the isolated rhodium single sites supported by porphyrin metal-organic framework showed high activity at room temperature during the photocatalytic reduction of CO2 with high selectivity toward formate-like species [132]. Interestingly, these isolated single sites did not show any activity toward methane formation.
5.1.2. Ru-Single-Atom Catalysts
For supported Ru catalysts, it was recently also reported by Aitbekova and coworkers that Ru nanoparticles on CeO2 can be dispersed to Ru single atoms upon exposure to an oxidative treatment at relatively low temperature (230 °C) [59]. Notably, upon transition from Ru nanoparticles to Ru single atoms the activity is switching from CO2 methanation to CO formation via RWGS. Furthermore, the exposure to a sequence of low temperature oxidation treatment followed by a reduction step at 230 or 530 °C with much higher selectivity for CO could be seen compared to only exposure to oxidative treatment at 230 °C before the catalytic reaction. This behavior is strongly analogous to the behavior of Rh single sites supported on TiO2, which are also selective to CO formation. Similar results were also observed when Al2O3 and TiO2 were used as supports for Ru, although the response of TiO2 to the pretreatments is more dependent on the temperature than for CeO2 (see Figure 7).
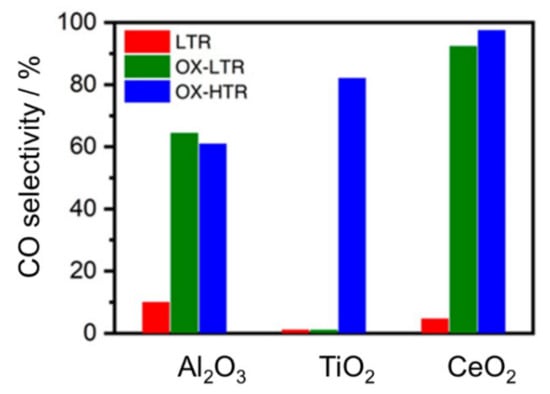
Figure 7.
Selectivity of CO2 reduction over oxide supported Ru catalysts (powder form) toward CO formation in CO2/H2 gas mixture at 240 °C upon exposure to different treatments (LTR: exposure to 5% H2/Ar at 230 °C for 30 min; OX-LTR: oxidation in 5% O2/Ar at 230 °C for 30 min, then 5% H2/Ar at 230 °C for 30 min; OX-HTR: oxidation in 5% O2/Ar at 230 °C for 30 min, then 5% H2/Ar at 530 °C for 30 min). All catalytic measurements were carried out at a constant GHSV using a layer of 20 mg of catalyst powder diluted (1:10) catalytically inactive Al2O3, sandwiched between two layers of granular acid-washed quartz (reprinted from Ref [59] © 2018, American Chemical Society Inc, Washington, DC, USA).
The long-term stability of these species with time-on-stream is, however, questionable. The quick reduction of RuOx species during reaction in CO/CO2/H2 feed gases have been studied with time-resolved XANES/EXAFS measurements for different supports including zeolites [76,77], Al2O3 [67,77], ZrO2 [71], and TiO2 [72,133]. Based on these findings, it has been established that the tiny RuOx species on different oxides can be quickly reduced during the first few minutes of reaction and grow further to form larger NPs. The persistence of small fraction of oxidic Ru species cannot, however, be excluded. Therefore, a clear judgement of the stability of these single-atom catalysts needs elaborate deactivation experiments with time-on-stream at different temperatures. This would include also the examination of the impact of catalyst pretreatment as well as the catalyst preparation method and its structural characteristics, which are decisive for these structural changes.
In contrast to the findings of Aitbekova et al. [59], a recent study by Fan et al., depicted that atomically dispersed Ru into porous hexagonal boron nitride supports shows high selectivity toward methanation of CO2 with extended time-on-stream stability. These catalysts are surprisingly more active than supported Ru nanoparticles [134]. The stability of these catalysts during reaction was attributed to the coordination of Ru by B and N atoms into defects sites of these 2D materials. It should be noted that the coordination with B and N also reduces the valency of Ru (Ru is more reducible), which presumably increases the local charge density needed to activate hydrogen. These observations underline the impact of the support material on the properties of SACs. Therefore, it cannot be accepted as a consensus that the SACs of the metals Rh or Ru should only be active for the RWGS.
Stable Ru-SAC catalysts could also be prepared using mesoporous silica supports. As an example, stable Ru-SACs were prepared by interaction of Ru3+ ions and CTA+ surfactant species inside the micro-cages of the mesoporous MCM-41 silica. This Ru SAC was found active and selective for the water-free reduction of CO2 to formic acid by interaction with molecular hydrogen. Any formation of CO or CH4 was not monitored leading to 100% selectivity toward formic acid [135]. The presence of surfactant into the micropores of MCM-41 was found to stabilize Ru3+ ions against singeing in the presence of hydrogen and has not made them inactive. Similarly, mononuclear Ru3+ sites coordinated with a N, P containing polymer were found active for CO2 reduction to formats [136]. This study showed that the stabilization of the mononuclear Ru single sites by nitrogen-donor groups (phosphazene-N and pyridine-N) leads to an increase of the local electron density on these sites and therefore promoted H2 dissociation and subsequently led to an enhanced catalytic activity.
5.1.3. Pt-Single-Atom Catalysts
For supported Pt catalysts, Wang et al. recently showed a comparison of the behavior of CeO2-supported Pt nanoparticles and CeO2-supported Pt single atoms for the thermocatalytic reduction of CO2 at ambient pressure [128]. These authors prepared Pt SACs by the impregnation of a CeO2 support but with limited concentration of Pt (0.05 wt.%) [128]. For comparison, 2 wt.% of Pt nanoparticles were loaded onto CeO2. In both cases, the catalysts were post-treated by calcination in air at 450 °C. Catalytic performance of both catalysts was examined between 150 and 500 °C and results indicated that Pt-SACs can be entirely selective for CO formation. However, the supported Pt NPs require higher reaction temperature to show this feature since they start to switch selectivity from CH4 to CO at temperatures ≥300 °C (see Figure 8a). The total reaction rate for CO2 conversion normalized by the Pt mass is higher for the Pt-SCA compared to the Pt-NP catalyst (see Figure 8b). This is an impressive example of how SACs can be used to selectively control reactions with a significantly reduced amount of precious metal. In contrast, supported Pt single atoms by triazine covalent organic framework (COF) were found highly active to methanation under visible light illumination [137]. These results confirm again the concept that the activity of single atoms toward a specific product is strongly dependent on the support material, reaction conditions, and regime of catalysts used (e.g., photocatalysis versus thermocatalytic reactions). Interestingly, the activity of Pt-supported triazine COF catalysts is significantly lower than that detected on the Pt SAC catalyst [137]. These aspects should be examined more in depth in the future, in particular the effect of support material on the activity and selectivity toward a specific product. There seems to be a common pattern for different metals as discussed above, especially for the Pt and Ru SACs.
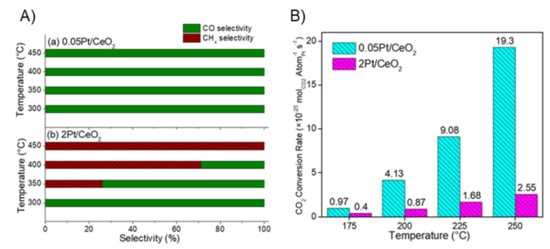
Figure 8.
(A) CO2 reduction selectivity of (a) 0.05 Pt/CeO2 and (b) 2 Pt/CeO2 toward CO and CH4; (B) CO2 conversion rate for 0.05 Pt/CeO2 and 2 Pt/CeO2 catalysts evaluated based on exposed Pt atoms in the temperature range from 175 to 250 °C. Catalytic measurements were done on 50 mg of powder catalyst at a total flow of 40 mL min−1, resulting in a GHSV of ca. 48,000 mLg−1 h−1 (reprinted from ref [128] © 2018, American Chemical Society Inc).
Overall, it is clear that most of the methanation active metals supported on typical metal oxides can be converted to RWGS-active catalyst by transforming the metal nanoparticles into atomically dispersed species on oxide supports. This cannot, however, be considered a general rule of thumb. Exceptions include metal single atoms supported by nonconventional supports such as carbon nitrides, and covalent organic frameworks, which were active toward CO2 methanation rather than CO formation, in contrast to what had been observed for CeO2 or TiO2 supports. Deep characterization of these species under reaction conditions and clear understanding of the coordination environments of these supports are indispensable for easy control of their selectivity for CO2 reduction.
5.2. Controlling the Metal-Support Interaction and Impact on CO2 Reduction Selectivity
The activity of different catalytic materials toward CO2 reduction are much influenced by the chemical nature of support, which in turn affects the metal-support-interactions (MSIs). This phenomena refers commonly to the classical strong metal-support-interaction effects (SMSI) reported in the late seventies and early eighties by Tauster et al. [138,139]. According to their model, SMSI was defined by the overgrowth of metal nanoparticles, in particular Pt and Ru, by partially reduced metal oxide species (i.e., partial encapsulation of metal NPs by reduced metal oxide support) at relatively high temperatures (> 300 °C). This results in the shrinkage of the accessible active metal surface area. This effect was reported recently to affect the activity of Ru/TiO2 catalysts for both CO and CO2 methanation [66]. Switching the selectivity for CO2 reduction between CO formation via RWGS or to CH4 formation via the Sabatier reaction is even more interesting aspect connected to changes in the MSIs. In the coming few paragraphs, we will show two recently reported key examples on the role of metal-support interactions in tuning the activity of supported metal nanoparticles for CO2 reduction.
The first example was reported by Matsubu et al. for supported Rh catalysts [91]. These authors found out that the selectivity of CO2 reduction at ambient pressure can be reversibly switched from methane-selective to CO-selective pathways by modulating the electronic properties of the catalysts (i.e., its tendency for O-vacancy formation). This approach was achieved by inducing a special kind of metal support interaction, conveniently referred to as “adsorbate-mediated strong metal-support interaction” (A-SMSI). It involves the deposition of reactant permeable CHx adlayer on the Rh nanoparticles by treatment of the catalyst in 20% CO2/2% H2 gas mixture at 250 °C, which results in the decay of the selectivity for methanation down to <15% compared to fully reduced catalyst, which exhibits about 100% selectivity for methane. The change in selectivity is the outcome of decay of CH4 formation rate and the concomitant increase of the rate of formation of CO, due to a change of the intrinsic activity of these catalysts toward CH4 and CO formation. In comparison with the standard catalyst reduced in hydrogen, these A-SMSI effects resulted in a strong modification of the electronic/adsorption properties of Rh NPs, which was deciphered by observing a strong red-shift of the COad-Rh signal as a consequence of inducing A-SMSI compared to hydrogen-reduced sample. These observations were monitored for Rh/TiO2 and Ru/Ta2O5 supported catalysts, while other irreducible supports such as Rh/Al2O3 did not show similar results. Despite CeO2 being a highly reducible oxide and having higher tendency of O-vacancy formation than other oxides such as TiO2, the Rh/CeO2 did not show similar behavior compared to Rh/TiO2 and Ru/Nb2O5. This point opens the door for further studies to identify the dependence of these A-SMSI on the chemical nature of the support, and not only its reducibility. In total, we believe that this approach is a very agile route for modification of the reactivity and selectivity of CO2 reduction catalysts and further studies on other metals are also needed to optimize its use for the control of the selectivity for CO2 reduction.
The other example of modification of the selectivity of CO2 reduction in CO/CO2/H2 and CO2/H2 gas mixtures (via modulation of MSI was reported recently by Abdel-Mageed et al. [70,121]). These authors found out that the systematic variation of the specific surface area of the TiO2 supports of Ru/TiO2 catalysts in the range from 20 to 230 m2g−1, which correlate to a change in the TiO2 particle size from about 24 to 9 nm, results in a strong variation of the activity toward methane and CO formation at ambient pressure in the temperature range between 190 and 350 °C, and therefore can be used to control the selectivity for CO2 reduction.
For Ru/TiO2 catalysts with specific surface < 120 m2g−1, the catalysts showed almost 100% selectivity for methane in the temperature range from 190 to 350 °C [66], while catalysts with larger specific surface area (SSA; >200 m2g−1) showed initially a limited methanation activity and no CO formation in the temperature range from 190 to 250 °C (see Figure 9a–d) [70,121]. At T ≥ 250 °C, the catalyst became inactive for methane formation and instead showed only CO formation (CO selectivity of 100%) with the reaction rate increasing continuously with rising the temperature up to 350 °C. These high SSA Ru/TiO2 catalysts remained CO selective upon returning back to 190 °C after reaction at 350 °C (see Figure 9f), while the lower SSA Ru/TiO2 catalysts showed only methane, but with higher reaction rate (see Figure 9e). These observations were explained by a strong modulation of the metal-support interactions with the change in the support TiO2 particle size. With increasing the SSA (decreasing TiO2 particle size), it could be observed that the concentration of O-vacancy defects increases significantly.
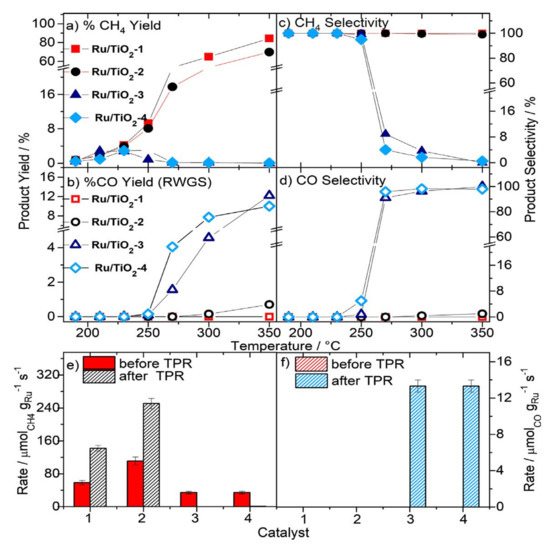
Figure 9.
CO2 conversion to CH4 (a) and CO (b) during a temperature programmed reaction sequence (temperature range 190–350 °C) under close to steady-state conditions (15.5% CO2, 80% H2, N2 balance) on different Ru/TiO2 catalysts (1: Ru/TiO2-1 (64 m2g−1); 2: Ru/TiO2-2 (120 m2g−1); 3: Ru/TiO2-3 (235 m2g−1); 4: Ru/TiO2-4 (225m2g−1)). (c,d) Selectivity for CO and CH4 formation over the entire temperature range. (e,f) Ru- mass-normalized rates (steady-state conditions) for CH4 formation (e) and CO formation (f) at 190 °C before and after the temperature programmed reaction (TPR) described in (a,b). All catalytic measurements were done on powder samples using 200 mg of Ru/TiO2 catalyst diluted by thermally stable and catalytically inactive α–Al2O3 (1:10–Ru/TiO2: α–Al2O3) using a flow of 41.6 Nml min−1 at a constant GHSV of 18,000 h−1 (reprinted from ref [121] © 2021, Elsevier Inc).
In their model, the authors claimed that a moderate reduction of the support enhances the CO2 methanation activity to some extent, while over-reduction, which is associated with small TiO2 crystallites (large SSA), changes the selectivity from 100% methanation to 100% CO formation via the RWGS reaction. Note that on the high SSA Ru/TiO2 catalysts large fraction of isolated Ru3+ ions were detected, which may match in part with findings reported on the switch of selectivity from CH4 to CO upon dispersion of Ru NPs into isolated Ru sites on CeO2 supports as demonstrated in the findings by Aitbekova et al. [59].
One challenge to reconcile this postulate is the persistence of a large fraction of the Ru NPs on these Ru/TiO2 catalysts after the high temperature TPR in addition to the different nature of CeO2 used as support by Aitbekova et al., compared to TiO2 oxides with large surface area. This apparent contradiction between these two studies would also be explained by the presence of Ru single sites in both cases in a different electronic structure (valency), which would definitely affect their activity.
In total, these two approaches (atomic dispersion of metals and modulation of MSI) allow for facile control of the selectivity for CO2 reduction either to syngas or to methane. In several cases, in particular for the high surface area Ru/TiO2 catalysts (BET > 200 m2 g−1), the catalyst can activate CO2 to syngas at much lower reaction temperatures than those used on the commercial Ni catalysts. This is needed to save energy and also enhances the safety measures of the whole process.
6. Outlook, Challenges, and Requirements for Power-to-Gas Applications
As outlined in detail in the introduction, the ambient pressure CO2 reduction can be envisaged as one of the rings in the chain of renewable energy, either for syngas production or for the production of synthetic natural gas. The implementation of this process would thus rely on production of highly active and selective catalysts on the one hand, and on the other hand high stability of these catalysts toward the fluctuation in the renewable energy supply, i.e., catalyst adaptability toward dynamic operations [78,79]. This kind of operation would include specifically the following incidents/circumstance that may affect the catalyst performance and its lifetime.
- (i)
- reactor abrupt shutdowns due to loss of power, which leads to a rapid reduction in reactor temperature, and thus leads to the condensation of water vapor, existing as a byproduct of either syngas or methanation pathways.
- (ii)
- hydrogen shortage during periods of low electricity production, which is intimately coupled with seasonal changes in temperature or speed of wind. This would lead to the decrease of the H2/CO2 ratios.
- (iii)
- considering that the anticipated supply of carbon dioxide would rely on the harvesting from air or from the outlet of industrial off-gases, any fluctuations in these two supplies would cause similar problems for the performance and stability of the catalyst. In this way, the reactor would operate instead at high H2/CO2 ratios.
- (iv)
- considering the collection of carbon dioxide from air the presence of some impurities of oxygen and water are highly possible in the CO2 feed, which may also affect the stability of these catalysts.
- (v)
- any loss of selectivity toward either syngas or methane would indispensably complicate the practical applications, especially for small scale uses, e.g., for methanation reactors applied for domestic uses (e.g., house heating or fueling farms), the formation of syngas as a byproduct would impost extra costs for elimination and may also result in safety risks.
These aspects can occur especially in the decentralized small-scale plants, which are in the center of the use of the renewable energy cycle. Therefore, catalysts intended for these two processes should fulfil certain criteria and be optimized and investigated under conditions relevant to the anticipated use. We do not need only highly active catalyst, but more importantly they have to be strictly selective toward one of the products and highly stable under dynamic changes in the temperature, reaction gas composition (e.g., CO2/H2 ratios), and tolerant toward the leak of small amounts of oxygen and water condensation during reaction. Based on this overview and considering the strengths and weaknesses of the different catalytic systems discussed in previous sections, we would like to refer to some selected examples that would have technical potential for methane and syngas production.
6.1. Candidates for CO2 to Methanation
Among the different catalytic systems described in previous sections, we see two important examples that can serve as excellent methanation catalysts in the gas-to-power applications. The first example is Ru nanoparticles supported on metal oxides, in particular the reducible oxides, which exhibit high selectivity toward methane and high activity at rather low reaction temperatures (<200 °C). These catalysts can be suitable for practical uses in small scale applications to convert hydrogen and carbon dioxide to methane, and they are highly stable under dynamic operation conditions as discussed before [78,79]. One of the challenges of using Ru/MOx is the relatively high price of Ru. This would need in the long term the optimization of smart synthetic methods using limited amounts of Ru nanoclusters or alloying Ru with cheaper metals. The second example is the NiFe catalysts, which turned out recently as very promising selective CO2 methanation catalysts. These catalysts showed very high stability and rather high activity at already lower temperature range (200–400 °C) than that used for the commercial NiAlOx catalysts. Although it is still much less active than the supported Ru catalysts, the NiFe alloys are a much cheaper choice and the tradeoff between price, stability, and high selectivity makes them an excellent option, especially for large scale production of methane, e.g., in industrial complexes where huge amounts of CO2 can be easily collected and diverted into methane. Extensive studies of stability under dynamic conditions including changes in reactor load and other parameters, which may affect their structural stability on stream, are still needed.
6.2. Candidates for CO2 to Syngas
For the production of syngas, one would first refer to metal single atoms supported on oxides. Successful examples such as Pt1/CeO2 [29], Ru1/CeO2 [59], and Rh1/TiO2 showed rather high selectivity toward CO at relatively moderate temperatures (≤250 °C). It should be noted that all these SAC catalysts are based on reducible oxide supports. The second candidate is the supported Ru on high specific surface area TiO2 supports (>200 m2g−1), which showed high activity and up to 100% selectivity toward CO formation at rather low temperatures (190 °C) after activation at moderate temperatures (190–350 °C). The long-term stability of these catalysts, especially under dynamic conditions remains unclear and further studies are needed before they are implemented in real applications.
7. Conclusions
In total, the increasing demand in energy, nowadays mainly produced from burning hydrocarbons and coal, which goes along with the enormous increase in CO2 emissions, requires strict and well-defined solutions and technologies for the production, storage, distribution, and sustainable use of green hydrogen, entirely generated from renewable resources. The reduction of CO2 serves as an immediate approach for the conversion of the green hydrogen, which can be used in the production of (i) methane that can be easily stored and distributed in the gas grid and (ii) syngas that can be used to produce a wide range of chemical commodities such as olefins and liquid fuels (via FT synthesis). This is feasible in the near future due to the well-established catalytic systems for these processes. This target needs, however, optimization of running conditions encountered in realistic operations involving dynamic changes of reactant supplies, which is coupled with the natural fluctuation in electric power generation from renewables. These aspects would need not only excellent catalytic materials, but also suitable engineering solutions.
Funding
This research received no external funding.
Data Availability Statement
Data discussed in the paper are reproduced from published works by the author and others.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kerr, R.A. Global warming is changing the world. Science 2007, 316, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Peters, G.P.; Andrew, R.M.; Boden, T.; Canadell, J.G.; Ciais, P.; Le Quéré, C.; Marland, G.; Raupach, M.R.; Wilson, C. The challenge to keep global warming below 2 C. Nat. Clim. Chang. 2013, 3, 4–6. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Schlögl, R. Chemistry’s Role in Regenerative Energy. Angew. Chem. Int. Ed. 2011, 50, 6424–6426. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Prakash, G.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—Closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.B.; Denton, W.H.; Nicholls, C.M. Technology and Uses of Liquid Hydrogen; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef] [Green Version]
- McGrath, K.M.; Prakash, G.S.; Olah, G.A. Direct methanol fuel cells. J. Ind. Eng. Chem. 2004, 10, 1063–1080. [Google Scholar]
- Al-Saydeh, S.A.; Zaidi, S.J. Carbon Dioxide Conversion to Methanol: Opportunities and Fundamental Challenges; Carbon Dioxide Chemistry, Capture and Oil Recovery. 2018. Available online: https://www.semanticscholar.org/paper/Carbon-Dioxide-Conversion-to-Methanol%3A-and-Al-Saydeh-Zaidi/5f2e7b783c722bc21c338c7170ff1b9ca9b995c2 (accessed on 19 December 2021).
- Fujiwara, N.; Tada, S.; Kikuchi, R. Power-to-gas systems utilizing methanation reaction in solid oxide electrolysis cell cathodes: A model-based study. Sustain. Energy Fuels 2020, 4, 2691–2706. [Google Scholar] [CrossRef]
- Sabatier, P.; Senderens, J. New methane synthesis. CR Acad. Sci. Paris 1902, 134, 514–516. [Google Scholar]
- Sabatier, P. The method of direct hydrogenation by catalysis. Nobel Lect. 1912, 11, 1901–1921. [Google Scholar]
- Mills, G.A.; Steffgen, F.W. Catalytic methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Schiebahn, S.; Grube, T.; Robinius, M.; Tietze, V.; Kumar, B.; Stolten, D. Power to gas: Technological overview, systems analysis and economic assessment for a case study in Germany. Int. J. Hydrogen Energy 2015, 40, 4285–4294. [Google Scholar] [CrossRef]
- Sterner, M.; Specht, M. Power-to-Gas and Power-to-X—The History and Results of Developing a New Storage Concept. Energies 2021, 14, 6594. [Google Scholar] [CrossRef]
- Snel, R. Olefins from syngas. Catal. Rev. Sci. Eng. 1987, 29, 361–445. [Google Scholar] [CrossRef]
- Lee, S. Methanol synthesis from syngas. In Handbook of Alternative Fuel Technologies; CRC Press: Boca Raton, FL, USA, 2007; pp. 313–338. [Google Scholar]
- Schulz, H. Short history and present trends of Fischer—Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported noble metal catalysts: Effects of the nature of the metallic phase on catalytic performance. Appl. Catal. A Gen. 2008, 344, 45–54. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Song, C.; Ji, P.; Wang, N.; Wang, W.; Cui, L. Recent advances in supported metal catalysts and oxide catalysts for the reverse water-gas shift reaction. Front. Chem. 2020, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, P. How I Have Been Led to the Direct Hydrogenation Method by Metallic Catalysts1. Ind. Eng. Chem. 1926, 18, 1005–1008. [Google Scholar] [CrossRef]
- Fischer, F.; Tropsch, H. Über die direkte Synthese von Erdöl-Kohlenwasserstoffen bei gewöhnlichem Druck.(Erste Mitteilung). Ber. Dtsch. Chem. Ges. 1926, 59, 830–831. [Google Scholar] [CrossRef]
- Ponec, V. Some aspects of the mechanism of methanation and Fischer-Tropsch synthesis. Catal. Rev. Sci. Eng. 1978, 18, 151–171. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Kester, K.B.; Zagli, E.; Falconer, J.L. Methanation of carbon monoxide and carbon dioxide on Ni/Al2O3 catalysts: Effects of nickel loading. Appl. Catal. 1986, 22, 311–319. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, T.-C.; Wu, J.-H.; Pao, C.-W.; Chen, C.-S. Influence of sodium-modified Ni/SiO2 catalysts on the tunable selectivity of CO2 hydrogenation: Effect of the CH4 selectivity, reaction pathway and mechanism on the catalytic reaction. J. Colloid Interface Sci. 2021, 586, 514–527. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Q.; Liang, L.; Ouyang, J. Surface hydroxyls mediated CO2 methanation at ambient pressure over attapulgite-loaded Ni-TiO2 composite catalysts with high activity and reuse ability. J. CO2 Util. 2021, 47, 101489. [Google Scholar] [CrossRef]
- Zhou, R.; Rui, N.; Fan, Z.; Liu, C.-j. Effect of the structure of Ni/TiO2 catalyst on CO2 methanation. Int. J. Hydrogen Energy 2016, 41, 22017–22025. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.; Li, Z.; Song, Y.; Zhang, J.; Wang, J.; He, X.; Wang, C.; Lin, W. Highly Dispersed Ni Catalyst on Metal–Organic Framework-Derived Porous Hydrous Zirconia for CO2 Methanation. ACS Appl. Mater. Interfaces 2020, 12, 17436–17442. [Google Scholar] [CrossRef] [PubMed]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, Y.H.; Moon, D.H.; Ahn, J.Y.; Nguyen, D.D.; Chang, S.W.; Kim, S.S. Reaction mechanism and catalytic impact of Ni/CeO2–x catalyst for low-temperature CO2 methanation. Ind. Eng. Chem. Res. 2019, 58, 8656–8662. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Xie, H.; Jiao, Z.; Zhang, G.; Xiong, K.; Zheng, X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrogen Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl. Catal. B Environ. 2019, 243, 262–272. [Google Scholar] [CrossRef]
- Patel, R.; Al-Fatesh, A.S.; Fakeeha, A.H.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Al-Zahrani, S.A.; Abasaeed, A.E.; Srivastava, V.K.; Kumar, R. Impact of ceria over WO3–ZrO2 supported Ni catalyst towards hydrogen production through dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 25015–25028. [Google Scholar] [CrossRef]
- Chatla, A.; Abu-Rub, F.; Prakash, A.V.; Ibrahim, G.; Elbashir, N.O. Highly stable and coke-resistant Zn-modified Ni-Mg-Al hydrotalcite derived catalyst for dry reforming of methane: Synergistic effect of Ni and Zn. Fuel 2022, 308, 122042. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Characterization and evaluation of mesoporous high surface area promoted Ni-Al2O3 catalysts in CO2 methanation. J. Energy Inst. 2020, 93, 482–495. [Google Scholar] [CrossRef]
- Ewald, S.; Kolbeck, M.; Kratky, T.; Wolf, M.; Hinrichsen, O. On the deactivation of Ni-Al catalysts in CO2 methanation. Appl. Catal. A Gen. 2019, 570, 376–386. [Google Scholar] [CrossRef]
- Munnik, P.; Velthoen, M.E.; De Jongh, P.E.; De Jong, K.P.; Gommes, C.J. Nanoparticle growth in supported nickel catalysts during methanation reaction—Larger is better. Angew. Chem. 2014, 126, 9647–9651. [Google Scholar] [CrossRef]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Reverse water gas shift reaction and CO2 mitigation: Nanocrystalline MgO as a support for nickel based catalysts. J. Environ. Chem. Eng. 2018, 6, 4945–4952. [Google Scholar] [CrossRef]
- Ranjbara, A.; Aghamiri, F.; Irankhah, A. Effect of MgAl2O4 catalyst support synthesis method on the catalytic activity of nickel Nano catalyst in reverse water gas shift reaction. Iran. J. Chem. Eng. 2019, 16, 58–69. [Google Scholar]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B Environ. 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Chen, X.; Su, X.; Su, H.-Y.; Liu, X.; Miao, S.; Zhao, Y.; Sun, K.; Huang, Y.; Zhang, T. Theoretical insights and the corresponding construction of supported metal catalysts for highly selective CO2 to CO conversion. ACS Catal. 2017, 7, 4613–4620. [Google Scholar] [CrossRef]
- Chen, C.-S.; Budi, C.S.; Wu, H.-C.; Saikia, D.; Kao, H.-M. Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO2 hydrogenation. ACS Catal. 2017, 7, 8367–8381. [Google Scholar] [CrossRef]
- Liu, M.-H.; Chen, H.-A.; Chen, C.-S.; Wu, J.-H.; Wu, H.-C.; Yang, C.-M. Tiny Ni particles dispersed in platelet SBA-15 materials induce high efficiency for CO2 methanation. Nanoscale 2019, 11, 20741–20753. [Google Scholar] [CrossRef]
- Wu, H.; Chang, Y.; Wu, J.; Lin, J.; Lin, I.; Chen, C. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdőhelyi, A. Hydrogenation of CO2 to CH4 over alumina-supported noble metals. J. Mol. Catal. 1980, 8, 471–474. [Google Scholar] [CrossRef] [Green Version]
- Rynkowski, J.M.; Paryjczak, T.; Lewicki, A.; Szynkowska, M.I.; Maniecki, T.P.; Jóźwiak, W.K. Characterization of Ru/CeO2-Al2O3 catalysts and their performance in CO2 methanation. React. Kinet. Catal. Lett. 2000, 71, 55–64. [Google Scholar] [CrossRef]
- Traa, Y.; Weitkamp, J. Kinetics of the methanation of carbon dioxide over ruthenium on titania. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process. Eng.-Biotechnol. 1999, 22, 291–293. [Google Scholar] [CrossRef]
- Li, D.; Ichikuni, N.; Shimazu, S.; Uematsu, T. Hydrogenation of CO2 over sprayed Ru/TiO2 fine particles and strong metal–support interaction. Appl. Catal. A Gen. 1999, 180, 227–235. [Google Scholar] [CrossRef]
- Jiménez, V.; Sánchez, P.; Panagiotopoulou, P.; Valverde, J.L.; Romero, A. Methanation of CO, CO2 and selective methanation of CO, in mixtures of CO and CO2, over ruthenium carbon nanofibers catalysts. Appl. Catal. A Gen. 2010, 390, 35–44. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J.N. CO2 reduction on supported Ru/Al2O3 catalysts: Cluster size dependence of product selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Shi, H.; Szanyi, J. Kinetic modeling and transient DRIFTS–MS studies of CO2 methanation over Ru/Al2O3 catalysts. J. Catal. 2016, 343, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Sheng, W.; GAO, D.-N.; WANG, S.-D. Effect of support calcination temperature on the catalytic properties of Ru/Ce0.8Zr0.2O2 for methanation of carbon dioxide. J. Fuel Chem. Technol. 2014, 42, 1440–1446. [Google Scholar]
- Proaño, L.; Tello, E.; Arellano-Trevino, M.A.; Wang, S.; Farrauto, R.J.; Cobo, M. In-situ DRIFTS study of two-step CO2 capture and catalytic methanation over Ru,“Na2O”/Al2O3 Dual Functional Material. Appl. Surf. Sci. 2019, 479, 25–30. [Google Scholar] [CrossRef]
- Dongapure, P.; Bagchi, S.; Mayadevi, S.; Devi, R.N. Variations in activity of Ru/TiO2 and Ru/Al2O3 catalysts for CO2 hydrogenation: An investigation by in-situ infrared spectroscopy studies. Mol. Catal. 2020, 482, 110700. [Google Scholar] [CrossRef]
- Aitbekova, A.; Wu, L.; Wrasman, C.J.; Boubnov, A.; Hoffman, A.S.; Goodman, E.D.; Bare, S.R.; Cargnello, M. Low-temperature restructuring of CeO2-supported Ru nanoparticles determines selectivity in CO2 catalytic reduction. J. Am. Chem. Soc. 2018, 140, 13736–13745. [Google Scholar] [CrossRef]
- Aitbekova, A.; Goodman, E.D.; Wu, L.; Boubnov, A.; Hoffman, A.S.; Genc, A.; Cheng, H.; Casalena, L.; Bare, S.R.; Cargnello, M. Engineering of Ruthenium–Iron Oxide Colloidal Heterostructures: Improved Yields in CO2 Hydrogenation to Hydrocarbons. Angew. Chem. Int. Ed. 2019, 58, 17451–17457. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrogen Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Nagase, H.; Naito, R.; Tada, S.; Kikuchi, R.; Fujiwara, K.; Nishijima, M.; Honma, T. Ru nanoparticles supported on amorphous ZrO2 for CO2 methanation. Catal. Sci. Technol. 2020, 10, 4522–4531. [Google Scholar] [CrossRef]
- Ruterana, P.; Buffat, P.; Thampi, K.; Graetzel, M. Selective Dispersion of the Ru-RuOx/TiO2 Catalyst for Methanation of CO2 at Room Temperature and Atmospheric Pressure. MRS Online Proc. Libr. 1989, 139, 327. [Google Scholar] [CrossRef]
- Abe, T.; Tanizawa, M.; Watanabe, K.; Taguchi, A. CO2 methanation property of Ru nanoparticle-loaded TiO2 prepared by a polygonal barrel-sputtering method. Energy Environ. Sci. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Brckner, A.; Behm, R., Jr. Encapsulation of Ru Nanoparticles: Modifying the Reactivity Toward CO and CO2 Methanation on Highly Active Ru/TiO2 Catalysts. Appl. Catal. B Environ 2020, 270, 118846. [Google Scholar] [CrossRef]
- Chen, S.; Abdel-Mageed, A.M.; Dyballa, M.; Parlinska-Wojtan, M.; Bansmann, J.; Pollastri, S.; Olivi, L.; Aquilanti, G.; Behm, R.J. Raising the COx Methanation Activity of a Ru/Al2O3 Catalyst by Activated Modification of Metal–Support Interactions. Angew. Chem. Int. Ed. 2020, 59, 22763–22770. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Abdel-Mageed, A.M.; Gauckler, C.; Olesen, S.E.; Chorkendorff, I.; Behm, R.J. Selective CO Methanation on Isostructural Ru Nanocatalysts: The Role of Support Effects. J. Catal. 2019, 373, 103–115. [Google Scholar] [CrossRef]
- Falbo, L.; Visconti, C.G.; Lietti, L.; Szanyi, J. The effect of CO on CO2 methanation over Ru/Al2O3 catalysts: A combined steady-state reactivity and transient DRIFT spectroscopy study. Appl. Catal. B Environ. 2019, 256, 117791. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Widmann, D.; Olesen, S.E.; Chorkendorff, I.; Biskupek, J.; Behm, R.J. Selective CO Methanation on Ru/TiO2 Catalysts: Role and Influence of Metal.Support Interactions. ACS Catal 2015, 5, 6753–6763. [Google Scholar] [CrossRef]
- Chen, S.; Abdel-Mageed, A.M.; Li, M.; Cisneros, S.; Bansmann, J.; Rabeah, J.; Brückner, A.; Groß, A.; Behm, R.J. Electronic metal-support interactions and their promotional effect on CO2 methanation on Ru/ZrO2 catalysts. J. Catal. 2021, 400, 407–420. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Widmann, D.; Olesen, S.E.; Chorkendorff, I.; Behm, R.J. Selective CO Methanation on Highly Active Ru/TiO2 Catalysts: Identifying the Physical Origin of the Observed Activation/Deactivation and Loss in Selectivity. ACS Catal. 2018, 8, 5399–5414. [Google Scholar] [CrossRef] [Green Version]
- Cisneros, S.; Chen, S.; Diemant, T.; Bansmann, J.; Abdel-Mageed, A.M.; Goepel, M.; Olesen, S.E.; Welter, E.S.; Parlinska-Wojtan, M.; Gläser, R. Effects of SiO2-doping on high-surface-area Ru/TiO2 catalysts for the selective CO methanation. Appl. Catal. B Environ. 2021, 282, 119483. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M. Operando investigations of particle size and support effects during the selective CO methanation over oxide supported Ru nanoparticles in idealized and realistic H2 feed gases, Universität Ulm. 2016. Available online: https://oparu.uni-ulm.de/xmlui/handle/123456789/4003 (accessed on 19 December 2021).
- Abdel-Mageed, A.M.; Widmann, D.; Eckle, S.; Behm, R.J. Improved performance of Ru/α–Al2O3 catalysts in the selective methanation of CO in CO2-rich reformate gases upon transient exposure to water containing reaction gas. ChemSusChem 2015, 8, 3869–3881. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, A.M.; Eckle, S.; Behm, R.J. Water assisted dispersion of Ru nanoparticles: The impact of water on the activity and selectivity of supported Ru catalysts during the selective methanation of CO in CO2-rich reformate. J. Catal. 2016, 335, 79–94. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Eckle, S.; Behm, R.J. High selectivity of supported Ru catalysts in the Selective CO Methanation—Water makes the difference. J. Am. Chem. Soc. 2015, 137, 8672–8675. [Google Scholar] [CrossRef]
- Stiegler, T.; Meltzer, K.; Tremel, A.; Baldauf, M.; Wasserscheid, P.; Albert, J. Development of a structured reactor system for CO2 methanation under dynamic operating conditions. Energy Technol. 2019, 7, 1900047. [Google Scholar] [CrossRef]
- Falbo, L.; Martinelli, M.; Visconti, C.G.; Lietti, L.; Bassano, C.; Deiana, P. Kinetics of CO2 methanation on a Ru-based catalyst at process conditions relevant for Power-to-Gas applications. Appl. Catal. B Environ. 2018, 225, 354–363. [Google Scholar] [CrossRef]
- Eckle, S.; Denkwitz, Y.; Behm, R.J. Activity, selectivity, and adsorbed reaction intermediates/reaction side products in the selective methanation of CO in reformate gases on supported Ru catalysts. J. Catal. 2010, 269, 255–268. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdöhelyi, A.; Kocsis, M. Surface interaction between H2 and CO2 on RhAl2O3, studied by adsorption and infrared spectroscopic measurements. J. Catal. 1980, 65, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Solymosi, F.; Knozinger, H. Infrared spectroscopic study of the adsorption and reactions of CO2 on K-modified Rh/SiO2. J. Catal. 1990, 122, 166–177. [Google Scholar] [CrossRef]
- Solymosi, F.; Tombácz, I.; Koszta, J. Effects of variation of electric properties of TiO2 support on hydrogenation of CO and CO2 over Rh catalysts. J. Catal. 1985, 95, 578–586. [Google Scholar] [CrossRef]
- Erdőhelyi, A. Hydrogenation of carbon dioxide on supported Rh catalysts. Catalysts 2020, 10, 155. [Google Scholar] [CrossRef] [Green Version]
- Solymosi, F.; Tombacz, I. Photocatalytic reaction of H2O+ CO2 over pure and doped Rh/TiO2. Catal. Lett. 1994, 27, 61–65. [Google Scholar] [CrossRef]
- Erdohelyi, A.; Cserényi, J.; Solymosi, F. Activation of CH4 and its reaction with CO2 over supported Rh catalysts. J. Catal. 1993, 141, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Au, C. Carbon dioxide reforming of methane to syngas over SiO2-supported rhodium catalysts. Appl. Catal. A Gen. 1997, 155, 239–252. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Efstathiou, A.M.; Verykios, X.E. Transient Kinetic Study of the Oxidation and Hydrogenation of Carbon Species Formed during CH4/He, CO2/He, and CH4/CO2Reactions over Rh/Al2O3Catalyst. J. Catal. 1996, 161, 31–42. [Google Scholar] [CrossRef]
- Stevens, R.W.; Chuang, S.S. In situ IR study of transient CO2 reforming of CH4 over Rh/Al2O3. J. Phys. Chem. B 2004, 108, 696–703. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Yang, V.N.; Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 2015, 137, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Matsubu, J.C.; Zhang, S.; DeRita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 2017, 9, 120–127. [Google Scholar] [CrossRef]
- Andersson, M.P.; Bligaard, T.; Kustov, A.; Larsen, K.E.; Greeley, J.; Johannessen, T.; Christensen, C.H.; Nørskov, J.K. Toward computational screening in heterogeneous catalysis: Pareto-optimal methanation catalysts. J. Catal. 2006, 239, 501–506. [Google Scholar] [CrossRef]
- De Masi, D.; Asensio, J.M.; Fazzini, P.F.; Lacroix, L.M.; Chaudret, B. Engineering iron–nickel nanoparticles for magnetically induced CO2 methanation in continuous flow. Angew. Chem. Int. Ed. 2020, 59, 6187–6191. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Perez, L.; Haycock, B.J.; Villora-Picó, J.J.; Sepulveda-Escribano, A.; Reina, T.R. Switchable catalysts for chemical CO2 recycling: A step forward in the methanation and reverse water–Gas shift reactions. ACS Sustain. Chem. Eng. 2020, 8, 4614–4622. [Google Scholar] [CrossRef]
- Raseale, S.; Marquart, W.; Jeske, K.; Prieto, G.; Claeys, M.; Fischer, N. Supported FexNiy catalysts for the co-activation of CO2 and small alkanes. Faraday Discuss. 2021, 229, 208–231. [Google Scholar] [CrossRef]
- Meshkini Far, R.; Ischenko, O.V.; Dyachenko, A.G.; Bieda, O.; Gaidai, S.V.; Lisnyak, V.V. CO2 hydrogenation into CH4 over Ni–Fe catalysts. Funct. Mater. Lett. 2018, 11, 1850057. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.; Antonucci, P. The role of Gadolinia Doped Ceria support on the promotion of CO2 methanation over Ni and NiFe catalysts. Int. J. Hydrogen Energy 2017, 42, 26828–26842. [Google Scholar] [CrossRef]
- Huynh, H.L.; Tucho, W.M.; Yu, X.; Yu, Z. Synthetic natural gas production from CO2 and renewable H2: Towards large-scale production of Ni–Fe alloy catalysts for commercialization. J. Clean. Prod. 2020, 264, 121720. [Google Scholar] [CrossRef]
- Tian, D.; Liu, Z.; Li, D.; Shi, H.; Pan, W.; Cheng, Y. Bimetallic Ni–Fe total-methanation catalyst for the production of substitute natural gas under high pressure. Fuel 2013, 104, 224–229. [Google Scholar] [CrossRef]
- Pandey, D.; Ray, K.; Bhardwaj, R.; Bojja, S.; Chary, K.; Deo, G. Promotion of unsupported nickel catalyst using iron for CO2 methanation. Int. J. Hydrogen Energy 2018, 43, 4987–5000. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Carbon dioxide methanation over Ni-M/Al2O3 (M: Fe, CO, Zr, La and Cu) catalysts synthesized using the one-pot sol-gel synthesis method. Int. J. Hydrogen Energy 2018, 43, 16522–16533. [Google Scholar] [CrossRef]
- Huynh, H.L.; Zhu, J.; Zhang, G.; Shen, Y.; Tucho, W.M.; Ding, Y.; Yu, Z. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J. Catal. 2020, 392, 266–277. [Google Scholar] [CrossRef]
- Serrer, M.-A.; Gaur, A.; Jelic, J.; Weber, S.; Fritsch, C.; Clark, A.H.; Saraçi, E.; Studt, F.; Grunwaldt, J.-D. Structural dynamics in Ni–Fe catalysts during CO2 methanation–role of iron oxide clusters. Catal. Sci. Technol. 2020, 10, 7542–7554. [Google Scholar] [CrossRef]
- Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.-A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.-D. Potential of an alumina-supported Ni3Fe catalyst in the methanation of CO2: Impact of alloy formation on activity and stability. ACS Catal. 2017, 7, 6802–6814. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zhao, G.; Yang, H.; Yuan, M.; An, X.; Zhou, H.; Qiao, Y.; Tian, Y. CO2 methanation over ordered mesoporous NiRu-doped CaO-Al2O3 nanocomposites with enhanced catalytic performance. Int. J. Hydrogen Energy 2018, 43, 239–250. [Google Scholar] [CrossRef]
- Lange, F.; Armbruster, U.; Martin, A. Heterogeneously-Catalyzed Hydrogenation of Carbon Dioxide to Methane using RuNi Bimetallic Catalysts. Energy Technol. 2015, 3, 55–62. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R.; Takagaki, A.; Sugawara, T.; Oyama, S.T.; Urasaki, K.; Satokawa, S. Study of RuNi/TiO2 catalysts for selective CO methanation. Appl. Catal. B Environ. 2013, 140, 258–264. [Google Scholar] [CrossRef]
- Chen, A.; Miyao, T.; Higashiyama, K.; Yamashita, H.; Watanabe, M. High catalytic performance of ruthenium-doped mesoporous nickel–aluminum oxides for selective CO methanation. Angew. Chem. 2010, 122, 10091–10094. [Google Scholar] [CrossRef]
- Qadir, M.I.; Weilhard, A.; Fernandes, J.A.; de Pedro, I.; Vieira, B.J.; Waerenborgh, J.C.; Dupont, J. Selective carbon dioxide hydrogenation driven by ferromagnetic RuFe nanoparticles in ionic liquids. ACS Catal. 2018, 8, 1621–1627. [Google Scholar] [CrossRef]
- Panaritis, C.; Edake, M.; Couillard, M.; Einakchi, R.; Baranova, E.A. Insight towards the role of ceria-based supports for reverse water gas shift reaction over RuFe nanoparticles. J. CO2 Util. 2018, 26, 350–358. [Google Scholar] [CrossRef]
- Schay, Z.; Guczi, L. Catalytic hydrogenation of carbon monoxide on RuFe/SiO2 catalysts. React. Kinet. Catal. Lett. 1980, 14, 207–212. [Google Scholar] [CrossRef]
- Proaño, L.; Arellano-Treviño, M.A.; Farrauto, R.J.; Figueredo, M.; Jeong-Potter, C.; Cobo, M. Mechanistic assessment of dual function materials, composed of Ru-Ni, Na2O/Al2O3 and Pt-Ni, Na2O/Al2O3, for CO2 capture and methanation by in-situ DRIFTS. Appl. Surf. Sci. 2020, 533, 147469. [Google Scholar] [CrossRef]
- Pawelec, B.; Damyanova, S.; Arishtirova, K.; Fierro, J.G.; Petrov, L. Structural and surface features of PtNi catalysts for reforming of methane with CO2. Appl. Catal. A Gen. 2007, 323, 188–201. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Stołecki, K.; Rarog-Pilecka, W.; Miśkiewicz, E.; Wilczkowska, E.; Karpiński, Z. Supported ruthenium catalysts for selective methanation of carbon oxides at very low COx/H2 ratios. Appl. Catal. A Gen. 2008, 342, 35–39. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported Ru catalysts. Appl. Catal. B Environ. 2009, 88, 470–478. [Google Scholar] [CrossRef]
- Vance, C.K.; Bartholomew, C.H. Hydrogenation of carbon dioxide on group viii metals: III, Effects of support on activity/selectivity and adsorption properties of nickel. Appl. Catal. 1983, 7, 169–177. [Google Scholar] [CrossRef]
- Li, M.; Amari, H.; van Veen, A.C. Metal-oxide interaction enhanced CO2 activation in methanation over ceria supported nickel nanocrystallites. Appl. Catal. B Environ. 2018, 239, 27–35. [Google Scholar] [CrossRef]
- Mutz, B.; Carvalho, H.W.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdöhelyi, A.; Bánsági, T. Methanation of CO2 on supported rhodium catalyst. J. Catal. 1981, 68, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Büchel, R.; Baiker, A.; Pratsinis, S.E. Effect of Ba and K addition and controlled spatial deposition of Rh in Rh/Al2O3 catalysts for CO2 hydrogenation. Appl. Catal. A Gen. 2014, 477, 93–101. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Hauble, A.; Bansmann, J.; Rabeah, J.; Parlinska-Wojtan, M.; Brückner, A.; Behm, R.J. Steering the selectivity in CO2 reduction on highly active Ru/TiO2 catalysts: Support particle size effects. J. Catal. 2021, 401, 160–173. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of support composition on the performance of nickel catalysts in CO2 methanation reaction. Catal. Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential role of the support for nickel-based CO2 methanation catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Effect of support on the catalytic activity of supported Ni–Fe catalysts for the CO2 methanation reaction. J. Ind. Eng. Chem. 2016, 33, 99–107. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-Atom Catalysts: Emerging Multifunctional Materials in Heterogeneous Catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Aguey-Zinsou, K.-F.; Amal, R. Single atom and nanoclustered Pt catalysts for selective CO2 reduction. ACS Appl. Energy Mater. 2018, 1, 6781–6789. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Yu, P.; Yuan, Y.; Shahbazian-Yassar, R.; Sheng, Y.; Wu, S.; Tu, W.; Liu, G.; Kraft, M. Isolated Ni single atoms in nitrogen doped ultrathin porous carbon templated from porous g-C3N4 for high-performance CO2 reduction. Nano Energy 2020, 77, 105158. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Tang, Y.; Asokan, C.; Xu, M.; Graham, G.W.; Pan, X.; Christopher, P.; Li, J.; Sautet, P. Rh single atoms on TiO2 dynamically respond to reaction conditions by adapting their site. Nat. Commun. 2019, 10, 4488. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fan, Y.-Z.; Li, X.; Wei, Z.; Xu, Y.-W.; Zhang, L.; Su, C.-Y. A porous rhodium (III)-porphyrin metal-organic framework as an efficient and selective photocatalyst for CO2 reduction. Appl. Catal. B Environ. 2018, 231, 173–181. [Google Scholar] [CrossRef]
- Chen, S.; Abdel-Mageed, A.M.; Li, D.; Bansmann, J.; Cisneros, S.; Biskupek, J.; Huang, W.; Behm, R.J. Morphology-Engineered Highly Active and Stable Ru/TiO2 Catalysts for Selective CO Methanation. Angew. Chem. Int. Ed. 2019, 58, 10732–10736. [Google Scholar] [CrossRef]
- Fan, M.; Jimenez, J.D.; Shirodkar, S.N.; Wu, J.; Chen, S.; Song, L.; Royko, M.M.; Zhang, J.; Guo, H.; Cui, J. Atomic Ru immobilized on porous h-BN through simple vacuum filtration for highly active and selective CO2 methanation. ACS Catal. 2019, 9, 10077–10086. [Google Scholar] [CrossRef]
- Wang, Q.; Santos, S.; Urbina-Blanco, C.A.; Hernández, W.Y.; Impéror-Clerc, M.; Vovk, E.I.; Marinova, M.; Ersen, O.; Baaziz, W.; Safonova, O.V. Solid micellar Ru single-atom catalysts for the water-free hydrogenation of CO2 to formic acid. Appl. Catal. B Environ. 2021, 290, 120036. [Google Scholar] [CrossRef]
- Chen, B.; Dong, M.; Liu, S.; Xie, Z.; Yang, J.; Li, S.; Wang, Y.; Du, J.; Liu, H.; Han, B. CO2 Hydrogenation to Formate Catalyzed by Ru Coordinated with a N, P-Containing Polymer. ACS Catal. 2020, 10, 8557–8566. [Google Scholar] [CrossRef]
- Huang, G.; Niu, Q.; Zhang, J.; Huang, H.; Chen, Q.; Bi, J.; Wu, L. Platinum single-atoms anchored covalent triazine framework for efficient photoreduction of CO2 to CH4. Chem. Eng. J. 2022, 427, 131018. [Google Scholar] [CrossRef]
- Tauster, S.; Fung, S.; Baker, R.; Horsley, J. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauster, S.; Fung, S.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).