Highly Active TiO2 Photocatalysts for Hydrogen Production through a Combination of Commercial TiO2 Material Selection and Platinum Co-Catalyst Deposition Using a Colloidal Approach with Green Reductants
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of TiO2 Catalysts
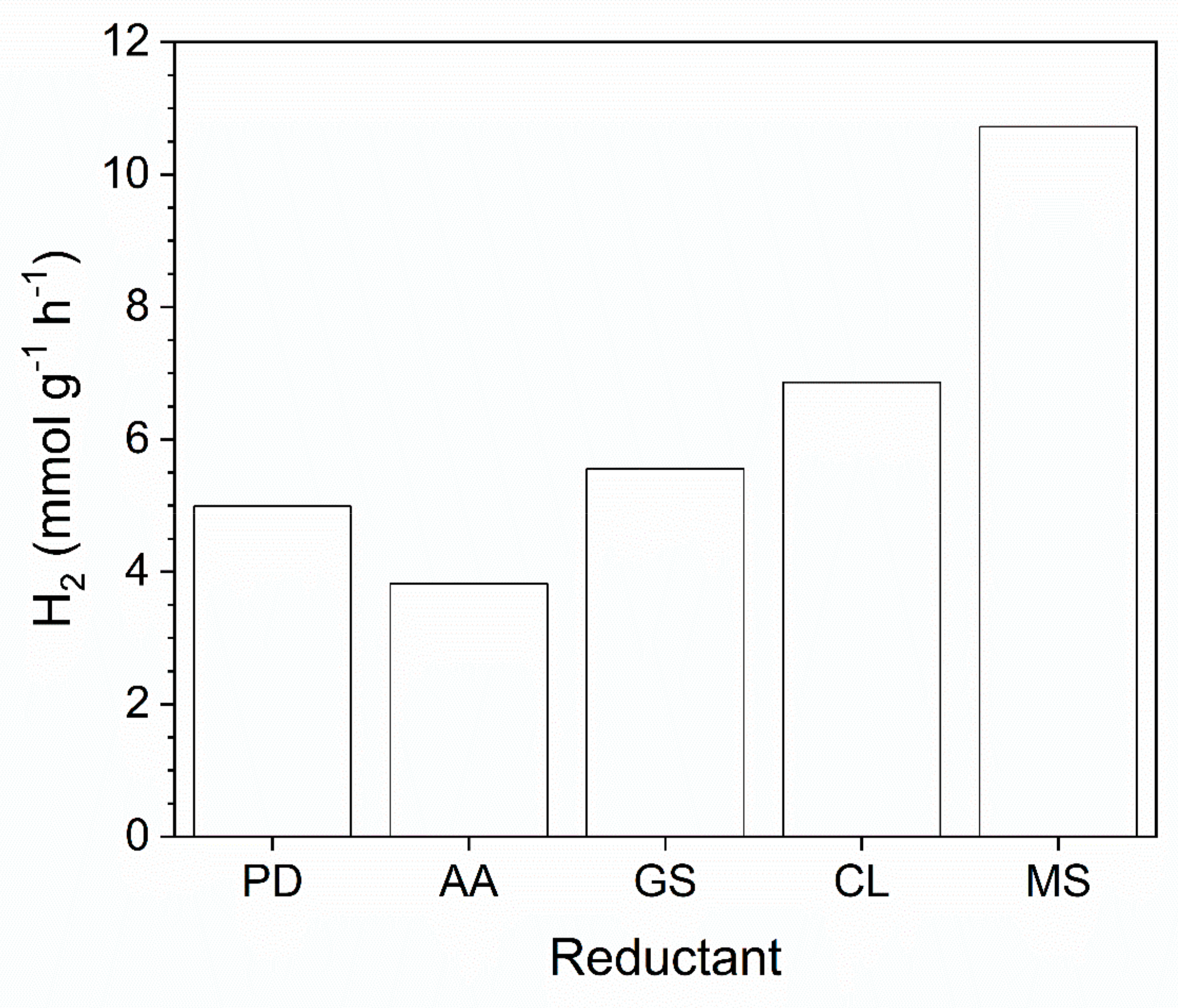
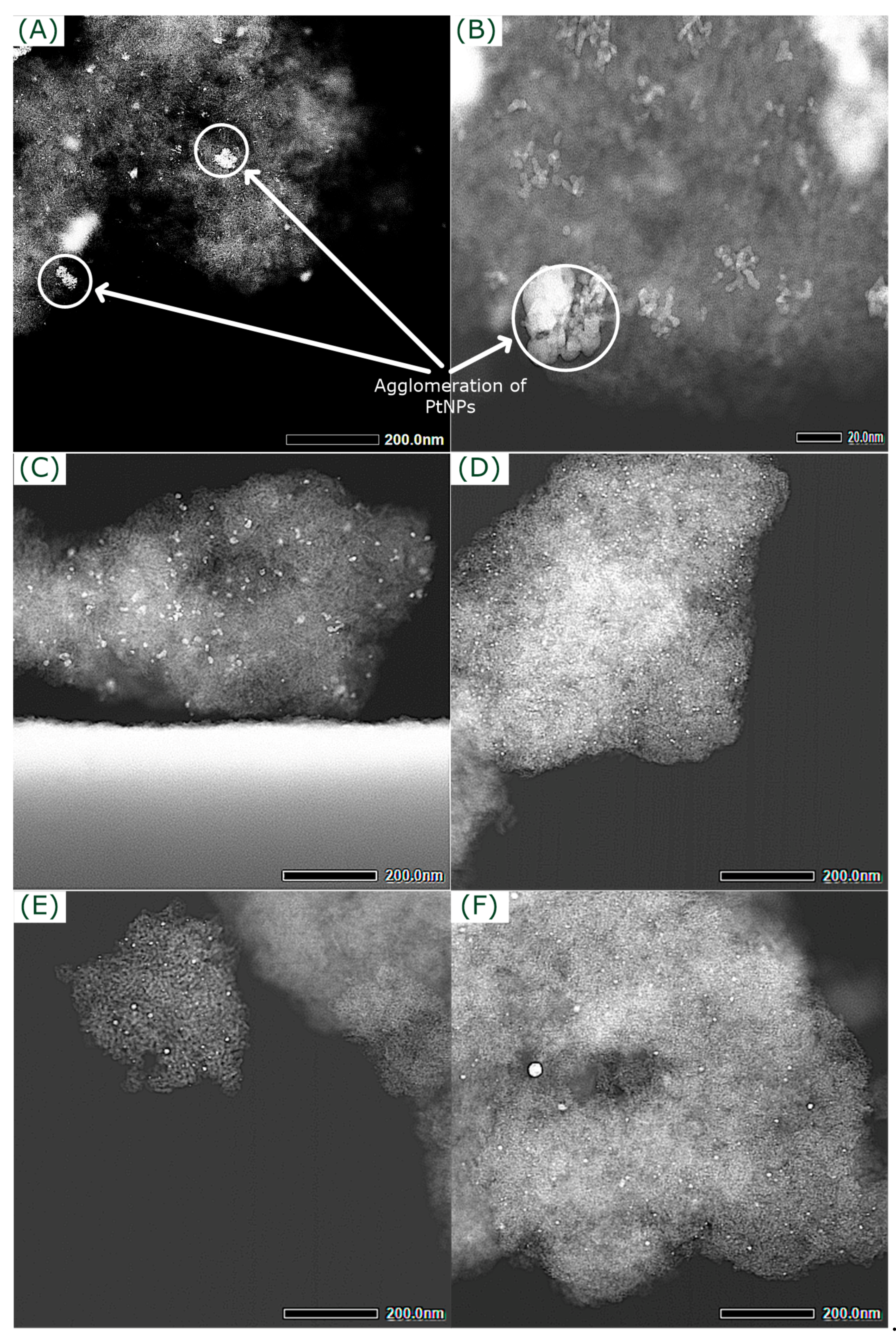
2.2. Impact of Pt Immobilization
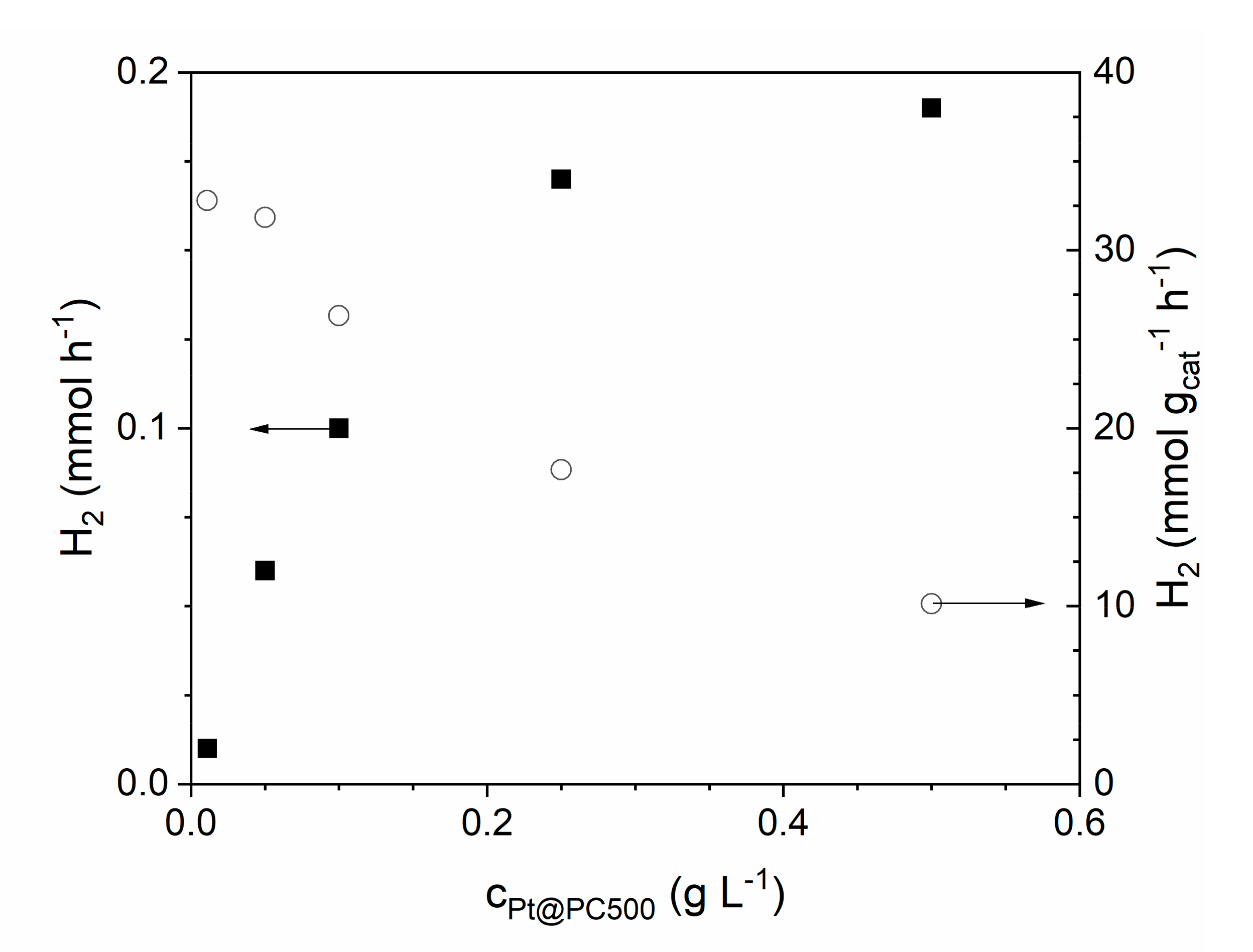
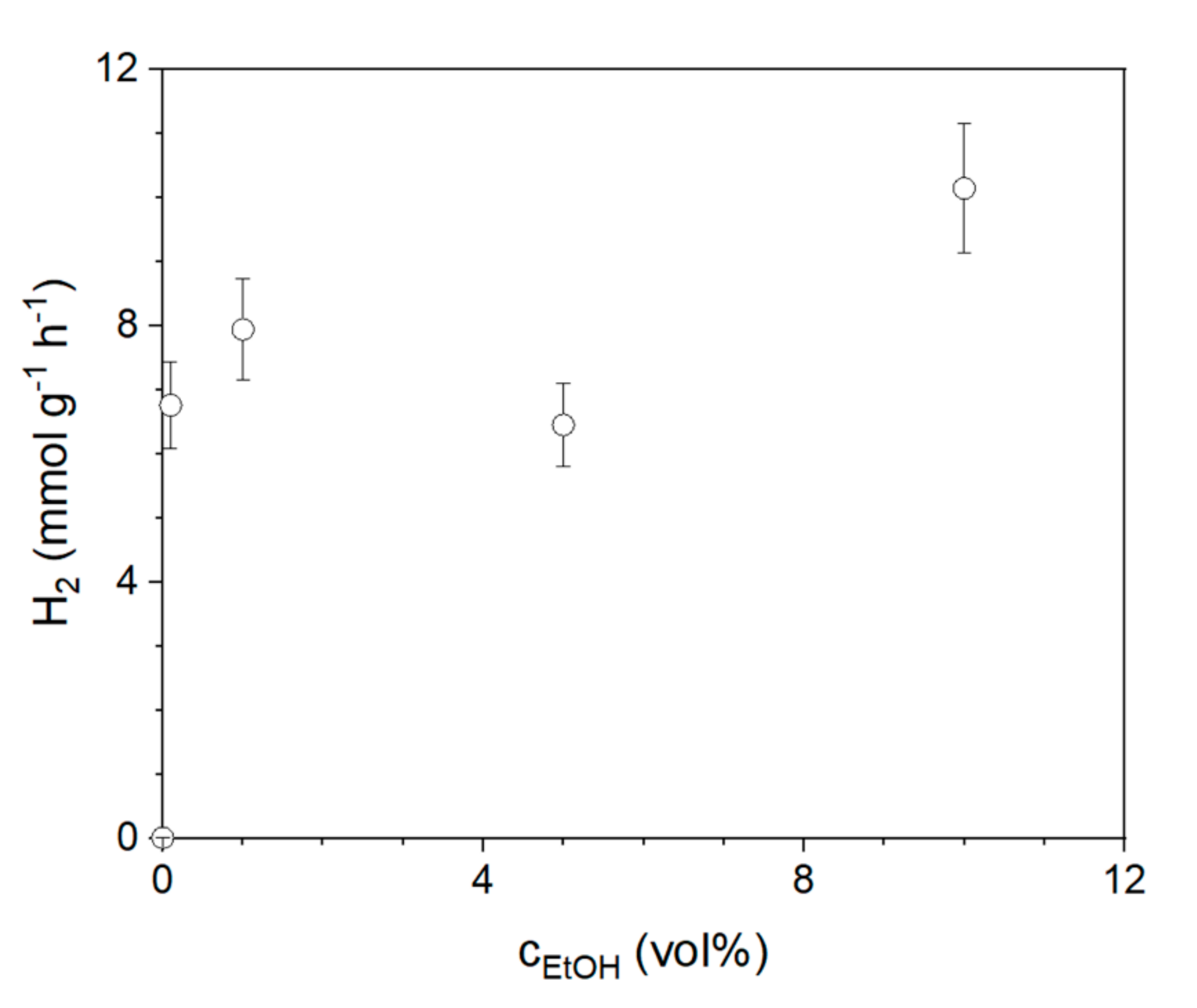
2.3. Variation of Reaction Conditions
3. Experimental
3.1. Chemicals
3.2. Colloidal Deposition
3.3. Photodeposition
3.4. Catalyst Characterization
3.5. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Hossain, A.; Sakthipandi, K.; Atique Ullah, A.K.M.; Roy, S. Recent Progress and Approaches on Carbon-Free Energy from Water Splitting. Nano-Micro Lett. 2019, 11, 103. [Google Scholar] [CrossRef]
- Tentu, R.D.; Basu, S. Photocatalytic water splitting for hydrogen production. Curr. Opin. Electrochem. 2017, 5, 56–62. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Sun, C.; Yan, X.; Wang, X.; Chen, Z.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Band-to-band visible-light photon excitation and photoactivity induced by homogeneous nitrogen doping in layered titanates. Chem. Mater. 2009, 21, 1266–1274. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Ilie, M.; Cojocaru, B.; Parvulescu, V.I.; Garcia, H. Improving TiO2 activity in photo-production of hydrogen from sugar industry wastewaters. Int. J. Hydrogen Energy 2011, 36, 15509–15518. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef]
- Maeda, K.; Masuda, H.; Domen, K. Effect of electrolyte addition on activity of (Ga1−xZnx)(N1−xOx) photocatalyst for overall water splitting under visible light. Catal. Today 2009, 147, 173–178. [Google Scholar] [CrossRef]
- Hirai, T.; Maeda, K.; Yoshida, M.; Kubota, J.; Ikeda, S.; Matsumura, M.; Domen, K. Origin of Visible Light Absorption in GaN-Rich (Ga1−xZnx)(N1−xOx) Photocatalysts. J. Phys. Chem. C 2007, 111, 18853–18855. [Google Scholar] [CrossRef]
- Maeda, K.; Hashiguchi, H.; Masuda, H.; Abe, R.; Domen, K. Photocatalytic Activity of (Ga1−xZnx)(N1−xOx) for Visible-Light-Driven H2 and O2 Evolution in the Presence of Sacrificial Reagents. J. Phys. Chem. C 2008, 112, 3447–3452. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Pattanaik, P.; Sahoo, M.K. TiO2 photocatalysis: Progress from fundamentals to modification technology. Desalin. Water Treat. 2013, 52, 6567–6590. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; Shea, K.O.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Mital, G.S.; Manoj, T. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Parapat, R.Y.; Parwoto, V.; Schwarze, M.; Zhang, B.; Su, D.S.; Schomäcker, R. A new method to synthesize very active and stable supported metal Pt catalysts: Thermo-destabilization of microemulsions. J. Mater. Chem. 2012, 22, 11605. [Google Scholar] [CrossRef]
- Parapat, R.Y.; Wijaya, M.; Schwarze, M.; Selve, S.; Willinger, M.; Schomäcker, R. Particle shape optimization by changing from an isotropic to an anisotropic nanostructure: Preparation of highly active and stable supported Pt catalysts in microemulsions. Nanoscale 2013, 5, 796–805. [Google Scholar] [CrossRef][Green Version]
- Parapat, R.Y.; Saputra, O.H.I.; Ang, A.P.; Schwarze, M.; Schomäcker, R. Support effect in the preparation of supported metal catalysts via microemulsion. RSC Adv. 2014, 4, 50955–50963. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Xu, L.; Yang, J. Immobilizing Ultrafine PtNi Nanoparticles within Graphitic Carbon Nanosheets toward High-Performance Hydrogenation Reaction. ACS Omega 2018, 3, 16436–16442. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, R.; Wu, H.; Wang, H.; He, X.; Wei, H.; Wang, S.; Zhang, R.; Lei, M.; Guo, W.; et al. Direct immobilization of an atomically dispersed Pt catalyst by suppressing heterogeneous nucleation at −40 °C. J. Mater. Chem. A 2019, 7, 25779–25784. [Google Scholar] [CrossRef]
- Yin, X.; Xie, X.; Wu, X.; An, X. Catalytic performance of nickel immobilized on organically modified montmorillonite in the steam reforming of ethanol for hydrogen production. J. Fuel Chem. Technol. 2016, 44, 689–697. [Google Scholar] [CrossRef]
- Yang, T.; Du, M.; Zhu, H.; Zhang, M.; Zou, M. Immobilization of Pt Nanoparticles in Carbon Nanofibers: Bifunctional Catalyst for Hydrogen Evolution and Electrochemical Sensor. Electrochim. Acta 2015, 167, 48–54. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Makise, Y.; Shuto, T.; Hu, J.; Tominaga, A.; Shironita, S.; Tanabe, S. Immobilization of noble metal nanoparticles on the surface of TiO2 by the sonochemical method: Photocatalytic production of hydrogen from an aqueous solution of ethanol. Ultrason. Sonochem. 2007, 14, 387–392. [Google Scholar] [CrossRef]
- Schröder, M.; Kailasam, K.; Rudi, S.; Fündling, K.; Rieß, J.; Lublow, M.; Thomas, A.; Schomäcker, R.; Schwarze, M. Applying thermo-destabilization of microemulsions as a new method for co-catalyst loading on mesoporous polymeric carbon nitride—Towards large scale applications. RSC Adv. 2014, 4, 50017–50026. [Google Scholar] [CrossRef]
- Parapat, R.Y.; Yudatama, F.A.; Musadi, M.R.; Schwarze, M.; Schomäcker, R. Antioxidant as Structure Directing Agent in Nanocatalyst Preparation. Case Study: Catalytic Activity of Supported Pt Nanocatalyst in Levulinic Acid Hydrogenation. Ind. Eng. Chem. Res. 2019, 58, 2460–2470. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Thomas, A.; Janaky, C. Photocatalytic activity of inorganic semiconductor surfaces: Myths, hype, and reality. J. Phys. Chem. Lett. 2015, 6, 139–147. [Google Scholar] [CrossRef]
- Kete, M.; Pavlica, E.; Fresno, F.; Bratina, G.; Štangar, U.L. Highly active photocatalytic coatings prepared by a low-temperature method. Environ. Sci. Pollut. Res. 2014, 21, 11238–11249. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between Surface Area and Photocatalytic Activity for Acetaldehyde Decomposition over Bismuth Tungstate Particles with a Hierarchical Structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Zhao, Y.; Han, X. Effect of phase composition, morphology, and specific surface area on the photocatalytic activity of TiO2 nanomaterials. RSC Adv. 2014, 4, 47031–47038. [Google Scholar] [CrossRef]
- Kailasam, K.; Epping, J.D.; Thomas, A.; Losse, S.; Junge, H. Mesoporous carbon nitride-silica composites by a combined sol-gel/thermal condensation approach and their application as photocatalysts. Energy Environ. Sci. 2011, 4, 4668. [Google Scholar] [CrossRef]
- Tasbihi, M.; Kocí, K.; Edelmannová, M.; Troppová, I.; Reli, M.; Schomäcker, R. Pt/TiO2 photocatalysts deposited on commercial support for photocatalytic reduction of CO2. J. Photochem. Photobiol. A Chem. 2018, 366, 72–80. [Google Scholar] [CrossRef]
- Alghamdi, H.; Idriss, H. Study of the modes of adsorption and electronic structure of hydrogen peroxide and ethanol over TiO2 rutile (110) surface within the context of water splitting. Surf. Sci. 2018, 669, 103–113. [Google Scholar] [CrossRef]
- Jiang, Z.; Isaacs, M.A.; Huang, Z.W.; Shangguan, W.; Deng, Y.; Lee, A.F. Active Site Elucidation and Optimization in Pt Co-catalysts for Photocatalytic Hydrogen Production over Titania. ChemCatChem 2017, 9, 4268–4274. [Google Scholar] [CrossRef]
- Shearer, C.; Alvino, J.; Batmunkh, M.; Metha, G. Pt Nanocluster Co-Catalysts for Photocatalytic Water Splitting. C 2018, 4, 64. [Google Scholar] [CrossRef]
- Ping, Y.; Zhang, J.; Xing, T.; Chen, G.; Tao, R.; Choo, K.-H. Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of Direct Orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Liu, Q.-m.; Yasunami, T.; Kuruda, K.; Okidom, M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. T. Nonferr. Met. Soc. 2012, 22, 2198–2203. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Depciuch, J.; Fryc, B.; Kus-Liskiewicz, M. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using clove eugenol. Appl. Organomet. Chem. 2018, 32, e4276. [Google Scholar] [CrossRef]
- Tekin, V.; Kozgus Guldu, O.; Dervis, E.; Yurt Kilcar, A.; Uygur, E.; Biber Muftuler, F.Z. Green synthesis of silver nanoparticles by using eugenol and evaluation of antimicrobial potential. Appl. Organomet. Chem. 2019, 33, e4969. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Fawzy, I.M.; Saleh, B.M.; Issa, M.Y.; Bakowsky, U.; Azzazy, H.M.E.-S. Green Synthesis of Platinum and Palladium Nanoparticles Using Peganum harmala L. Seed Alkaloids: Biological and Computational Studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Morazzoni, P.; Vanzani, P.; Santinello, S.; Gucciardi, A.; Zennaro, L.; Miotto, G.; Ursini, F. Grape seeds proanthocyanidins: Advanced technological preparation and analytical characterization. Antioxidants 2021, 10, 418. [Google Scholar] [CrossRef]
- Ismail, E.H.; Khalil, M.M.H.; Al Seif, F.A.; El-Magdoub, F. Biosynthesis of Gold Nanoparticles Using Extract of Grape (Vitis Vinifera) Leaves and Seeds. Progr. Nanotechn. Nanomat. 2014, 3, 1–12. [Google Scholar]
- Shan, Y.; Zhang, W. Preparative separation of major xanthones from mangosteen pericarp using high-performance centrifugal partition chromatography. J. Sep. Sci. 2010, 33, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Nishanthi, R.; Malathi, S.; John Paul, S.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar] [CrossRef]
- Tasbihi, M.; Schwarze, M.; Edelmannová, M.; Spöri, C.; Strasser, P.; Schomäcker, R. Photocatalytic reduction of CO2 to hydrocarbons by using photodeposited Pt nanoparticles on carbon-doped titania. Catal. Today 2019, 328, 8–14. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Schwarze, M.; Stellmach, D.; Schröder, M.; Kailasam, K.; Reske, R.; Thomas, A.; Schomäcker, R. Quantification of photocatalytic hydrogen evolution. Phys. Chem. Chem. Phys. 2013, 15, 3466–3472. [Google Scholar] [CrossRef] [PubMed]
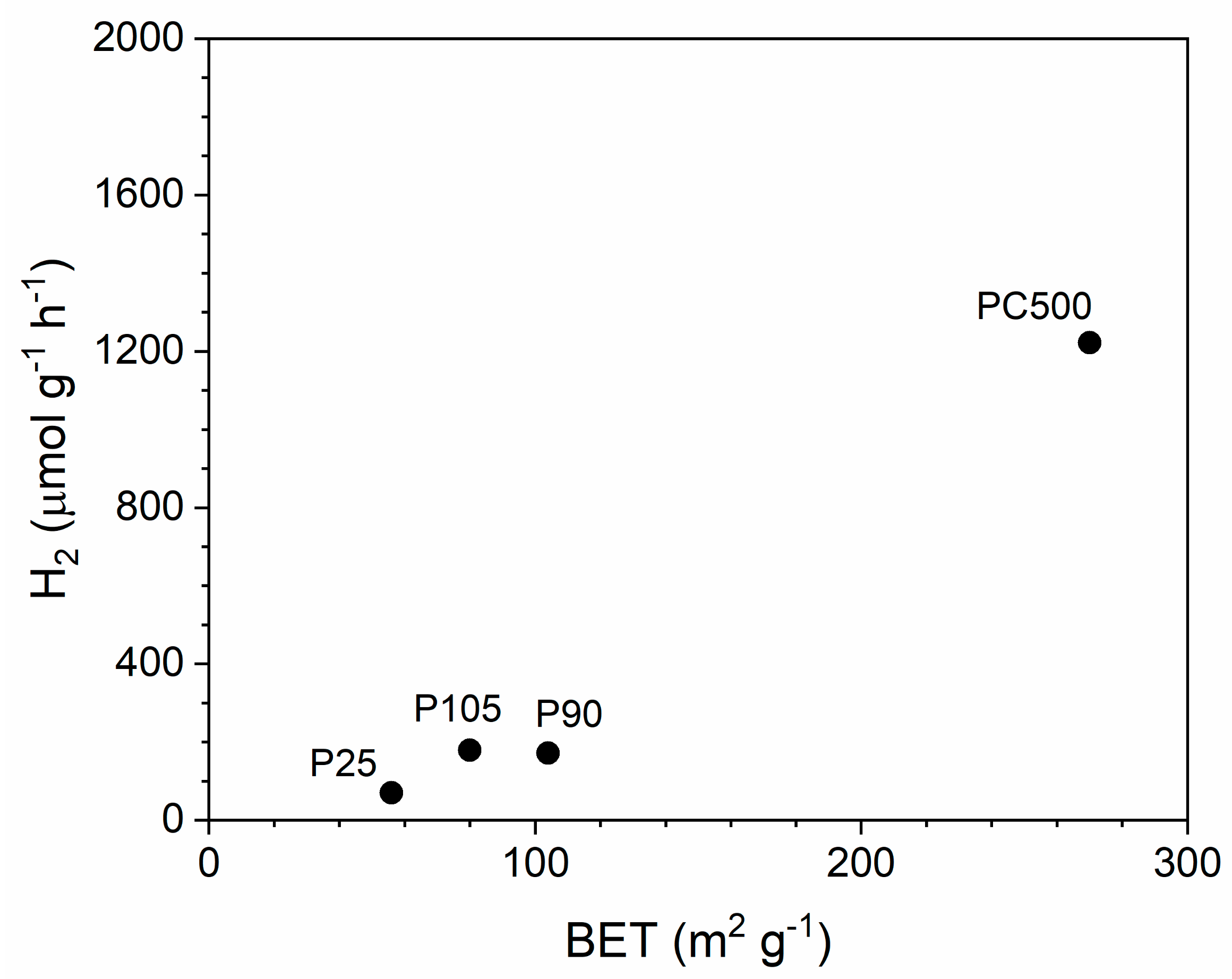

| TiO2 | CS (nm) | %Anatase | BGE (eV) | SA (m2 g−1) | H2 (μmol g−1 h−1) | H2/SA (μmol m−2 h−1) |
|---|---|---|---|---|---|---|
| P25 | 21.3 | 82 | 3.19 | 56 | 48 | 0.86 |
| P90 | 12.6 | 87 | 3.20 | 104 | 118 | 1.13 |
| PC105 | 20.9 | 100 | 3.33 | 80 | 123 | 1.54 |
| PC500 | 6.0 | 100 | 3.28 | 270 | 1222 | 4.52 |
| Entry | Method | Nominal Loading (wt.%) | ICP-OES Loading (wt.%) | SA (m2 g−1) | H2 | H2 |
|---|---|---|---|---|---|---|
| 1 | without Pt | - | - | 270 | 1.4 | - |
| 2 | PD | 1.0 | 0.49 | 275 | 5.0 | 1.0 |
| 3 | AA | 1.0 | 0.31 | 239 | 3.8 | 1.2 |
| 4 | CL | 1.0 | 0.37 | 305 | 6.9 | 1.9 |
| 5 | GS | 1.0 | 0.36 | 247 | 5.6 | 1.5 |
| 6 | MS | 1.0 | 0.37 | 309 | 10.1 | 2.7 |
| Entry | Method | NPtNPs (-) | (nm) | σ1 (nm) | (nm) | σ2 (nm) |
|---|---|---|---|---|---|---|
| 1 | PD * | 22 | 12.5 ± 1.3 | 10 | - | - |
| 2 | AA | 62 | 8.8 ± 0.3 | 2.5 | 9.3 ± 0.5 | 2.6 |
| 3 | CL | 62 | 5.4 ± 0.1 | 1.1 | 5.88 ± 0.02 | 1.1 |
| 4 | GS | 62 | 4.2 ± 0.3 | 2.6 | 3.1 ± 0.6 | 0.4 |
| 5 | MS | 62 | 5.1 ± 0.3 | 2.2 | 4.81 ± 0.11 | 1.5 |
| Entry | Reducing Agent | Active Compund | Active Compound in NP Synthesis |
|---|---|---|---|
| 1 | AA | AA | Ag [43], Ag, Au [44], Cu [45] |
| 2 | CL | Eugenol [46,47] | Ag [46,47], Pt, Pd [48] |
| 3 | GS | Oligomeric proanthocyanidin complexes (OCP) [49] | Ag, Pd, Pt, Ru [33], Ag [43], Au [50] |
| 4 | MS | Alpha-mangostin [51] | Ag, Au, Pt [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarze, M.; Klingbeil, C.; Do, H.U.; Kutorglo, E.M.; Parapat, R.Y.; Tasbihi, M. Highly Active TiO2 Photocatalysts for Hydrogen Production through a Combination of Commercial TiO2 Material Selection and Platinum Co-Catalyst Deposition Using a Colloidal Approach with Green Reductants. Catalysts 2021, 11, 1027. https://doi.org/10.3390/catal11091027
Schwarze M, Klingbeil C, Do HU, Kutorglo EM, Parapat RY, Tasbihi M. Highly Active TiO2 Photocatalysts for Hydrogen Production through a Combination of Commercial TiO2 Material Selection and Platinum Co-Catalyst Deposition Using a Colloidal Approach with Green Reductants. Catalysts. 2021; 11(9):1027. https://doi.org/10.3390/catal11091027
Chicago/Turabian StyleSchwarze, Michael, Charly Klingbeil, Ha Uyen Do, Edith Mawunya Kutorglo, Riny Yolandha Parapat, and Minoo Tasbihi. 2021. "Highly Active TiO2 Photocatalysts for Hydrogen Production through a Combination of Commercial TiO2 Material Selection and Platinum Co-Catalyst Deposition Using a Colloidal Approach with Green Reductants" Catalysts 11, no. 9: 1027. https://doi.org/10.3390/catal11091027
APA StyleSchwarze, M., Klingbeil, C., Do, H. U., Kutorglo, E. M., Parapat, R. Y., & Tasbihi, M. (2021). Highly Active TiO2 Photocatalysts for Hydrogen Production through a Combination of Commercial TiO2 Material Selection and Platinum Co-Catalyst Deposition Using a Colloidal Approach with Green Reductants. Catalysts, 11(9), 1027. https://doi.org/10.3390/catal11091027







