Catalytic CO Oxidation and H2O2 Direct Synthesis over Pd and Pt-Impregnated Titania Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis, Exfoliation and Nanotube Formation
2.2. Nanotube Structure with Pd or Pt Impregnation
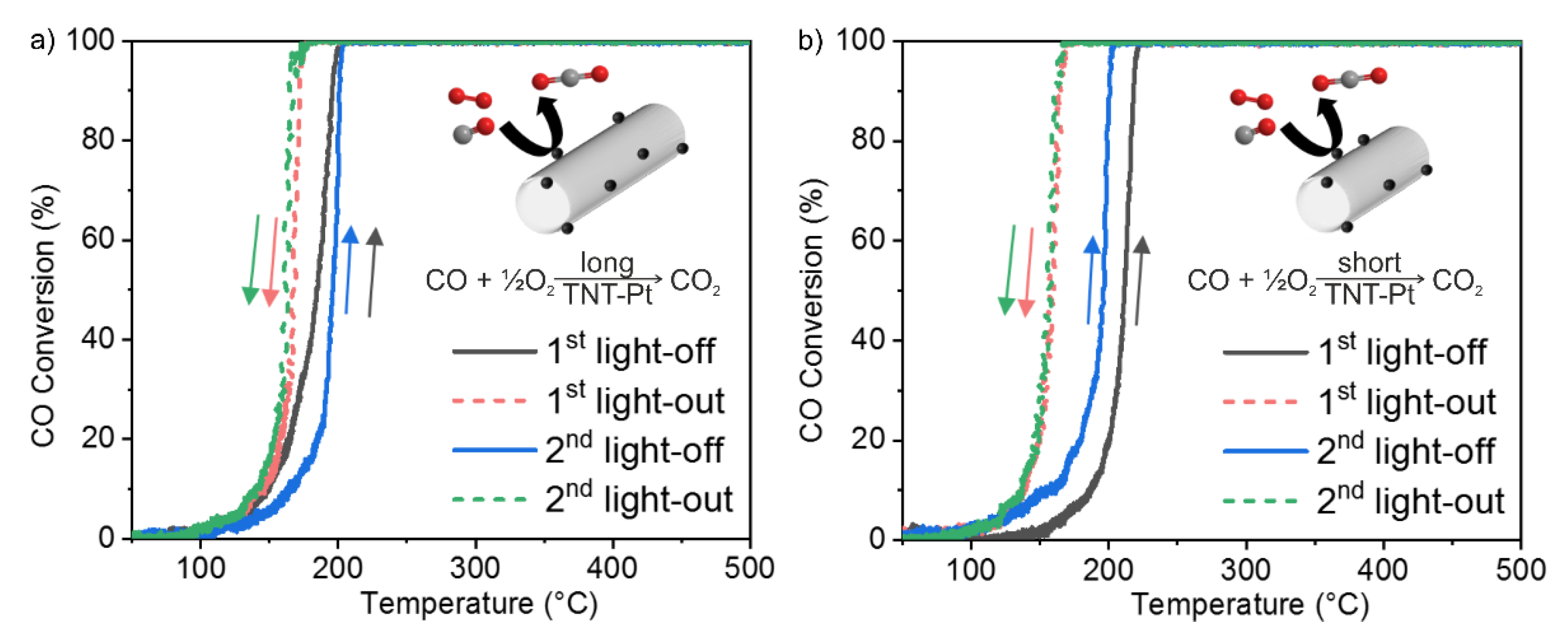
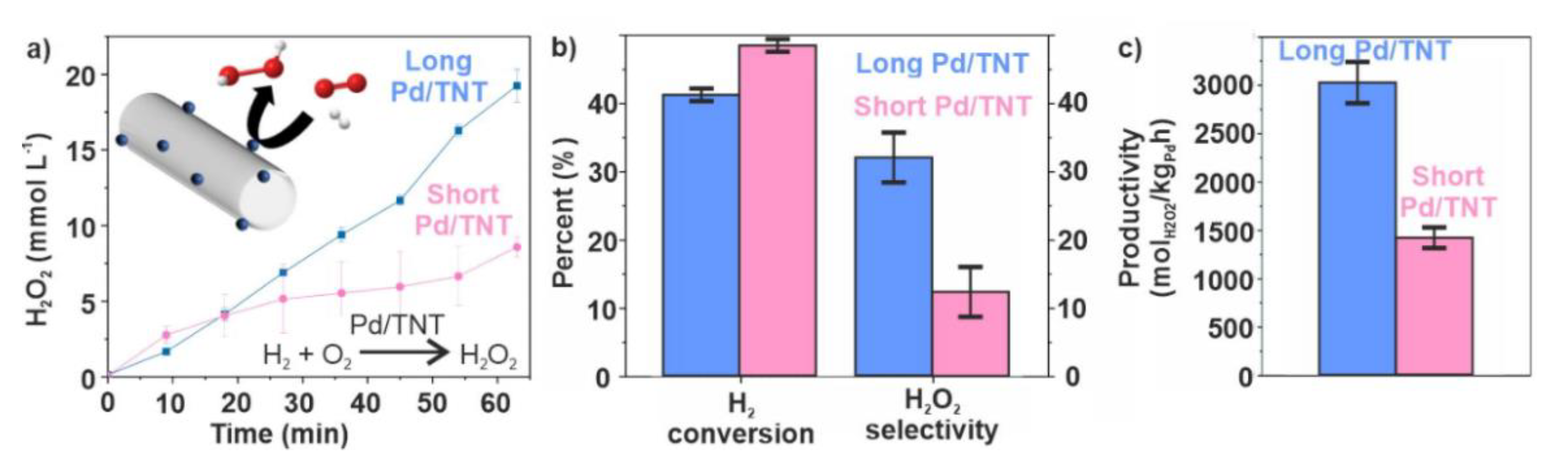
2.3. Catalytic CO Oxidation and H2O2 Direct Synthesis
3. Materials and Methods
3.1. Synthesis
3.2. Analytical Equipment
3.3. Catalysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ertl, G.; Knözinger, H.; Weitkamp, J. (Eds.) Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997; Volume 2. [Google Scholar]
- Trovarelli, A.; Llorca, J. Ceria catalysts at nanoscale: How do crystal shapes shape catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Janssen, A.; Nguyen, Q.; Xia, Y. Colloidal metal nanocrystals with metastable crystal structures. Angew. Chem. Int. Ed. 2021, 60, 12192–12203. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Yang, X.; Xie, S.; Zhao, M.; Qin, D.; Xia, Y. Shape-controlled synthesis of colloidal metal nanocrystals by replicating the surface atomic structure on the seed. Adv. Mater. 2018, 30, 1706312. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Fernandez-Garcia, M.; Colon, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Zhang, S.; Plessow, P.N.; Willis, J.J.; Dai, S.; Xu, M.; Graham, G.W.; Cargnello, M.; Abild-Pedersen, F.; Pan, X. Dynamical observation and detailed description of catalysts under strong metal–support interaction. Nano Lett. 2016, 16, 4528–4534. [Google Scholar] [CrossRef]
- Musselwhite, N.; Somorjai, G.A. Investigations of structure sensitivity in heterogeneous catalysis: From single crystals to monodisperse nanoparticles. Top. Catal. 2013, 56, 1277–1283. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 919–9986. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-G.; Tang, L.; Tang, Y.-K.; Li, C.-X.; Li, M.-L.; Zhou, J.-J.; Chen, W.; Zhu, F.; Jiang, J. Selective electrocatalytic water oxidation to produce H2O2 using a C,N codoped TiO2 electrode in an acidic electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, K.; Hou, G.; Zhang, H.; Cao, H. A highly active Pt nanocatalysts supported on RuO2 modified TiO2-NTs for methanol electrooxidation with excellent CO tolerance. Int. J. Hydrog. Energy 2019, 44, 31506–31514. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, Z.; Chen, J.; Yu, J.; Zhou, L. Synthesis, characterization, and photocatalytic activity of porous La-N-co-doped TiO2 nanotubes for gaseous chlorobenzene oxidation. J. Environ. Sci. 2016, 46, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Camposeco, R.; Hinojosa-Reyes, M.; Castillo, S.; Nava, N.; Zanella, R. Synthesis and characterization of highly dispersed bimetallic Au-Rh nanoparticles supported on titanate nanotubes for CO oxidation reaction at low temperature. Environ. Sci. Pollut. Res. 2021, 28, 10734–10748. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, Q.; Zhong, S.; Zhu, B.; Huang, W.; Zhang, S. Au/M-TiO2 nanotube catalysts (M = Ce, Ga, Co, Y): Preparation, characterization and their catalytic activity for CO oxidation. J. Sol-Gel Sci. Technol. 2018, 86, 699–710. [Google Scholar] [CrossRef]
- Krick, C.; Grabau, M.; Yoo, J.E.; Killian, M.; Schmuki, P.; Steinrueck, H.P.; Papp, C. Reactivity of TiO2 nanotube-supported Pt particles in the CO oxidation reaction. ChemCatChem 2017, 9, 564–572. [Google Scholar] [CrossRef]
- Andersson, S.; Wadsley, A.D. The crystal structure of Na2Ti3O7. Acta Crystallogr. 1961, 14, 1245–1249. [Google Scholar] [CrossRef] [Green Version]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Ling, Y.; Zhang, M.; Li, X.; Zheng, J.; Xu, J. Formation of uniform mesoporous TiO2@C-Ni hollow hybrid composites. Dalton Trans. 2018, 47, 10093–10101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, L.; Liu, H.; Yu, X.; Yin, Y.; Gao, C.A. Unique disintegration-reassembly route to mesoporous titania nanocrystalline hollow spheres with enhanced photocatalytic activity. Adv. Funct. Mater. 2018, 28, 1704208. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Shen, D.; Zhao, D.; Wang, G. Graphitic carbon conformal coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 2015, 137, 13161–13166. [Google Scholar] [CrossRef]
- Bu, D. Biotemplated synthesis of high specific surface area copper-doped hollow spherical titania and its photocatalytic research for degradating chlorotetracycline. Appl. Surf. Sci. 2013, 265, 677–685. [Google Scholar] [CrossRef]
- Weng, S.; Zhao, X.; Liu, G.; Guan, Y.; Wu, F.; Luo, Y. Synthesis, characterization, antibacterial activity in dark and in vitro cytocompatibility of Ag-incorporated TiO2 microspheres with high specific surface area. J. Mater. Sci. 2018, 29, 50. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, T.V.; Evdokimova, O.L.; Kraev, A.S.; Ivanov, V.K.; Agafonov, A.V. Micro-mesoporous anatase TiO2 nanorods with high specific surfacearea possessing enhanced adsorption ability and photocatalyticactivity. Microporous Mesoporous Mater. 2016, 235, 185–194. [Google Scholar] [CrossRef]
- Mitrofanov, A.; Brandes, S.; Herbst, F.; Rigolet, S.; Bessmertnykh-Lemeune, A.; Beletskaya, I. Immobilization of copper complexes with (1,10-phenanthrolinyl)phosphonates on titania supports for sustainable catalysis. J. Mater. Chem. A 2017, 5, 12216–12235. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Yang, H.; Xie, Y.; Sun, S.; Guo, X. Hierarchically porous titania xerogel monoliths: Synthesis, characterization and electrochemical properties. Mater. Res. Bull. 2016, 73, 48–55. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Teng, H. Regulation of the physical characteristics of titania nanotube aggregates synthesized from hydrothermal treatment. Chem. Mater. 2004, 16, 4352–4358. [Google Scholar] [CrossRef]
- Luxa, J.; Vosecký, P.; Mazánek, V.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Cation-controlled electrocatalytical activity of transition-metal disulfides. ACS Catal. 2018, 8, 2774–2781. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Carrera-Crespo, J.E.; González, F.; González, I. Effect of heat treatment on the crystal phase composition, semiconducting properties and photoelectrocatalytic color removal efficiency of TiO2 nanotubes arrays. Electrochim. Acta 2014, 140, 564–571. [Google Scholar] [CrossRef]
- Grigorieva, A.V.; Goodilin, E.A.; Derlyukova, L.E.; Anufrieva, T.A.; Tarasov, A.B.; Dobrovolskii, Y.A.; Tretyakov, Y.D. Titania nanotubes supported platinum catalyst in CO oxidation process. Appl. Catal. A 2009, 362, 20–25. [Google Scholar] [CrossRef]
- Deutschmann, O.; Grunwaldt, J.-D. Exhaust gas aftertreatment in mobile systems: Status, challenges, and perspectives. Chem. Ing. Tech. 2013, 85, 595–617. [Google Scholar] [CrossRef]
- Ranganathan, S.; Sieber, V. Recent advances in the direct synthesis of hydrogen peroxide using chemical Catalysis—A review. Catalysts 2018, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Dittmeyer, R.; Grunwaldt, J.-D.; Pashkova, A. A review of catalyst performance and novel reaction engineering concepts in direct synthesis of hydrogen peroxide. Catal. Today 2015, 248, 149–159. [Google Scholar] [CrossRef]
- Casapu, M.; Fischer, A.; Gänzler, A.M.; Popescu, R.; Crone, M.; Gerthsen, D.; Türk, M.; Grunwaldt, J.D. Origin of the normal and inverse hysteresis behavior during CO oxidation over Pt/Al2O3. ACS Catal. 2017, 7, 343–355. [Google Scholar] [CrossRef]
- Maurer, F.; Jelic, J.; Wang, J.; Gänzler, A.; Dolcet, P.; Wöll, C.; Wang, Y.; Studt, F.; Casapu, M.; Grunwaldt, J.D. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 2020, 3, 824–833. [Google Scholar] [CrossRef]
- Wan, Q.; Hu, S.; Dai, J.; Chen, C.; Li, W.X. Influence of crystal facet and phase of titanium dioxide on ostwald ripening of supported Pt nanoparticles from first-principles kinetics. J. Phys. Chem. C 2019, 123, 11020–11031. [Google Scholar] [CrossRef]
- Zhou, Y.; Doronkin, D.E.; Chen, M.; Wei, S.; Grunwaldt, J.D. Interplay of Pt and crystal facets of TiO2:CO oxidation activity and operando XAS/DRIFTS studies. ACS Catal. 2016, 6, 7799–7809. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Boubnov, A.; Müller, O.; Conrad, S.; Lichtenberg, H.; Grunwaldt, J.D. Operando spatially and time-resolved X-ray absorption spectroscopy and infrared thermography during oscillatory CO oxidation. J. Catal. 2015, 328, 216–224. [Google Scholar] [CrossRef]
- Naina, V.R.; Wang, S.; Sharapa, D.I.; Zimmermann, M.; Hähsler, M.; Niebl-Eibenstein, L.; Wang, J.; Wöll, C.; Wang, Y.; Singh, S.K.; et al. Shape-selective synthesis of intermetallic Pd3Pb nanocrystals and enhanced catalytic properties in the direct synthesis of hydrogen peroxide. ACS Catal. 2021, 11, 2288–2301. [Google Scholar] [CrossRef]

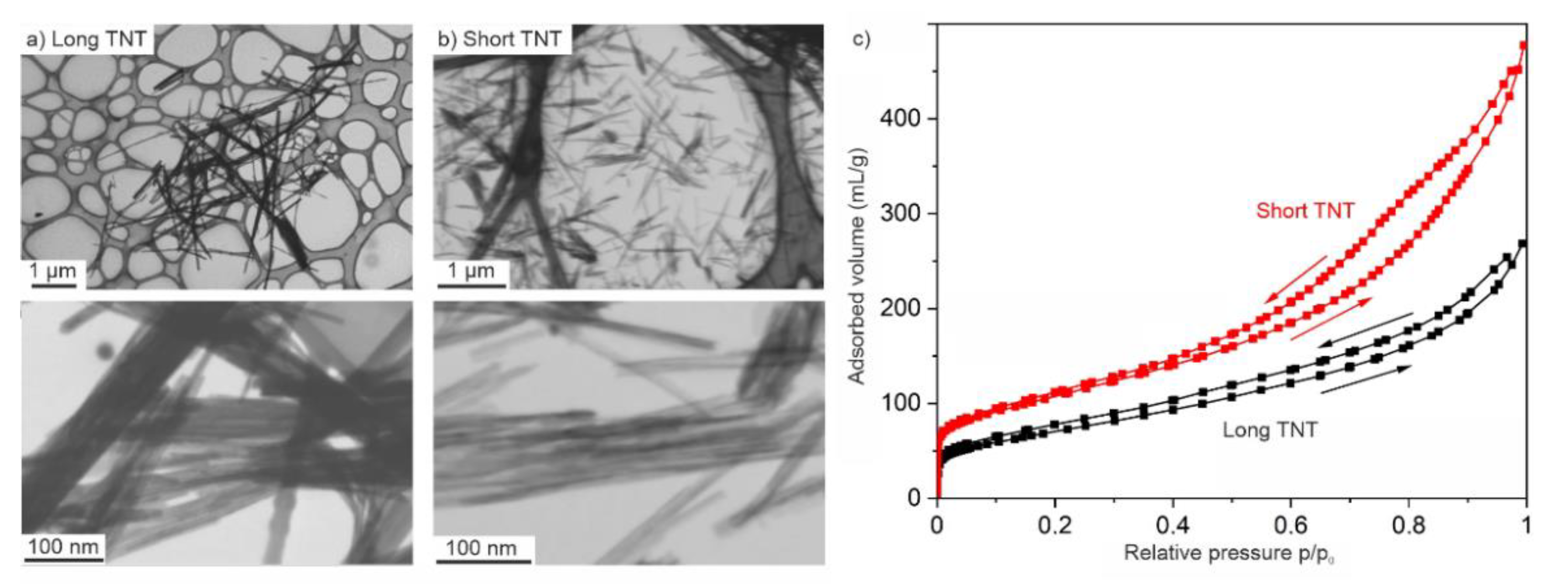
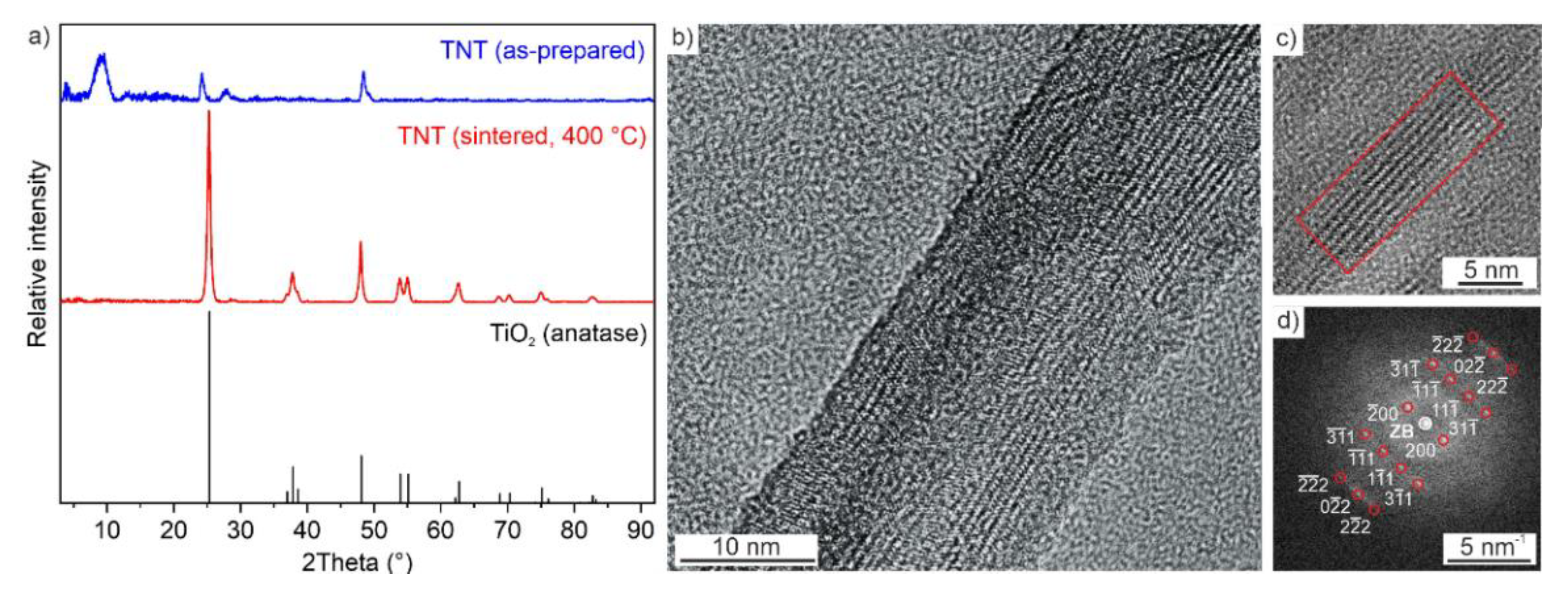
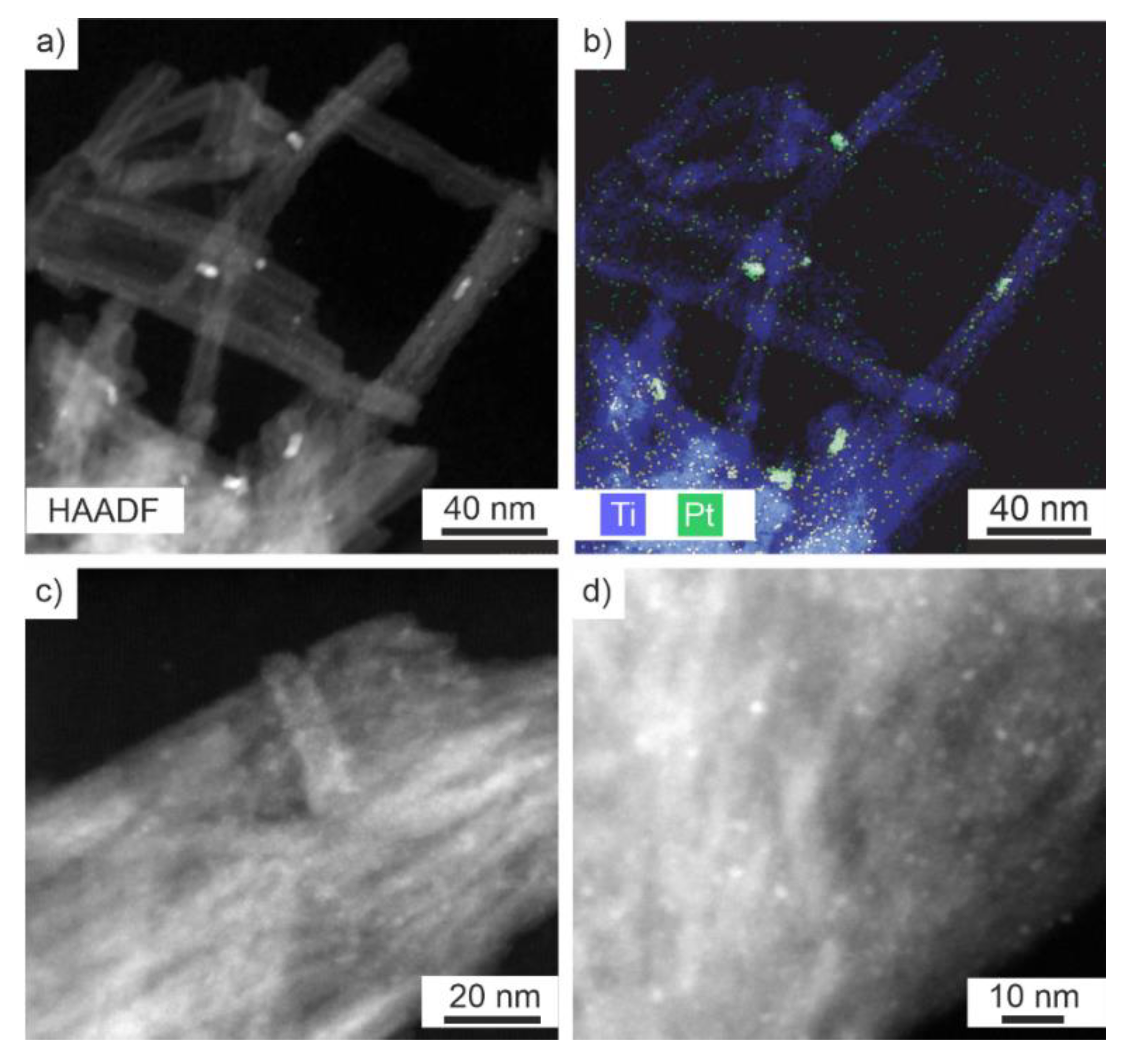
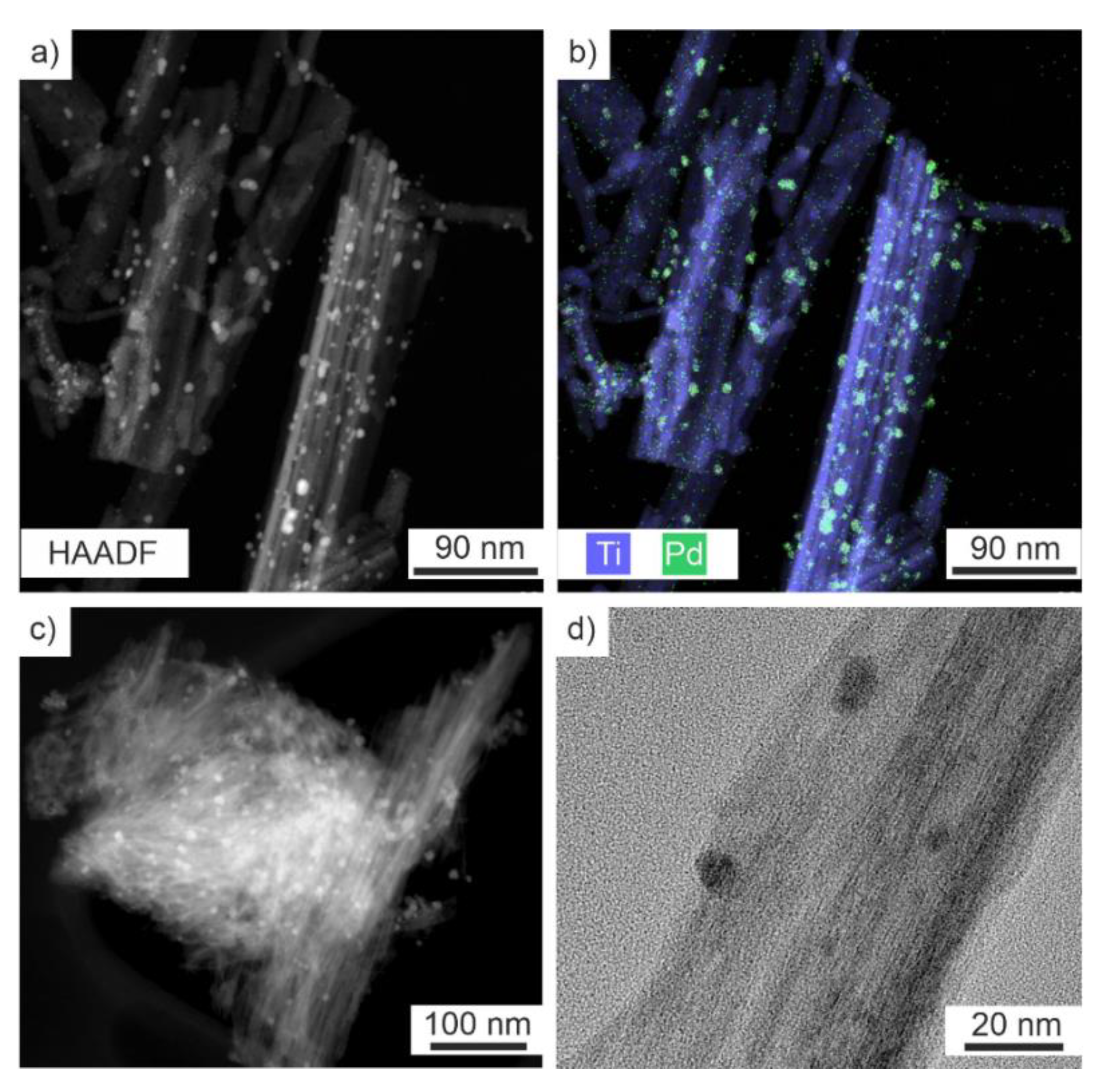
| Material | Specific Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| TNTs (long) 1 | 214 | 0.237 |
| Pt/TNTs (long) 1 | 201 | 0.640 |
| Pd/TNTs (long) 1 | 193 | 0.467 |
| TNTs (short) 2 | 387 | 0.695 |
| Pt/TNTs (short) 2 | 379 | 0.941 |
| Pd/TNTs (short) 2 | 369 | 0.830 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warmuth, L.; Nails, G.; Casapu, M.; Wang, S.; Behrens, S.; Grunwaldt, J.-D.; Feldmann, C. Catalytic CO Oxidation and H2O2 Direct Synthesis over Pd and Pt-Impregnated Titania Nanotubes. Catalysts 2021, 11, 949. https://doi.org/10.3390/catal11080949
Warmuth L, Nails G, Casapu M, Wang S, Behrens S, Grunwaldt J-D, Feldmann C. Catalytic CO Oxidation and H2O2 Direct Synthesis over Pd and Pt-Impregnated Titania Nanotubes. Catalysts. 2021; 11(8):949. https://doi.org/10.3390/catal11080949
Chicago/Turabian StyleWarmuth, Lucas, Gülperi Nails, Maria Casapu, Sheng Wang, Silke Behrens, Jan-Dierk Grunwaldt, and Claus Feldmann. 2021. "Catalytic CO Oxidation and H2O2 Direct Synthesis over Pd and Pt-Impregnated Titania Nanotubes" Catalysts 11, no. 8: 949. https://doi.org/10.3390/catal11080949
APA StyleWarmuth, L., Nails, G., Casapu, M., Wang, S., Behrens, S., Grunwaldt, J.-D., & Feldmann, C. (2021). Catalytic CO Oxidation and H2O2 Direct Synthesis over Pd and Pt-Impregnated Titania Nanotubes. Catalysts, 11(8), 949. https://doi.org/10.3390/catal11080949








