Abstract
The use of water splitting has been investigated as a good alternate for storing electrical energy. While the general interest in developing non-toxic, high-performance, and economically feasible catalysts for oxygen evolution reaction (OER) is noteworthy, there is also significant interest in water splitting research. Recently, perovskite-type oxides have performed as an alternative to non-precious metal catalysts and can act as a new class of effective catalysts in water splitting systems. Herein, a perovskite-structured FeTiO3 was prepared via a facile one-step solvothermal method using ionic liquid as templates. The results of structural and morphological studies have supported the formation of FeTiO3 perovskite. Furthermore, FeTiO3 perovskite demonstrated OER activity with a lower onset potential of 1.45 V vs. RHE and Tafel slope value of 0.133 V.dec−1 at 1 M KOH solution using mercury/mercurous oxide (Hg/HgO) were used as working electrodes.
1. Introduction
Hydrogen has long been considered as an alternative renewable energy carrier to replace fossil-fuel-based energy, with its zero-emission energy source. The electrocatalytic water splitting, which includes both an oxygen evolution reaction (OER) and a hydrogen evolution reaction (HER), is regarded as a significant opportunity for the zero-carbon hydrogen synthesis from the water [1]. CO2 radioactive forcing from fossil fuel ignition exalted thermal discharge on large proportion due to its longer lifetime [2]. Considering environmental protection and in order to safeguard the depletion of non-renewable energy resources, interest in clean alternative energies has been intensively focused. In recent years, the carbon-neutral energy system received impressive advancements in the field of renewable energy due to its clean repository. Attainment in the production of hydrogen is directly proportional to the efficiency of hydrogen produced and the use of hydrogen in energy conversion devices [3]. Comparably, electrochemical water electrolysis delivers an eco-friendly method to produce hydrogen as one of the energy transporters in the form of a chemical bond between two hydrogens and oxygen [4]. An alkaline condition of water splitting results in large quantities of pure hydrogen production, where it can be converted into storable combustible fuel in metal-air batteries and H2/O2 fuel cells to produce energy [5,6].
The development of highly operative, noble metal-free and reliable OER catalyst is a challenging one. A potent electrocatalyst relies on an increase in the reaction rate and control of the electron transfer kinetic rate. So far, only a few potential catalysts such as Pt, IrO2 and RuO2 were identified as efficient functional materials for OER activity catalyst [7,8]. However, the inadequateness of noble metals and the high cost of the main blocks affect practical development. Thus, it is important to develop an alternate electrocatalyst with a lower cost, higher abundance and with greater efficiency [9]. Exceptional interest in nanostructured spinel oxides has been found to be a favorable electrode material for various electrochemical applications due to their superior electrochemical activity and chemical stability compared to the monometal oxide counterparts [10]. A transition metal ferrite, with a general formula of MFe2O4 (M = Co, Ni, Cu, etc.), constitutes an important class of spinel oxides, due to the fascinating wide range of desirable surfaces, dimensions, and chemical compositions, which can produce versatile catalytic, electrical, optical, and mechanical properties for the electrocatalytic applications. The application of electrocatalysts is still faced with several limitations, among which the ferrites nanoparticles (NPs) tend to aggregate in the electrochemical redox process, which decreases the number of active sites for electrocatalysis and thereby causes an inadequate use of active materials. As a result, the overall performance of the electrocatalysts and their long-term structure stability is diminished [11].
Recently, perovskite-type oxides have obtained the significant consideration due to their solid crystal structure and have high electronic conductivity and catalytic activity. Perovskite materials are arranged in the matrix of crystalline structure with ABO3, where A is a large cation usually an alkali or alkaline earth metal and B is a small cation of transition metals [12]. Titanium dioxide (TiO2) has received great interest due to its superior chemical stability, nontoxicity, low cost and abundant nature. These properties also tend to produce conceivable material for photocatalytic degradation of organic dyes, solar cells, drug carriers, sodium-ion capacitors, sensors [13,14,15,16,17]. However, TiO2 is inactive for OER due to lack of oxidation species, but it can be support for ilmenite type mixed oxides. The MTiO3 nanomaterials can be synthesized via thermal treatment, solvothermal, chemical precipitation methods using various templates such as zeolites, ionic liquids. Obviously, ionic liquids (ILs) are preferred in the field of green chemistry due to their distinctive features such as recyclability, substantial temperature, considerable thermal stability and superior ionic conductivity. The recent advancement in ILs is desirable for the synthesis of nanomaterials with unique morphology via the solvothermal method [18]. Iron-based titanium oxides with formulating Z-scheme heterostructures of anodes have received great importance due to their strong electronic, magnetic and catalytic behaviors [19,20]. Moreover, the FeTiO3 group of the crystalline structure is similar to corundum (Al2O3), which has trigonal symmetry and an R3 ilmenite crystal structure. This structure is distinguished by its ferroelectric properties, results from the degree of distortion experienced by octahedral clusters and the asymmetry between both the cations A and B along with the c axis. This will lead to a high active surface area of the material and superior electrochemical oxidation of water. In this present study, FeTiO3 perovskite was formulated via a simple solvothermal reaction, the physical and chemical characteristics of the obtained samples were tested using analytical techniques. Their electrocatalytic activity was also calculated towards OER in an alkaline media, the response of TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 perovskite measured in order to attain ideal electrocatalytic activity.
2. Results and Discussion
Figure 1 illustrates the powder XRD patterns of TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 perovskite. The planes of TiO2 is clearly seen at a 2θ of 35.3°, 38.6°, 40.3°, 53.0°, 63.2°, 70.2°, and 76.0 corresponding to (1 0 1)-TiO, (2 0 0)-Ti, (1 1 1)-TiO, (2 1 1)-Ti, (3 1 0)-Ti, (3 1 1)-Ti and (2 0 2)-TiO planes, respectively, as shown in Figure 1a [15]. Figure 1b shows the major XRD peaks presented at 24.1° (0 1 2), 33.1° (1 0 4), 35.5° (1 1 0), 40.7° (1 1 3), 49.2° (0 2 4), 53.8° (1 1 6), 57.4° (1 3 1), 62.2° (2 1 4) and 63.8° (3 0 0) of α-Fe2O3 NPs (JCPDS data Card No.: 00-001-1053). Furthermore, the XRD peaks of FeTiO3 perovskite are obtained as displayed in Figure 1c. In the perovskite, the α-Fe2O3 NPs peak of 24.1° shifted toward 24.4° (0 0 2), and the intensity of 40.7° and 62.2° peaks were decreased due to composite of TiO2 with α-Fe2O3 (JCPDS data Card No.: 00-047-0465) [21].
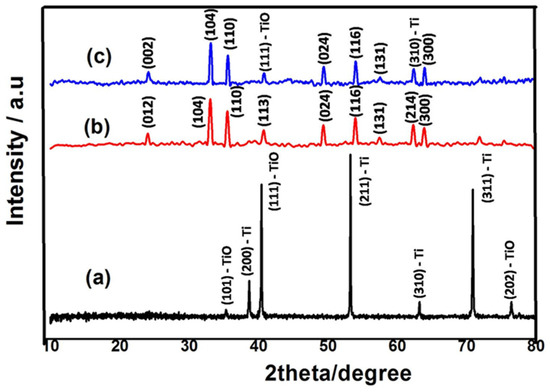
Figure 1.
XRD patterns of TiO2 nanorod (a), α-Fe2O3 NPs (b), and FeTiO3 (c).
In order to investigate compositional and oxidation information, the XPS analysis was carried out for the FeTiO3. Here, Figure 2 represents the high-resolution spectra of Ti, Fe and O for FeTiO3. The doublet peaks at 458.6 eV (Ti 2p3/2) and 464.4 eV (Ti 2p1/2) as shown in Figure 2a [22]. The additional peaks of Ti 2p1/2 at binding energies of 460.2, 461.3 and 462.4 eV are corresponding to Ti3+ in FeTiO3. This designates the presence of TiO2 in the film. The high-resolution spectrum of Fe is shown in Figure 2b. The peaks are appeared at 710 and 715 eV for Fe 2p3/2 and Fe 2p1/2, respectively. The binding energy at 714.5 and 711.5 eV proceeded with the formation of Fe2+, and the peaks appeared at 715.2 eV for Fe3+ in FeTiO3 perovskite. Hence, the result confirmed the formation of Fe2O3 in the perovskite. Figure 2c shows the high-resolution spectrum of O 1S. The deconvolution peaks are presented at 530.2, 531.5 and 533.5 eV correspond to Ti–O–Ti, Fe–O–Ti and O–C, which further confirmed the formation of FeTiO3 perovskite.
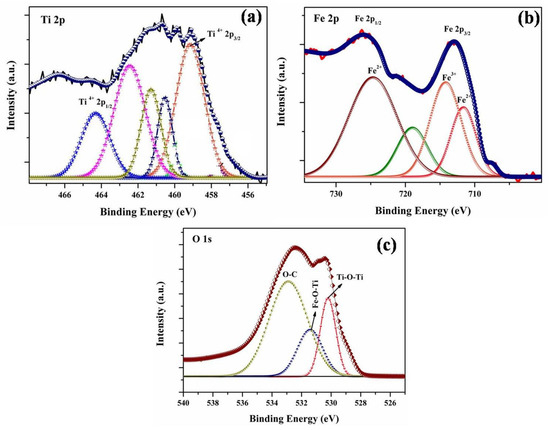
Figure 2.
Represents high resolution XPS spectra of (a) Ti2p, (b) Fe2p and (c) O1s of FeTiO3 perovskite.
Figure 3 shows the surface morphology and microstructure analysis of the TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 perovskite. Figure 3a reveals that TiO2 is formed in the rod shape, Fe2O3 is formed as microspheres structure (Figure 3b), and FeTiO3 showed a mixed structure of rods and spherical particles (Figure 3c). The energy dispersive X-ray analysis (EDAX) spectra for TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 show the presence of Ti, Fe and O Figure 3a1–c1. Figure 3a1 confirmed the presence of Ti and O in TiO2 nanorods. Further, the EDAX spectrum of α-Fe2O3 NPs showed the presence of Fe and O elements (Figure 3b1). Moreover, Fe, Ti and O elements are observed in the EDAX spectrum of FeTiO3 (Figure 3c1), which confirmed the formation of NPs.
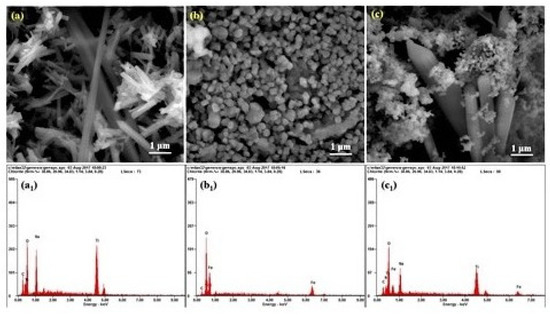
Figure 3.
Shows FE-SEM images of (a) TiO2 nanorod, (b) α- Fe2O3 NPs, (c) FeTiO3 NPs, and (a1–c1) their corresponding EDAX spectrum.
The TEM image of TiO2 nanorod is revealed in Figure 4a and the subsequently selected area electron diffraction (SAED) pattern is shown in Figure 4a1. The SAED pattern represents a highly crystalline TiO2 nanorod with 10 nm thickness and few micrometers in length. For α-Fe2O3 NPs, a fine surface with ~100 nm size as shown in Figure 4b and polycrystalline directly envisioned in the SAED pattern (Figure 4b1), while in FeTiO3 a mixed structure of rod and spherical with aggregate morphology is observed as shown in Figure 4c. Furthermore, the SAED pattern confirmed the formation of polycrystalline (Figure 4c1).
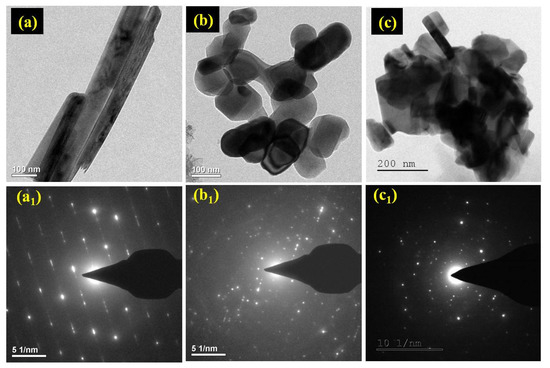
Figure 4.
Shows TEM images of (a) TiO2 nanorod, (b) α- Fe2O3 NPs, (c) FeTiO3 perovskite, and (a1–c1) their corresponding SAED pattern.
The OER activities of TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 perovskite were investigated using linear sweep voltammetry (LSV) in a three-electrode system as shown in Figure 5. The FeTiO3 perovskite nanoparticles coated on Toray carbon sheet, platinum (Pt) rod, and mercury/mercurous oxide (Hg/HgO) were used as working, counter, and reference electrodes, respectively. In all the measurements, the potential of Hg/HgO was converted into reversible hydrogen electrode (RHE) using the formula [23],
E(RHE) = E0(Hg/HgO) + E(observed)+ 0.059pH V
η = E(RHE) − 1.23 V
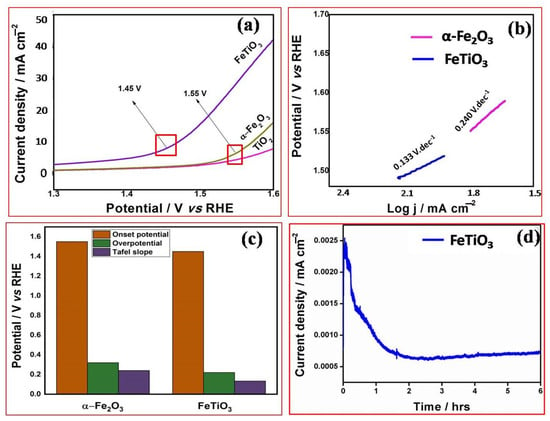
Figure 5.
Shows (a) LSV of TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 perovskite, (b) Tafel slop values of α-Fe2O3 NPs and FeTiO3, (c) comparison plot TiO2, α-Fe2O3 and FeTiO3 perovskite and (d) chronoamperometry study FeTiO3 perovskite.
Noticeably, FeTiO3 perovskite exhibits superior OER performance than TiO2 nanorod and α-Fe2O3 NPs (Figure 5a). The FeTiO3 shows the lowest onset potential of 1.45 V and the current density of 30 mA/cm2 (Figure 5a). Furthermore, the overpotential (η) of FeTiO3 is 0.22 V, which is very low potential compared to α-Fe2O3 NPs (1.55 V), and TiO2 nanorod is inactive. FeTiO3 also displays less Tafel slope value of 0.133 V·dec–1 compare to α-Fe2O3 NPs (0.240 V dec−1) (Figure 5b). Besides, TiO2 nanorod and α-Fe2O3 NPs as supports have less activity as shown in Figure 5a. The onset potential of 1.55 V is gradually increased and no further activity is observed for α-Fe2O3 NPs and TiO2 when using the single-channel electrocatalysts, as shown in Figure 5a,b. It is clear from this finding that the formation is inherently more efficient at improving the electron transport and improving the interaction between Fe and TiO2 by the higher electronic conductivity of Ti and surface area [24]. The better performance of FeTiO3 is due to Ti, which can be supported to avoid the further oxidation of Fe during the OER process. Figure 5c represents the comparison plot of onset potential, over potential and Tafel slope value for α-Fe2O3 NPs and FeTiO3. Moreover, the stability test was measured by the chronoamperometry technique at 1.5 V. FeTiO3 shows high stability for 6 h which is comparable for IrO2 as shown in Figure 5d [24,25]. After 6 h, the catalyst current was slightly declined due to the disturbance of catalyst surface morphology. The evolution of O2 was disturbed the interaction between nanoparticles and nanorods. Table 1 shows a comparison of OER activity for various catalysts in an alkaline medium.

Table 1.
Comparison of onset potential of various catalysts for OER.
3. Materials and Methods
3.1. Materials
Ferric chloride hexahydrate (FeCl3·6H2O), ferrous sulfate (FeSO4·7H2O), titanium (IV) isopropoxide Ti(OCH(CH3)2)4 (TIP) were supplied by Sigma-Aldrich Bengaluru, Karnataka 560099, India, 1-butyl-3-methylimidazolium bromide (C8H15BrN2) (IL) was purchased from TCI Chemicals (Chennai, Tamil Nadu 600045, India) and, ethylene glycol (HOCH2CH2OH) (EG) and sodium hydroxide (NaOH) were obtained from Merck (Mumbai- 400079, India). All of the chemicals used were of the highest analytical quality, and no further purification was required.
3.2. Preparation of TiO2 Nanorod
The pure TiO2 was synthesized by an effortless solvothermal process. In brief, 0.1 M of Ti (OCH(CH3)2)4 as a source of TiO2 was first dissolved in 50 mL of ethylene glycol and 0.015 M of C8H15BrN2 was mixed with the above solution under continuous stirring for 2 h. Then, 1.0 M/L NaOH solution was added dropwise to adjust pH to 12.0. The solution was transferred into 100 mL volume of a Teflon-lined stainless-steel autoclave and then it was heated at 180 °C for 24 h. Subsequently cool down to room temperature, then the resultant suspensions were washed several times with water and ethanol to remove the unreacted species, and then the final product was dried at 100 °C for 2 h. Finally, TiO2 nanorods were obtained by calcined in air at 850 °C for 5 h.
3.3. Synthesis of FeTiO3 Perovskite
α-Fe2O3 NPs and FeTiO3 perovskite were prepared by facile solvothermal method. The detailed process is explained as follows: 0.050 M of FeCl3·6H2O and 0.050 M of FeSO4·7H2O (α-Fe2O3 sources) were dissolved in ethylene glycol under magnetic stirring for 30 min at room temperature, additionally 0.1 M of Ti (OCH(CH3)2)4 was further added to form the homogenous solution. Thereafter, 0.015 M of C8H15BrN2 was added to the above mixture under vigorous stirring. The pH value was adjusted to 12 by using NaOH. Subsequently, the mixture was transferred into a Teflon-lined stainless-steel autoclave and heated in an electric oven at 180 °C for 24 h. The resultant suspensions were washed several times with water and ethanol to remove the unreacted species in the final product and then dried at 60 °C for 12 h. Finally, FeTiO3 perovskite was obtained by calcination in air at 850 °C for 5 h as shown in Scheme 1. The same producer was used for the synthesis of α-Fe2O3 NPs without adding titanium sources.
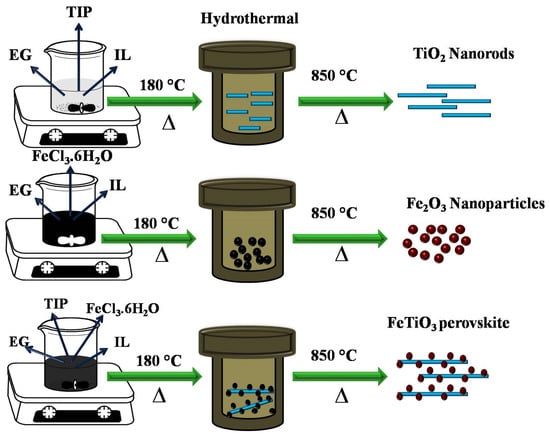
Scheme 1.
Schematic representation of TiO2 nanorod, α-Fe2O3 NPs and FeTiO3 synthesis via a one-step solvothermal method.
3.4. Electrochemical Studies
The electrochemical studies were investigated using a Bio-Logic instrument (Seyssinet-Pariset, France) with the three electrode systems for OER in 1 M KOH. Linear sweep voltammogram (LSV) was observed at 10 mV s−1 to reduce the capacitive current, which is directly proportional to the scan rate. The stability test was measured by the chronoamperometry technique at 1.5 V. FeTiO3 perovskite nanoparticles coated on a Toray carbon sheet, platinum (Pt) rod, and mercury/mercurous oxide (Hg/HgO) were used as working, counter, and reference electrodes, respectively.
3.5. Characterization
The X-ray diffraction (XRD) was measured for all the samples using Cu-Kα radiation with the wavelength of 1.54060 Å by PAN analytical (Malvern Panalytical, Malvern, England) (XPERT-PRO) diffractometer in the 2 theta range from 10° to 80°. The morphology of prepared samples was recorded using field emission scanning electron microscopy (FE-SEM: Model Hitachi S–4500, Carl Zeiss NTS Ltd. Jena, Germany) equipped with the energy-dispersive X-ray spectroscopy (EDAX). The transmission electron microscopy (TEM) and selected area electron diffraction (SAED) were carried out to investigate the morphology and crystalline nature of the samples (Tecnai Instruments). The X-ray photoelectron spectroscopy (XPS) was carried out using the XPS instrument (Carl Zeiss). The spectra were at pressure using an ultra-high vacuum with Al Kα excitation wavelength at 250 W. The electrochemical studies were investigated by using a Bio-Logic instrument with the three electrode systems for OER in 1 M KOH.
4. Conclusions
FeTiO3 perovskite was developed using a one-step solvothermal method. The proposed method facilitated in reducing the onset potential and increased the current density for OER in an alkaline solution. The formation of FeTiO3 was confirmed using XRD, XPS and TEM studies. FeTiO3 demonstrated a high OER activity compared to the bare TiO2 nanorod and α-Fe2O3 NPs. The onset potential of FeTiO3 was 1.45 V with a current density of 30 mA/cm2, which were comparable to the benchmark catalyst of IrO2. Furthermore, the catalyst was highly stable for 6 h in 1M KOH solution. Therefore, the prepared FeTiO3 perovskite could be considered as an effective catalyst for water electrolyzer technology in the future.
Author Contributions
P.K. and M.P.K. contributed to methodology, investigation, writing—original draft preparation; R.V.M. contributed to validation, data curation, formal analysis; U.W.H. contributed to data curation, formal analysis; M.S. contributed to software, data curation; R.V. and M.R.K. contributed to formal analysis, data curation; J.R.R. contributed to formal analysis; S.G.P. contributed to formal analysis, validation; G.M. conceptualized the overall idea of this research, supervision, writing—review and editing, visualization, data curation. Finally, G.M. reviewed, edited and submitted the manuscript in the final form. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
The author G.M. thanks the Chancellor, President, and Vice Chancellor, Sathyabama Institute of Science and Technology, Chennai for the support and encouragement. The author J.R.R. thanks the Researchers Supporting Project Number (RSP-2021/354), King Saud University, Riyadh, Saudi Arabia for the financial support. The author S.G.Peera also thank research support from the National Research Foundation of Korea (NRF) funded by the Korean government, Ministry of Science and ICT (MSIT) (No. 2021R1F1A1046648), Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, A.; Xie, Y.; Ma, H.; Tian, C.; Gu, Y.; Yan, H.; Zhang, X.; Yang, G.; Fu, H. Integrating the active OER and HER components as the heterostructures for the efficient overall water splitting. Nano Energy 2018, 44, 353–363. [Google Scholar] [CrossRef]
- Zhang, X.; Caldeira, K. Time scales and ratios of climate forcing due to thermal versus carbon dioxide emissions from fossil fuels. Geophys. Res. Lett. 2015, 42, 4548–4555. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, M.R.; Dinh, C.T.; Béland, F.; Do, T.O. Nanocomposite heterostructures as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Lu, M.; Yang, S.; Liu, Z.; Lee, J.Y. The origin of catalytic activity of nickel phosphate for oxygen evolution in alkaline solution and its further enhancement by iron substitution. ChemElectroChem 2016, 3, 615–621. [Google Scholar] [CrossRef]
- Zaffora, A.; Santamaria, M.; Di Franco, F.; Habazaki, H.; Di Quarto, F. Photoelectrochemical evidence of inhomogeneous composition at nm length scale of anodic films on valve metals alloys. Electrochim. Acta. 2016, 201, 333–339. [Google Scholar] [CrossRef]
- Koza, J.A.; He, Z.; Miller, A.S.; Switze, J.A. Electrodeposition of crystalline Co3O4-A Catalyst for the Oxygen Evolution Reaction. Chem. Mater. 2012, 24, 3567–3573. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Dutta, A.; Pradhan, N. Developments of metal phosphides as efficient OER precatalysts. J. Phys. Chem. Lett. 2017, 8, 144–152. [Google Scholar] [CrossRef]
- Kaleeswarran, P.; Sriram, B.; Wang, S.F.; Baby, J.N.; Arumugam, A.; Bilgrami, A.L.; Hashsham, S.A.; Sayegh, F.A.; Liu, C.J. Electrochemical detection of antipsychotic drug in water samples based on nano/sub-microrod-like CuBi2−xInxO4electrocatalysts. Microchem. J. 2021, 163, 105886. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Liu, X.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Horn, Y.S. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Murugadoss, G.; Jayavel, R.; Kumar, M.R. Systematic investigation of structural and morphological studies on doped TiO2 nanoparticles for solar cell applications. Superlattices Microstruct. 2014, 76, 349–361. [Google Scholar] [CrossRef]
- Murugadoss, G.; Thangamuthu, R.; Vijayaraghavan, S.; Kanda, H.; Ito, S. Caesium-Methyl Ammonium Mixed-Cation Lead Iodide Perovskite Crystals: Analysis and Application for Perovskite Solar Cells. Electrochim. Acta 2017, 257, 267–280. [Google Scholar] [CrossRef]
- Murugadoss, G.; Kanda, H.; Tanaka, S.; Nishino, H.; Ito, S.; Imahoric, H.; Umeyama, T. An efficient electron transport material of tin oxide for planar structure perovskite solar cells. J. Power Sources 2016, 307, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Manibalan, G.; Govindaraj, Y.; Yesuraj, J.; Kuppusami, P.; Murugadoss, G.; Murugavel, R.; Kumar, M.R. Facile synthesis of NiO@Ni(OH)2-α-MoO3 nanocomposite for enhanced solid-state symmetric supercapacitor application. J. Colloid Interface Sci. 2021, 585, 505–518. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Kumar, R.M.; Jayavel, R. Facile synthesis of heterostructure CeO2-TiO2 nanocomposites for enhanced electrochemical sensor and solar cell applications. J. Alloy. Compd. 2019, 773, 449–461. [Google Scholar] [CrossRef]
- Zwara, J.; Paszkiewicz-Gawron, M.; Łuczak, J.; Pancielejko, A.; Lisowski, W.; Trykowski, G.; Zaleska-Medynska, A.; Grabowsk, E. The effect of imidazolium ionic liquid on the morphology of Pt nanoparticles deposited on the surface of SrTiO3 and photoactivity of Pt-SrTiO3 composite in the H2 generation reaction. Int. J. Hydrogen Energy 2019, 44, 26308–26321. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Chen, L.; Pan, D.; Guo, Z.; Xia, S. Porous TiO2–FeTiO3@Carbon nanocomposites as anode for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 858, 157635. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R. FeTiO3nanohexagons based electrochemical sensor for the detection of dopamine in presence of uric acid. Mater. Chem. Phys. 2019, 233, 319–328. [Google Scholar] [CrossRef]
- Gou, H.P.; Zhang, G.H.; Chou, K.C. Influence of Pre-oxidation on Carbothermic Reduction Process of Ilmenite Concentrate. ISIJ Int. 2015, 55, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Praveen Kumar, M.; Jagannathan, R.; Ravichandran, S. Photoelectrochemical system for unassisted high-efficiency water-splitting reactions using N-doped TiO2 Nanotubes. Energy Fuels 2020, 34, 9030–9036. [Google Scholar] [CrossRef]
- Praveen Kumar, M.; Murugesan, P.; Vivek, S.; Ravichandran, S. NiWO3 Nanoparticles Grown on Graphitic Carbon Nitride (g-C3N4) Supported Toray Carbon as an Efficient Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Part. Part. Syst. Charact. 2017, 34, 1700043–1700052. [Google Scholar] [CrossRef]
- Badreldin, A.; Abusrafa, A.E.; Abdel-Wahab, A. Oxygen-deficient perovskites for oxygen evolution reaction in alkaline media: A review. Emergent Mater. 2020, 3, 567–590. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 1–7. [Google Scholar]
- Han, T.; Chen, Y.; Tian, G.; Wang, J.Q.; Ren, Z.; Zhou, W.; Fu, H. Hierarchical FeTiO3-TiO2 hollow spheres for efficient simulated sunlight-driven water oxidation. Nanoscale 2015, 7, 15924–15934. [Google Scholar] [CrossRef] [PubMed]
- Luque-Centeno, J.M.; Martínez-Huerta, M.V.; Sebastián, D.; Pardo, J.I.; Lázaro, M.J. CoTiO3/NrGO nanocomposites for oxygen evolution and oxygen reduction reactions: Synthesis and electrocatalytic performance. Electrochem. Acta 2020, 331, 135396. [Google Scholar] [CrossRef]
- Vineesh, T.V.; Praveen Kumar, M.; Takahashi, C.; Kalita, G.; Alwarappan, S.; Pattanayak, D.K.; Narayanan, T.N. Bifunctional Electrocatalytic Activity of Boron-Doped Graphene Derived from Boron Carbide. Adv. Energy Mater. 2015, 5, 1500658–1500665. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).