NH3-Selective Catalytic Reduction of NOx to N2 over Ceria Supported WOx Based Catalysts: Influence of Tungsten Content
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
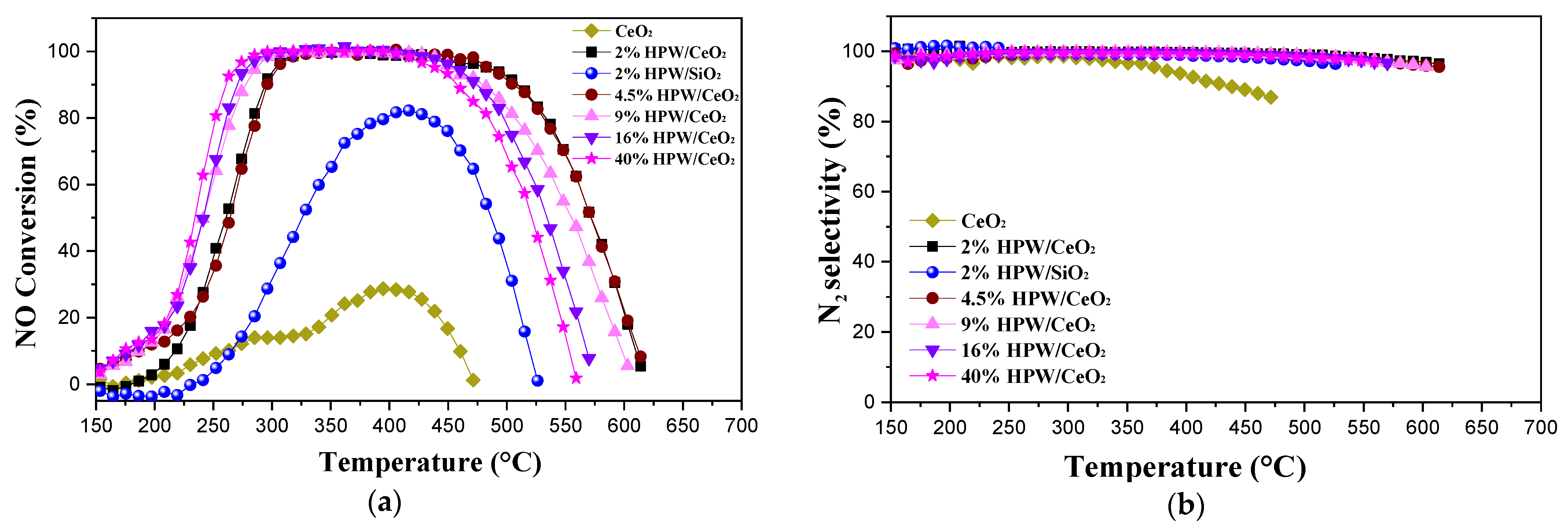
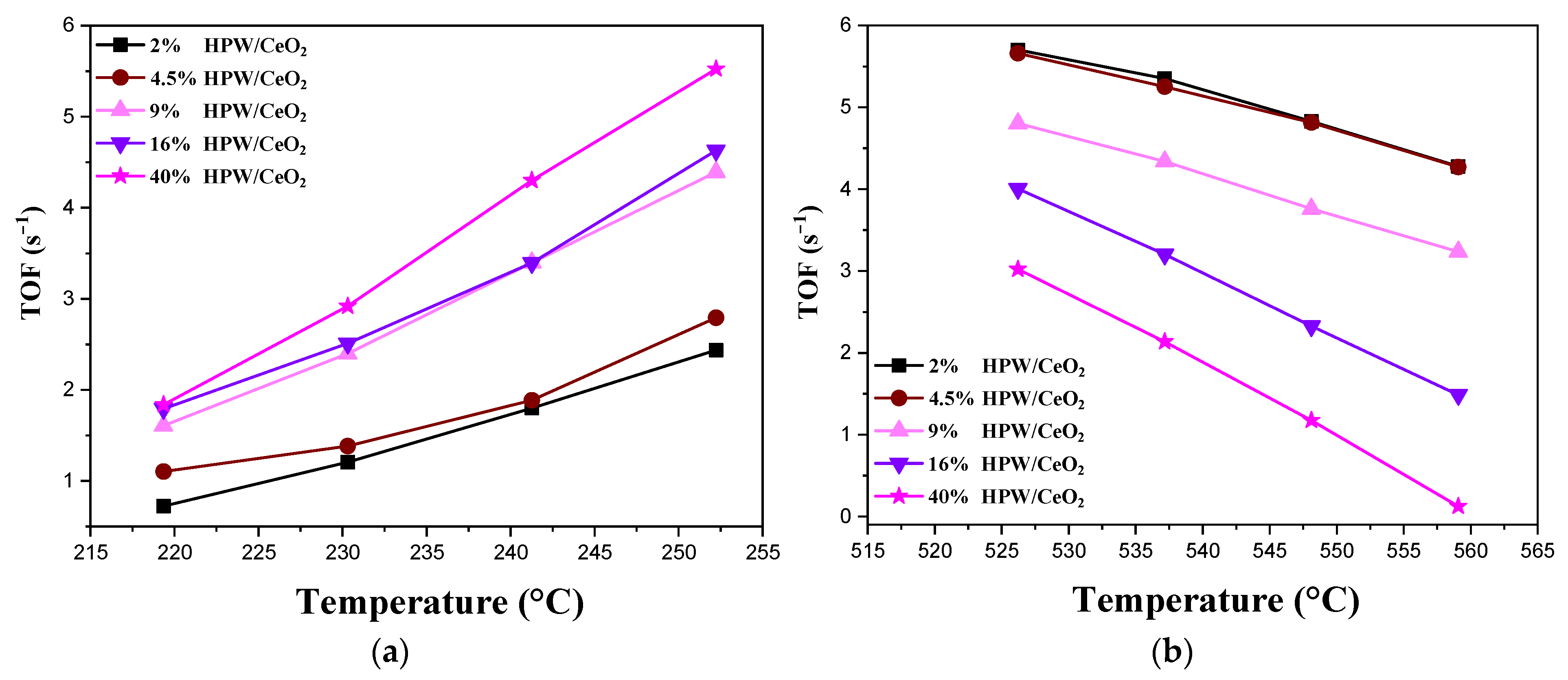
2.2. Catalytic Activity
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalysts Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franco, M.C.; Rocha, R.C.; Costa, M. Characteristics of NH3/H2/air flames in a combustor fired by a swirl and bluff-body stabilized burner. Proc. Combust. Inst. 2021, 38, 5129–5138. [Google Scholar] [CrossRef]
- Ariemma, G.B.; Sabia, P.; Sorrentino, G.; Bozza, P.; De Joannon, M.; Ragucci, R. Influence of water addition on MILD ammonia combustion performances and emissions. Proc. Combust. Inst. 2021, 38, 5147–5154. [Google Scholar] [CrossRef]
- Funke, H.H.; Beckmann, N.; Abanteriba, S. An overview on dry low NO x micromix combustor development for hydrogen-rich gas turbine applications. Int. J. Hydrogen Energy 2019, 44, 6978–6990. [Google Scholar] [CrossRef]
- Sorrentino, G.; Sabia, P.; Bozza, P.; Joannon, M. De Low-NOx conversion of pure ammonia in a cyclonic burner under locally diluted and preheated conditions. Appl. Energy 2019, 254, 113676. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N. Towards the development of an efficient low-NOx ammonia combustor for a micro gas turbine. Proc. Combust. Inst. 2018, 37, 4597–4606. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Wei, Y.; Zhang, R.; Royer, S. Morphology-Oriented ZrO2-Supported vanadium oxide for the NH3-SCR process importance of structural and textural properties. ACS Appl. Mater. Interfaces 2019, 11, 22240–22254. [Google Scholar] [CrossRef]
- Shimizu, K.; Satsuma, A.; Hattori, T. Catalytic performance of Ag-Al2O3 catalyst for the selective catalytic reduction of NO by higher hydrocarbons. Appl. Catal. B Environ. 2000, 25, 239–247. [Google Scholar] [CrossRef]
- Valanidou, L.; Theologides, C.; Zorpas, A.A.; Savva, P.G.; Costa, C.N. A novel highly selective and stable Ag MgO-CeO2-Al2O3 catalyst for the low-temperature ethanol-SCR of NO. Appl. Catal. B Environ. 2011, 107, 164–176. [Google Scholar] [CrossRef]
- Popovych, N.; Kirienko, P.; Soloviev, S.; Orlyk, S. Selective catalytic reduction of NOx by C2H5OH over Ag/Al2O3/cordierite: Effect of the surface concentration of silver. Catal. Today 2012, 191, 38–41. [Google Scholar] [CrossRef]
- Hamada, H.; Haneda, M. A review of selective catalytic reduction of nitrogen oxides with hydrogen and carbon monoxide. Appl. Catal. A Gen. 2012, 421, 1–13. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Ciardelli, C.; Nova, I.; Tronconi, E.; Chatterjee, D.; Bandl-konrad, B.; Weibel, M.; Krutzsch, B. Reactivity of NO/NO2–NH3 SCR system for diesel exhaust aftertreatment: Identification of the reaction network as a function of temperature and NO2 feed content. Appl. Catal. B Environ. 2007, 70, 80–90. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Qiu, W.; Chen, Y.; Cheng, J.; Sun, Y.; Bai, G. Effect of organic assistant on the performance of ceria-based catalysts for the selective catalytic. Catalyst 2019, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Jin, Q.; Tao, X.; Pan, Y.; Gu, S. Novel W-Zr-Ox/TiO2 catalyst for selective catalytic reduction of NO by NH3 at high temperature. Catal. Today 2019, 358, 254–262. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. Influence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. A Gen. 2015, 497, 160–166. [Google Scholar] [CrossRef]
- Li, C.; Shen, M.; Wang, J.; Wang, J.; Yanping, Z. New Insights into the Role of WO3 in Improved Activity and Ammonium Bisulfate Resistance for NO Reduction with NH3 over V−W-Ce-Ti Catalyst. Ind. Eng. Chem. Res. 2018, 57, 8424–8435. [Google Scholar] [CrossRef]
- Rui, Y.; Zhenchao, Z.; Shi, C.; Zhang, W. Insight into the synergic effect of Fe-SSZ-13 zeolite and FeMnTiZrOx catalyst with enhanced reactivity in NH -SCR of NOx. J. Phys. Chem. C 2019, 123, 2216–2227. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, X.; Li, Y.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Effect of W on the acidity and redox performance of the Cu0.02Fe0.2WaTiOx (a = 0.01, 0.02, 0.03) catalysts for NH3-SCR of NO. Appl. Catal. B Environ. 2019, 248, 226–238. [Google Scholar]
- Wang, X.; Li, X.; Zhao, Q.; Sun, W.; Tade, M.; Liu, S. Improved activity of W-modified MnOx-TiO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2016, 288, 216–222. [Google Scholar] [CrossRef]
- Trovarelli, A. Structural and oxygen storage/release properties of CeO2-based solid solutions. J. Crit. Discuss. Curr. Lit. 1999, 20, 263–284. [Google Scholar] [CrossRef]
- Liang, C.; Junhua, L.; Maofa, G. DRIFT Study on Cerium-Tungsten/Titiania catalyst for selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar]
- Geng, Y.; Xiong, S.; Li, B.; Liao, Y.; Xiao, X.; Yang, S. H3PW12O40 grafted on CeO2: A High-performance catalyst for the selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 856–866. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, S.H.; Nam, K.B.; Hong, S.C. A study on the structure of tungsten by the addition of ceria: Effect of monomeric structure over W/Ce/TiO2 catalyst on the SCR reaction. Appl. Srface Sci. 2020, 507, 145064. [Google Scholar] [CrossRef]
- Narasimharao, K.; Brown, D.R.; Lee, A.F.; Newman, A.D.; Siril, P.F.; Tavener, S.J.; Wilson, K. Structure-activity relations in Cs-doped heteropolyacid catalysts for biodiesel production. J. Catal. 2007, 248, 226–234. [Google Scholar] [CrossRef]
- Weng, X.; Dai, X.; Zeng, Q.; Liu, Y.; Wu, Z. DRIFT studies on promotion mechanism of H3PW12O40 in selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2016, 461, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, Q.; Ma, Y.; Liu, Q.; Ning, P.; Liu, X. Mechanism-dependent on the different CeO2 supports of phosphotungstic acid modification CeO2 catalysts for the selective catalytic reduction of NO with NH3. J. Taiwan Inst. Chem. Eng. 2017, 71, 277–284. [Google Scholar] [CrossRef]
- García-fernández, S.; Gandarias, I.; Requies, J.; Güemez, M.B.; Bennici, S.; Auroux, A.; Arias, P.L. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1, 3-propanediol. J. Catal. 2015, 323, 65–75. [Google Scholar] [CrossRef]
- Ramasamy, V.; Mohana, V.; Rajendran, V. Characterization of Ca doped CeO2 quantum dots and their applications in photocatalytic degradation. OpenNano 2018, 3, 38–47. [Google Scholar] [CrossRef]
- Kannan, S.K.; Sundrarajan, M.; Green, A. A green approach for the synthesis of a cerium oxide nanoparticle: Characterization and antibacterial activity. Int. J. Nanosci. 2014, 13, 1450018. [Google Scholar] [CrossRef]
- Kim, D.S.; Ostromecki, M.; Wachs, I.E.; Kohler, S.D.; Ekerdt, J.G. Preparation and characterization of WO3/SiO2 catalysts. Catal. Lett. 1995, 33, 209–215. [Google Scholar] [CrossRef]
- Javidi, J.; Esmaeilpour, M.; Rahiminezhad, Z.; Dodeji, F.N. Synthesis and characterization of H3PW12O40 and H3PMo12O40 nanoparticles by a simple method. J. Clust. Sci. 2014, 25, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, Y.; Xu, F.; Lu, D.; Xu, B.; Hu, Z.; Chen, Y. A study on the surface properties of ceria-supported tungsten and copper oxides. J. Phys. Chem. B 2000, 104, 78–85. [Google Scholar] [CrossRef]
- Marcì, G.; García-lópez, E.I.; Rita, F.; Liotta, L.F.; Palmisano, L. General Enhanced (photo) catalytic activity of Wells-Dawson (H6P2W18O62) in comparison to Keggin (H3PW12O40) heteropolyacids for 2-propanol dehydration in gas-solid regime. Appl. Catal. A Gen. 2016, 528, 113–122. [Google Scholar] [CrossRef]
- Castro, D.; Sigoli, F.A.; Mazali, I.O. Reversible Oxygen Vacancy Generation on Pure CeO2 Nanorods Evaluated by in Situ Raman Spectroscopy. J. Phys. Chem. C 2017, 121, 12928–12935. [Google Scholar]
- Farias, M.D.D.; Nguyen-thanh, D.; Fraga, M.A. Environmental Discussing the use of modified ceria as support for Pt catalysts on water-gas shift reaction. Appl. Catal. B Environ. 2010, 93, 250–258. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Sun, J.; Oh, R.; Feng, J.; Shi, D.; Zhao, W.; Liu, S. Influence of CeO2 morphology on WO3/CeO2 catalyzed NO selective catalytic reduction by NH3. J. Energy Inst. J. 2020, 93, 1–8. [Google Scholar] [CrossRef]
- Howell, J.; Li, Y.; Bell, A.T. Propene metathesis over supported tungsten oxide catalysts: A study of active site formation. ACS Catal. 2016, 6, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, J.; Liu, S.; Ren, X.; Ma, J.; Su, W. Ultra hydrothermal stability of CeO2-WO3/TiO2 for NH3-SCR of NO compared to traditional V2O5-WO3/TiO2 catalyst. Catal. Today 2015, 258, 11–16. [Google Scholar] [CrossRef]
- Gu, Y.; Cai, T.; Gao, X.; Xia, H.; Sun, W.; Zhao, J.; Dai, Q.; Wang, X. Catalytic combustion of chlorinated aromatics over WOx /CeO2 catalysts at low temperature. Appl. Catal. B Environ. 2019, 248, 264–276. [Google Scholar] [CrossRef]
- Larabi, C.; Chen, C.; Merle, N.; Charlin, M.; Szeto, K.C.; De Mallmann, A.; Benayad, A.; Ben Tayeb, K.; Kaddouri, A.; Nguyen, H.P.; et al. Well-defined surface tungstenocarbyne complex through the reaction of [W(≡CtBu)(CH2tBu)3] with CeO2: A highly and stable precatalyst for NOx reduction with NH3. New J. Chem. 2021, 45, 12024–12032. [Google Scholar] [CrossRef]
- Ma, Z.; Weng, D.; Wu, X.; Si, Z. Effects of WOx modification on the activity, adsorption and redox properties of CeO2 catalyst for NOx reduction with ammonia. J. Environ. Sci. 2012, 24, 1305–1316. [Google Scholar] [CrossRef]
- Higashi, A.; Shougo, B. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2019, 2, 590–602. [Google Scholar]
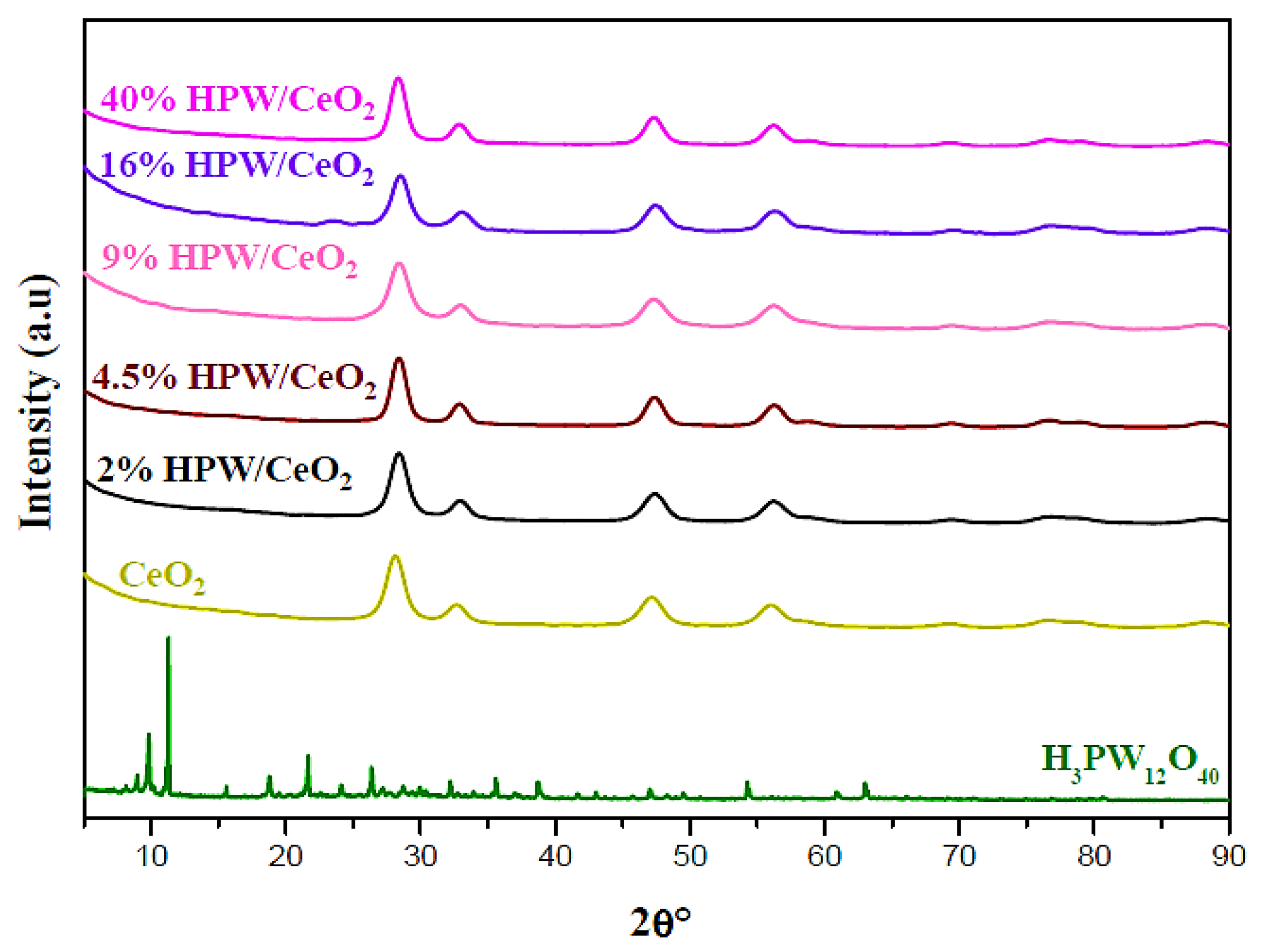
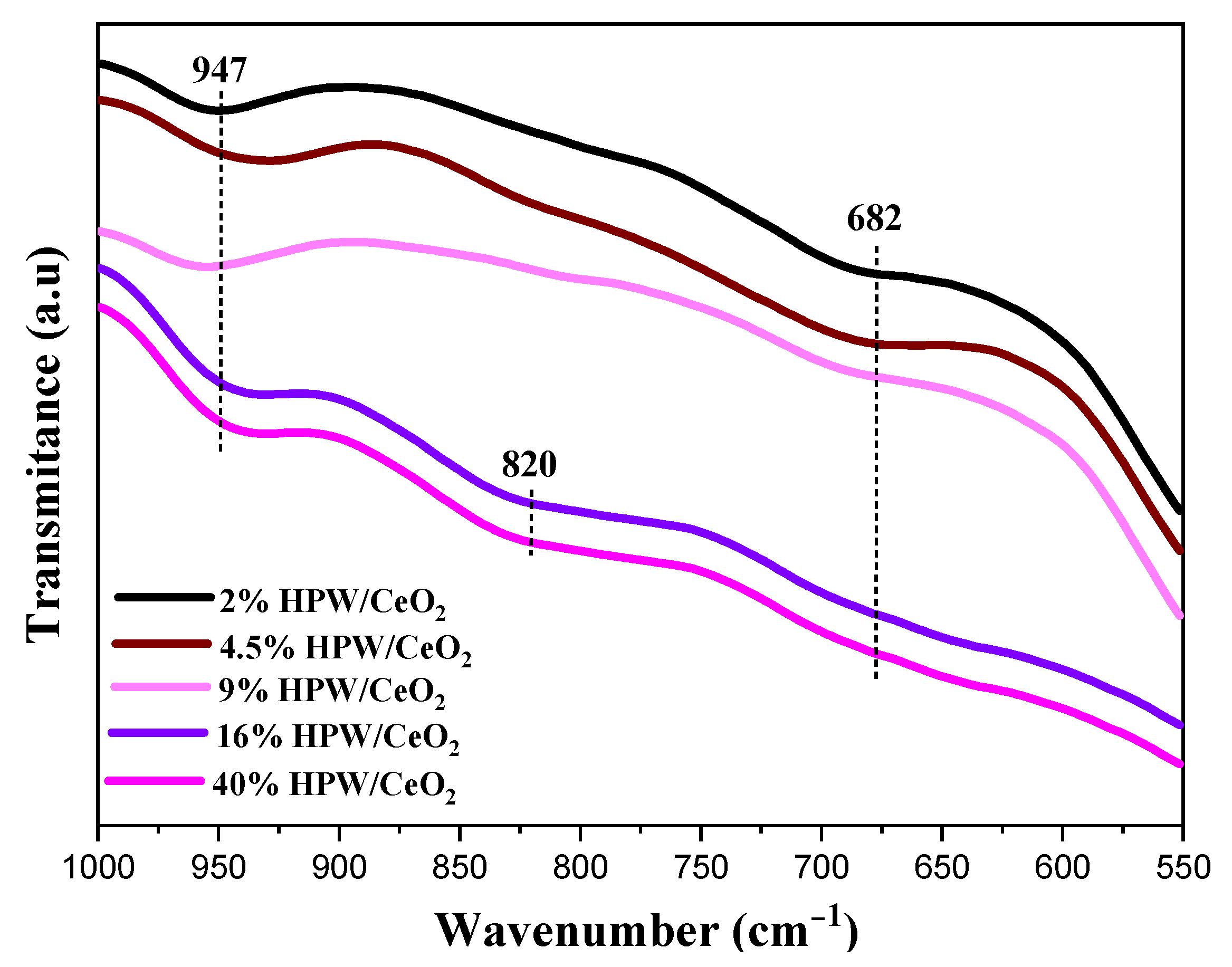
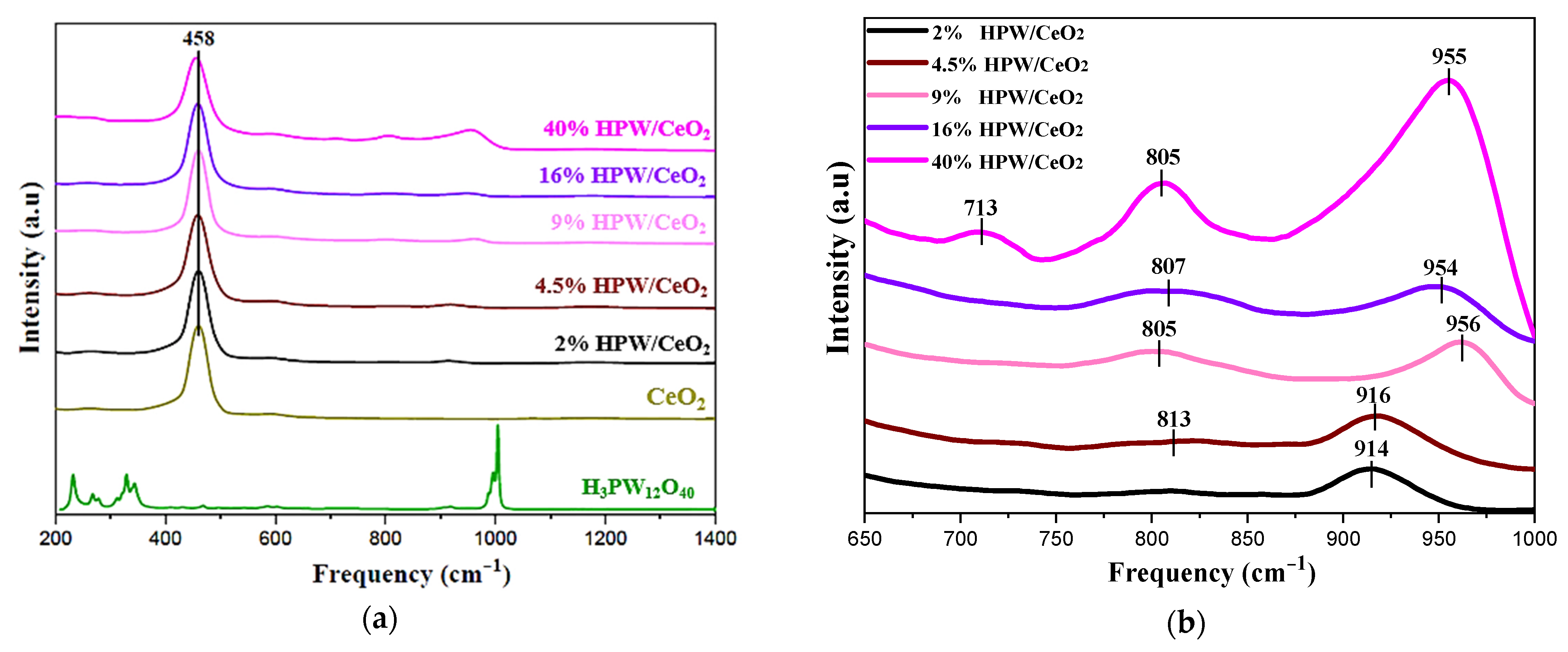
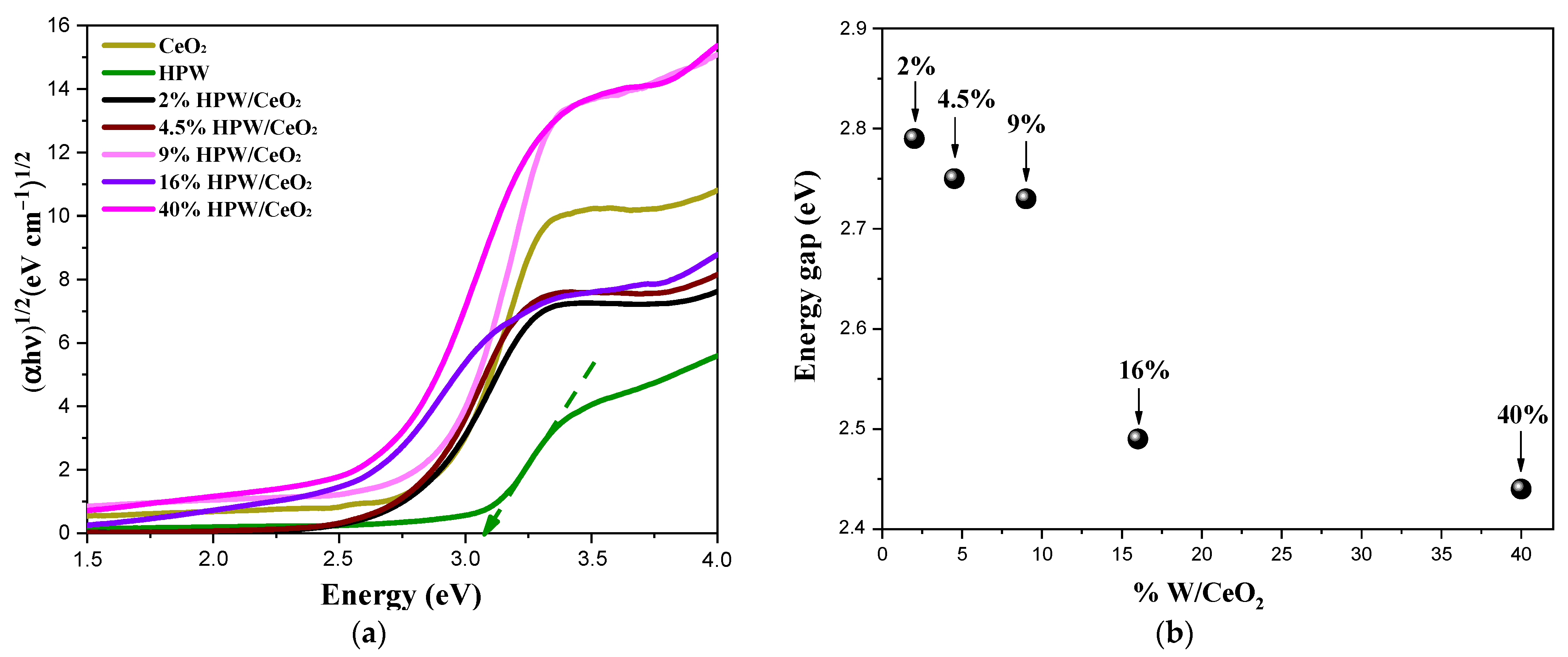
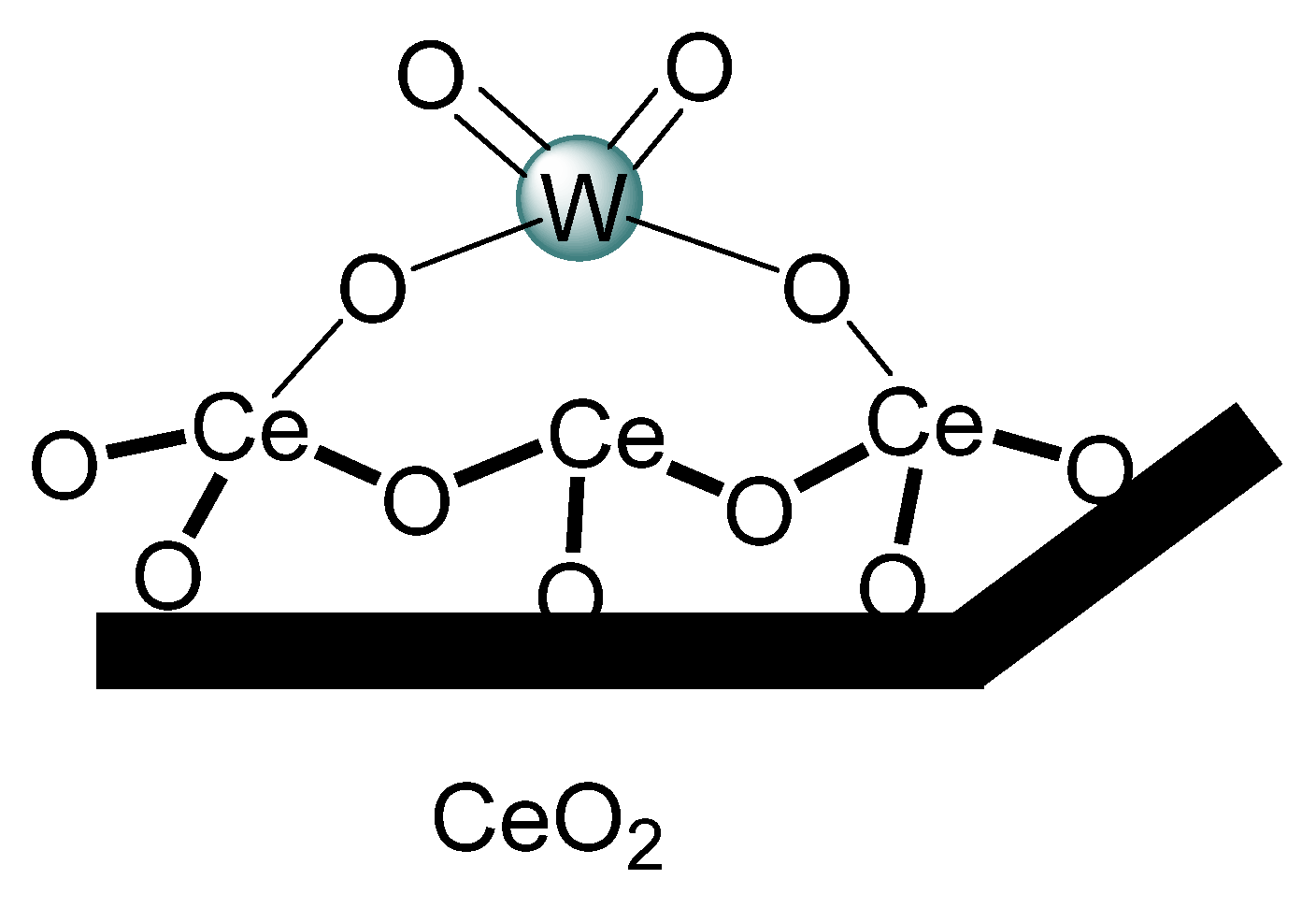
| Catalysts | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Surface Density (W at/nm2) | Average Crystallite Sizes (A°) |
|---|---|---|---|---|
| H3PW12O40 | 9 | 0.019 | - | |
| CeO2 | 247 | 0.18 | - | 56 |
| 2 % HPW/CeO2 | 221 | 0.17 | 0.3 | 57 |
| 4.5 % HPW/CeO2 | 156 | 0.16 | 0.95 | 67 |
| 9 % HPW/CeO2 | 141 | 0.15 | 2.08 | 60 |
| 16 % HPW/CeO2 | 92 | 0.13 | 5.69 | 64 |
| 40 % HPW/CeO2 | 85 | 0.08 | 15.4 | 58 |
| Catalysts | W Monomeric/W Mono + Oligo 1 | WO3 Nanoparticles 2 (IA1g/IF2g) | Estimation of W Monomeric Proportion |
|---|---|---|---|
| 2% HPW/CeO2 | 1 | 0 | 100% |
| 4.5% HPW/CeO2 | 1 | 0.04 | 96% |
| 9% HPW/CeO2 | 0.67 | 0.05 | 64% |
| 16% HPW/CeO2 | 0.47 | 0.06 | 44% |
| 40% HPW/CeO2 | 0.42 | 0.17 | 36% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Arrouji, I.; Chen, C.; Toyir, J.; Larabi, C.; Szeto, K.C.; de Mallmann, A.; Taoufik, M.; Oulmekki, A. NH3-Selective Catalytic Reduction of NOx to N2 over Ceria Supported WOx Based Catalysts: Influence of Tungsten Content. Catalysts 2021, 11, 950. https://doi.org/10.3390/catal11080950
El Arrouji I, Chen C, Toyir J, Larabi C, Szeto KC, de Mallmann A, Taoufik M, Oulmekki A. NH3-Selective Catalytic Reduction of NOx to N2 over Ceria Supported WOx Based Catalysts: Influence of Tungsten Content. Catalysts. 2021; 11(8):950. https://doi.org/10.3390/catal11080950
Chicago/Turabian StyleEl Arrouji, Imane, Cuirong Chen, Jamil Toyir, Cherif Larabi, Kai C. Szeto, Aimery de Mallmann, Mostafa Taoufik, and Abdallah Oulmekki. 2021. "NH3-Selective Catalytic Reduction of NOx to N2 over Ceria Supported WOx Based Catalysts: Influence of Tungsten Content" Catalysts 11, no. 8: 950. https://doi.org/10.3390/catal11080950
APA StyleEl Arrouji, I., Chen, C., Toyir, J., Larabi, C., Szeto, K. C., de Mallmann, A., Taoufik, M., & Oulmekki, A. (2021). NH3-Selective Catalytic Reduction of NOx to N2 over Ceria Supported WOx Based Catalysts: Influence of Tungsten Content. Catalysts, 11(8), 950. https://doi.org/10.3390/catal11080950







