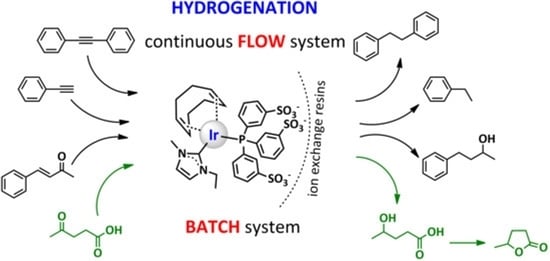
Immobilization of an Iridium(I)-NHC-Phosphine Catalyst for Hydrogenation Reactions under Batch and Flow Conditions
Abstract
1. Introduction
2. Results and Discussion
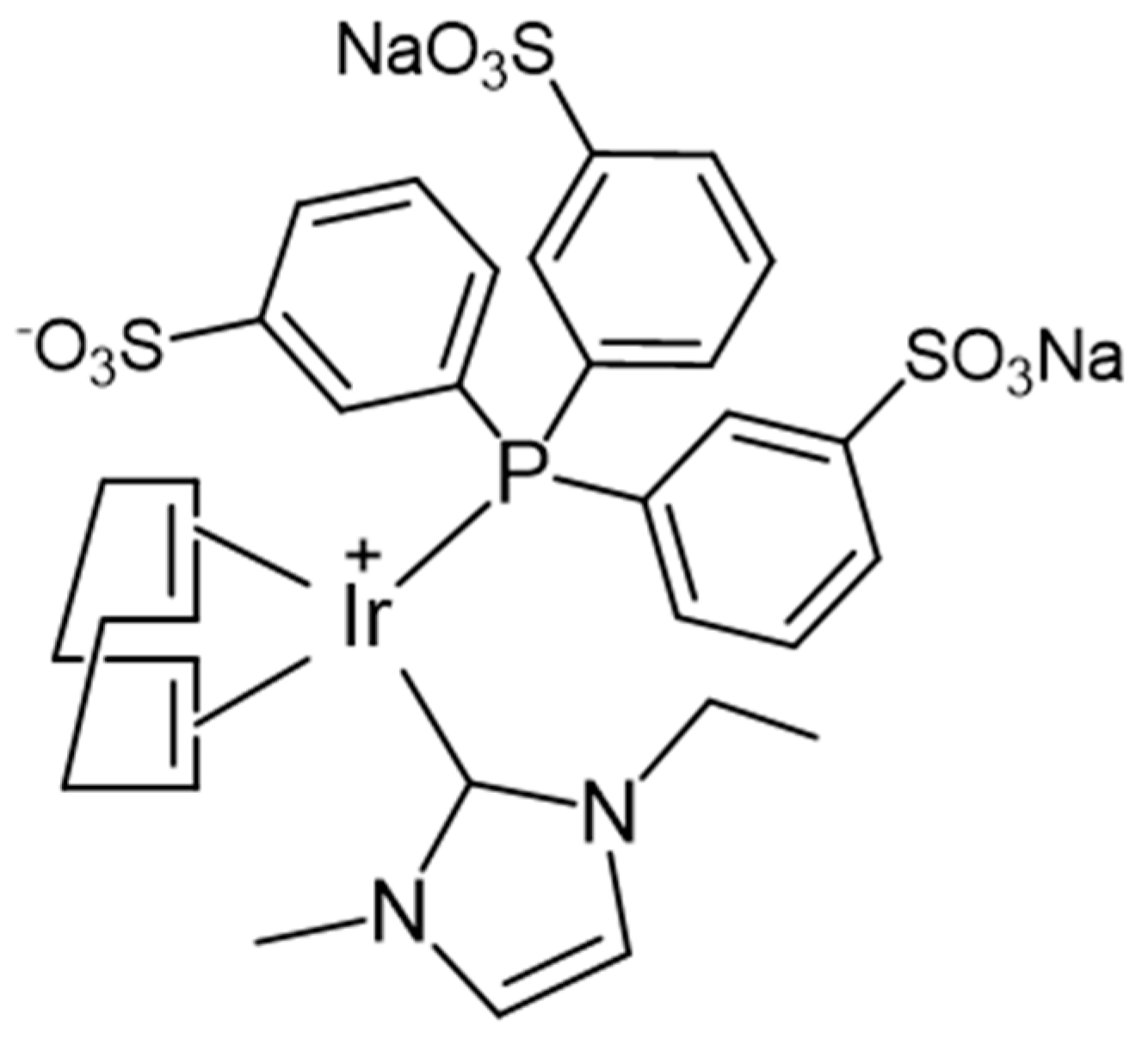
2.1. Heterogenization of Na2[Ir(cod)(emim)(mtppts)] (1) and Leaching Tests
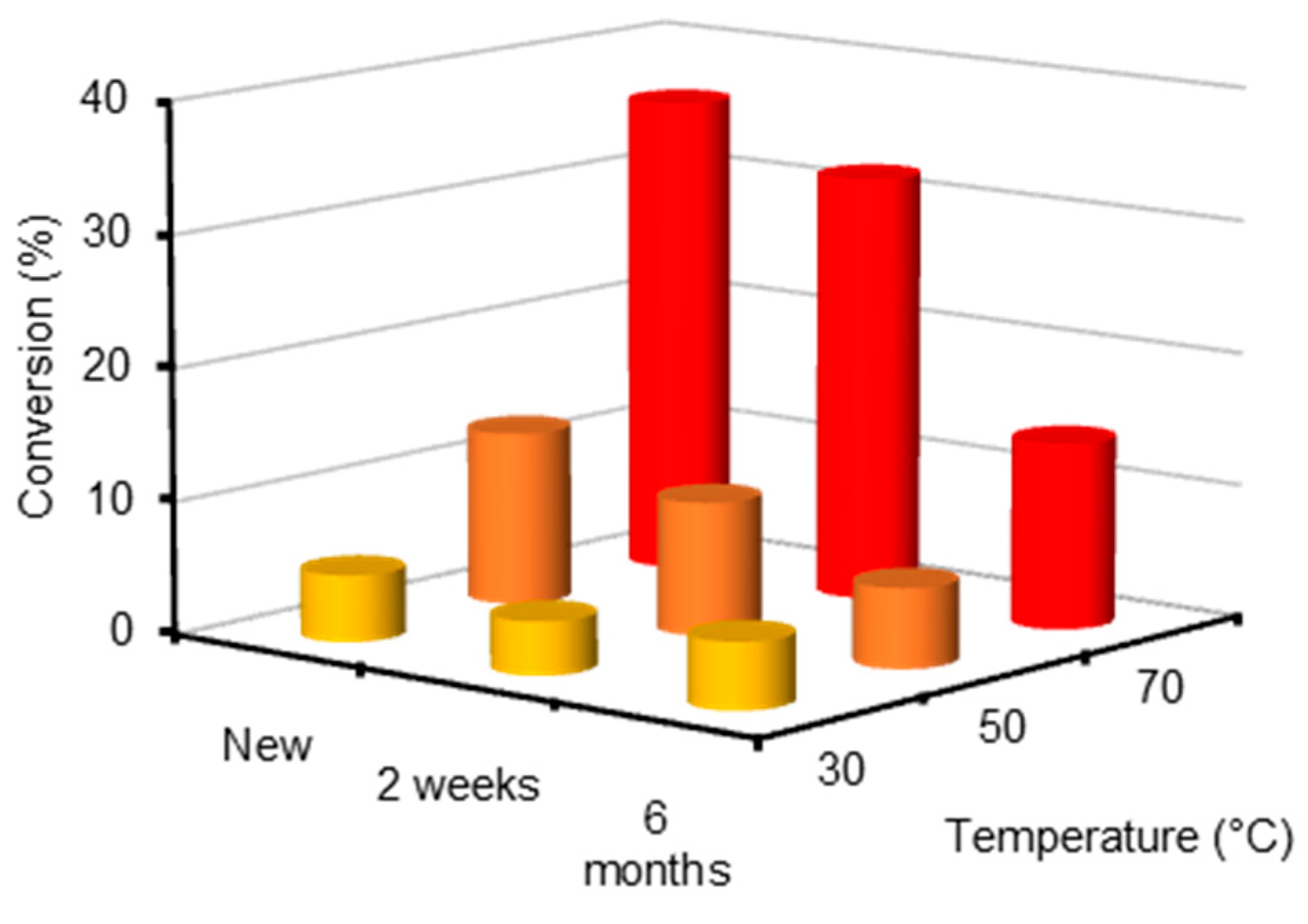
2.2. Reaction of 1@Lewatit with Hydrogen
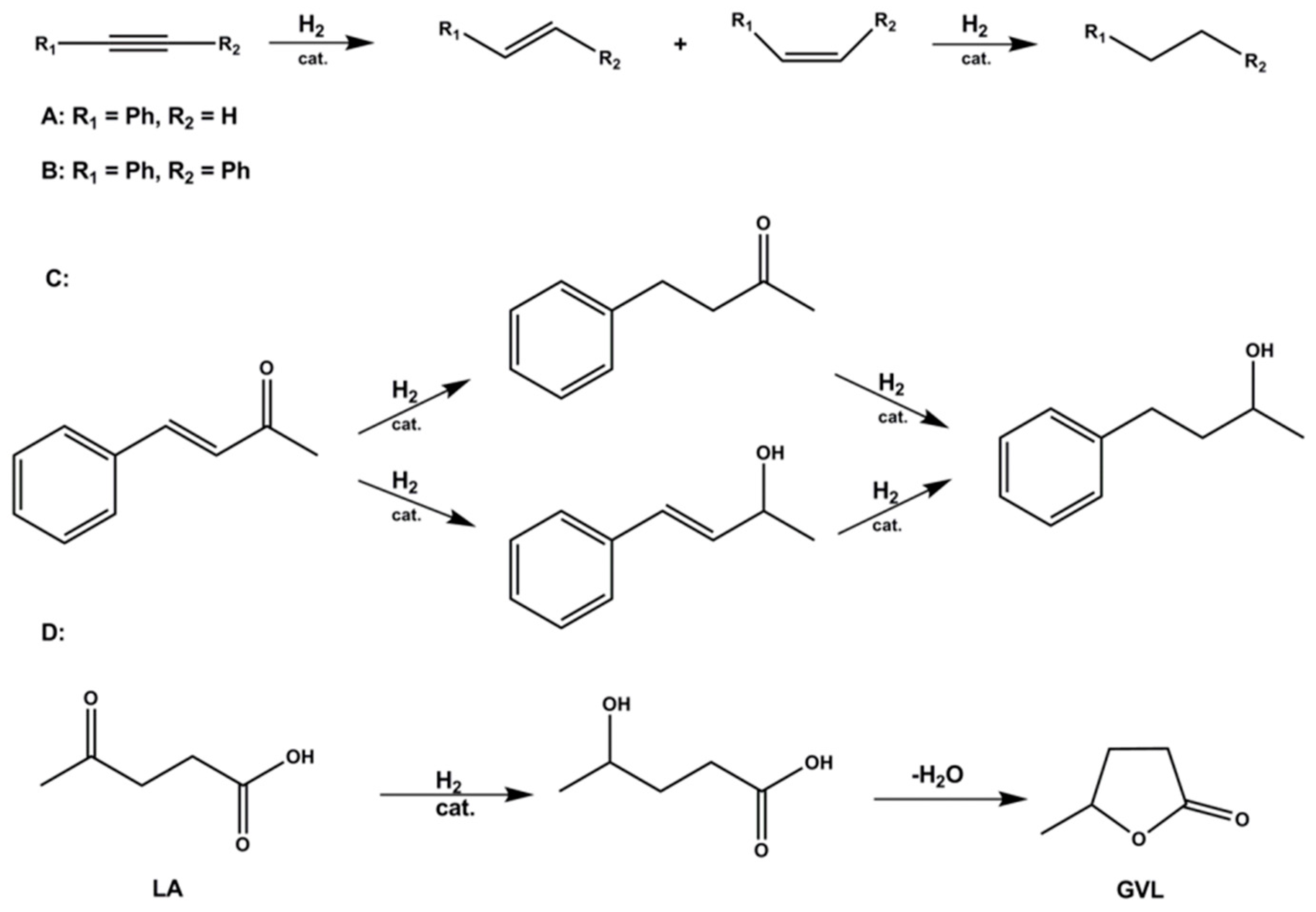
2.3. Catalytic Hydrogenation Reactions with 1@Lewatit Supported Catalyst
2.3.1. Hydrogenations with Soluble and Heterogenized Na2[Ir(cod)(emim)(mtppts)] in Batch Reactions
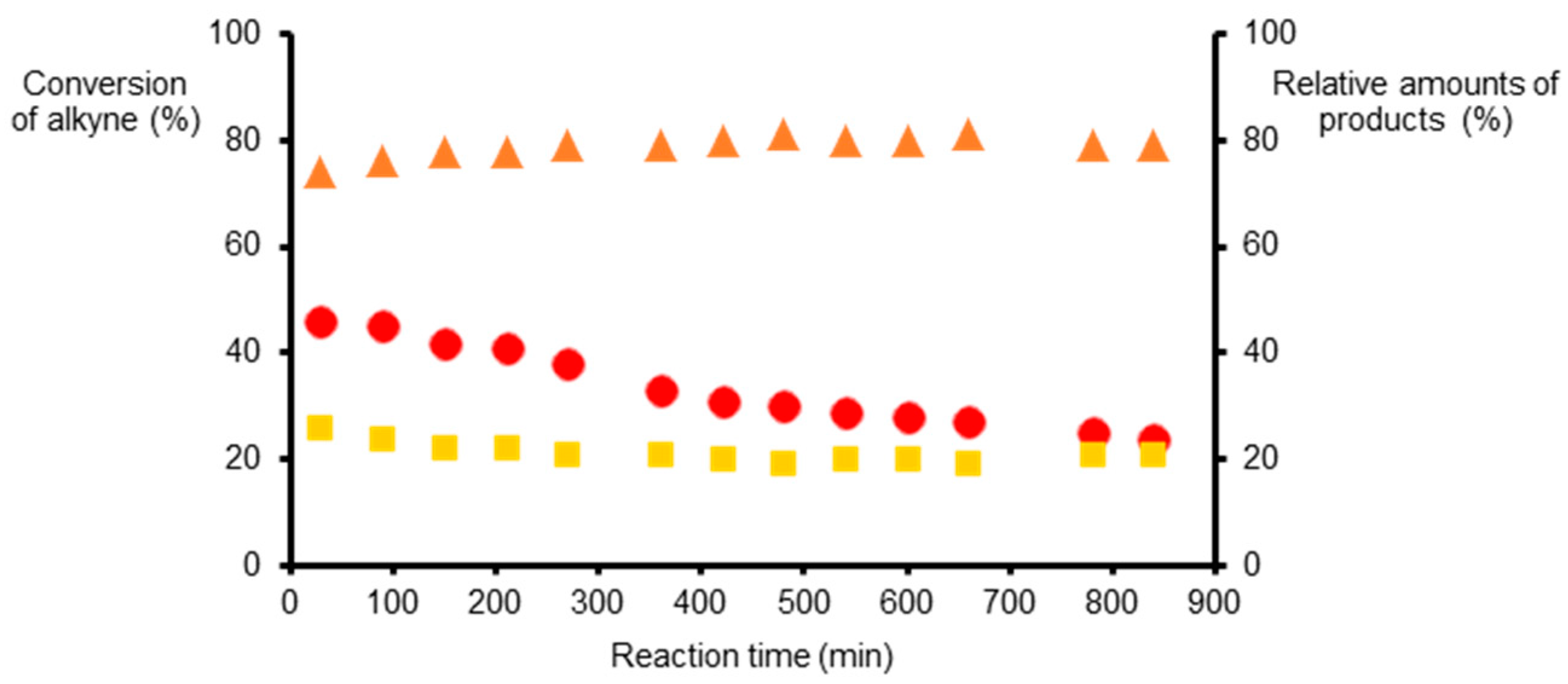
2.3.2. Hydrogenations under Flow Conditions
3. Materials and Methods
3.1. Anchoring of Na2[Ir(cod)(emim)(mtppts)] on Solid Supports
3.2. Spectrophotometric Study of the Immobilization Process and Leaching Tests
3.3. Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, C.C.; Baker, J.R.; Cossar, P.J.; McCluskey, A. Recent developments in the use of flow hydrogenation in the field of medicinal chemistry. In New Advances in Hydrogenation Processes—Fundamentals and Applications; InTechOpen: London, UK, 2017. [Google Scholar]
- Cossar, P.J.; Hizartzidis, L.; Simone, M.I.; McCluskey, A.; Gordon, C.P. The expanding utility of continuous flow hydrogenation. Org. Biomol. Chem. 2015, 13, 7119–7130. [Google Scholar] [CrossRef]
- Barbaro, P. Recycling asymmetric hydrogenation catalysts by their immobilization onto ion-exchange resins. Chem. Rev. 2006, 12, 5666–5675. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F. Ion exchange resins: Catalyst recovery and recycle. Chem. Rev. 2009, 109, 515–529. [Google Scholar] [CrossRef] [PubMed]
- James, B.R. Homogeneous Hydrogenation; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Chaloner, P.A.; Esteruelas, M.A.; Joó, F.; Oro, L.A. Homogeneous Hydrogenation; Kluwer: Dordrecht, The Netherlands, 1994; Volume 15. [Google Scholar]
- de Vries, J.G.; Elsevier, C.J. Handbook of Homogeneous Hydrogenation; Wiley: Weinheim, Germany, 2007. [Google Scholar]
- Tóth, I.; van Geem, P.C. Immobilization techniques. In Handbook of Homogeneous Hydrogenation; Wiley: Weinheim, Germany, 2007; pp. 1421–1467. [Google Scholar]
- Crabtree, R.H. Iridium. In Handbook of Homogeneous Hydrogenation; Wiley: Weinheim, Germany, 2007; pp. 31–44. [Google Scholar]
- Andersson, P.G. Iridium Catalysis; Springer: Berlin/Heildelberg, Germany, 2011. [Google Scholar]
- Vaska, L.; Rhodes, R.E. Hydrido complexes of iridium. J. Am. Chem. Soc. 1961, 83, 756. [Google Scholar] [CrossRef]
- Eberhardt, G.G.; Vaska, L.J. Homogeneous catalysis with trans-chlorocarbonylbis (triphenylphosphine) iridium (I). J. Catal. 1967, 8, 183–188. [Google Scholar] [CrossRef]
- James, B.R.; Memon, N.A. Kinetic study of iridium (I) complexes as homogeneous hydrogenation catalysts. Can. J. Chem. 1968, 46, 217–223. [Google Scholar] [CrossRef]
- Spogliarich, R.; Farnetti, E.; Kašpar, J.; Graziani, M.; Cesarotti, E. Selective hydrogenation of benzylideneacetone catalyzed by iridium diphosphine complexes. J. Mol. Catal. 1989, 50, 19–29. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic hydrogenation of carbon dioxide using Ir (III)-pincer complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef]
- Albrecht, M.; Miecznikowski, J.R.; Samuel, A.; Faller, A.J.W.; Crabtree, R.H. Chelated Iridium (III) Bis-carbene complexes as air-stable catalysts for transfer hydrogenation. Organometallics 2002, 21, 3596–3604. [Google Scholar] [CrossRef]
- Jiménez, M.V.; Fernández-Tornos, J.; Pérez-Torrente, J.J.; Modrego, F.J.; García-Orduña, P.; Oro, L.A. Mechanistic insights into transfer hydrogenation catalysis by [Ir(cod)(NHC)2]+ complexes with functionalized N-heterocyclic carbene ligands. Organometallics 2015, 34, 926–940. [Google Scholar] [CrossRef]
- García, N.; Jaseer, E.A.; Munarriz, J.; Miguel, P.J.S.; Polo, V.; Iglesias, M.; Oro, L.A. An insight into transfer hydrogenation reactions catalysed by iridium (III) Bis-N-heterocyclic carbenes. Eur. J. Inorg. Chem. 2015, 2015, 4388–4395. [Google Scholar] [CrossRef]
- Papp, G.; Horváth, H.; Joó, F. A simple and efficient procedure for Rh (I)- and Ir (I)-complex catalyzed para -hydrogenation of alkynes and alkenes in aqueous media resulting in strong PHIP effects. ChemCatChem 2019, 11, 3000–3003. [Google Scholar] [CrossRef]
- Song, S.; Zhu, S.-F.; Li, Y.; Zhou, Q.-L. Iridium-catalyzed enantioselective hydrogenation of α,β-unsaturated carboxylic acids with tetrasubstituted olefins. Org. Lett. 2013, 15, 3722–3725. [Google Scholar] [CrossRef]
- Malacea, R.; Poli, R.; Manoury, E. Asymmetric hydrosilylation, transfer hydrogenation and hydrogenation of ketones catalyzed by iridium complexes. Coord. Chem. Rev. 2010, 254, 729–752. [Google Scholar] [CrossRef]
- Horváth, H.; Kathó, Á.; Udvardy, A.; Papp, G.; Szikszai, D.; Joó, F. New water-soluble Iridium (I)-N-heterocyclic carbene-tertiary phosphine mixed-ligand complexes as catalysts of hydrogenation and redox isomerization. Organometallics 2014, 33, 6330–6340. [Google Scholar] [CrossRef]
- Brown, J.A.; Cochrane, A.R.; Irvine, S.; Kerr, W.J.; Mondal, B.; Parkinson, J.; Paterson, L.C.; Reid, M.; Tuttle, T.; Andersson, S.; et al. The synthesis of highly active Iridium (I) complexes and their application in catalytic hydrogen isotope exchange. Adv. Synth. Catal. 2014, 356, 3551–3562. [Google Scholar] [CrossRef]
- Jones, J.H. The Cativa™ process for the manufacture of acetic acid: Iridium catalyst improves productivity in an established industrial process. Platin. Met. Rev. 2000, 44, 94–105. [Google Scholar]
- Blaser, H.-U. The Chiral switch of (S)-Metolachlor: A personal account of an industrial Odyssey in asymmetric catalysis. Adv. Synth. Catal. 2002, 344, 17–31. [Google Scholar] [CrossRef]
- Huang, Z.; Brookhart, M.; Goldman, A.S.; Kundu, S.; Ray, A.; Scott, S.L.; Vicente, B.C. Highly active and recyclable heterogeneous iridium pincer catalysts for transfer dehydrogenation of alkanes. Adv. Synth. Catal. 2009, 351, 188–206. [Google Scholar] [CrossRef]
- Xu, Z.; McNamara, N.D.; Neumann, G.T.; Schneider, W.; Hicks, J.C. Catalytic hydrogenation of CO2 to formic acid with silica-tethered iridium catalysts. ChemCatChem 2013, 5, 1769–1771. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kobayashi, T.; Suenobu, T. Unusually large tunneling effect on highly efficient generation of hydrogen and hydrogen isotopes in pH-selective decomposition of formic acid catalyzed by a heterodinuclear iridium-ruthenium complex in water. J. Am. Chem. Soc. 2010, 132, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.F.; Himeda, Y.; Wang, W.-H.; Hashiguchi, B.G.; A Periana, R.; Szalda, D.J.; Muckerman, J.T.; Fujita, E. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 2012, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Czaun, M.; Kothandaraman, J.; Goeppert, A.; Yang, B.; Greenberg, S.; May, R.B.; Olah, G.A.; Prakash, G.K.S. Iridium-catalyzed continuous hydrogen generation from formic acid and its subsequent utilization in a fuel cell: Toward a carbon neutral chemical energy storage. ACS Catal. 2016, 6, 7475–7484. [Google Scholar] [CrossRef]
- Papp, G.; Ölveti, G.; Horváth, H.; Kathó, Á.; Joó, F. Highly efficient dehydrogenation of formic acid in aqueous solution catalysed by an easily available water-soluble Iridium (III) dihydride. Dalton Trans. 2016, 45, 14516–14519. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, H.; Himeda, Y.; Laurenczy, G. Formic acid as a hydrogen carrier for fuel cells toward a sustainable energy system. Adv. Inorg. Chem. 2017, 70, 395–427. [Google Scholar] [CrossRef]
- Horváth, H.; Papp, G.; Szabolcsi, R.; Kathó, Á.; Joó, F. Water-soluble iridium-NHC-phosphine complexes as catalysts for chemical hydrogen batteries based on formate. ChemSusChem 2015, 8, 3036–3038. [Google Scholar] [CrossRef] [PubMed]
- Fehér, P.P.; Horváth, H.; Joó, F.; Purgel, M. DFT study on the mechanism of hydrogen storage based on the formate-bicarbonate equilibrium catalyzed by an Ir-NHC Complex: An elusive intramolecular C-H activation. Inorg. Chem. 2018, 57, 5903–5914. [Google Scholar] [CrossRef]
- Horváth, H.; Papp, G.; Kovács, H.; Kathó, Á.; Joó, F. Iridium (I) NHC-phosphine complex-catalyzed hydrogen generation and storage in aqueous formate/bicarbonate solutions using a flow reactor—Effective response to changes in hydrogen demand. Int. J. Hydrogen. Energy 2019, 44, 28527–28532. [Google Scholar] [CrossRef]
- Denney, M.C.; Pons, V.; Hebden, T.J.; Heinekey, A.D.M.; Goldberg, K.I. Efficient catalysis of Ammonia borane dehydrogenation. J. Am. Chem. Soc. 2006, 128, 12048–12049. [Google Scholar] [CrossRef]
- Dietrich, B.L.; Goldberg, K.I.; Heinekey, D.M.; Autrey, T.; Linehan, J.C. Iridium-catalyzed dehydrogenation of substituted amine boranes: Kinetics, thermodynamics, and implications for hydrogen storage. Inorg. Chem. 2008, 47, 8583–8585. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Wada, T.; Shiraishi, T. Reversible Interconversion between 2,5-Dimethylpyrazine and 2,5-Dimethylpiperazine by Iridium-catalyzed hydrogenation/dehydrogenation for efficient hydrogen storage. Angew. Chem. Int. Ed. 2017, 56, 10886–10889. [Google Scholar] [CrossRef]
- Ventura-Espinosa, D.; Sabater, S.; Carretero-Cerdán, A.; Baya, M.; Mata, J.A. High production of hydrogen on demand from silanes catalyzed by iridium complexes as a versatile hydrogen storage system. ACS Catal. 2018, 8, 2558–2566. [Google Scholar] [CrossRef]
- Onoda, M.; Nagano, Y.; Fujita, K.-I. Iridium-catalyzed dehydrogenative lactonization of 1,4-butanediol and reversal hydrogenation: New hydrogen storage system using cheap organic resources. Int. J. Hydrogen. Energy 2019, 44, 28514–28520. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Prakash, N.; Jung, K.-D.; Yoon, S. A highly efficient heterogenized iridium complex for the catalytic hydrogenation of carbon dioxide to formate. ChemSusChem 2015, 8, 3410–3413. [Google Scholar] [CrossRef] [PubMed]
- Corral-Pérez, J.J.; Billings, A.; Stoian, D.; Urakawa, A. Continuous hydrogenation of carbon dioxide to formic acid and methyl formate by a molecular iridium complex stably heterogenized on a covalent triazine framework. ChemCatChem 2019, 11, 4725–4730. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Maegawa, Y.; Onishi, N.; Kanega, R.; Waki, M.W.M.; Himeda, Y.; Inagaki, S. Catalytic disproportionation of formic acid to methanol by an iridium complex immobilized on bipyridine-periodic Mesoporous Organosilica. ChemCatChem 2019, 11, 4797–4802. [Google Scholar] [CrossRef]
- Haag, W.O.; Whitehurst, D.D. Improvements Relating to Organic Reactions Catalyzed by Resin-Metal Complexes; DE 1 800 371; Mobil Oil Corp.: Irving, TX, USA, 1969. [Google Scholar]
- Hartley, F.R. Supported Metal Complexes; D. Riedel Publishing: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Gan, W.; Dyson, P.J.; Laurenczy, G. Heterogeneous silica-supported ruthenium phosphine catalysts for selective formic acid decomposition. ChemCatChem 2013, 5, 3124–3130. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Anion exchange resins as effective sorbents for acidic dye removal from aqueous solutions and wastewaters. Solvent Extr. Ion Exch. 2012, 30, 507–523. [Google Scholar] [CrossRef]
- Andriollo, A.; Esteruelas, M.A.; Meyer, U.; Oro, L.A.; Sánchez-Delgado, R.A.; Sola, E.; Valero, C.; Werner, H. Kinetic and mechanistic investigation of the sequential hydrogenation of phenylacetylene catalyzed by OsHCl (CO)(PR3) 2 [PR3 = PMe-tert-Bu2 and P-i-Pr3]. J. Am. Chem. Soc. 1989, 111, 7431–7437. [Google Scholar] [CrossRef]
- Orosz, K.; Papp, G.; Kathó, Á.; Joó, F.; Horváth, H. Strong solvent effects on catalytic transfer hydrogenation of ketones with [Ir(cod)(NHC)(PR3)] catalysts in 2-propanol-water mixtures. Catalysts 2019, 10, 17. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Horváth, H.H.; Papp, G.; Csajági, C.; Joó, F. Selective catalytic hydrogenations in a microfluidics-based high throughput flow reactor on ion-exchange supported transition metal complexes: A modular approach to the heterogenization of soluble complex catalysts. Catal. Commun. 2007, 8, 442–446. [Google Scholar] [CrossRef]
- Vázquez-Serrano, L.D.; Owens, B.T.; Buriak, J.M. Catalytic olefinhydrogenation using N-heterocyclic carbene-phosphine complexes of iridium. Chem. Commun. 2002, 2518–2519. [Google Scholar] [CrossRef]
- Vazquez-Serrano, L.D.; Owens, B.T.; Buriak, J.M. The search for new hydrogenation catalyst motifs based on N-heterocyclic carbene ligands. Inorg. Chim. Acta 2006, 359, 2786–2797. [Google Scholar] [CrossRef]
- Zsigmond, Á.; Notheisz, F. Selective syntheses on heterogenized metal complexes. Curr. Org. Chem. 2006, 10, 1655–1680. [Google Scholar] [CrossRef]

| Type of Support | Name | Time a (min) | Leaching b (%) | |
|---|---|---|---|---|
| 0.1 M H3PO4 | 0.1 M Na3PO4 | |||
| Cellulose | DEAE-Cellulose | 4 | 36 | 45 |
| Dextrane | Molselect DEAE-25 | 4 | 15 | 27 |
| Polymethacrylate | Relisorb QA405/EB | 8 | 16 | 23 |
| PS/DVB | DIAION HPA25 | 12 | 4 | 8 |
| PS/DVB | Amberlite IRA-900 | 20 | 5 | 4 |
| PS/DVB | Lewatit MonoPlus MP 500 | 32 | 1 | 4 |
| Entry | Catalyst a | Substrate | T/°C | Conversion | Products b | |
|---|---|---|---|---|---|---|
| % | A/% | B/% | ||||
| 1 | H | Phenylacetylene | 5 | 74 | 75 | 25 |
| 2 | H | Phenylacetylene | 20 | 82 | 85 | 15 |
| 3 | H | Phenylacetylene | 50 | 100 | 0 | 100 |
| 4 | H | Phenylacetylene | 80 | 100 | 0 | 100 |
| 5 | S | Phenylacetylene | 20 | 35 | 75 | 25 |
| 6 | S | Phenylacetylene | 50 | 90 | 85 | 15 |
| 7 | S | Phenylacetylene | 80 | 100 | 10 | 90 |
| 8 | H | 1-Hexyne | 20 | 42 | 82 | 18 |
| 9 | H | 1-Hexyne | 50 | 90 | 23 | 77 |
| 10 | H | 1-Hexyne | 80 | 93 | 4 | 96 |
| 11 | S | 1-Hexyne | 50 | 10 | 82 | 18 |
| 12 | S | 1-Hexyne | 80 | 82 | 55 | 45 |
| Entry | Substrate | T/°C | Conversion | Products a | |
|---|---|---|---|---|---|
| A/% | B/% | ||||
| 1 | Phenylacetylene | 60 | 22 | 78 | 22 |
| 2 | Phenylacetylene | 80 | 47 | 80 | 20 |
| 3 | Diphenylacetylene b | 60 | 16 | 80 | 20 |
| 4 | Diphenylacetylene b | 80 | 34 | 82 | 18 |
| 5 | 1-Hexyne | 60 | 13 | 55 | 45 |
| 6 | 1-Hexyne | 80 | 38 | 57 | 43 |
| 7 | Benzylideneacetone c | 60 | 37 | 92 | 7 |
| 8 | Benzylideneacetone c | 80 | 48 | 93 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, H.; Orosz, K.; Papp, G.; Joó, F.; Horváth, H. Immobilization of an Iridium(I)-NHC-Phosphine Catalyst for Hydrogenation Reactions under Batch and Flow Conditions. Catalysts 2021, 11, 656. https://doi.org/10.3390/catal11060656
Kovács H, Orosz K, Papp G, Joó F, Horváth H. Immobilization of an Iridium(I)-NHC-Phosphine Catalyst for Hydrogenation Reactions under Batch and Flow Conditions. Catalysts. 2021; 11(6):656. https://doi.org/10.3390/catal11060656
Chicago/Turabian StyleKovács, Henrietta, Krisztina Orosz, Gábor Papp, Ferenc Joó, and Henrietta Horváth. 2021. "Immobilization of an Iridium(I)-NHC-Phosphine Catalyst for Hydrogenation Reactions under Batch and Flow Conditions" Catalysts 11, no. 6: 656. https://doi.org/10.3390/catal11060656
APA StyleKovács, H., Orosz, K., Papp, G., Joó, F., & Horváth, H. (2021). Immobilization of an Iridium(I)-NHC-Phosphine Catalyst for Hydrogenation Reactions under Batch and Flow Conditions. Catalysts, 11(6), 656. https://doi.org/10.3390/catal11060656