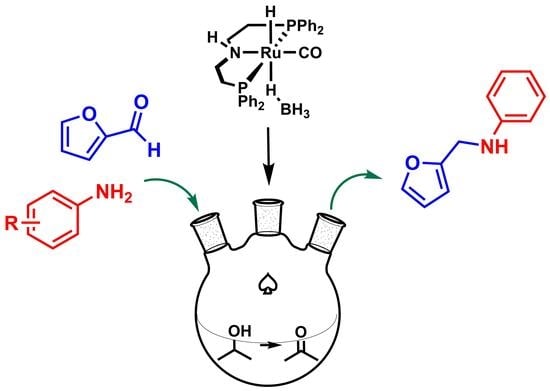
Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes
Abstract
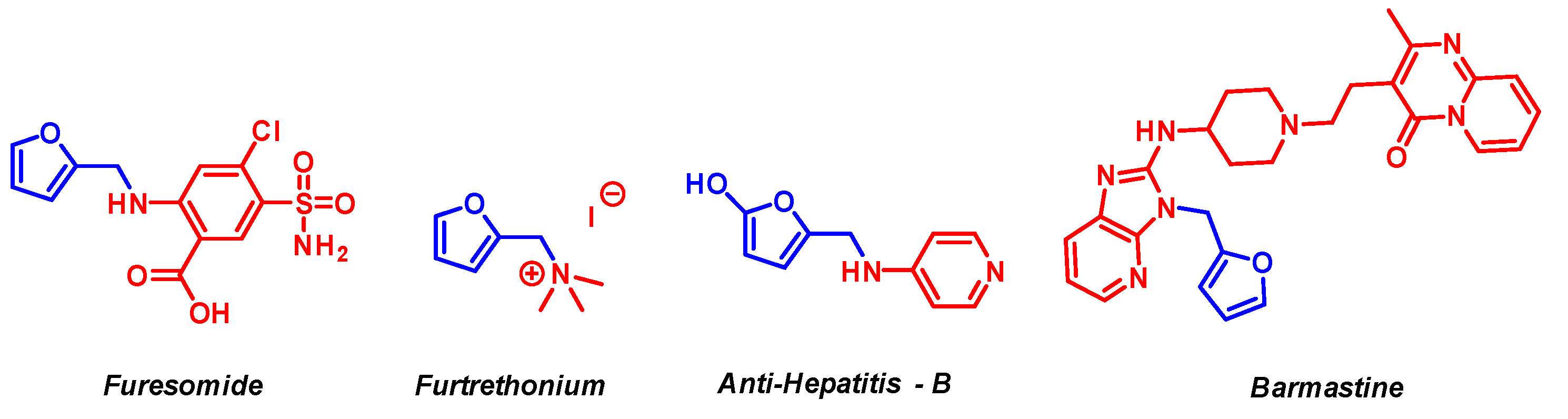
1. Introduction
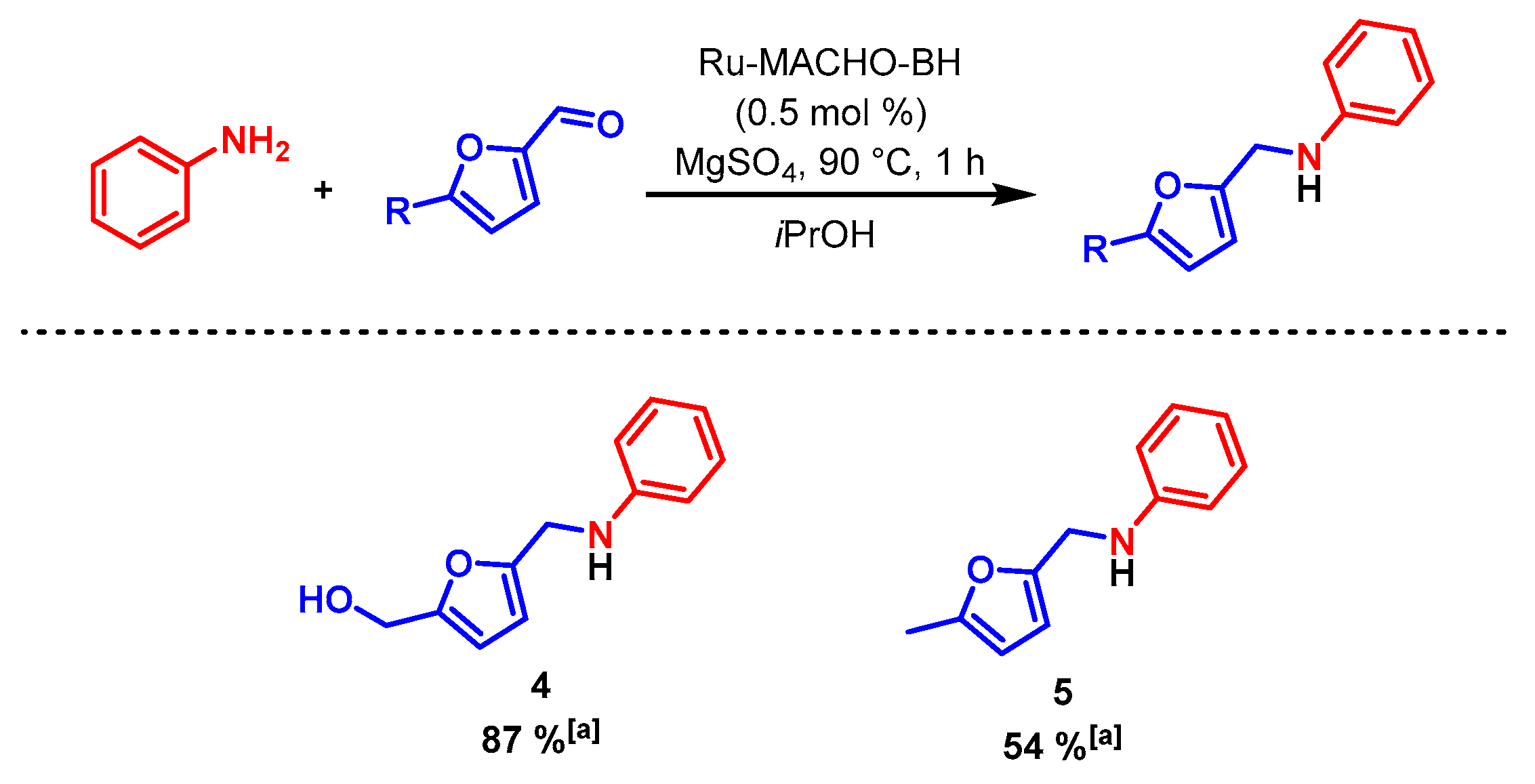
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Aldimine 1a
3.2.2. General Procedure for Transfer Hydrogenation of Aldimine 1a Catalyzed by Ru-PNP Complexes
3.2.3. General Procedure for One-Pot Reductive Amination of Furfural
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Caetano, J.A.T.; Fernandes, A.C. One-pot synthesis of amines from biomass resources catalyzed by HReO4. Green Chem. 2018, 20, 2494–2498. [Google Scholar] [CrossRef]
- Dunbabin, A.; Subrizi, F.; Ward, J.M.; Sheppard, T.D.; Hailes, H.C. Furfurylamines from biomass: Transaminase catalysed upgrading of furfurals. Green Chem. 2017, 19, 397–404. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádabaa, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Liu, S.; Song, K.; Yang, S.; Riisager, A. Sustainable access to renewable N-containing chemicals from reductive amination of biomass-derived platform compounds. Green Chem. 2020, 22, 6714–6747. [Google Scholar] [CrossRef]
- Chieffi, G.; Braun, M.; Esposito, D. Continuous reductive amination of biomass-derived molecules over carbonized filter paper-supported FeNi alloy. ChemSusChem 2015, 8, 3590–3594. [Google Scholar] [CrossRef]
- Deng, D.; Kita, Y.; Kamata, K.; Hara, M. Low-Temperature Reductive Amination of Carbonyl Compounds over Ru Deposited on Nb2O5 · nH2O. ACS Sustain. Chem. Eng. 2019, 7, 4692–4698. [Google Scholar] [CrossRef]
- Laroche, B.; Ishitani, H.; Kobayashi, S. Direct Reductive Amination of Carbonyl Compounds with H2 Using Heterogeneous Catalysts in Continuous Flow as an Alternative to N-Alkylation with Alkyl Halides. Adv. Synth. Catal. 2018, 360, 4699–4704. [Google Scholar] [CrossRef]
- Gould, N.S.; Landfield, H.; Dinkelacker, B.; Brady, C.; Yang, X.; Xu, B. Selectivity Control in Catalytic Reductive Amination of Furfural to Furfurylamine on Supported Catalysts. ChemCatChem 2020, 12, 2106–2115. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective Synthesis of Furfurylamine by Reductive Amination of Furfural over Raney Cobalt. ChemCatChem 2019, 11, 5562–5569. [Google Scholar] [CrossRef]
- Dong, C.; Wang, H.; Du, H.; Peng, J.; Cai, Y.; Guo, S.; Zhang, J.; Samart, C.; Ding, M. Ru/HZSM-5 as an efficient and recyclable catalyst for reductive amination of furfural to furfurylamine. Mol. Catal. 2020, 482, 110755. [Google Scholar] [CrossRef]
- Carrillo, A.I.; Llanes, P.; Pericàs, M.A. A versatile, immobilized gold catalyst for the reductive amination of aldehydes in batch and flow. React. Chem. Eng. 2018, 3, 714–721. [Google Scholar] [CrossRef]
- Maya, R.J.; Poulose, S.; John, J.; Varma, R.L. Direct Reductive Amination of Aldehydes via Environmentally Benign Bentonite-Gold Nanohybrid Catalysis. Adv.Synth. Catal. 2017, 359, 1177–1184. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Kalantari, M.; Boukherroub, R. Palladium oxide nanoparticles supported on graphene oxide: A convenient heterogeneous catalyst for reduction of various carbonyl compounds using triethylsilane. Appl. Organomet. Chem. 2019, 33, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Zhu, M.; Liu, Y.; He, H.; Cao, Y. Direct reductive amination of aldehydes with nitroarenes using bio-renewable formic acid as a hydrogen source. Green Chem. 2016, 18, 2507–2513. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Su, Y.; Hiraga, Y.; Fang, Z.; Watanabe, M.; Lee, R.; Smith, R.L., Jr.; Hensen, E.J.M. N-formyl-stabilizing quasi-catalytic species afford rapid and selective solvent-free amination of biomass-derived feedstocks. Nat. Commun. 2019, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Ramo, D.J.; Yus, M. Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds using Alcohols and Amines as Electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, T.; Kempe, R. Transition-metal-catalyzed reductive amination employing hydrogen. Chem. Rev. 2020, 120, 9583–9674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Furukawa, S.; Fu, X.; Yan, N. Organonitrogen chemicals from oxygen-containing feedstock over heterogeneous catalysts. ACS Catal. 2020, 10, 311–335. [Google Scholar] [CrossRef]
- Saberi, A.A. Recent advances in percolation theory and its applications. Phys. Rep. 2015, 578, 1–32. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Y.; Du, J.; Si, Z.; Tang, X.; Zeng, X.; Lin, L.; Liu, S.; Lei, T. Preparation of 5-(Aminomethyl)-2-furanmethanol by direct reductive amination of 5-Hydroxymethylfurfural with aqueous ammonia over the Ni/SBA-15 catalyst. J. Chem. Technol. Biotechnol. 2018, 93, 3028–3034. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarova, G.A. Cu-Al mixed oxide derived from layered double hydroxide as an efficient catalyst for continuous-flow reductive amination of aromatic aldehydes. J. Chem. Technol. Biotechnol. 2020, 95, 3292–3299. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Simonov, P.A.; Bukhtiyarova, G.A.; Eltsov, I.V.; Bukhtiyarov, V.I. Reductive amination of 5-acetoxymethylfurfural over Pt/Al2O3 catalyst in a flow reactor. Mol. Catal. 2021, 499, 111297. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(hydroxymethyl)furfural. ChemistryOpen 2020, 9, 1135–1148. [Google Scholar] [CrossRef]
- Lancien, A.; Wojcieszak, R.; Cuvelier, E.; Duban, M.; Dhulster, P.; Paul, S.; Dumeignil, F.; Froidevaux, R.; Heuson, E. Hybrid Conversion of 5-Hydroxymethylfurfural to 5-Aminomethyl-2-furancarboxylic acid: Toward New Bio-sourced Polymers. ChemCatChem 2021, 13, 247–259. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Hao, Y.C.; Hu, S.Q.; Zong, M.H.; Chen, Q.; Li, N. Direct Reductive Amination of Biobased Furans to N-Substituted Furfurylamines by Engineered Reductive Aminase. Adv. Synth. Catal. 2021, 363, 1033–1037. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Vidal, J.D.; Climent, M.J.; Concepción, P.; Corma, A.; Iborra, S. Chemicals from Biomass: Selective Synthesis of N-Substituted Furfuryl Amines by the One-Pot Direct Reductive Amination of Furanic Aldehydes. ACS Sustain. Chem. Eng. 2019, 7, 6243–6250. [Google Scholar] [CrossRef]
- Wei, D.; Bruneau-Voisine, A.; Dubois, M.; Bastin, S.; Sortais, J.B. Manganese-Catalyzed Transfer Hydrogenation of Aldimines. ChemCatChem 2019, 11, 5256–5259. [Google Scholar] [CrossRef]
- Tanaka, K.; Miki, T.; Murata, K.; Yamaguchi, A.; Kayaki, Y.; Kuwata, S.; Ikariya, T.; Watanabe, M. Reductive amination of ketonic compounds catalyzed by Cp*Ir(III) complexes bearing a picolinamidato ligand. J. Org. Chem. 2019, 84, 10962–10977. [Google Scholar] [CrossRef]
- Yang, M.L.; Wu, Y.X.; Liu, Y.; Qiu, J.J.; Liu, C.M. A novel bio-based AB2 monomer for preparing hyperbranched polyamides derived from levulinic acid and furfurylamine. Polym. Chem. 2019, 10, 6217–6226. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: An environmentally friendly approach. Green Chem. 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Piccirilli, L.; Pinheiro, D.L.J.; Nielsen, M. Recent progress with pincer transition metal catalysts for sustainability. Catalysts 2020, 10, 773. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Xiao, J. Broader, greener, and more efficient: Recent advances in asymmetric transfer hydrogenation. Chem. Asian J. 2008, 3, 1750–1770. [Google Scholar] [CrossRef]
- Farrar-tobar, R.A.; Dell’Acqua, A.; Tin, S.; de Vries, J.G. Metal-catalysed selective transfer hydrogenation of α,β-unsaturated carbonyl compounds to allylic alcohols. Green Chem. 2020, 22, 3323–3357. [Google Scholar] [CrossRef]
- Clapham, S.E.; Hadzovic, A.; Morris, R.H. Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord. Chem. Rev. 2004, 248, 2201–2237. [Google Scholar] [CrossRef]
- Werkmeister, S.; Neumann, J.; Junge, K.; Beller, M. Pincer-Type Complexes for Catalytic (De)Hydrogenation and Transfer (De)Hydrogenation Reactions: Recent Progress. Chem. Eur. J. 2015, 21, 12226–12250. [Google Scholar] [CrossRef]
- Farrar-Tobar, R.A.; Wozniak, B.; Savini, A.; Hinze, S.; Tin, S.; de Vries, J.G. Base-Free Iron Catalyzed Transfer Hydrogenation of Esters Using EtOH as Hydrogen Source. Angew. Chem. Int. Ed. 2019, 58, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Clarke, Z.E.; Maragh, P.T.; Dasgupta, T.P.; Gusev, D.G.; Lough, A.J.; Abdur-Rashid, K. A family of active iridium catalysts for transfer hydrogenation of ketones. Organometallics 2006, 25, 4113–4117. [Google Scholar] [CrossRef]
- Castellanos-blanco, N.; Arévalo, A.; García, J.J. Nickel-catalyzed transfer hydrogenation of ketones using ethanol as a solvent and a hydrogen donor. Dalt. Trans. 2016, 45, 13604–13614. [Google Scholar] [CrossRef]
- Aboo, A.H.; Begum, R.; Zhao, L.; Farooqi, Z.H.; Xiao, J. Methanol as hydrogen source: Chemoselective transfer hydrogena- tion of α,β -unsaturated ketones with a rhodacycle. Chin. J. Catal. 2019, 40, 1795–1799. [Google Scholar] [CrossRef]
- Farrar-tobar, R.A.; Wei, Z.; Jiao, H.; Hinze, S.; Vries, J.G. De Selective Base-free Transfer Hydrogenation of α,β-Unsaturated Carbonyl Compounds using iPrOH or EtOH as Hydrogen Source. Chem. Eur.J. 2018, 24, 2725–2734. [Google Scholar] [CrossRef]
- Padilla, R.; Koranchalil, S.; Nielsen, M. Efficient and selective catalytic hydrogenation of furanic aldehydes using well defined Ru and Ir pincer complexes. Green Chem. 2020, 22, 6767–6772. [Google Scholar] [CrossRef]
- Padilla, R.; Nielsen, M.; Jørgensen, M.S.B. Efficient catalytic hydrogenation of alkyl levulinates to γ-valerolactone. Green Chem. 2019, 21, 5195–5200. [Google Scholar] [CrossRef]
- Garbe, M.; Wei, Z.; Tannert, B.; Spannenberg, A.; Jiao, H.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. Enantioselective Hydrogenation of Ketones using Different Metal Complexes with a Chiral PNP Pincer Ligand. Adv. Synth. Catal. 2019, 361, 1913–1920. [Google Scholar] [CrossRef]
- Guan, C.; Pan, Y.; Ang, E.P.L.; Hu, J.; Yao, C.; Huang, M.H.; Li, H.; Lai, Z.; Huang, K.W. Conversion of CO2 from air into formate using amines and phosphorus-nitrogen PN3P-Ru(ii) pincer complexes. Green Chem. 2018, 20, 4201–4205. [Google Scholar] [CrossRef]
- Neumann, J.; Bornschein, C.; Jiao, H.; Junge, K.; Beller, M. Hydrogenation of Aliphatic and Aromatic Nitriles Using a Defined Ruthenium PNP Pincer Catalyst. Eur. J. Org. Chem. 2015, 2015, 5944–5948. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Van Putten, R.; Schulpen, E.N.; Hensen, E.J.M.; Pidko, E.A. Highly efficient reversible hydrogenation of carbon dioxide to formates using a ruthenium PNP-pincer catalyst. ChemCatChem 2014, 6, 1526–1530. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Hensen, E.J.M.; Pidko, E.A. Mechanism of CO2 hydrogenation to formates by homogeneous Ru-PNP pincer catalyst: From a theoretical description to performance optimization. Catal. Sci. Technol. 2014, 4, 3474–3485. [Google Scholar] [CrossRef]
- Oldenhuis, N.J.; Dong, V.M.; Guan, Z. Catalytic acceptorless dehydrogenations: Ru-Macho catalyzed construction of amides and imines. Tetrahedron 2014, 70, 4213–4218. [Google Scholar] [CrossRef]
- Agapova, A.; Alberico, E.; Kammer, A.; Junge, H.; Beller, M. Catalytic Dehydrogenation of Formic Acid with Ruthenium-PNP-Pincer Complexes: Comparing N-Methylated and NH-Ligands. ChemCatChem 2019, 11, 1910–1914. [Google Scholar] [CrossRef]
- Bertoli, M.; Choualeb, A.; Lough, A.J.; Moore, B.; Spasyuk, D.; Gusev, D.G. Osmium and ruthenium catalysts for dehydrogenation of alcohols. Organometallics 2011, 30, 3479–3482. [Google Scholar] [CrossRef]
- Alberico, E.; Lennox, A.J.J.; Vogt, L.K.; Jiao, H.; Baumann, W.; Drexler, H.J.; Nielsen, M.; Spannenberg, A.; Checinski, M.P.; Junge, H.; et al. Unravelling the Mechanism of Basic Aqueous Methanol Dehydrogenation Catalyzed by Ru-PNP Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 14890–14904. [Google Scholar] [CrossRef]
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef]
- Sponholz, P.; Mellmann, D.; Cordes, C.; Alsabeh, P.G.; Li, B.; Li, Y.; Nielsen, M.; Junge, H.; Dixneuf, P.; Beller, M. Efficient and Selective Hydrogen Generation from Bioethanol using Ruthenium Pincer-type Complexes. ChemSusChem 2014, 7, 2419–2422. [Google Scholar] [CrossRef]
- Li, Y.; Nielsen, M.; Li, B.; Dixneuf, P.H.; Junge, H.; Beller, M. Ruthenium-catalyzed hydrogen generation from glycerol and selective synthesis of lactic acid. Green Chem. 2015, 17, 193–198. [Google Scholar] [CrossRef]
- Nielsen, M.; Junge, H.; Kammer, A.; Beller, M. Towards a green process for bulk-scale synthesis of ethyl acetate: Efficient acceptorless dehydrogenation of ethanol. Angew. Chem. Int. Ed. 2012, 51, 5711–5713. [Google Scholar] [CrossRef]
- Dub, P.A.; Gordon, J.C. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat. Rev. Chem. 2018, 2, 396–408. [Google Scholar] [CrossRef]
- Kuriyama, W.; Matsumoto, T.; Ogata, O.; Ino, Y.; Aoki, K.; Tanaka, S.; Ishida, K.; Kobayashi, T.; Sayo, N.; Saito, T. Catalytic Hydrogenation of Esters. Development of an Efficient Catalyst and Processes for Synthesising (R)-1,2-Propanediol and 2-(l-Menthoxy)ethanol. Org. Process Res. Dev. 2012, 16, 166–171. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Q.; Yang, J.; Xiong, L.; Qiu, J.; Jiang, J.; Peng, Y.; Li, T.; Qiu, Z.; Yang, W. Catalytic Synthesis of N-(5-Methylfurfuryl)aniline from Bio-Derived Carbohydrates. Asian J. Org. Chem. 2019, 8, 328–334. [Google Scholar] [CrossRef]
- Zubkov, F.I.; Nikitina, E.V.; Galeev, T.R.; Zaytsev, V.P.; Khrustalev, V.N.; Novikov, R.A.; Orlova, D.N.; Varlamov, A.V. General synthetic approach towards annelated 3a,6-epoxyisoindoles by tandem acylation/IMDAF reaction of furylazaheterocycles. Scope and limitations. Tetrahedron 2014, 70, 1659–1690. [Google Scholar] [CrossRef]
- Wu, J.; Darcel, C. Iron-Catalyzed Hydrogen Transfer Reduction of Nitroarenes with Alcohols: Synthesis of Imines and Aza Heterocycles. J. Org. Chem. 2021, 86, 1023–1036. [Google Scholar] [CrossRef]
- Ge, C.; Sang, X.; Yao, W.; Zhang, L.; Wang, D. Unsymmetrical indazolyl-pyridinyl-triazole ligand-promoted highly active iridium complexes supported on hydrotalcite and its catalytic application in water. Green Chem. 2018, 20, 1805–1812. [Google Scholar] [CrossRef]
- Weickmann, D.; Frey, W.; Plietker, B. Synchronizing steric and electronic effects in {RuII(NNNN,P)} complexes: The catalytic dehydrative alkylation of anilines by using alcohols as a case study. Chem. Eur. J. 2013, 19, 2741–2748. [Google Scholar] [CrossRef]
- Iovel, I.; Golomba, L.; Popelis, J.; Grinberga, S.; Lukevics, E. Synthesis and hydrosilylation of furan and thiophene N- methylenefluoroanilines in the presence of Pd(I) complex. Chem. Heterocycl. Compd. 2005, 41, 1112–1118. [Google Scholar] [CrossRef]
- Lim, C.H.; Kudisch, M.; Liu, B.; Miyake, G.M. C-N Cross-Coupling via Photoexcitation of Nickel-Amine Complexes. J. Am. Chem. Soc. 2018, 140, 7667–7673. [Google Scholar] [CrossRef]
- Ware, R.W.; Hinkley, L.A.; Hardeman, K.P.; Jenks, M.G. Substituted Quinoline and Quinazoline Inhibitors of Quinone Reductase 2. U.S. Patent Application No. WO2006034235A3, 6 April 2006. [Google Scholar]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarov, V.I. Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor. Molecules 2020, 25, 4771. [Google Scholar] [CrossRef]
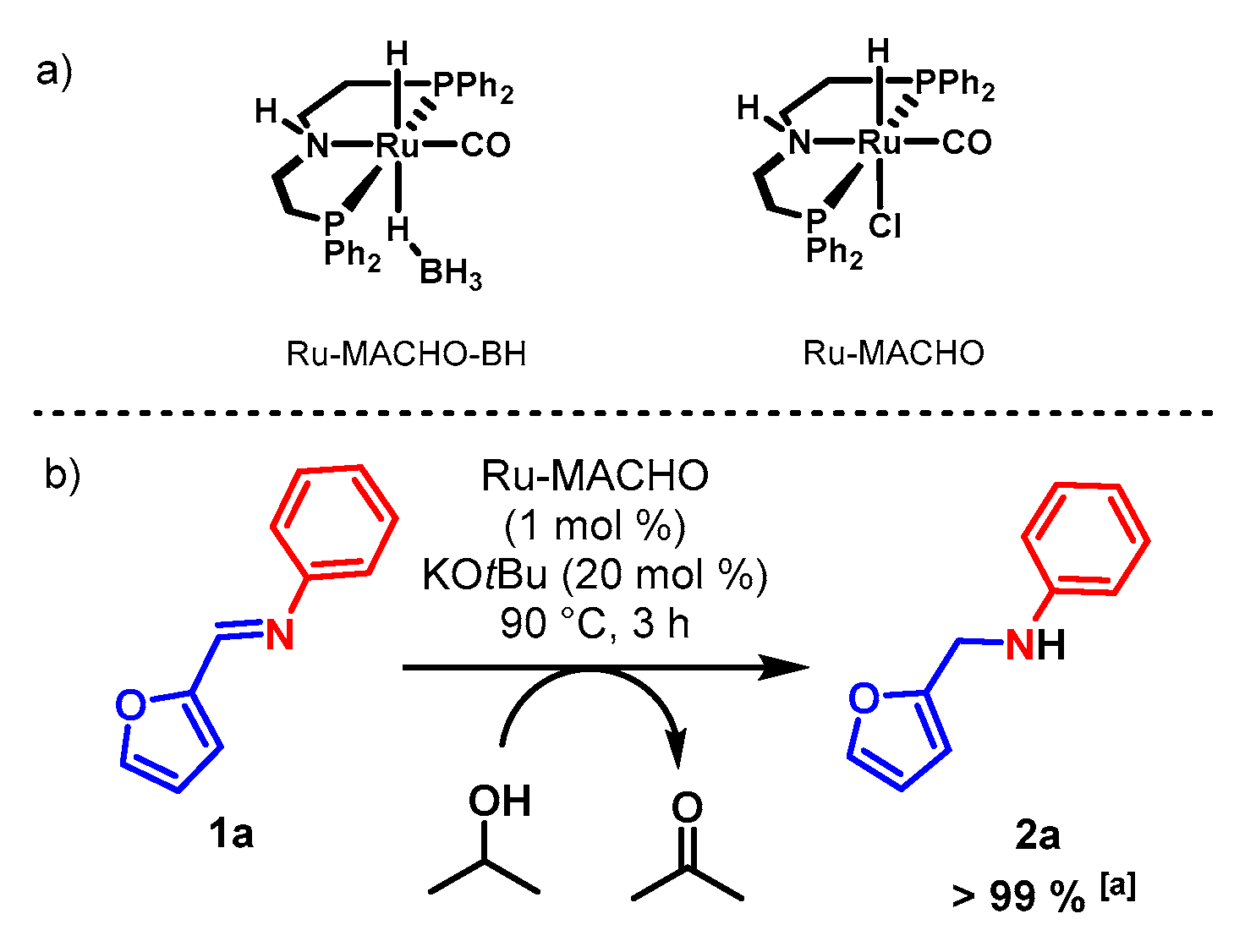


| Entry a | Catalyst (mol%) | Additive b | Time | Conv. c (%) |
|---|---|---|---|---|
| 1 | Ru-MACHO (0.5) | KOtBu | 1 h | >99 |
| 2 | Ru-MACHO (0.5) | KOtBu | 30 min | >99 |
| 3 | Ru-MACHO (0.5) | KOtBu | 15 min | >99 |
| 4 | Ru-MACHO (0.5) | KOtBu | 5 min | 51 |
| 5 | Ru-MACHO (0.5) | NaOH | 15 min | 18 |
| 6 | Ru-MACHO (0.1) | KOtBu | 15 min | <5 |
| 7 | Ru-MACHO-BH (0.5) | - | 15 min | <5 |
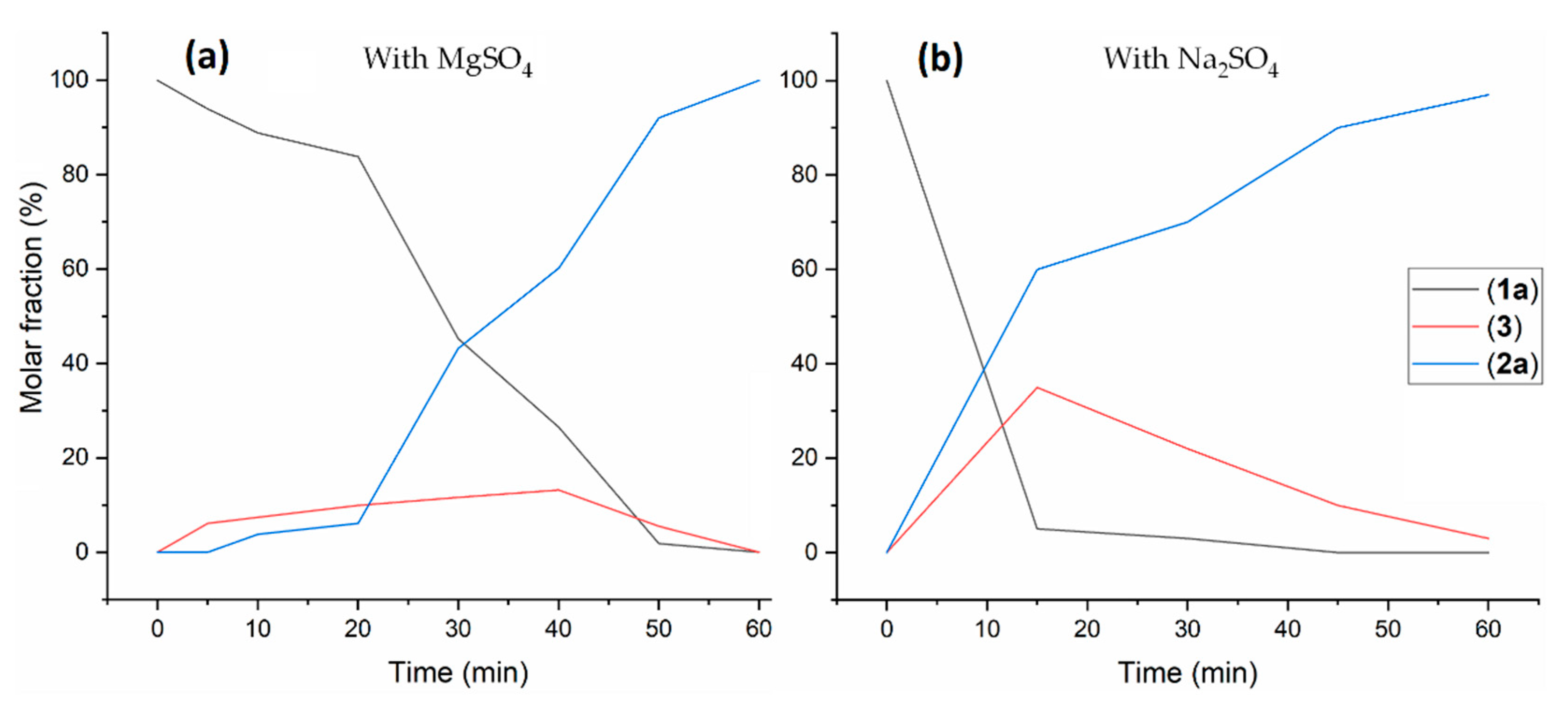
| Entry a | Catalyst (mol%) | Additive b | Temperature (°C) | Time | Conversion c (%) | 2a c (%) | 3 c (%) |
|---|---|---|---|---|---|---|---|
| 1 | Ru-MACHO (0.5) | KOtBu | 90 | 18 h | >99 | 70 | 30 |
| 2 | Ru-MACHO (0.5) | KOtBu + MgSO4 | 90 | 3 h | >99 | >99 | - |
| 3 | Ru-MACHO (0.5) | KOtBu + MgSO4 | 90 | 1 h | >99 | 93 | 7 |
| 4 | Ru-MACHO-BH (0.5) | MgSO4 | 90 | 1 h | 93 | >99 | - |
| 5 d | Ru-MACHO-BH (0.5) | MgSO4 | 90 | 1 h | >99 | >99 | - |
| 6 d | Ru-MACHO-BH (0.5) | MgSO4 | 90 | 45 min | 75 | 86 | 14 |
| 7 d | Ru-MACHO-BH (0.5) | MgSO4 | 90 | 30 min | 30 | 73 | 27 |
| 8 d | Ru-MACHO-BH (0.5) | - | 90 | 30 min | 15 | 52 | 48 |
| 9 d | Ru-MACHO-BH (0.25) | MgSO4 | 90 | 1 h | 11 | - | >99 |
| 10 d | Ru-MACHO-BH (0.5) | MgSO4 | 70 | 1 h | <5 | - | - |
| 11 d | Ru-MACHO-BH (0.5) | MgSO4 | 120 | 30 min | >99 | >99 | - |
| 12 d | Ru-MACHO-BH (0.5) | Na2SO4 | 90 | 1 h | >99 | 97 | 3 |
| 13 d | Ru-MACHO-BH (0.5) | Na2SO4 | 90 | 45 min | >99 | 90 | 10 |
| 14 d | Ru-MACHO-BH (0.5) | Na2SO4 | 90 | 30 min | >99 | 76 | 24 |
| 15 d | Ru-MACHO-BH (0.5) | Na2SO4 | 90 | 15 min | >99 | 57 | 42 |
| 16 d | Ru-MACHO-BH (0.5) | Na2SO4 | 70 | 1 h | 17 | 72 | 28 |
| 17 d | Ru-MACHO-BH (0.5) | MS 4 Å | 90 | 1 h | >99 | 94 | 6 |
| 18 d | Ru-MACHO-BH (0.5) | MS 4 Å | 90 | 15 min | >99 | 71 | 29 |
| 19 d | Ru-MACHO-BH (0.5) | MS 4 Å | 70 | 1 h | 71 | 96 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, D.L.J.; Nielsen, M. Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes. Catalysts 2021, 11, 558. https://doi.org/10.3390/catal11050558
Pinheiro DLJ, Nielsen M. Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes. Catalysts. 2021; 11(5):558. https://doi.org/10.3390/catal11050558
Chicago/Turabian StylePinheiro, Danielle Lobo Justo, and Martin Nielsen. 2021. "Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes" Catalysts 11, no. 5: 558. https://doi.org/10.3390/catal11050558
APA StylePinheiro, D. L. J., & Nielsen, M. (2021). Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes. Catalysts, 11(5), 558. https://doi.org/10.3390/catal11050558