Ru Catalysts Supported on Commercial and Biomass-Derived Activated Carbons for the Transformation of Levulinic Acid into γ-Valerolactone under Mild Conditions
Abstract
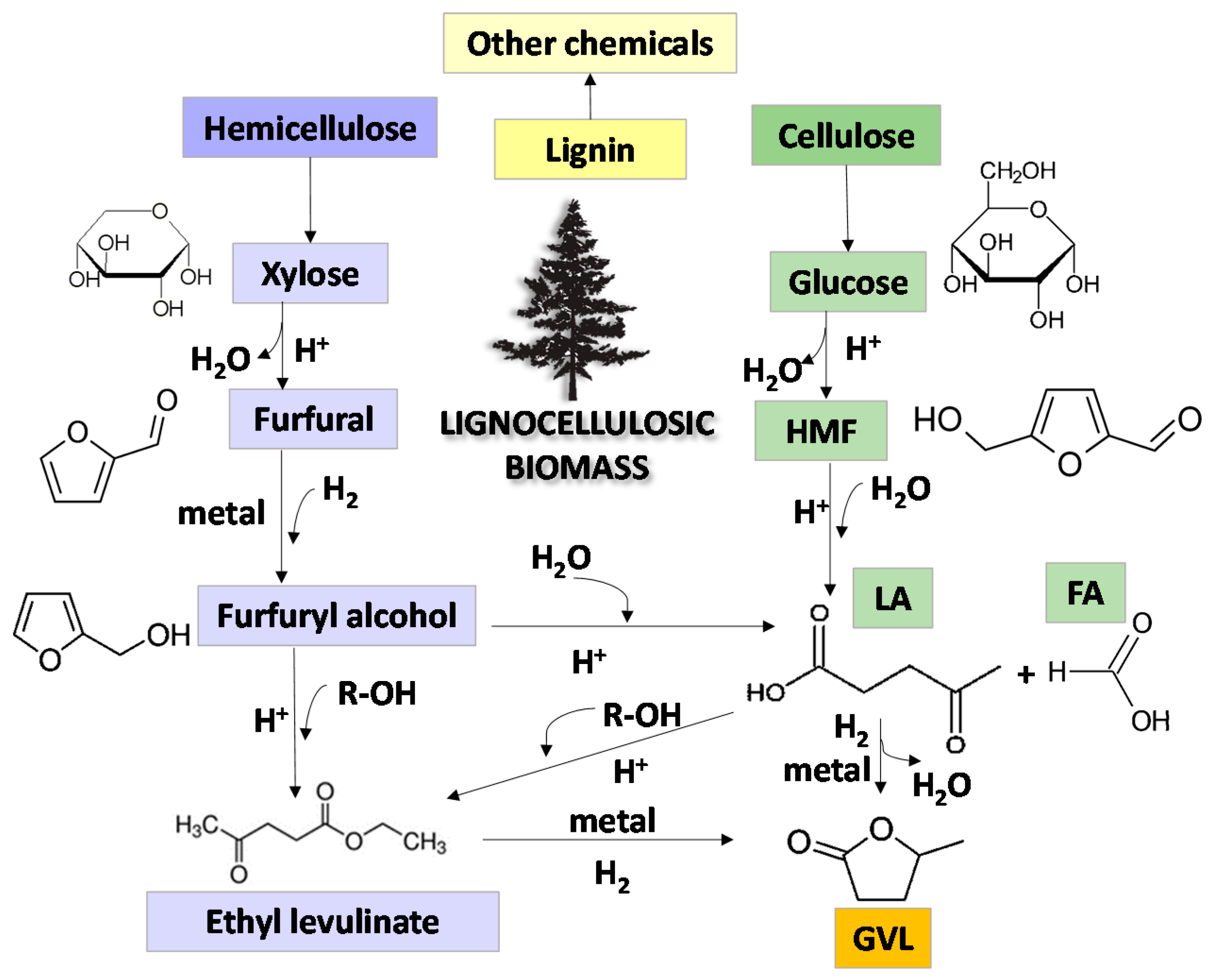
1. Introduction

2. Results and Discussion
2.1. Textural Properties of Supports and Catalysts
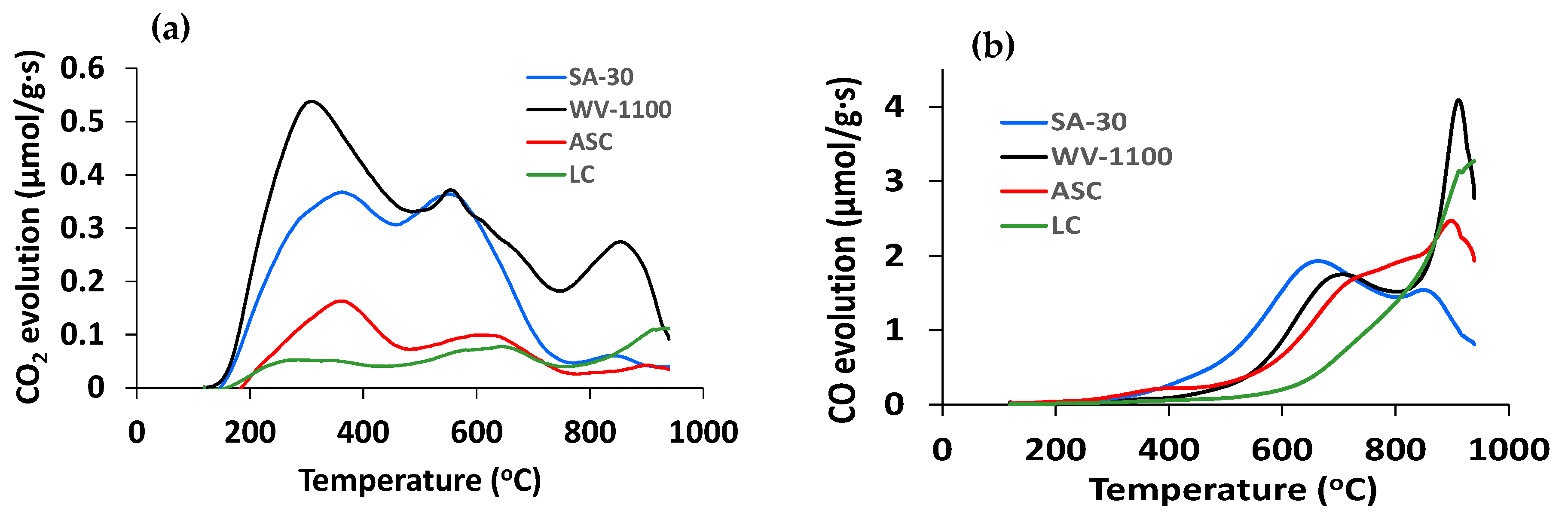
2.2. Surface Chemistry of Supports and Catalysts
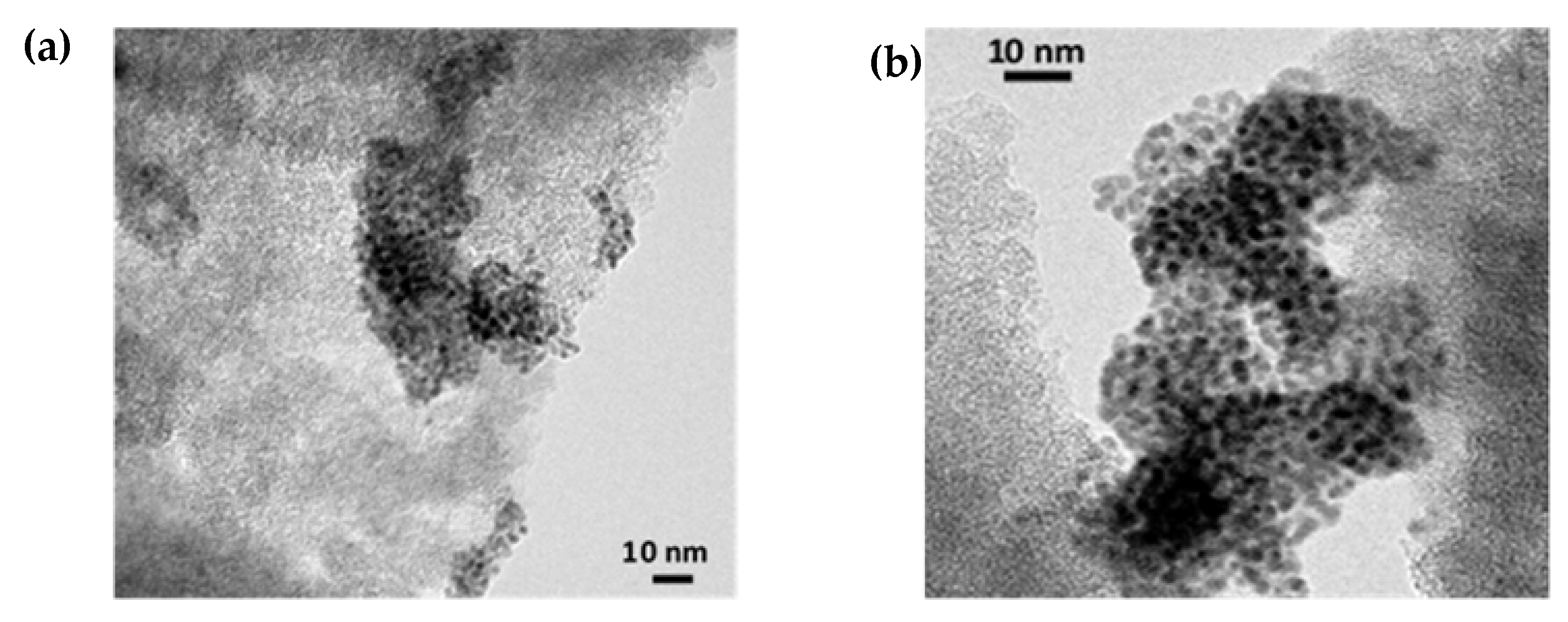
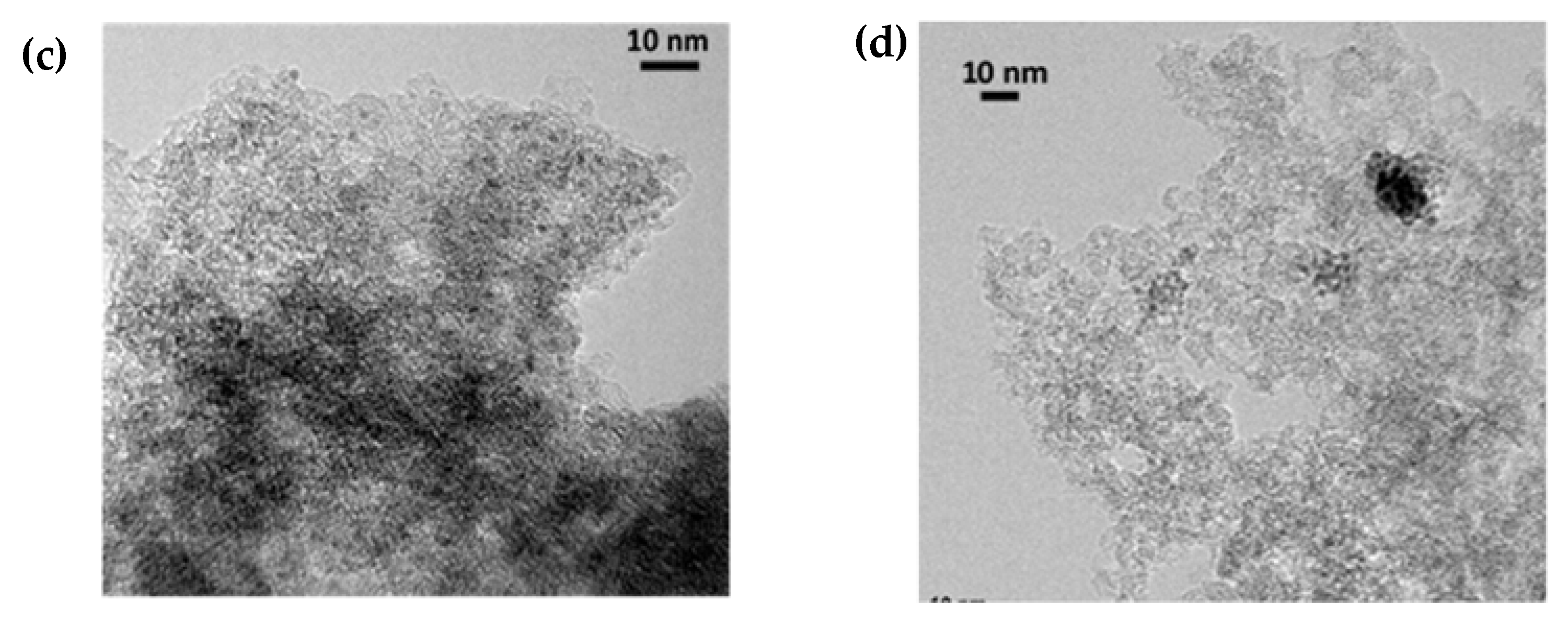
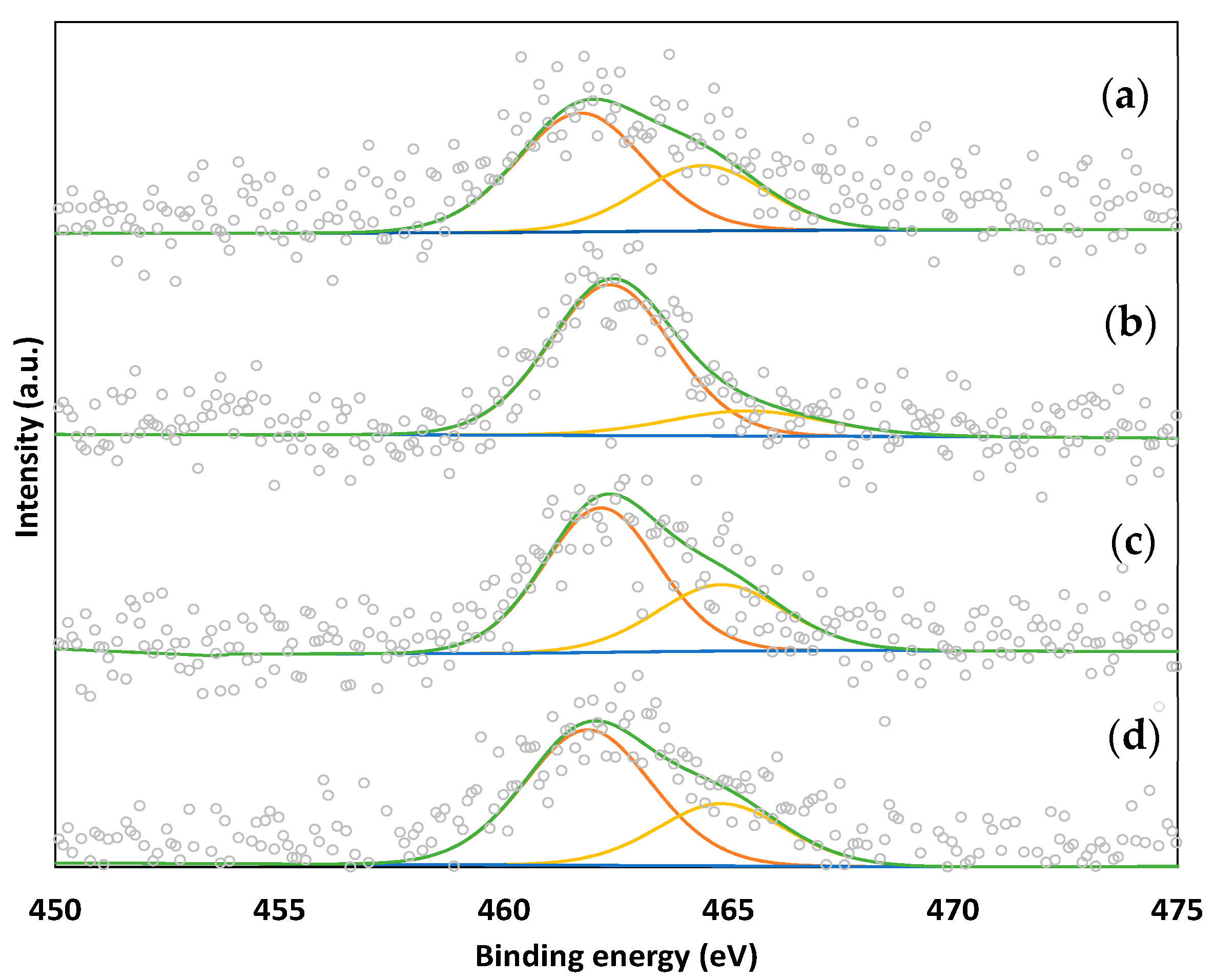
2.3. TEM and XPS Analysis of Ru Catalysts
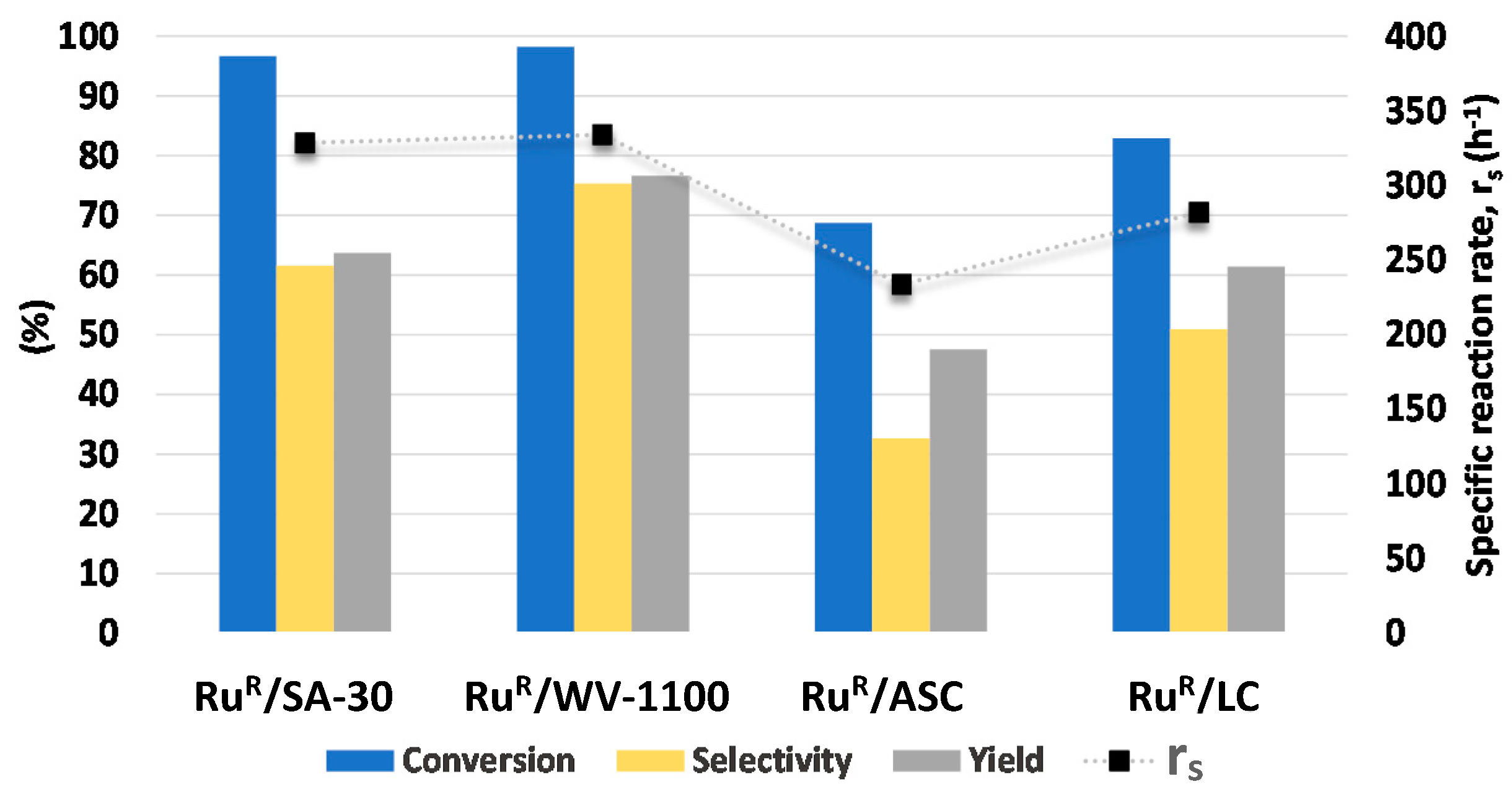
2.4. Catalytic Activity Tests
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Characterization of Supports and Catalysts
3.4. Catalytic Activity Tests
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef]
- Anjali, K.; Venkatesha, N.J.; Christopher, J.; Sakthivel, A. Rhodium porphyrin molecule-based catalysts for the hydrogenation of biomass derived levulinic acid to biofuel additive γ-valerolactone. New J. Chem. 2020, 44, 11064–11075. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Du, X.L.; Bi, Q.Y.; Liu, Y.M.; Cao, Y.; Fan, K.N. Conversion of biomass-derived levulinate and formate esters into γ-valerolactone over supported gold catalysts. ChemSusChem 2011, 4, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Licursi, D.; Di Nasso, N.N.O. Levulinic acid production from waste biomass. BioResources 2012, 7, 1824–1835. [Google Scholar] [CrossRef]
- Wang, Y.; Vernières-Hassimi, L.; Casson-Moreno, V.; Hébert, J.P.; Leveneur, S. Thermal risk assessment of levulinic acid hydrogenation to γ-valerolactone. Org. Process Res. Dev. 2018, 22, 1092–1100. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Mafokoane, M.; Seguel, J.; García, R.; Díaz de León, J.N.; Sepúlveda, C.; Escalona, N. Conversion of levulinic acid using CuO/WO3(x)-Al2O3 catalysts. Catal. Today 2020, 367, 310–319. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Carvalho, H.W.P.; Lu, C.; Kleist, W.; Grunwaldt, J.D. Synthesis of γ-valerolactone by hydrogenation of levulinic acid over supported nickel catalysts. Appl. Catal. A Gen. 2015, 502, 18–26. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Heyden, A.; Bond, J.Q. Analysis of kinetics and reaction pathways in the aqueous-phase hydrogenation of levulinic acid to form γ-Valerolactone over Ru/C. ACS Catal. 2014, 4, 1171–1181. [Google Scholar] [CrossRef]
- Wright, W.R.H.; Palkovits, R. Development of heterogeneous catalysts for the conversion of levulinic acid to γ-valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, E.V.; Turova, O.V.; Vinogradov, M.G.; Gorshkova, L.S.; Ferapontov, V.A. Enantioselective hydrogenation of levulinic acid esters in the presence of the RuII-BINAP-HCl catalytic system. Russ. Chem. Bull. 2005, 54, 2374–2378. [Google Scholar] [CrossRef]
- Mehdi, H.; Fábos, V.; Tuba, R.; Bodor, A.; Mika, L.T.; Horváth, I.T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass: From sucrose to levulinic acid, γ-valerolactone, 1,4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes. Top. Catal. 2008, 48, 49–54. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Selva, M.; Gottardo, M.; Perosa, A. Upgrade of biomass-derived levulinic acid via Ru/C-catalyzed hydrogenation to γ-valerolactone in aqueous-organic-ionic liquids multiphase systems. ACS Sustain. Chem. Eng. 2013, 1, 180–189. [Google Scholar] [CrossRef]
- Feng, J.; Gu, X.; Xue, Y.; Han, Y.; Lu, X. Production of γ-valerolactone from levulinic acid over a Ru/C catalyst using formic acid as the sole hydrogen source. Sci. Total Environ. 2018, 633, 426–432. [Google Scholar] [CrossRef]
- Glotov, A.; Novikov, A.; Stavitskaya, A.; Nedolivko, V.; Kopitsyn, D.; Kuchierskaya, A.; Ivanov, E.; Stytsenko, V.; Vinokurov, V.; Lvov, Y. Nanoreactors based on hydrophobized tubular aluminosilicates decorated with ruthenium: Highly active and stable catalysts for aromatics hydrogenation. Catal. Today 2020. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Nedolivko, V.; Zasypalov, G.; Stytsenko, V.; Karakhanov, E.; Vinokurov, V. Ruthenium catalysts templated on mesoporous MCM-41 type silica and natural clay nanotubes for hydrogenation of benzene to cyclohexane. Catalysts 2020, 10, 537. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Soszka, E.; Goscianska, J.; Kozanecki, M.; Grams, J.; Ruppert, A.M. The influence of carbon nature on the catalytic performance of Ru/C in levulinic acid hydrogenation with Internal Hydrogen Source. Molecules 2020, 25, 5362. [Google Scholar] [CrossRef]
- Yan, Z.P.; Lin, L.; Liu, S. Synthesis of γ-valerolactone by hydrogenation of biomass- derived levulinic acid over Ru/C catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Bourne, R.A.; Stevens, J.G.; Ke, J.; Poliakoff, M. Maximising opportunities in supercritical chemistry: The continuous conversion of levulinic acid to γ-valerolactone in CO2. Chem. Commun. 2007, 44, 4632–4634. [Google Scholar] [CrossRef] [PubMed]
- Hengne, A.M.; Rode, C.V. Cu–ZrO2 nanocomposite catalyst for selective hydrogenation of levulinic acid and its ester to γ-valerolactone. Green Chem. 2012, 14, 1064–1072. [Google Scholar] [CrossRef]
- Primo, A.; Concepción, P.; Corma, A. Synergy between the metal nanoparticles and the support for the hydrogenation of functionalized carboxylic acids to diols on Ru/TiO2. Chem. Commun. 2011, 47, 3613–3615. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Z.; Zhao, S.; Wang, D.; Wu, Z.; Zhang, M. A stable and effective Ru/polyethersulfone catalyst for levulinic acid hydrogenation to γ-valerolactone in aqueous solution. Catal. Today 2014, 234, 245–250. [Google Scholar] [CrossRef]
- Sudhakar, M.; Lakshmi Kantam, M.; Swarna Jaya, V.; Kishore, R.; Ramanujachary, K.V.; Venugopal, A. Hydroxyapatite as a novel support for Ru in the hydrogenation of levulinic acid to γ-valerolactone. Catal. Commun. 2014, 50, 101–104. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Jȩdrzejczyk, M.; Sneka-Płatek, O.; Keller, N.; Dumon, A.S.; Michel, C.; Sautet, P.; Grams, J. Ru catalysts for levulinic acid hydrogenation with formic acid as a hydrogen source. Green Chem. 2016, 18, 2014–2028. [Google Scholar] [CrossRef]
- Song, W.; Lozano-Martin, M.C.; Gallegos-Suarez, E.; Ramirez-Barria, C.; Weng, W.; Yi, X.; Bachiller-Baeza, B.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I. New insights in the development of carbon supported ruthenium catalysts for hydrogenation of levulinic acid. Curr. Catal. 2018, 7, 129–137. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Szymański, G.S.; Karpiński, Z.; Biniak, S.; Światkowski, A. The effect of the gradual thermal decomposition of surface oxygen species on the chemical and catalytic properties of oxidized activated carbon. Carbon 2002, 40, 2627–2639. [Google Scholar] [CrossRef]
- Román-Martínez, M.C.; Cazorla-Amorós, D.; Linares-Solano, A.; Salinas-Martínez de Lecea, C. TPD and TPR characterization of carbonaceous supports and Pt/C catalysts. Carbon 1993, 31, 895–902. [Google Scholar] [CrossRef]
- Azar, F.-Z.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Cellulose hydrolysis catalysed by mesoporous activated carbons functionalized under mild conditions. SN Appl. Sci. 2019, 1, 1739. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T. Role of surface phosphorus complexes on the oxidation of porous carbons. Fuel Process. Technol. 2017, 157, 116–126. [Google Scholar] [CrossRef]
- Peng, G.; Gramm, F.; Ludwig, C.; Vogel, F. Effect of carbon surface functional groups on the synthesis of Ru/C catalysts for supercritical water gasification. Catal. Sci. Technol. 2015, 5, 3658–3666. [Google Scholar] [CrossRef]
- Grigorev, M.E.; Mikhailov, S.P.; Bykov, A.V.; Tiamina, I.Y.; Nikoshvili, L.Z.; Sulman, M.G.; Vasiliev, A.L.; Sidorov, A.I.; dos Santos, T.V.; Meneghetti, M.R.; et al. Surface interactions with the metal oxide surface control Ru nanoparticle formation and catalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125722. [Google Scholar] [CrossRef]
- Folkesson, B.; Bjorøy, M.; Pappas, J.; Skaarup, S.; Aaltonen, R.; Swahn, C.-G. ESCA Studies on the charge distribution in some dinitrogen complexes of Rhenium, Iridium, Ruthenium, and Osmium. Acta Chem. Scand. 1973, 27, 287–302. [Google Scholar] [CrossRef]
- Shen, J.Y.; Adnot, A.; Kaliaguine, S. An ESCA study of the interaction of oxygen with the surface of ruthenium. Appl. Surf. Sci. 1991, 51, 47–60. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Auroux, A.; Gervasini, A. Microcalorimetric study of the acidity and basicity of metal oxide surfaces. J. Phys. Chem. 1990, 94, 6371–6379. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B.; Gnanakumar, E.S.; Martínez-Arias, A.; Gengler, R.; Rudolf, P.; Rothenberg, G.; Shiju, N.R. Highly selective hydrogenation of levulinic acid to γ-Valerolactone Over Ru/ZrO2 Catalysts. Catal. Lett. 2017, 147, 1744–1753. [Google Scholar] [CrossRef]
- Seretis, A.; Diamantopoulou, P.; Thanou, I.; Tzevelekidis, P.; Fakas, C.; Lilas, P.; Papadogianakis, G. Recent advances in Ruthenium-catalyzed hydrogenation reactions of renewable biomass-derived levulinic acid in aqueous media. Front. Chem. 2020, 8, 221. [Google Scholar] [CrossRef]
- Piskun, A.S.; van de Bovenkamp, H.H.; Rasrendra, C.B.; Winkelman, J.G.M.; Heeres, H.J. Kinetic modeling of levulinic acid hydrogenation to γ-valerolactone in water using a carbon supported Ru catalyst. Appl. Catal. A Gen. 2016, 525, 158–167. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Chaparro-Garnica, J.; Navlani-García, M.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D. Highly stable N-doped carbon-supported Pd-based catalysts prepared from biomass waste for H2 production from formic acid. ACS Sustain. Chem. Eng. 2020, 8, 15030–15043. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Salinas-Martínez de Lecea, C.; Alcañiz-Monge, J.; Cazorla-Amorós, D. Further advances in the characterization of microporous carbons by physical adsorption of gases. TANSO 1998, 185, 316–325. [Google Scholar] [CrossRef]
- Cazorla-Amorós, D.; Alcañiz-Monge, J.; Linares-Solano, A. Characterization of activated carbon fibers by CO2 adsorption. Langmuir 1996, 12, 2820–2824. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Linares-Solano, A. Microporous Structure of Activated Carbons as Revealed by Adsorption Methods; Thrower, P.A., Ed.; Chemistry and Physics of Carbon: New York, NY, USA, 1989; Volume 21, pp. 2–146. ISBN 0-8247-7939-8. [Google Scholar]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Xiao, C.; Goh, T.W.; Qi, Z.; Goes, S.; Brashler, K.; Perez, C.; Huang, W. Conversion of levulinic acid to γ-valerolactone over few-layer graphene-supported ruthenium catalysts. ACS Catal. 2016, 6, 593–599. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | VDR, N2 (cm3/g) | Vmeso (cm3/g) | VT (cm3/g) | VDR, CO2 (cm3/g) | Vsuper micro (cm3/g) |
|---|---|---|---|---|---|---|
| SA-30 | 1587 | 0.68 | 0.57 | 1.51 | 0.35 | 0.33 |
| Ru/SA-30 | 1465 | 0.63 | 0.51 | 1.36 | 0.33 | 0.30 |
| RuR/SA-30 | 1505 | 0.64 | 0.51 | 1.35 | 0.31 | 0.33 |
| WV-1100 | 1713 | 0.70 | 0.38 | 1.15 | 0.37 | 0.33 |
| Ru/WV-1100 | 1671 | 0.68 | 0.39 | 1.16 | 0.38 | 0.30 |
| RuR/WV-1100 | 1771 | 0.73 | 0.40 | 1.20 | 0.38 | 0.35 |
| ASC | 1916 | 0.79 | 0.49 | 1.51 | 0.43 | 0.36 |
| Ru/ASC | 1918 | 0.79 | 0.49 | 1.50 | 0.43 | 0.36 |
| RuR/ASC | 1323 | 0.55 | 0.60 | 1.44 | 0.27 | 0.28 |
| LC | 831 | 0.33 | 0.41 | 0.98 | 0.19 | 0.12 |
| Ru/LC | 761 | 0.30 | 0.39 | 0.95 | 0.17 | 0.13 |
| RuR/LC | 1238 | 0.51 | 0.30 | 0.92 | 0.17 | 0.34 |
| Sample | CO2 (µmol/g) | CO (µmol/g) | CO2/CO | O total (wt.%) |
|---|---|---|---|---|
| SA-30 | 539 | 2065 | 0.26 | 5.0 |
| WV-1100 | 735 | 2203 | 0.33 | 5.9 |
| ASC | 276 | 2024 | 0.14 | 4.1 |
| LC | 146 | 1455 | 0.10 | 2.8 |
| Sample | Ru 3p3/2 B.E. (eV) | Atomic Ratio | |||
|---|---|---|---|---|---|
| Ru0 | RuOx/Ru0 | Ru/C | Cl/C | Cl/Ru | |
| RuR/SA-30 | 461.70 (63%) | 464.42 (37%) | 0.0026 | 0.0005 | 0.19 |
| RuR/WV-1100 | 462.36 (83%) | 465.42 (17%) | 0.0032 | 0.0032 | 1.00 |
| RuR/ASC | 462.15 (66%) | 464.83 (34%) | 0.0032 | 0.0035 | 1.10 |
| RuR/LC | 461.87 (68%) | 464.84 (32%) | 0.0031 | 0.0011 | 0.36 |
| Catalyst | LA Conversion (%) | GVL Yield (%) | GVL Selectivity (%) | rs ** (h−1) |
|---|---|---|---|---|
| RuR/SA-30 | 100 | 82 | 82 | 340 |
| RuR/WV-1100 | 100 | 87 | 87 | 340 |
| RuR/ASC | 96 | 81 | 84 | 327 |
| RuR/LC | 98 | 92 | 94 | 334 |
| Catalyst | Conversion (%) | GVL Yield (%) | GVL Selectivity (%) |
|---|---|---|---|
| Ru/SA-30 run 1 | 100 | 82 | 82 |
| Ru/SA-30 run 2 | 100 | 78 | 78 |
| Ru/ASC run 1 | 96 | 81 | 84 |
| Ru/ASC run 2 | 100 | 84 | 84 |
| Ru/LC run 1 | 98 | 92 | 94 |
| Ru/LC run 2 | 100 | 94 | 94 |
| Entry | Support | Ru wt.% | S/C a | Solvent | T (°C) | P (bar) | t (min) | Conv. (%) | Sel. (%) | rs * (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C (not specified) | 5 | 348 | methanol | 130 | 12 | 160 | 92 | 91 | 129 | [25] |
| 2 | C (not specified) | 5 | -- | dioxane | 150 | 55 | 120 | 80 | 72 | -- | [19] |
| 3 | C (not specified) | 5 | -- | dioxane | 150 | 34 | 240 | 100 | 97 | -- | [19] |
| 4 | C-DARCO b | 5 | 58 | water | 190 | 10 | 60 | 87 | 75 | 49 | [31] |
| 5 | C c–h | 5 | 58 | water | 190 | 10 | 60 | 80–95 | 80–87 | 44–55 | [24] |
| 6 | AC i | 4 | 2256 | water | 100 | 50 | 180 | 43 | 91 | 329 | [32] |
| 7 | rGO j | 4 | 2256 | water | 100 | 50 | 180 | 100 | 80 | 764 | [32] |
| 8 | C (Table 2) | 1 | 338 | water | 170 | 15 | 60 | 96–100 | 82–94 | 327–340 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Bernal, Z.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. Ru Catalysts Supported on Commercial and Biomass-Derived Activated Carbons for the Transformation of Levulinic Acid into γ-Valerolactone under Mild Conditions. Catalysts 2021, 11, 559. https://doi.org/10.3390/catal11050559
Ruiz-Bernal Z, Lillo-Ródenas MÁ, Román-Martínez MdC. Ru Catalysts Supported on Commercial and Biomass-Derived Activated Carbons for the Transformation of Levulinic Acid into γ-Valerolactone under Mild Conditions. Catalysts. 2021; 11(5):559. https://doi.org/10.3390/catal11050559
Chicago/Turabian StyleRuiz-Bernal, Zaira, María Ángeles Lillo-Ródenas, and María del Carmen Román-Martínez. 2021. "Ru Catalysts Supported on Commercial and Biomass-Derived Activated Carbons for the Transformation of Levulinic Acid into γ-Valerolactone under Mild Conditions" Catalysts 11, no. 5: 559. https://doi.org/10.3390/catal11050559
APA StyleRuiz-Bernal, Z., Lillo-Ródenas, M. Á., & Román-Martínez, M. d. C. (2021). Ru Catalysts Supported on Commercial and Biomass-Derived Activated Carbons for the Transformation of Levulinic Acid into γ-Valerolactone under Mild Conditions. Catalysts, 11(5), 559. https://doi.org/10.3390/catal11050559








