Multi-Scale Biosurfactant Production by Bacillus subtilis Using Tuna Fish Waste as Substrate
Abstract
1. Introduction
2. Results and Discussion
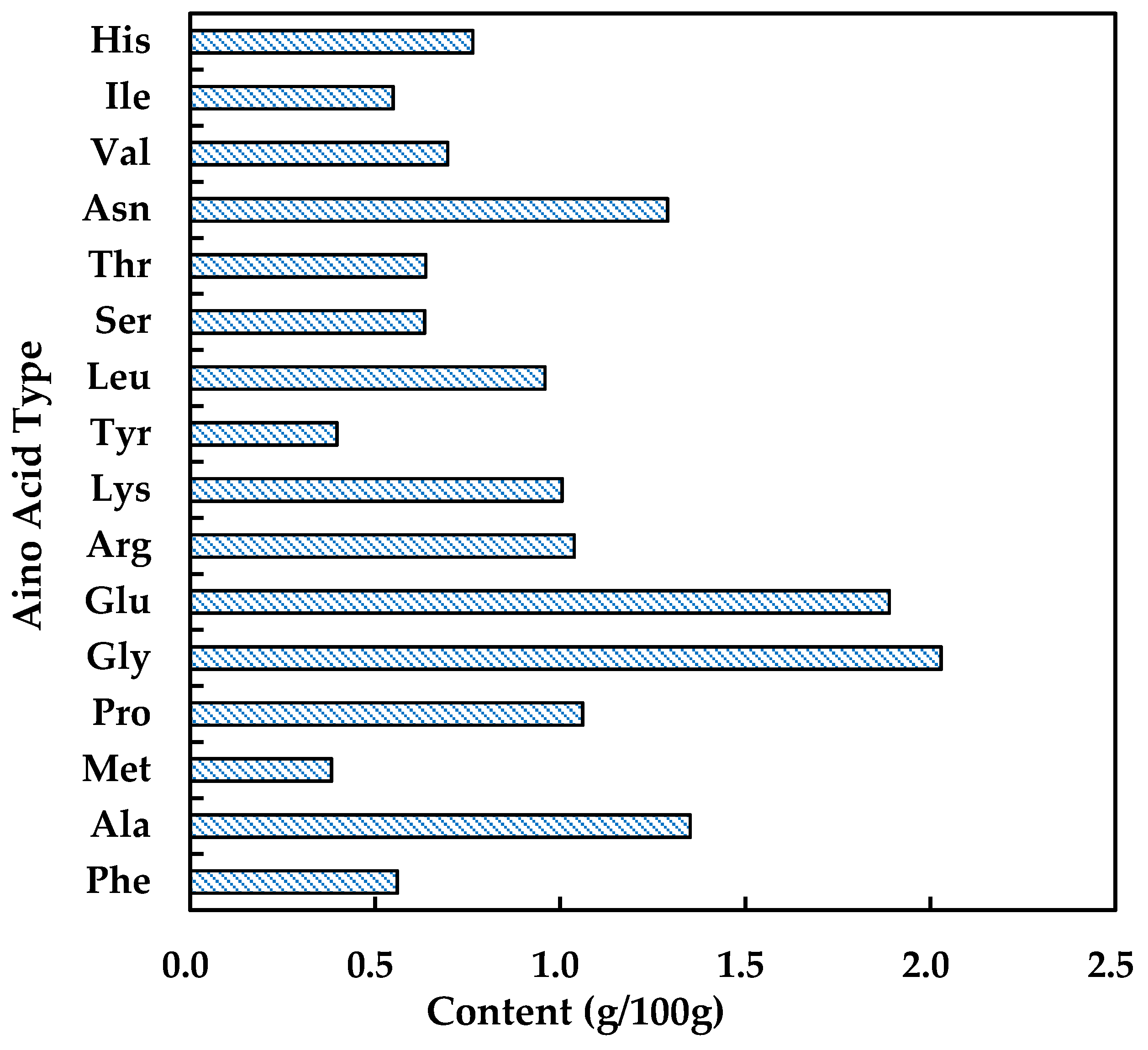
2.1. Characterization of Hydrolyzed Peptones
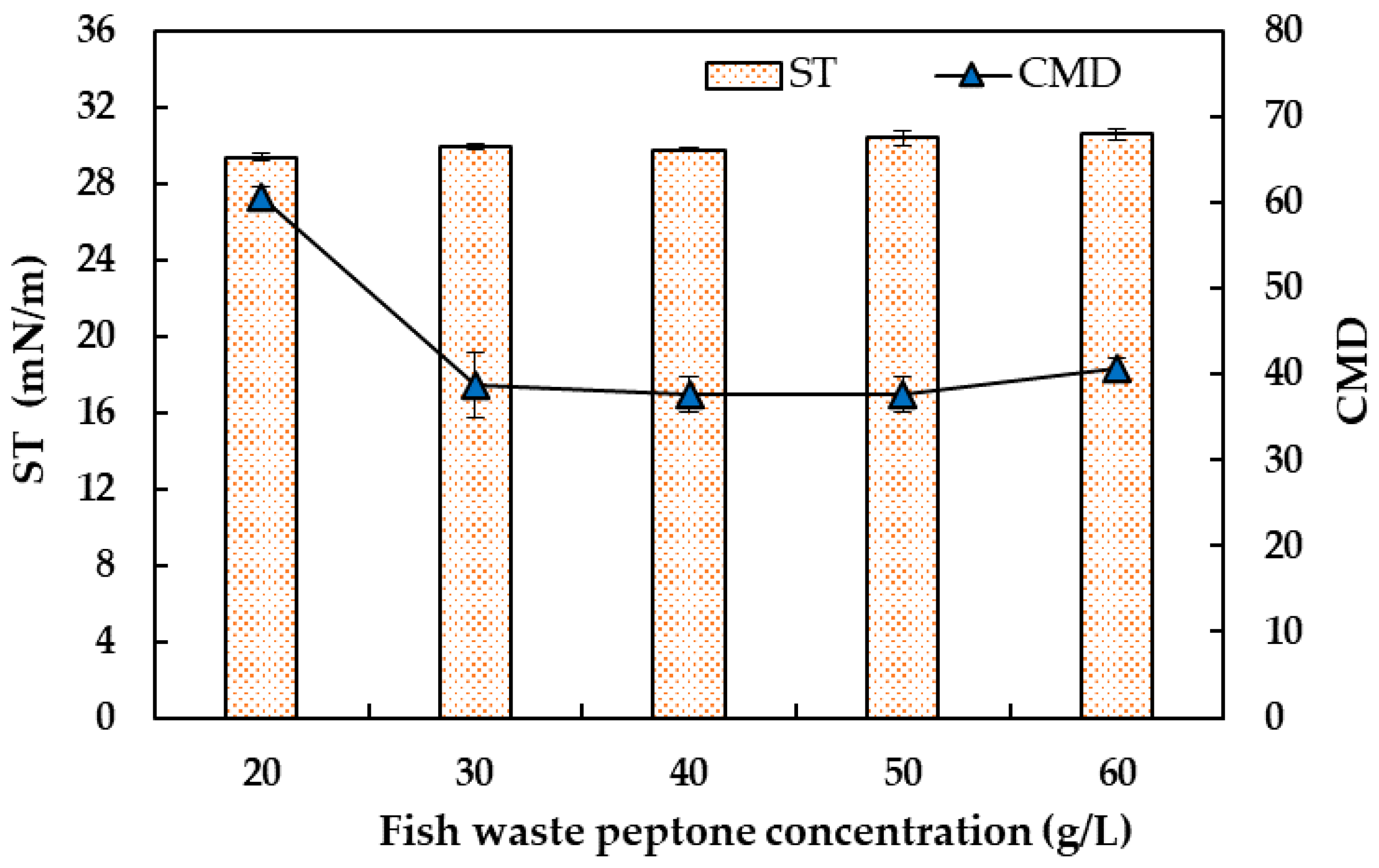
2.2. Bench-Scale Production of Biosurfactants
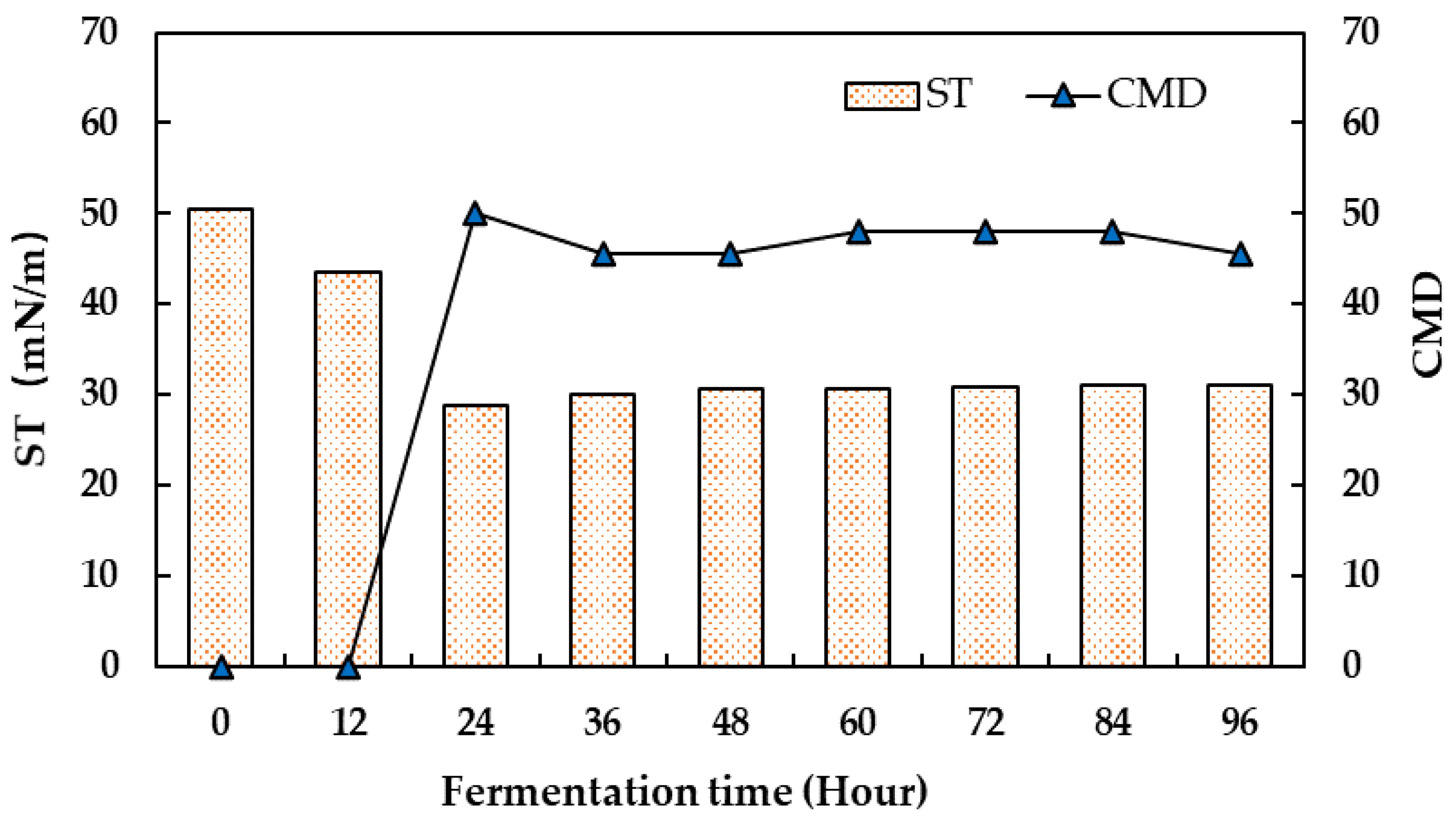
2.3. Batch-Scale Production of Biosurfactants
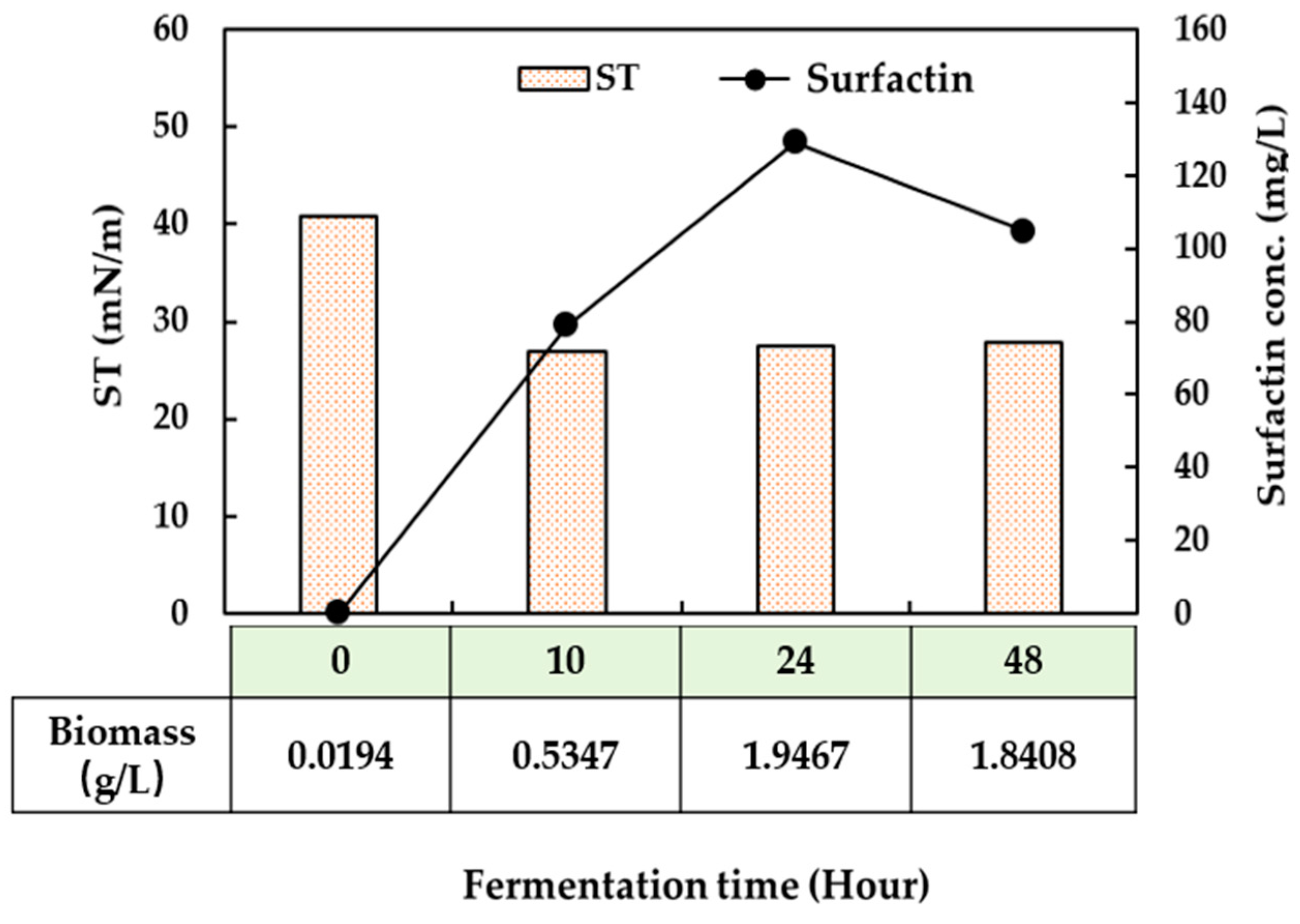
2.4. Pilot-Scale Biosurfactant Production Experiments
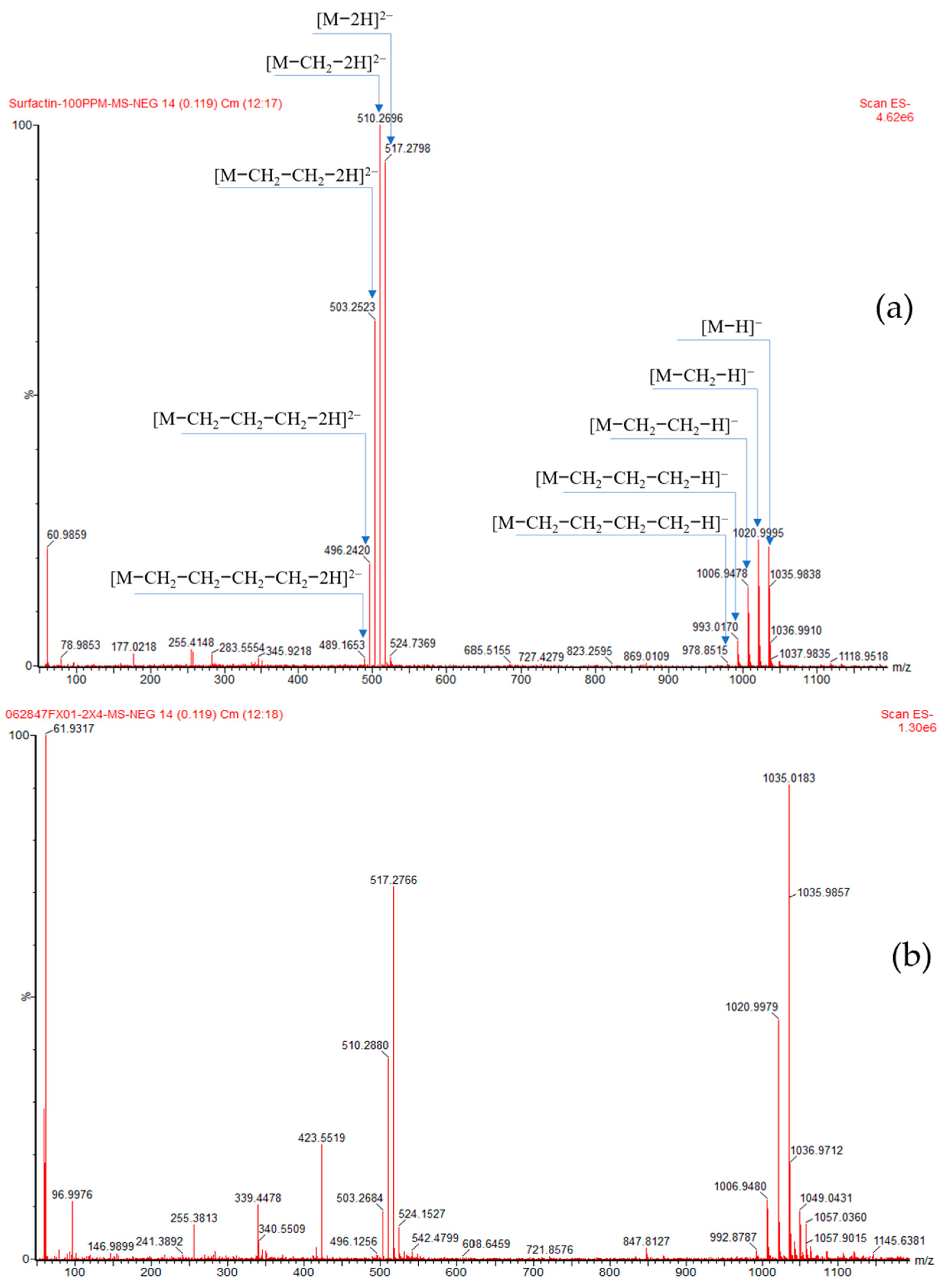
2.5. Characterization of Biosurfactant Production
3. Methodology
3.1. Materials
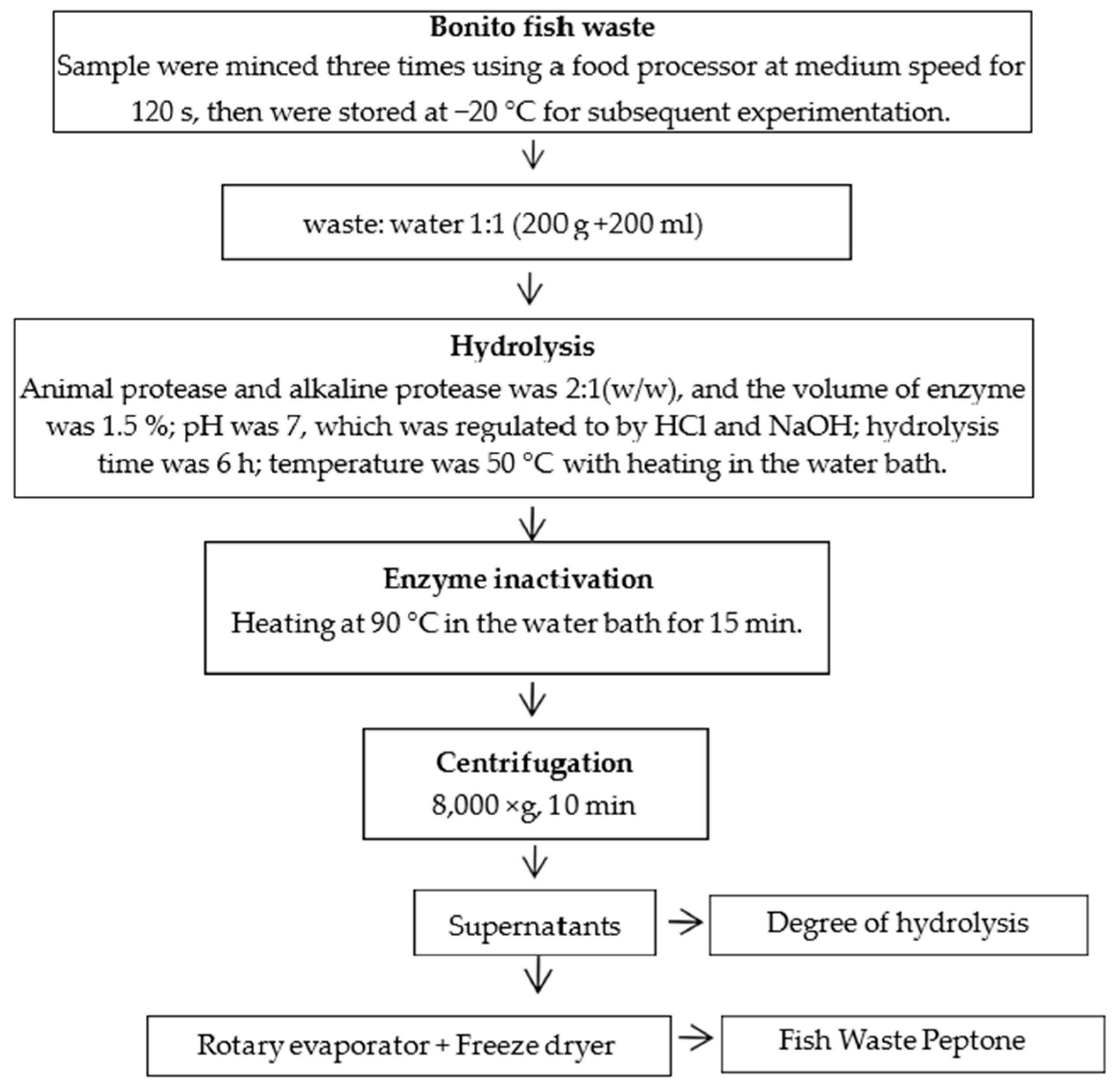
3.2. Enzymatic Hydrolysis for Generating a Fish Waste-Based Substrate
3.3. Bench-Scale Biosurfactant Production (20 mL)
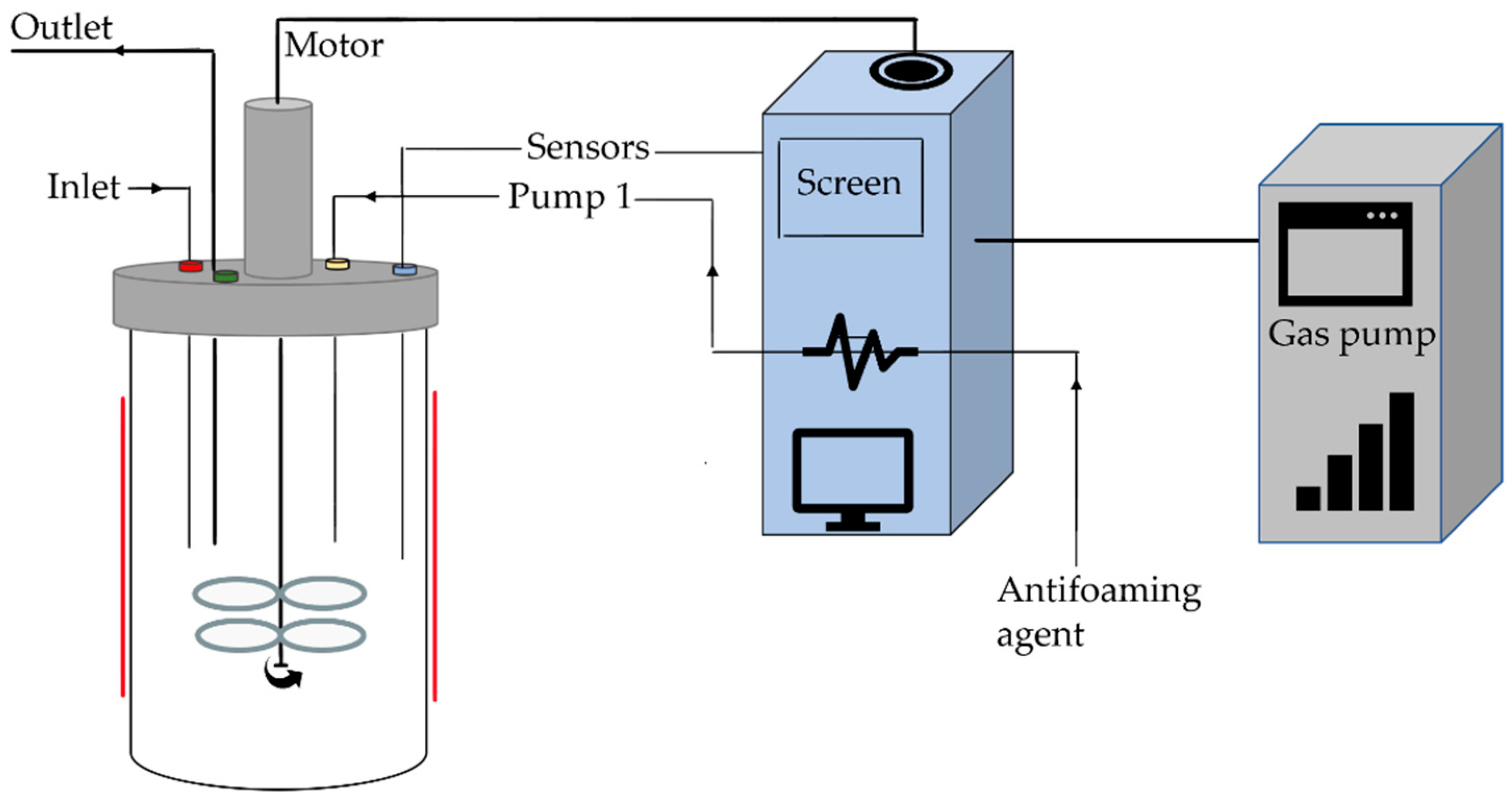
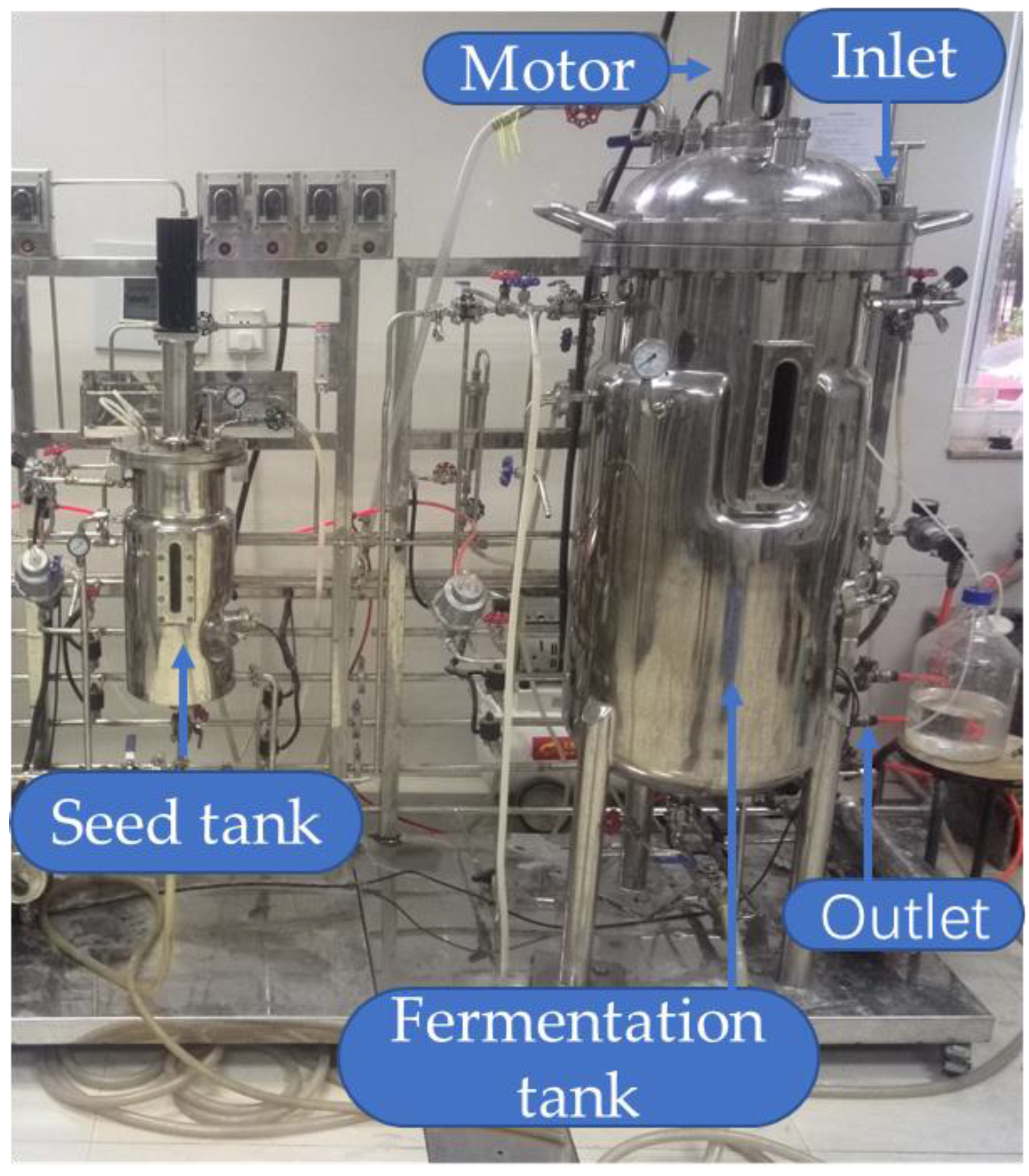
3.4. Batch-Scale Biosurfactant Production (7 L)
3.5. Pilot-Scale Biosurfactant Production (100 L)
3.6. Evaluation of Biosurfactant Production Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Zhang, B.; Chen, B.; Ling, J.; Cai, Q.; Husain, T. Fly ash based robust biocatalyst generation: A sustainable strategy towards enhanced green biosurfactant production and waste utilization. RSC Adv. 2019, 9, 20216–20225. [Google Scholar] [CrossRef]
- Georgiou, G.; Lin, S.-C.; Sharma, M.M. Surface–active compounds from microorganisms. Biotechnology 1992, 10, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; You, Y.; Zhao, R.; Sun, D.; Zhang, P.; Jiang, J.; Zhu, A.; Liu, W. Biosurfactant production from Pseudomonas taiwanensis L1011 and its application in accelerating the chemical and biological decolorization of azo dyes. Ecotoxicol. Environ. Saf. 2017, 145, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. [Google Scholar] [CrossRef]
- Aparna, A.; Srinikethan, G.; Smitha, H. Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf. B Biointerfaces 2012, 95, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Unás, J.H.; de Alexandria Santos, D.; Azevedo, E.B.; Nitschke, M. Brevibacterium luteolum biosurfactant: Production and structural characterization. Biocatal. Agric. Biotechnol. 2018, 13, 160–167. [Google Scholar] [CrossRef]
- Khaje Bafghi, M.; Fazaelipoor, M.H. Application of rhamnolipid in the formulation of a detergent. J. Surfactants Deterg. 2012, 15, 679–684. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Hasan, H.A.; Idris, M.; Abdullah, S.R.S.; Anuar, N. Potential application of a biosurfactant in phytoremediation technology for treatment of gasoline-contaminated soil. Ecol. Eng. 2015, 84, 113–120. [Google Scholar] [CrossRef]
- Rogers, R.E.; Kothapalli, C.; Lee, M.S.; Woolsey, J.R. Catalysis of Gas Hydrates by Biosurfactants in Seawater-Saturated Sand/Clay. Can. J. Chem. Eng. 2003, 81, 973–980. [Google Scholar] [CrossRef]
- Arora, A.; Cameotra, S.S.; Kumar, R.; Balomajumder, C.; Singh, A.K.; Santhakumari, B.; Kumar, P.; Laik, S. Biosurfactant as a promoter of methane hydrate formation: Thermodynamic and kinetic studies. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Jiao, J.; Xin, X.; Wang, X.; Xie, Z.; Xia, C.; Pan, W. Self-assembly of biosurfactant–inorganic hybrid nanoflowers as efficient catalysts for degradation of cationic dyes. RSC Adv. 2017, 7, 43474–43482. [Google Scholar] [CrossRef]
- Castelletto, V.; Edwards-Gayle, C.J.; Hamley, I.W.; Pelin, J.N.; Alves, W.A.; Aguilar, A.M.; Seitsonen, J.; Ruokolainen, J. Self-assembly of a catalytically active lipopeptide and its incorporation into cubosomes. ACS Appl. Bio Mater. 2019, 2, 3639–3647. [Google Scholar] [CrossRef]
- Sen, R.; Swaminathan, T. Application of response-surface methodology to evaluate the optimum environmental conditions for the enhanced production of surfactin. Appl. Microbiol. Biotechnol. 1997, 47, 358–363. [Google Scholar] [CrossRef]
- Jing, L.; Qian, Y. Research progress on biocontrol Bacillus subtilis. J. Anhui Agric. Sci. 2008, 36, 106. [Google Scholar]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–494. [Google Scholar] [CrossRef]
- Peypoux, F.; Bonmatin, J.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef]
- Mandal, S.M.; Barbosa, A.E.; Franco, O.L. Lipopeptides in microbial infection control: Scope and reality for industry. Biotechnol. Adv. 2013, 31, 338–345. [Google Scholar] [CrossRef]
- Altenbuchner, J. Editing of the Bacillus subtilis genome by the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2016, 82, 5421–5427. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, J.; Wang, W.; Huang, H.; Li, S. Production of surfactin isoforms by Bacillus subtilis BS-37 and its applicability to enhanced oil recovery under laboratory conditions. Biochem. Eng. J. 2015, 93, 31–37. [Google Scholar] [CrossRef]
- Santos, V.S.V.; Silveira, E.; Pereira, B.B. Toxicity and applications of surfactin for health and environmental biotechnology. J. Toxicol. Environ. Health Part B 2018, 21, 382–399. [Google Scholar] [CrossRef]
- Fei, D.; Liu, F.-F.; Gang, H.-Z.; Liu, J.-F.; Yang, S.-Z.; Ye, R.-Q.; Mu, B.-Z. A new member of the surfactin family produced by Bacillus subtilis with low toxicity on erythrocyte. Process Biochem. 2020, 94, 164–171. [Google Scholar] [CrossRef]
- Nitschke, M.; Araújo, L.; Costa, S.; Pires, R.; Zeraik, A.; Fernandes, A.; Freire, D.; Contiero, J. Surfactin reduces the adhesion of food-borne pathogenic bacteria to solid surfaces. Lett. Appl. Microbiol. 2009, 49, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, I.M.; Vaz, D.A.; Banat, I.M.; Marchant, R.; Alameda, E.J.; Román, M.G. Hydrolysis of olive mill waste to enhance rhamnolipids and surfactin production. Bioresour. Technol. 2016, 205, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chaprão, M.J.; Rita De Cássia, F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments. Ecotoxicology 2018, 27, 1310–1322. [Google Scholar] [CrossRef]
- Santos, D.K.; Brandão, Y.B.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Santos, V.A.; Sarubbo, L.A. Optimization of cultural conditions for biosurfactant production from Candida lipolytica. Biocatal. Agric. Biotechnol. 2014, 3, 48–57. [Google Scholar] [CrossRef]
- Zouari, R.; Ellouze-Chaabouni, S.; Ghribi, D. Use of butter milk and poultry-transforming wastes for enhanced production of Bacillus SPB1 biosurfactant in submerged fermentation. J. Microbiol. Biotechnol. Food Sci. 2019, 2019, 462–466. [Google Scholar] [CrossRef]
- Kadam, D.; Savant, D. Biosurfactant Production from Shrimp Shell Waste by Pseudomonas stutzeri; NISCAIR-CSIR: New Delhi, India, 2019. [Google Scholar]
- Jia, J.; Zhou, Y.; Lin, S. Nutritional components analysis of Thunnus albacares bone. Sci. Technol. Food Ind. 2013, 34, 334–337. [Google Scholar]
- Boopathy, R.; Bonvillain, C.; Fontenot, Q.; Kilgen, M. Biological treatment of low-salinity shrimp aquaculture wastewater using sequencing batch reactor. International Biodeterior. Biodegrad. 2007, 59, 16–19. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, B.; Cai, Q.; Ling, J.; Lee, K.; Chen, B. Fish waste based lipopeptide production and the potential application as a bio-dispersant for oil spill control. Front. Bioeng. Biotechnol. 2020, 8, 734. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Docasal, S.; Prieto, M.; González, M.P.; Murado, M. Growth and metabolic features of lactic acid bacteria in media with hydrolysed fish viscera. An approach to bio-silage of fishing by-products. Bioresour. Technol. 2008, 99, 6246–6257. [Google Scholar] [CrossRef]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess Technol. 2012, 5, 73–79. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Xiao, X.; Jia, Y.; Fang, P.; Huang, J.-H.; Wu, H.-W.; Tang, Z.-J.; Chen, D.-Y. Simultaneous Removal of SO2 and NO by O3 Oxidation Combined with Wet Absorption. ACS Omega 2020, 5, 5844–5853. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-H.; Chu, I.-M. Enhancement of surfactin production in iron-enriched media by Bacillus subtilis ATCC 21332. Enzym. Microb. Technol. 1998, 22, 724–728. [Google Scholar] [CrossRef]
- Fox, S.L.; Bala, G.A. Production of surfactant from Bacillus subtilis ATCC 21332 using potato substrates. Bioresour. Technol. 2000, 75, 235–240. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Lai, C.-C.; Chang, J.-S. Using Taguchi experimental design methods to optimize trace element composition for enhanced surfactin production by Bacillus subtilis ATCC 21332. Process Biochem. 2007, 42, 40–45. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Loh, K.-C. Activated carbon enhanced anaerobic digestion of food waste–laboratory-scale and pilot-scale operation. Waste Manag. 2018, 75, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. Research of the Fish Protein Organic Liquid Fertilizer with Cadmium Enrichment Development from Katsuwonus Pelamis by Products. Master’s of Thesis, Ningbo University, Ningbo, China, 2013. [Google Scholar]
- Davis, D.; Lynch, H.; Varley, J. The production of surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzym. Microb. Technol. 1999, 25, 322–329. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S.; Lobianco, A.; Angelini, D.L.; Borghini, F.; Focardi, S.E. Degradation of fatty acids and production of biosurfactant as an added value, by a bacterial strain Pseudomonas aeruginosa DG2a isolated from aquaculture wastewaters. Water Air Soil Pollut. 2013, 224, 1–11. [Google Scholar] [CrossRef]
- Deshpande, K.L.; Katze, J.; Kane, J.F. Effect of glutamine on enzymes of nitrogen metabolism in Bacillus subtilis. J. Bacteriol. 1981, 145, 768–774. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.n.; Wang, Y.; Liu, J.; Lu, L. The positive effects of Mn2+ on nitrogen use and surfactin production by Bacillus subtilis ATCC 21332. Biotechnol. Biotechnol. Equip. 2015, 29, 381–389. [Google Scholar] [CrossRef]
- Reis, R.; Pacheco, G.; Pereira, A.; Freire, D. Biosurfactants: Production and Applications; Biodegradation-Life of Science; CRC Press: Boca Raton, FL, USA, 2013; pp. 31–61. [Google Scholar]
- Shaligram, N.S.; Singhal, R.S. Surfactin—A review on biosynthesis, fermentation, purification and applications. Food Technol. Biotechnol. 2010, 48, 119–134. [Google Scholar]
- Yeh, M.S.; Wei, Y.H.; Chang, J.S. Enhanced Production of Surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2005, 21, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Ariff, A.; Marziah, M.; Ali, A.; Lajis, N. Strategies to overcome foaming and wall-growth during the cultivation of Morinda elliptica cell suspension culture in a stirred-tank bioreactor. Plant Cell Tissue Organ Cult. 2000, 60, 205–212. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Guo, R.; Ma, G.; Li, S. Optimization of Culture Conditions for the Enhancement of Surfactin Production from Bacillus substilis B006. Chin. Biotechnol. Bull. 2017, 33, 214–221. [Google Scholar]
- Xu, N.; Liu, S.; Xu, L.; Zhou, J.; Xin, F.; Zhang, W.; Qian, X.; Li, M.; Dong, W.; Jiang, M. Enhanced rhamnolipids production using a novel bioreactor system based on integrated foam-control and repeated fed-batch fermentation strategy. Biotechnol. Biofuels 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, B.; Chen, B.; Zhu, Z.; Zhao, Y. A novel bioemulsifier produced by Exiguobacterium sp. strain N4-1P isolated from petroleum hydrocarbon contaminated coastal sediment. RSC Adv. 2017, 7, 42699–42708. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Q.; Jiang, L.; Huang, H.; Li, S. Polydiacetylene-based high-throughput screen for surfactin producing strains of Bacillus subtilis. PLoS ONE 2014, 9, e88207. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Luo, J.; Zhu, Z.; Chen, B.; Ye, X.; Zhu, P.; Zhang, B. Multi-Scale Biosurfactant Production by Bacillus subtilis Using Tuna Fish Waste as Substrate. Catalysts 2021, 11, 456. https://doi.org/10.3390/catal11040456
Hu J, Luo J, Zhu Z, Chen B, Ye X, Zhu P, Zhang B. Multi-Scale Biosurfactant Production by Bacillus subtilis Using Tuna Fish Waste as Substrate. Catalysts. 2021; 11(4):456. https://doi.org/10.3390/catal11040456
Chicago/Turabian StyleHu, Jiheng, Jie Luo, Zhiwen Zhu, Bing Chen, Xudong Ye, Peng Zhu, and Baiyu Zhang. 2021. "Multi-Scale Biosurfactant Production by Bacillus subtilis Using Tuna Fish Waste as Substrate" Catalysts 11, no. 4: 456. https://doi.org/10.3390/catal11040456
APA StyleHu, J., Luo, J., Zhu, Z., Chen, B., Ye, X., Zhu, P., & Zhang, B. (2021). Multi-Scale Biosurfactant Production by Bacillus subtilis Using Tuna Fish Waste as Substrate. Catalysts, 11(4), 456. https://doi.org/10.3390/catal11040456








