Low-Dimensional Nanostructured Photocatalysts for Efficient CO2 Conversion into Solar Fuels
Abstract
1. Introduction
2. The Main Fundamentals of CO2 Photoconversion into Solar Fuels and Hydrocarbon Species
2.1. Nature of CO2
2.2. CO2 Adsorption on the Surface of Photocatalysts
2.3. The Mechanism of Efficient CO2 Photoconversion
3. State-of-the-Art Progress in Low-Dimensional Nanostructured Photocatalyst
3.1. Low-Dimensional Semiconductor Materials in Photocatalytic Application
3.1.1. Zero-Dimensional Structure-Based Photocatalysts for CO2 Conversion
3.1.2. One-Dimensional Structure-Based Photocatalyst for CO2 Conversion
3.1.3. Two-Dimensional Structure-Based Photocatalysts for CO2 Conversion
3.2. Low-Dimensional Metal Materials for CO2 Conversion
4. Strategies for Enhancement in the Light-Driven CO2 Conversion over Low-Dimensional Photocatalysts
4.1. Construction of Junction Formed by Low-Dimensional Structures
4.2. Modification of Low-Dimensional Nanomaterials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Hemminger, J.; Carr, R.; Somorjai, G. The photoassisted reaction of gaseous water and carbon dioxide adsorbed on the SrTiO3 (111) crystal face to form methane. Chem. Phys. Lett. 1978, 57, 100–104. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Zeng, S.; Kar, P.; Thakur, U.K.; Shankar, K. A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials. Nanotechnology 2018, 29, 052001. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Xie, C.; Chen, C.; Qian, Q.; Han, B. Mo–Bi–Cd Ternary Metal Chalcogenides: Highly Efficient Photocatalyst for CO2 Reduction to Formic Acid Under Visible Light. ACS Sustain. Chem. Eng. 2018, 6, 5754–5759. [Google Scholar] [CrossRef]
- Bie, C.; Zhu, B.; Xu, F.; Zhang, L.; Yu, J. In situ grown monolayer N-doped graphene on CdS hollow spheres with seamless contact for photocatalytic CO2 reduction. Adv. Mater. 2019, 31, 1902868. [Google Scholar] [CrossRef] [PubMed]
- Billo, T.; Shown, I.; Kumar Anbalagan, A.; Effendi, T.A.; Sabbah, A.; Fu, F.-Y.; Chu, C.-M.; Woon, W.-Y.; Chen, R.-S.; Lee, C.-H. A mechanistic study of molecular CO2 interaction and adsorption on carbon implanted SnS2 thin film for photocatalytic CO2 reduction activity. Nano Energy 2020, 72, 104717. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Wang, C.; Pan, H.; Liu, W.; Wang, K.; Zeng, Q.; Wang, R.; Jiang, J. A scalable general synthetic approach toward ultrathin imine-linked two-dimensional covalent organic framework nanosheets for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2019, 141, 17431–17440. [Google Scholar] [CrossRef]
- Yang, C.; Huang, W.; da Silva, L.C.; Zhang, K.A.; Wang, X. Functional conjugated polymers for CO2 reduction using visible light. Chem. Eur. J. 2018, 24, 17454–17458. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Gao, W.; Bai, X.; Gao, Y.; Liu, J.; He, H.; Yang, Y.; Han, Q.; Wang, X.; Wu, X.; Wang, J. Anchoring of black phosphorus quantum dots onto WO3 nanowires to boost photocatalytic CO2 conversion into solar fuels. Chem. Comm. 2020, 56, 7777–7780. [Google Scholar] [CrossRef]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl. Catal. B 2020, 274, 119063. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, K.; Sun, X.; Wang, W. Enhanced photocatalytic CO2 reduction to methane over WO3· 0.33 H2O via Mo doping. Appl. Catal. B 2019, 243, 771–779. [Google Scholar] [CrossRef]
- Gao, M.; Yang, J.; Sun, T.; Zhang, Z.; Zhang, D.; Huang, H.; Lin, H.; Fang, Y.; Wang, X. Persian buttercup-like BiOBrxCl1-x solid solution for photocatalytic overall CO2 reduction to CO and O2. Appl. Catal. B 2019, 243, 734–740. [Google Scholar] [CrossRef]
- Samanta, S.; Yadav, R.; Kumar, A.; Sinha, A.K.; Srivastava, R. Surface modified C, O co-doped polymeric g-C3N4 as an efficient photocatalyst for visible light assisted CO2 reduction and H2O2 production. Appl. Catal. B 2019, 259, 118054. [Google Scholar] [CrossRef]
- Shi, R.; Chen, Y. Controlled formation of defective shell on TiO2 (001) facets for enhanced photocatalytic CO2 reduction. ChemCatChem 2019, 11, 2270–2276. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Razzaq, A.; In, S.-I. TiO2 based nanostructures for photocatalytic CO2 conversion to valuable chemicals. Micromachines 2019, 10, 326. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Guo, R.-T.; Pan, W.-G.; Tang, J.-Y.; Zhou, W.-G.; Liu, X.-Y.; Qin, H.; Jia, P.-Y. One-dimension TiO2 nanostructures with enhanced activity for CO2 photocatalytic reduction. Appl. Surf. Sci. 2019, 464, 534–543. [Google Scholar] [CrossRef]
- Patil, S.B.; Basavarajappa, P.S.; Ganganagappa, N.; Jyothi, M.; Raghu, A.; Reddy, K.R. Recent advances in non-metals-doped TiO2 nanostructured photocatalysts for visible-light driven hydrogen production, CO2 reduction and air purification. Int. J. Hydrog. Energy 2019, 44, 13022–13039. [Google Scholar] [CrossRef]
- Xu, H.-M.; Wang, H.-C.; Shen, Y.; Lin, Y.-H.; Nan, C.-W. Low-dimensional nanostructured photocatalysts. J. Adv. Ceram. 2015, 4, 159–182. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Markovits, A.; Fahmi, A.; Minot, C. A theoretical study of CO2 adsorption on TiO2. J. Mol. Struct. THEOCHEM 1996, 371, 219–235. [Google Scholar] [CrossRef]
- Sharma, N.; Das, T.; Kumar, S.; Bhosale, R.; Kabir, M.; Ogale, S. Photocatalytic activation and reduction of CO2 to CH4 over single phase nano Cu3SnS4: A combined experimental and theoretical study. ACS Appl. Energy Mater. 2019, 2, 5677–5685. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 activation over catalytic surfaces. ChemPhysChem 2017, 18, 3135–3141. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Sun, Z.; Talreja, N.; Tao, H.; Texter, J.; Muhler, M.; Strunk, J.; Chen, J. Catalysis of carbon dioxide photoreduction on nanosheets: Fundamentals and challenges. Angew. Chem. Int. Ed. 2018, 57, 7610–7627. [Google Scholar] [CrossRef] [PubMed]
- Yoffe, A.D. Low-dimensional systems: Quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 1993, 42, 173–262. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low dimensional carbon-based catalysts for efficient photocatalytic and photo/electrochemical water splitting reactions. Materials 2020, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Philo, D.; Li, Y.; Shi, L.; Chang, K.; Ye, J. Recent advances of low-dimensional phosphorus-based nanomaterials for solar-driven photocatalytic reactions. Coord. Chem. Rev. 2020, 424, 213516. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.; Zhang, F.; Zhang, G.; Fan, X. Roles of two-dimensional transition metal dichalcogenides as cocatalysts in photocatalytic hydrogen evolution and environmental remediation. Ind. Eng. Chem. Res. 2017, 56, 4611–4626. [Google Scholar] [CrossRef]
- Chowdhury, C.; Karmakar, S.; Datta, A. Monolayer group IV–VI monochalcogenides: Low-dimensional materials for photocatalytic water splitting. J. Phys. Chem. C 2017, 121, 7615–7624. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Huynh, K.A.; Nguyen, D.L.T.; Nguyen, V.H.; Vo, D.V.N.; Trinh, Q.T.; Nguyen, T.P.; Kim, S.Y.; Le, Q.V. Halide perovskite photocatalysis: Progress and perspectives. J. Chem. Technol. Biotechnol. 2020, 95, 2579–2596. [Google Scholar] [CrossRef]
- Khan, M.S.; Zhang, F.; Osada, M.; Mao, S.S.; Shen, S. Graphitic Carbon Nitride-Based Low-Dimensional Heterostructures for Photocatalytic Applications. Sol. RRL 2020, 4, 1900435. [Google Scholar] [CrossRef]
- Zhao, J.; Holmes, M.A.; Osterloh, F.E. Quantum confinement controls photocatalysis: A free energy analysis for photocatalytic proton reduction at CdSe nanocrystals. ACS Nano 2013, 7, 4316–4325. [Google Scholar] [CrossRef]
- Qamar, S.; Lei, F.; Liang, L.; Gao, S.; Liu, K.; Sun, Y.; Ni, W.; Xie, Y. Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 2016, 26, 692–698. [Google Scholar] [CrossRef]
- Li, A.; Wang, T.; Li, C.; Huang, Z.; Luo, Z.; Gong, J. Adjusting the reduction potential of electrons by quantum confinement for selective photoreduction of CO2 to methanol. Angew. Chem. Int. Ed. 2019, 58, 3804–3808. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cao, S.; Wu, Y.; Gao, Z.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Inorganic colloidal perovskite quantum dots for robust solar CO2 reduction. Chem. Eur. J. 2017, 23, 9481–9485. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; He, Y.; Li, J.; Yuan, C.; Huang, H.; Wang, S.; Sun, Y.; Wang, Z.; Dong, F. Identification of halogen-associated active sites on bismuth-based perovskite quantum dots for efficient and selective CO2-to-CO photoreduction. ACS Nano 2020, 14, 13103–13114. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.F.; Chen, B.X.; Kuang, D.B.; Su, C.Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, 1703762. [Google Scholar] [CrossRef] [PubMed]
- Que, M.; Zhao, Y.; Pan, L.; Yang, Y.; He, Z.; Yuan, H.; Chen, J.; Zhu, G. Colloidal formamidinium lead bromide quantum dots for photocatalytic CO2 reduction. Mater. Lett. 2021, 282, 128695. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, X.; Li, W.; Pu, Y.; Sun, J.; Tang, K.; Wan, H.; Ge, C.; Zou, W.; Dong, L. Crystal-Plane Effects of CeO2 {110} and CeO2 {100} on Photocatalytic CO2 Reduction: Synergistic Interactions of Oxygen Defects and Hydroxyl Groups. ACS Sustain. Chem. Eng. 2020, 8, 14397–14406. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-doped graphitic carbon nitride nanotubes with amino-rich surface for efficient CO2 capture, enhanced photocatalytic activity, and product selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B 2019, 256, 117854. [Google Scholar] [CrossRef]
- Di, J.; Zhu, C.; Ji, M.; Duan, M.; Long, R.; Yan, C.; Gu, K.; Xiong, J.; She, Y.; Xia, J. Defect-Rich Bi12O17Cl2 Nanotubes Self-Accelerating Charge Separation for Boosting Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 14847–14851. [Google Scholar] [CrossRef]
- Di, J.; Song, P.; Zhu, C.; Chen, C.; Xiong, J.; Duan, M.; Long, R.; Zhou, W.; Xu, M.; Kang, L. Strain-Engineering of Bi12O17Br2 Nanotubes for Boosting Photocatalytic CO2 Reduction. ACS Mater. Lett. 2020, 2, 1025–1032. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Wei, J.; Zhang, H.-X.; Wu, X.; Li, Y.; Li, C.; Zhang, J.; Ye, J. Integrating the g-C3N4 Nanosheet with B–H bonding decorated metal–organic framework for CO2 activation and photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhu, B.; Yu, J.; Cao, S.; Jaroniec, M. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Han, C.; Wang, B.; Wu, C.; Shen, S.; Zhang, X.; Sun, L.; Tian, Q.; Lei, Y.; Wang, Y. Ultrathin SiC nanosheets with high reduction potential for improved CH4 generation from photocatalytic reduction of CO2. ChemistrySelect 2019, 4, 2211–2217. [Google Scholar] [CrossRef]
- Di, J.; Zhao, X.; Lian, C.; Ji, M.; Xia, J.; Xiong, J.; Zhou, W.; Cao, X.; She, Y.; Liu, H. Atomically-thin Bi2MoO6 nanosheets with vacancy pairs for improved photocatalytic CO2 reduction. Nano Energy 2019, 61, 54–59. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Luo, S.; Hu, X.; Yang, L.; Zhang, S.; Li, B.; Luo, X.; Zou, J. WS2 quantum dots seeding in Bi2S3 nanotubes: A novel Vis-NIR light sensitive photocatalyst with low-resistance junction interface for CO2 reduction. Chem. Eng. J. 2020, 389, 123430. [Google Scholar] [CrossRef]
- Zeng, Z.; Yan, Y.; Chen, J.; Zan, P.; Tian, Q.; Chen, P. Boosting the photocatalytic ability of Cu2O nanowires for CO2 conversion by MXene quantum dots. Adv. Funct. Mater. 2019, 29, 1806500. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, Y.; Li, D.; Chen, L.; Meng, S.; Jiang, D.; Chen, M. Construction of CuO quantum Dots/WO3 nanosheets 0D/2D Z-scheme heterojunction with enhanced photocatalytic CO2 reduction activity under visible-light. J. Alloys Compd. 2021, 858, 157668. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Tan, Y.-C.; Tan, L.-L.; Li, N.; Ng, Y.H.; Wen, X.; Chai, S.-P. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study. Nano Res. 2017, 10, 1673–1696. [Google Scholar] [CrossRef]
- Shi, H.; Long, S.; Hu, S.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction. Appl. Catal. B 2019, 245, 760–769. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Sun, K.; Tang, H.; Liu, Q. Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction. Appl. Surf. Sci. 2019, 493, 1142–1149. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, N.; Du, X.; Ma, J.; He, C.; Li, Z. Direct Z-Scheme 0D/2D Heterojunction of CsPbBr3 Quantum Dots/Bi2WO6 Nanosheets for Efficient Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 31477–31485. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 1D/2D TiO2/MoS2 hybrid nanostructures for enhanced photocatalytic CO2 reduction. Adv. Opt. Mater. 2018, 6, 1800911. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Nawawi, M.; Hussain, M.; Muhammad, A. Cu-NPs embedded 1D/2D CNTs/pCN heterojunction composite towards enhanced and continuous photocatalytic CO2 reduction to fuels. Appl. Surf. Sci. 2019, 485, 450–461. [Google Scholar] [CrossRef]
- Bian, J.; Qu, Y.; Zhang, X.; Sun, N.; Tang, D.; Jing, L. Dimension-matched plasmonic Au/TiO2/BiVO4 nanocomposites as efficient wide-visible-light photocatalysts to convert CO2 and mechanistic insights. J. Mater. Chem. A 2018, 6, 11838–11845. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Kong, X.Y.; Lee, W.Q.; Mohamed, A.R.; Chai, S.-P. Effective steering of charge flow through synergistic inducing oxygen vacancy defects and pn heterojunctions in 2D/2D surface-engineered Bi2WO6/BiOI cascade: Towards superior photocatalytic CO2 reduction activity. Chem. Eng. J. 2019, 372, 1183–1193. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Yunus, M.A.C.; Mohamed, A.R.; Siraj, M.; Fatehmulla, A. 2D/2D Mt/m-CN composite with enriched interface charge transfer for boosting photocatalytic CO2 hydrogenation by H2 to CH4 under visible light. Appl. Surf. Sci. 2020, 520, 146296. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Zhao, Z.; Jiang, M.; Huang, J.; Wang, L.; Wang, Q. Construction of a two-dimensional composite derived from TiO2 and SnS2 for enhanced photocatalytic reduction of CO2 into CH4. ACS Sustain. Chem. Eng. 2018, 7, 650–659. [Google Scholar] [CrossRef]
- Lu, M.; Li, Q.; Zhang, C.; Fan, X.; Li, L.; Dong, Y.; Chen, G.; Shi, H. Remarkable photocatalytic activity enhancement of CO2 conversion over 2D/2D g-C3N4/BiVO4 Z-scheme heterojunction promoted by efficient interfacial charge transfer. Carbon 2020, 160, 342–352. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Wang, Z.; Dai, K.; Pan, C.; Liang, C. Efficient interfacial charge transfer of 2D/2D porous carbon nitride/bismuth oxychloride step-scheme heterojunction for boosted solar-driven CO2 reduction. J. Colloid Interface Sci. 2021, 585, 684–693. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Z.; Li, C.; Zuo, Y.; Zhou, Y. Monolithic g-C3N4/reduced graphene oxide aerogel with in situ embedding of Pd nanoparticles for hydrogenation of CO2 to CH4. Appl. Surf. Sci. 2019, 475, 953–960. [Google Scholar] [CrossRef]
- Wang, J.; Xia, T.; Wang, L.; Zheng, X.; Qi, Z.; Gao, C.; Zhu, J.; Li, Z.; Xu, H.; Xiong, Y. Enabling Visible-Light-Driven Selective CO2 Reduction by Doping Quantum Dots: Trapping Electrons and Suppressing H2 Evolution. Angew. Chem. Int. Ed. 2018, 57, 16447–16451. [Google Scholar] [CrossRef]
- Wang, C.; Thompson, R.L.; Ohodnicki, P.; Baltrus, J.; Matranga, C. Size-dependent photocatalytic reduction of CO2 with PbS quantum dot sensitized TiO2 heterostructured photocatalysts. J. Mater. Chem. 2011, 21, 13452–13457. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Sahm, C.D.; Neri, G.; Lee, J.R.; Orchard, K.L.; Cowan, A.J.; Reisner, E. ZnSe quantum dots modified with a Ni (cyclam) catalyst for efficient visible-light driven CO2 reduction in water. Chem. Sci. 2018, 9, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gatty, M.G.; Xu, B.; Pati, P.B.; Etman, A.S.; Tian, L.; Sun, J.; Hammarström, L.; Tian, H. Covalently linking CuInS2 quantum dots with a Re catalyst by click reaction for photocatalytic CO2 reduction. Dalton Trans. 2018, 47, 10775–10783. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, M.; Yang, J.; Li, Y.; Sivalingam, Y.; Shi, Q.; Xie, M.; Han, W. Synthesis of BiVO4 quantum dots/reduced graphene oxide composites for CO2 reduction. Mater. Sci. Semicond. Process. 2019, 102, 104578. [Google Scholar] [CrossRef]
- Qin, H.; Guo, R.-T.; Liu, X.-Y.; Shi, X.; Wang, Z.-Y.; Tang, J.-Y.; Pan, W.-G. 0D NiS2 quantum dots modified 2D g-C3N4 for efficient photocatalytic CO2 reduction. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124912. [Google Scholar] [CrossRef]
- Yadav, D.; Yadav, R.K.; Kumar, A.; Park, N.J.; Baeg, J.O. Functionalized Graphene Quantum Dots as Efficient Visible-Light Photocatalysts for Selective Solar Fuel Production from CO2. ChemCatChem 2016, 8, 3389–3393. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Ma, Z.; Xie, H.; Waterhouse, G.I.; Ye, L.; Zhang, T. Black phosphorus quantum dot/g-C3N4 composites for enhanced CO2 photoreduction to CO. Sci. China Mater. 2018, 61, 1159–1166. [Google Scholar] [CrossRef]
- Guo, R.-T.; Liu, X.-Y.; Qin, H.; Wang, Z.-Y.; Shi, X.; Pan, W.-G.; Fu, Z.-G.; Tang, J.-Y.; Jia, P.-Y.; Miao, Y.-F. Photocatalytic reduction of CO2 into CO over nanostructure Bi2S3 quantum dots/g-C3N4 composites with Z-scheme mechanism. Appl. Surf. Sci. 2020, 500, 144059. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Fernando, K.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Sun, Z.; Tang, Q.; Liu, Y.; Wang, L.; Wang, H.; Wu, Z. Enhanced CH4 selectivity in CO2 photocatalytic reduction over carbon quantum dots decorated and oxygen doping g-C3N4. Nano Res. 2019, 12, 2749–2759. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, S.; Tan, T.T.Y.; Xiao, F.-X. Graphene quantum dots (GQDs) and its derivatives for multifarious photocatalysis and photoelectrocatalysis. Catal. Today 2018, 315, 171–183. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jang, H.W. Lead-free all-inorganic halide perovskite quantum dots: Review and outlook. J. Korean Ceram. Soc. 2020, 57, 455–479. [Google Scholar]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of quantum dots, quantum dot films, and quantum dot light-emitting diodes for display applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef]
- Lee, H.; Horn, M.W. Sculptured platinum nanowire counter electrodes for dye-sensitized solar cells. Thin Solid Film. 2013, 540, 208–211. [Google Scholar] [CrossRef]
- Lee, H.; In, S.; Horn, M.W. Plasmonic enhancement of CO2 conversion to methane using sculptured copper thin films grown directly on TiO2. Thin Solid Films 2014, 565, 105–110. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-dimensional hybrid nanostructures for heterogeneous photocatalysis and photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Su, C.; Yang, X.; Hu, J.; Lin, X. Ag-AgBr nanoparticles loaded on TiO2 nanofibers as an efficient heterostructured photocatalyst driven by visible light. J. Mol. Catal. A Chem. 2015, 410, 226–234. [Google Scholar] [CrossRef]
- Wang, M.; Pang, X.; Zheng, D.; He, Y.; Sun, L.; Lin, C.; Lin, Z. Nonepitaxial growth of uniform and precisely size-tunable core/shell nanoparticles and their enhanced plasmon-driven photocatalysis. J. Mater. Chem. A 2016, 4, 7190–7199. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Kar, P.; Zeng, S.; Zhang, Y.; Vahidzadeh, E.; Manuel, A.; Kisslinger, R.; Alam, K.M.; Thakur, U.K.; Mahdi, N.; Kumar, P. High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B 2019, 243, 522–536. [Google Scholar] [CrossRef]
- Wu, J.; Feng, Y.; Li, D.; Han, X.; Liu, J. Efficient photocatalytic CO2 reduction by P–O linked g-C3N4/TiO2-nanotubes Z-scheme composites. Energy 2019, 178, 168–175. [Google Scholar] [CrossRef]
- Rambabu, Y.; Kumar, U.; Singhal, N.; Kaushal, M.; Jaiswal, M.; Jain, S.L.; Roy, S.C. Photocatalytic reduction of carbon dioxide using graphene oxide wrapped TiO2 nanotubes. Appl. Surf. Sci. 2019, 485, 48–55. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Rao, G.; Deng, W.; Li, Y. Enhancing photocatalytic CO2 reduction by coating an ultrathin Al2O3 layer on oxygen deficient TiO2 nanorods through atomic layer deposition. Appl. Surf. Sci. 2017, 404, 49–56. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Huang, Z.; Dong, P.; Nie, X.; Jin, Z.; Zhang, X. Synergistic effect of N-Ho on photocatalytic CO2 reduction for N/Ho co-doped TiO2 nanorods. Mater. Res. Bull. 2019, 118, 110502. [Google Scholar] [CrossRef]
- Ohno, T.; Higo, T.; Murakami, N.; Saito, H.; Zhang, Q.; Yang, Y.; Tsubota, T. Photocatalytic reduction of CO2 over exposed-crystal-face-controlled TiO2 nanorod having a brookite phase with co-catalyst loading. Appl. Catal. B 2014, 152, 309–316. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Gold-nanoparticle-modified TiO2 nanowires for plasmon-enhanced photocatalytic CO2 reduction with H2 under visible light irradiation. Appl. Surf. Sci. 2015, 356, 1289–1299. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Shi, J. Converting CO2 into fuels by graphitic carbon nitride-based photocatalysts. Nanotechnology 2018, 29, 412001. [Google Scholar] [CrossRef] [PubMed]
- Akhundi, A.; Habibi-Yangjeh, A.; Abitorabi, M.; Rahim Pouran, S. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. 2019, 61, 595–628. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium dioxide: An overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef]
- Khan, B.; Raziq, F.; Faheem, M.B.; Farooq, M.U.; Hussain, S.; Ali, F.; Ullah, A.; Mavlonov, A.; Zhao, Y.; Liu, Z. Electronic and nanostructure engineering of bifunctional MoS2 towards exceptional visible-light photocatalytic CO2 reduction and pollutant degradation. J. Hazard. Mater. 2020, 381, 120972. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Mao, L.; Cai, X.; Sun, C.; Zhu, M. CsPbBr3 perovskite nanocrystals anchoring on monolayer MoS2 nanosheets for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2020, 128077. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wang, L.; Liu, Y.; Sun, W.; Li, W.; Li, G. In situ growth of MoS2 nanosheet arrays and TS2 (T = Fe, Co, and Ni) nanocubes onto molybdate for efficient oxygen evolution reaction and improved hydrogen evolution reaction. ACS Omega 2018, 3, 464–471. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Yi, Z.-Q.; Au, C.-T.; Yin, S.-F. CdS nanorods coupled with WS2 nanosheets for enhanced photocatalytic hydrogen evolution activity. Ind. Eng. Chem. Res. 2016, 55, 8327–8333. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Zhou, T.; Jin, S.; Huang, J.; Ye, L.; Huang, Z.; Wang, F.; Zhou, Y. B–O Bonds in Ultrathin Boron Nitride Nanosheets to Promote Photocatalytic Carbon Dioxide Conversion. ACS Appl. Mater. Interfaces 2020, 12, 9935–9943. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Zhang, X.; Zhang, X.; Ali, S.; Zhao, L.; Shaheen, S.; Jing, L. Improved visible-light activities of g-C3N4 nanosheets by co-modifying nano-sized SnO2 and Ag for CO2 reduction and 2, 4-dichlorophenol degradation. Mater. Res. Bull. 2020, 122, 110676. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Li, J.; Lu, G.; Dong, F.; Majima, T.; Zhu, M. Immobilizing perovskite CsPbBr3 nanocrystals on Black phosphorus nanosheets for boosting charge separation and photocatalytic CO2 reduction. Appl. Catal. B 2020, 277, 119230. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Huang, H.; Li, Y.; Tang, Y.; Mei, D.; Zhong, C. Metal-free 2D/2D black phosphorus and covalent triazine framework heterostructure for CO2 photoreduction. ACS Sustain. Chem. Eng. 2020, 8, 5175–5183. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, S.; Yu, Q.; She, Y.; Yang, J.; Zhou, G.; Li, Q.; She, X.; Deng, J.; Li, H. In-situ hydroxyl modification of monolayer black phosphorus for stable photocatalytic carbon dioxide conversion. Appl. Catal. B 2020, 269, 118760. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Wang, Y.-F.; Li, Y.-Y.; Zhang, L.; Yao, H.-C.; Li, Z.-J. Enhanced photocatalytic CO2 reduction activity of Z-scheme CdS/BiVO4 nanocomposite with thinner BiVO4 nanosheets. J. CO2 Util. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Ma, Z.; Li, P.; Ye, L.; Zhou, Y.; Su, F.; Ding, C.; Xie, H.; Bai, Y.; Wong, P.K. Oxygen vacancies induced exciton dissociation of flexible BiOCl nanosheets for effective photocatalytic CO2 conversion. J. Mater. Chem. A 2017, 5, 24995–25004. [Google Scholar] [CrossRef]
- Ye, L.; Jin, X.; Liu, C.; Ding, C.; Xie, H.; Chu, K.H.; Wong, P.K. Thickness-ultrathin and bismuth-rich strategies for BiOBr to enhance photoreduction of CO2 into solar fuels. Appl. Catal. B 2016, 187, 281–290. [Google Scholar] [CrossRef]
- Vu, N.N.; Nguyen, C.C.; Kaliaguine, S.; Do, T.O. Reduced Cu/Pt–HCa2Ta3O10 Perovskite Nanosheets for Sunlight-Driven Conversion of CO2 into Valuable Fuels. Adv. Sustain. Syst. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Pan, A.; Ma, X.; Huang, S.; Wu, Y.; Jia, M.; Shi, Y.; Liu, Y.; Wangyang, P.; He, L.; Liu, Y. CsPbBr3 perovskite nanocrystal grown on MXene nanosheets for enhanced photoelectric detection and photocatalytic CO2 reduction. J. Phys. Chem. Lett. 2019, 10, 6590–6597. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, S.; Wang, R.; Zhang, H.; Xie, Y. Grain boundary engineering in atomically-thin nanosheets achieving bright white light emission. Chem. Sci. 2014, 5, 1328–1335. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Zhou, Y.; Su, H.; Wang, R.; Zhang, C.; Yan, S.; Xiao, M.; Zou, Z. Single-crystalline, ultrathin ZnGa2O4 nanosheet scaffolds to promote photocatalytic activity in CO2 reduction into methane. ACS Appl. Mater. Interfaces 2014, 6, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, B.; Liu, H.; Guo, H. Controlled assemble of hollow heterostructured g-C3N4@ CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction. Appl. Catal. B 2019, 243, 566–575. [Google Scholar] [CrossRef]
- He, Y.; Rao, H.; Song, K.; Li, J.; Yu, Y.; Lou, Y.; Li, C.; Han, Y.; Shi, Z.; Feng, S. 3D hierarchical ZnIn2S4 nanosheets with rich Zn vacancies boosting photocatalytic CO2 reduction. Adv. Funct. Mater. 2019, 29, 1905153. [Google Scholar] [CrossRef]
- Fu, J.; Bao, H.; Liu, Y.; Mi, Y.; Qiu, Y.; Zhuo, L.; Liu, X.; Luo, J. Oxygen Doping Induced by Nitrogen Vacancies in Nb4N5 Enables Highly Selective CO2 Reduction. Small 2020, 16, 1905825. [Google Scholar]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Xiong, J.; Ji, M.; Zhou, J.; Fu, Q.; Xu, M.; Hao, W. Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2019, 11, 30786–30792. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. Photocatalytic performance of different exposed crystal facets of BiOCl. Curr. Opin. Green Sustain. Chem. 2017, 6, 48–56. [Google Scholar] [CrossRef]
- Yao, Y.-C.; Dai, X.-R.; Hu, X.-Y.; Huang, S.-Z.; Jin, Z. Synthesis of Ag-decorated porous TiO2 nanowires through a sunlight induced reduction method and its enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 387, 469–476. [Google Scholar] [CrossRef]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazis, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B 2017, 206, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Schaadt, D.; Feng, B.; Yu, E. Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles. Appl. Phys. Lett. 2005, 86, 063106. [Google Scholar] [CrossRef]
- Kim, K.-H.; Husakou, A.; Herrmann, J. Linear and nonlinear optical characteristics of composites containing metal nanoparticles with different sizes and shapes. Opt. Express 2010, 18, 7488–7496. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Yu, J.; Ho, W. Enhanced Photocatalytic Activity and Selectivity for CO2 Reduction over a TiO2 Nanofibre Mat Using Ag and MgO as Bi-Cocatalyst. ChemCatChem 2019, 11, 465–472. [Google Scholar] [CrossRef]
- Lu, C.; Li, J.; Yan, J.; Li, B.; Huang, B.; Lou, Z. Surface plasmon resonance and defects on tungsten oxides synergistically boost high-selective CO2 reduction for ethylene. Appl. Mater. Today 2020, 20, 100744. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Rheinberger, T.; Ohm, D.; Zhumaev, U.E.; Domke, K.F. Extending surface plasmon resonance spectroscopy to platinum surfaces. Electrochim. Acta 2019, 314, 96–101. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, X.; Feng, X.; Li, Y. CO2 reduction by plasmonic Au nanoparticle-decorated TiO2 photocatalyst with an ultrathin Al2O3 Interlayer. J. Phys. Chem. C 2018, 122, 18949–18956. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D Heterostructured Photocatalysts: From Design and Unique Properties to Their Environmental Applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef]
- Li, B.; Cao, Z.; Wang, S.; Wei, Q.; Shen, Z. BiVO4 quantum dot-decorated BiPO4 nanorods 0D/1D heterojunction for enhanced visible-light-driven photocatalysis. Dalton Trans. 2018, 47, 10288–10298. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B 2018, 239, 586–598. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B 2020, 272, 119006. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shen, Q.; Guan, R.; Xue, J.; Liu, X.; Jia, H.; Li, Q.; Wu, Y. Oxygen vacancy self-doped black TiO2 nanotube arrays by aluminothermic reduction for photocatalytic CO2 reduction under visible light illumination. J. CO2 Util. 2020, 35, 205–215. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in Ti3+ self-doped nanostructured TiO2 visible light photocatalysts for environmental and energy applications. Chem. Eng. J. 2020, 382, 123011. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Niu, P.; Wang, S.; Shi, J.; Li, L. Porous two-dimensional materials for photocatalytic and electrocatalytic applications. Matter 2020, 2, 1377–1413. [Google Scholar] [CrossRef]
- Tian, N.; Xiao, K.; Zhang, Y.; Lu, X.; Ye, L.; Gao, P.; Ma, T.; Huang, H. Reactive sites rich porous tubular yolk-shell g-C3N4 via precursor recrystallization mediated microstructure engineering for photoreduction. Appl. Catal. B 2019, 253, 196–205. [Google Scholar] [CrossRef]
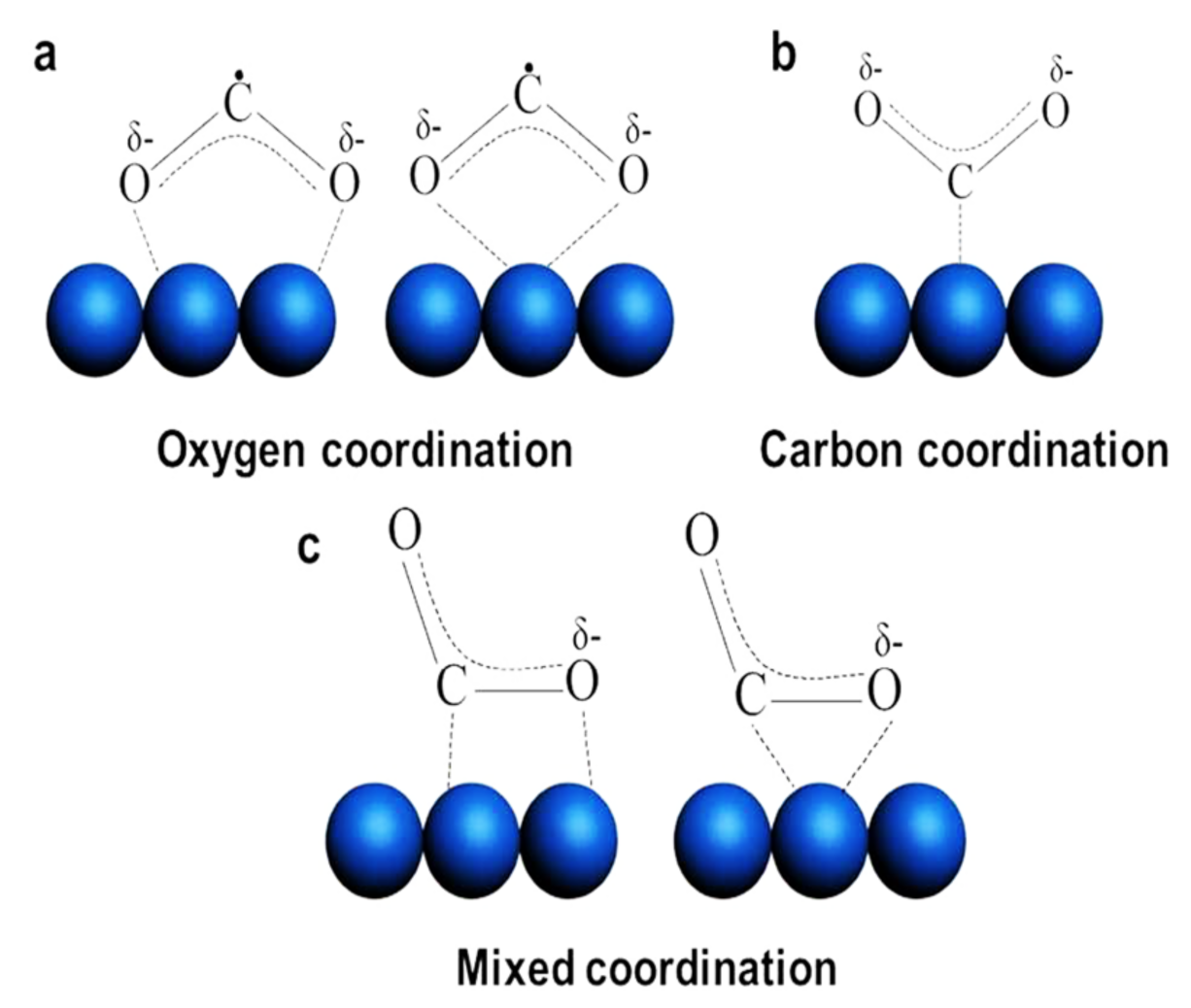
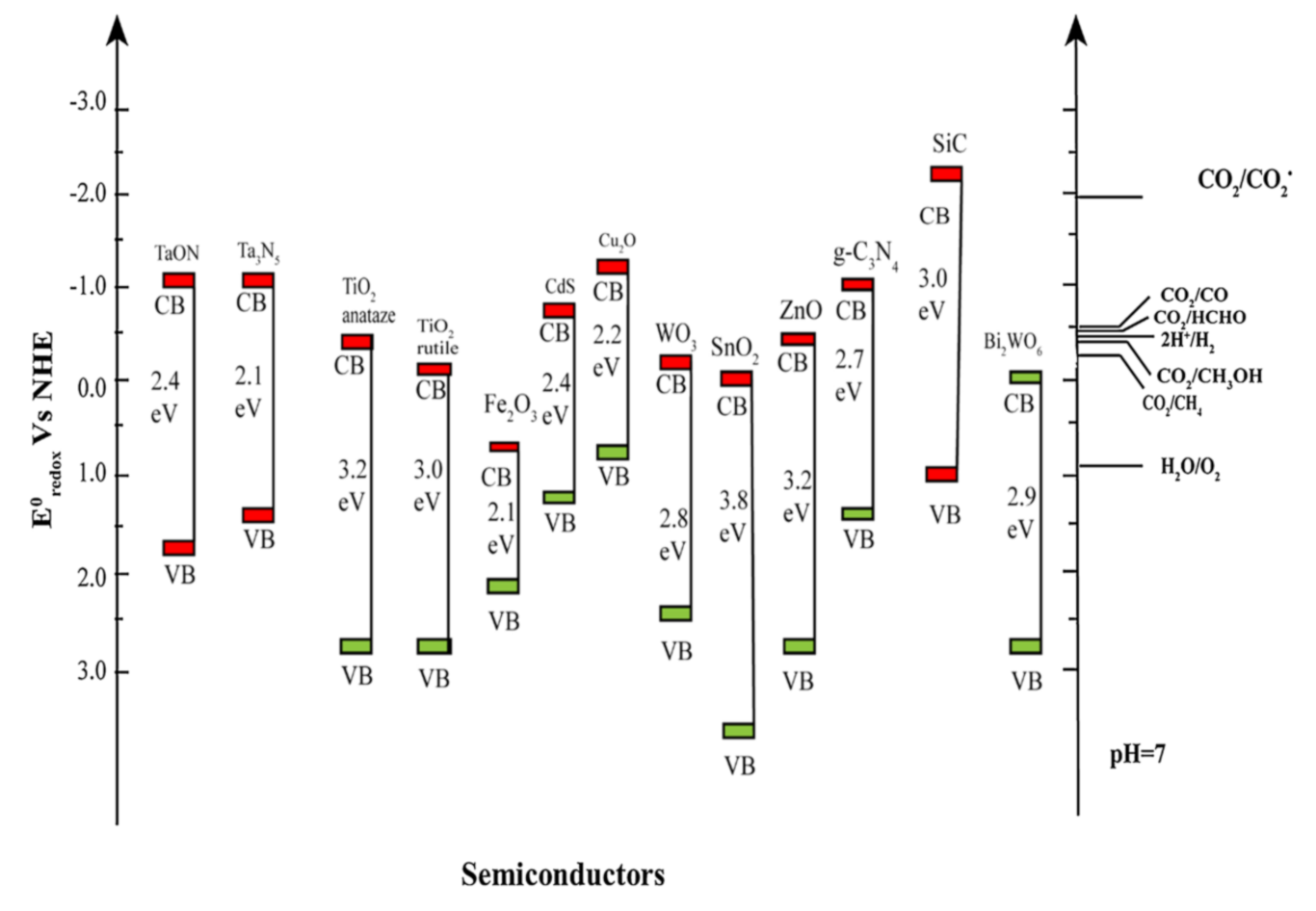


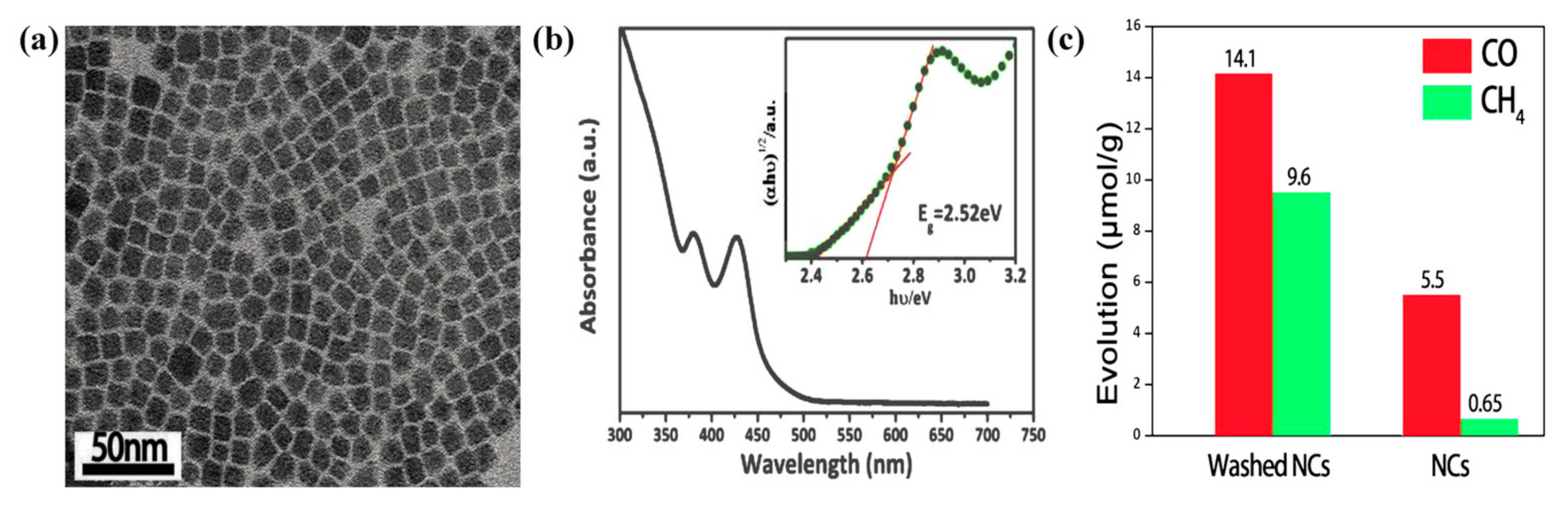
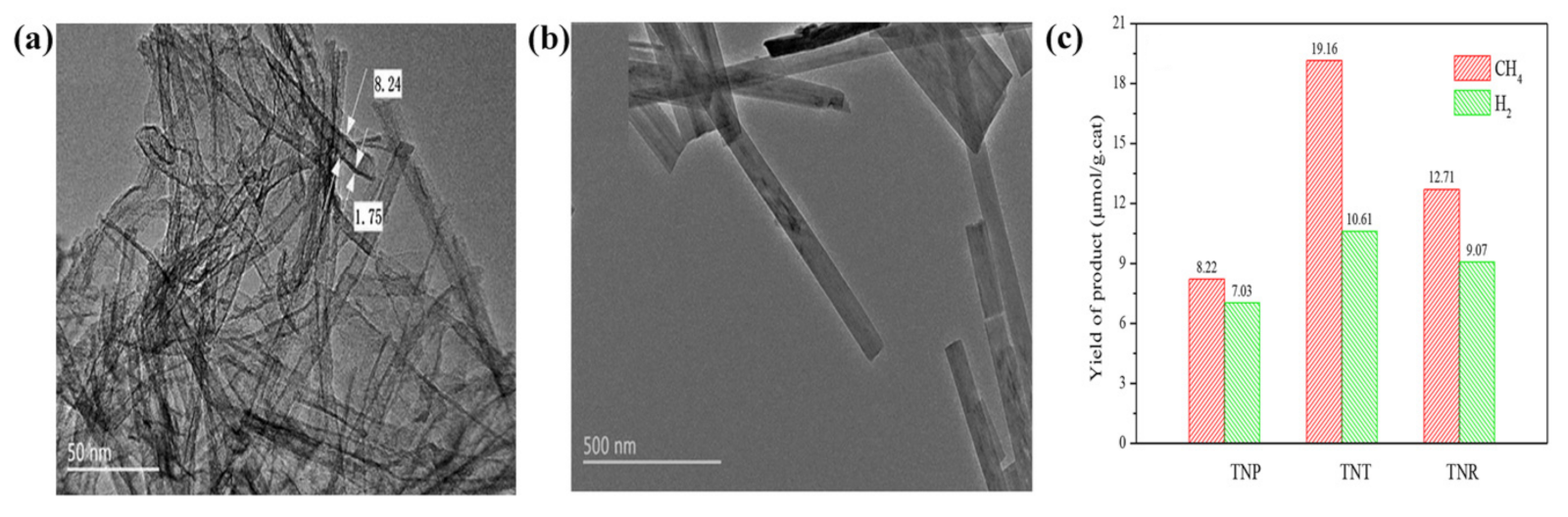
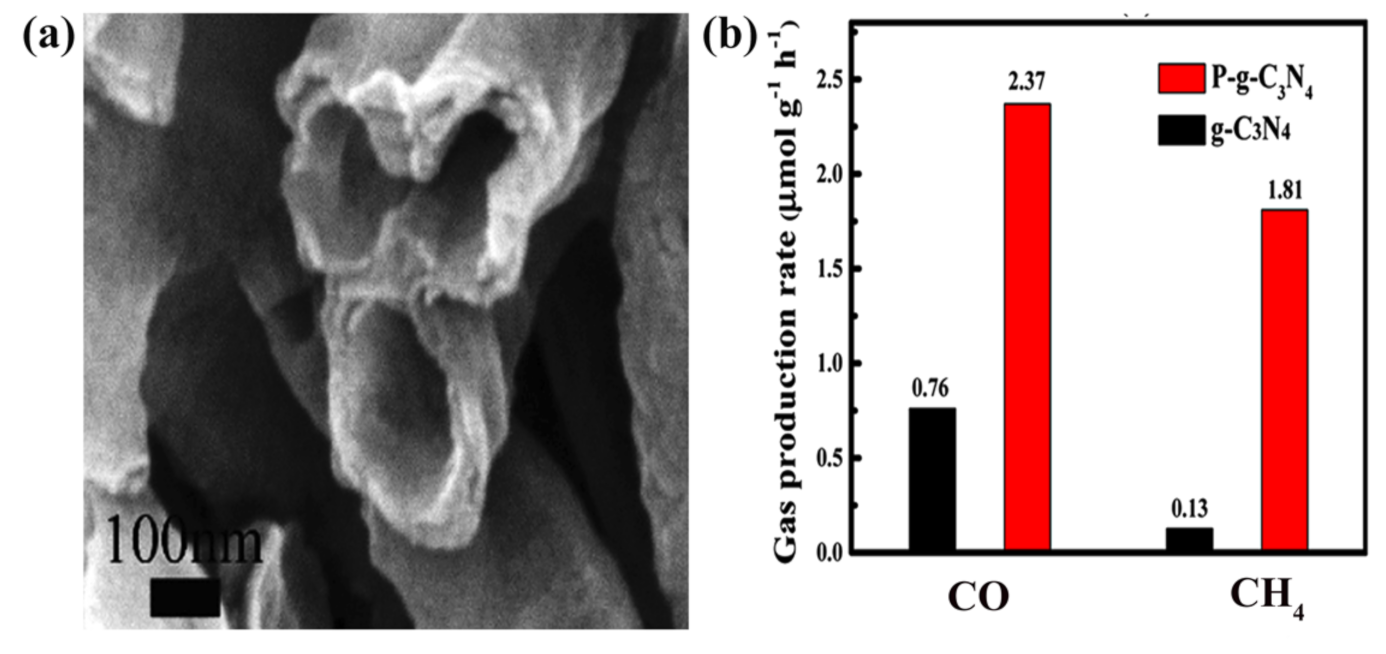
| Reaction | Eo at pH 7 (V vs. NHE) | Solar Fuel | |
|---|---|---|---|
| CO2 reduction | CO2 + 2H+ + 2e− → CO + H2O | −0.51 | CO |
| CO2 + 8H+ + 8e− → CH4 + 2H2O | −0.24 | CH4 | |
| CO2 + 6H+ + 6e− → CH3OH + H2O | −0.39 | CH3OH | |
| 2CO2 + 12H+ + 12e− → C2H5OH + 3H2O | −0.33 | C2H5OH | |
| CO2 + 2H+ + 2e− → HCOOH | −0.58 | HCOOH | |
| CO2 + 4H+ + 4e− → HCHO + H2O | −0.48 | HCHO | |
| H2O oxidation | 2H2O → O2 +4H+ | +0.81 | O2 |
| Dimensionality | Morphology | Photocatalyst | Light Source | Reducing Agent | Main Product | Activity [µmol∙g−1∙h−1] | Ref. |
|---|---|---|---|---|---|---|---|
| 0D | QD 3–12 nm | CsPbBr3 | 300 W Xe lamp | H2O | CO CH4 | 4.26 1.53 | [45] |
| QD 2.3 nm | Cs3Bi2I9 | 300 W Xe lamp | H2O | CO | 1.15 | [46] | |
| QD 2.9 nm | Cs3Bi2Br9 | 300 W Xe lamp | H2O | CO | 26.95 | ||
| QD 2.4 nm | Cs3Bi2Cl9 | 300 W Xe lamp | H2O | CO | 21.01 | ||
| NC 9.5 nm | Cs2AgBiBr6 | AM 1.5G | Ethyl acetate | CO CH4 | 2.35 1.6 | [47] | |
| QD 9.45 nm | FAPbBr3 | 300 W Xe arc lamp | H2O | CO CH4 | 181.25 16.9 | [48] | |
| QD 5.86 nm | GQD | 300 W Xe lamp 420 nm cutoff filter | H2O | CH3OH | 0.695 | [11] | |
| 1D | NR | CeO2 | 300 W Xe lamp | H2O | CO | 0.020 | [49] |
| NT | TiO2 | 300 W Xe arc lamp 320 nm < λ < 780 nm | H2O | CH4 | 2.128 | [21] | |
| NR | TiO2 | 300 W Xe arc lamp 320 nm < λ < 780 nm | H2O | CH4 | 1.41 | [21] | |
| NT | P-g-C3N4 | 300 W Xe lamp | H2O/TEOA | CO CH4 | 2.37 1.81 | [50] | |
| NT | PGCN | 300 W Xe lamp | H2O/MeCN /TEOA | CO | 103.6 | [51] | |
| NT | Bi12O17Cl2 | 300 W Xe lamp | H2O | CO | 48.6 | [52] | |
| NT | Bi12O17Br2 | 300 W Xe lamp | H2O | CO | 34.5 | [53] | |
| 2D | NS | g-C3N4 | 300 W Xe arc lamp | MeCN/TEOA (4:1) | CO CH4 | 5.407 1.549 | [54] |
| UTNS | g-C3N4 | 300 W Xe lamp | H2O | CH4 CH3OH | 1.39 1.87 | [55] | |
| UTNS | SiC | 300 W Xe lamp | H2O | CO CH4 | 1.29 3.11 | [56] | |
| UTNS | Bi2MoO6 | 300 W Xe lamp | H2O | CO | 3.62 | [57] | |
| 0D/1D | QD/NW | Black P/WO3 | 300 W Xenon arc lamp | H2O | CO C2H4 | ~ 135 ~ 11 | [13] |
| QD (10 nm)/NT | WS2/Bi2S3 | 300 W Xe arc lamp | H2O | CH3OH C2H5OH | 9.55 6.95 | [58] | |
| QD (3.5 nm)/NW | Ti3C2/Cu2O | 300 W Xe lamp | H2O | CH3OH | 78.50 | [59] | |
| 0D/2D | QD (1.6 nm)/NS | CuO/WO3 | 300 W Xe lamp λ > 400 nm | H2O | CO | 1.58 | [60] |
| ND (4.4 nm)/NS | CND/p-CN | Xe arc lamp | H2O | CO CH4 | 5.88 2.92 | [61] | |
| QD/NS | TiO2/g-C3N4 | 300 W Xe lamp λ > 400 nm | MeCN/TEOA | CO | 77.8 | [62] | |
| QD (5nm)/NS | Au/TiO2 | 300 W Xe arc lamp | H2O | CO CH4 | 19.75 70.34 | [63] | |
| QD (7nm) /NS | CsPbBr3/Bi2WO6 | 300 W Xe lamp λ > 400 nm | H2O | CO/CH4 | 503 µmol∙ g−1 | [64] | |
| 1D/2D | NF/NS | TiO2/MoS2 | 350 W Xe lamp | H2O | CH4 CH3OH | 2.86 2.55 | [65] |
| NT/NS | CNT/g-C3N4 | 200 W Hg and solar simulator | H2O | CO CH4 | 410 74 | [66] | |
| NR/NS/NF | Au/TiO2/BiVO4 | 300 W Xe lamp | H2O | CO CH4 | 2.5 7.5 | [67] | |
| 2D/2D | NS/NS | Ti3C2/Bi2WO6 | 300 W Xe lamp | H2O | CH4 CH3OH | 1.78 0.44 | [68] |
| NS/NS | Bi2WO6/BiOI | 500 W Xe arc lamp λ < 400 nm | H2O | CH4 | 2.92 | [69] | |
| NS/NS | Mt/m-CN | 35 W Xenon lamp | H2O/H2 | CO CH4 | 505 330 | [70] | |
| NS/NS | SnS2/TiO2 | 300 W Xe lamp | H2O | CH4 | 23 | [71] | |
| NS/NS | g-C3N4/BiVO4 | 300 W Xe lamp λ ≥420 nm | H2O | CO CH4 | 5.19 4.57 | [72] | |
| NS/NS | PGCN/Bi12O17Cl2 | 300 W xenon lamp | H2O | CH4 | 24.4 | [73] | |
| NP-NS/NS | Pd-g-C3N4 /RGOA | 300 W Xe lamp | H2O | CH4 | 6.4 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omr, H.A.E.; Horn, M.W.; Lee, H. Low-Dimensional Nanostructured Photocatalysts for Efficient CO2 Conversion into Solar Fuels. Catalysts 2021, 11, 418. https://doi.org/10.3390/catal11040418
Omr HAE, Horn MW, Lee H. Low-Dimensional Nanostructured Photocatalysts for Efficient CO2 Conversion into Solar Fuels. Catalysts. 2021; 11(4):418. https://doi.org/10.3390/catal11040418
Chicago/Turabian StyleOmr, Hossam A. E., Mark W. Horn, and Hyeonseok Lee. 2021. "Low-Dimensional Nanostructured Photocatalysts for Efficient CO2 Conversion into Solar Fuels" Catalysts 11, no. 4: 418. https://doi.org/10.3390/catal11040418
APA StyleOmr, H. A. E., Horn, M. W., & Lee, H. (2021). Low-Dimensional Nanostructured Photocatalysts for Efficient CO2 Conversion into Solar Fuels. Catalysts, 11(4), 418. https://doi.org/10.3390/catal11040418





