Application Prospect of K Used for Catalytic Removal of NOx, COx, and VOCs from Industrial Flue Gas: A Review
Abstract
1. Introduction
2. The Removal of NOx
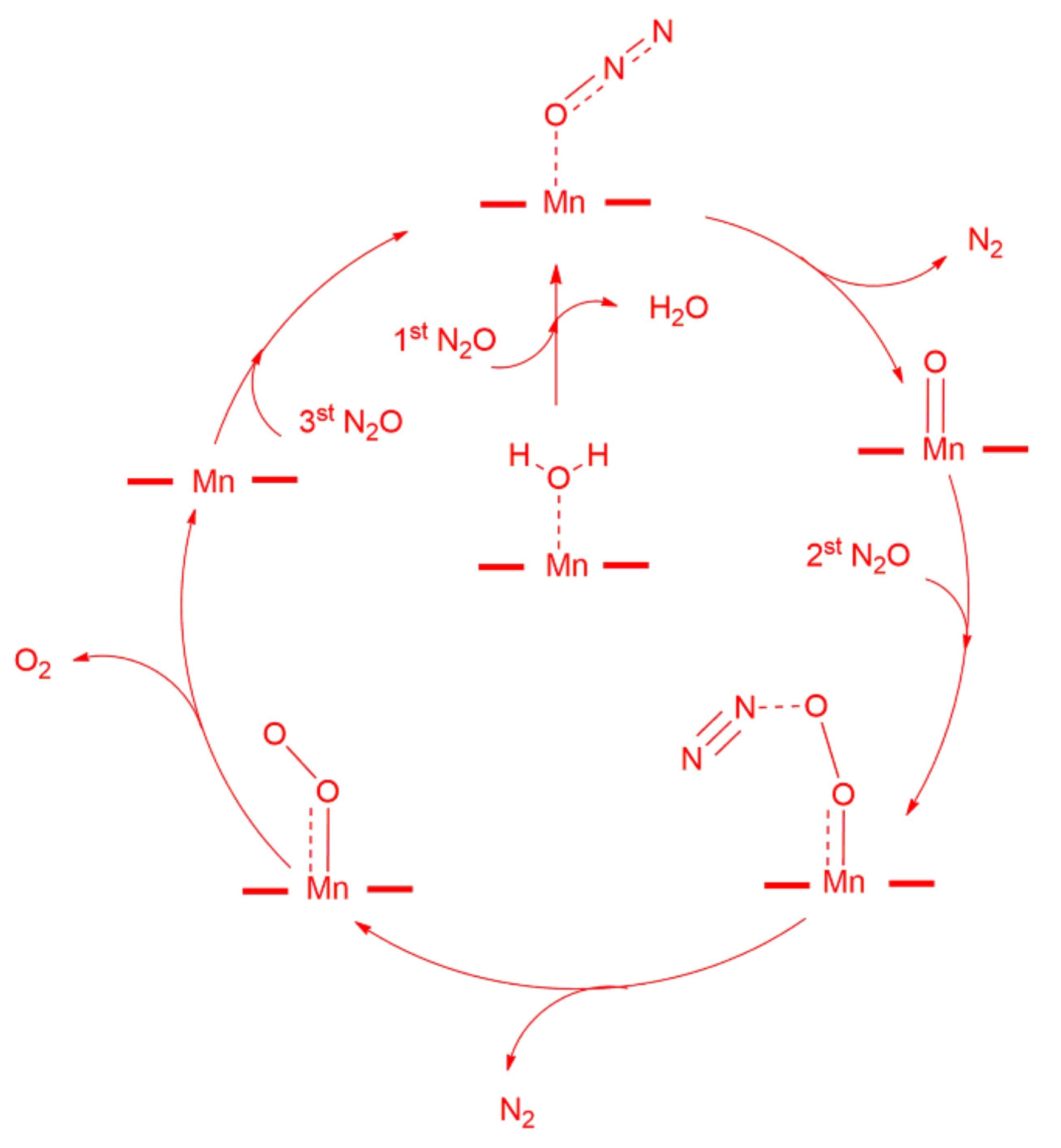
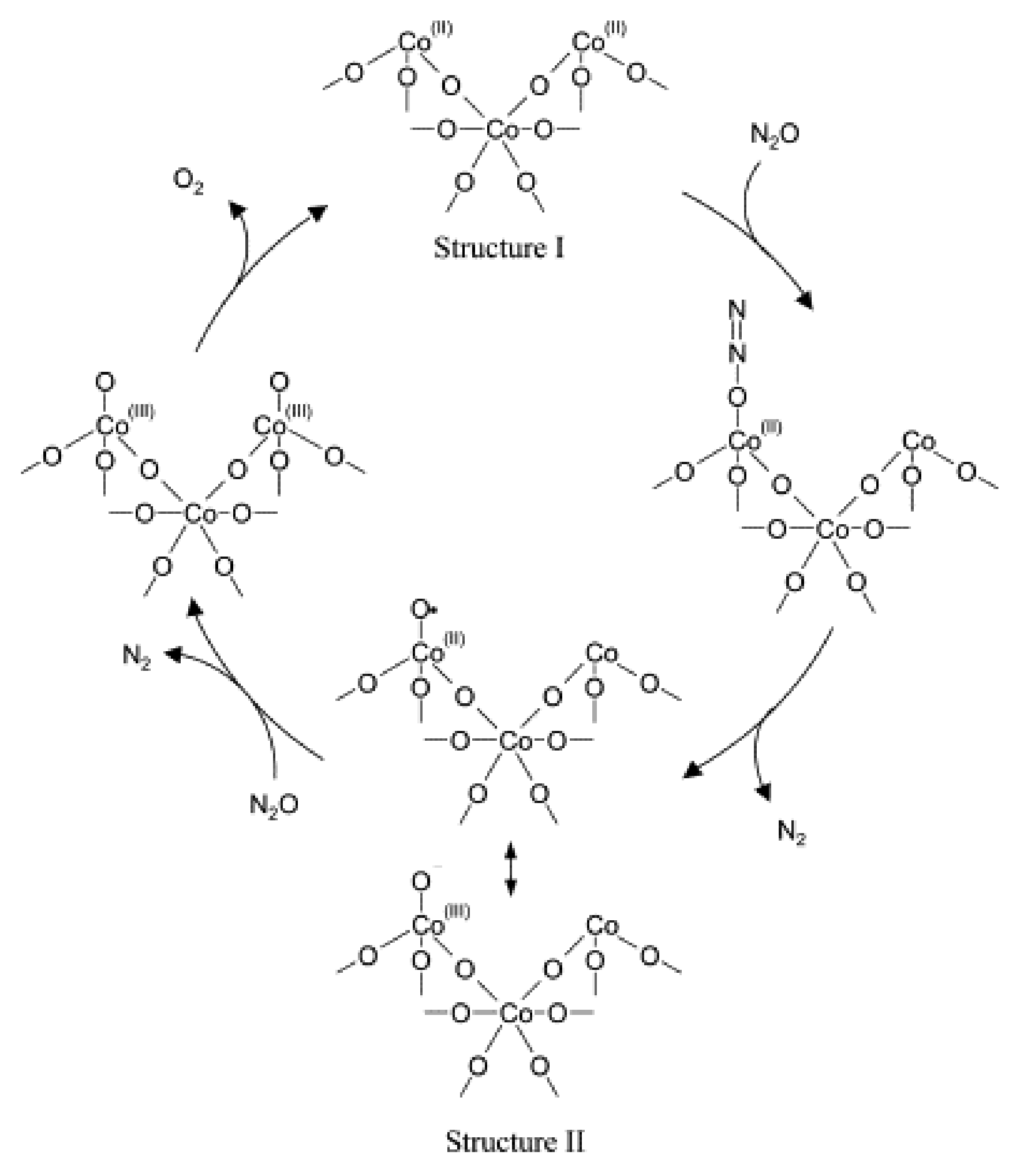
2.1. N2O Decomposition
2.1.1. Promotion Effects of K on Reaction Process
2.1.2. Promotion Effects of K on Catalyst Properties
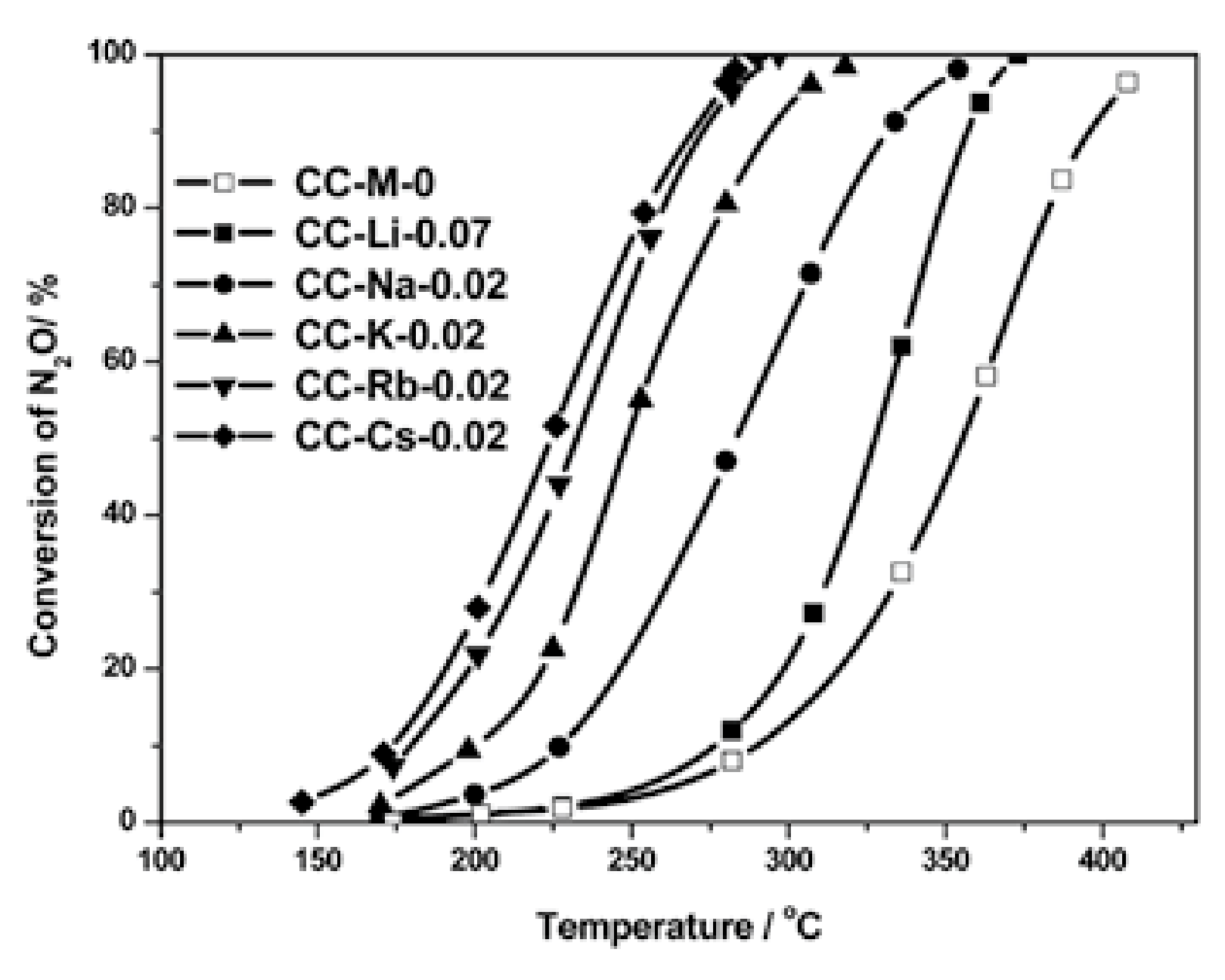
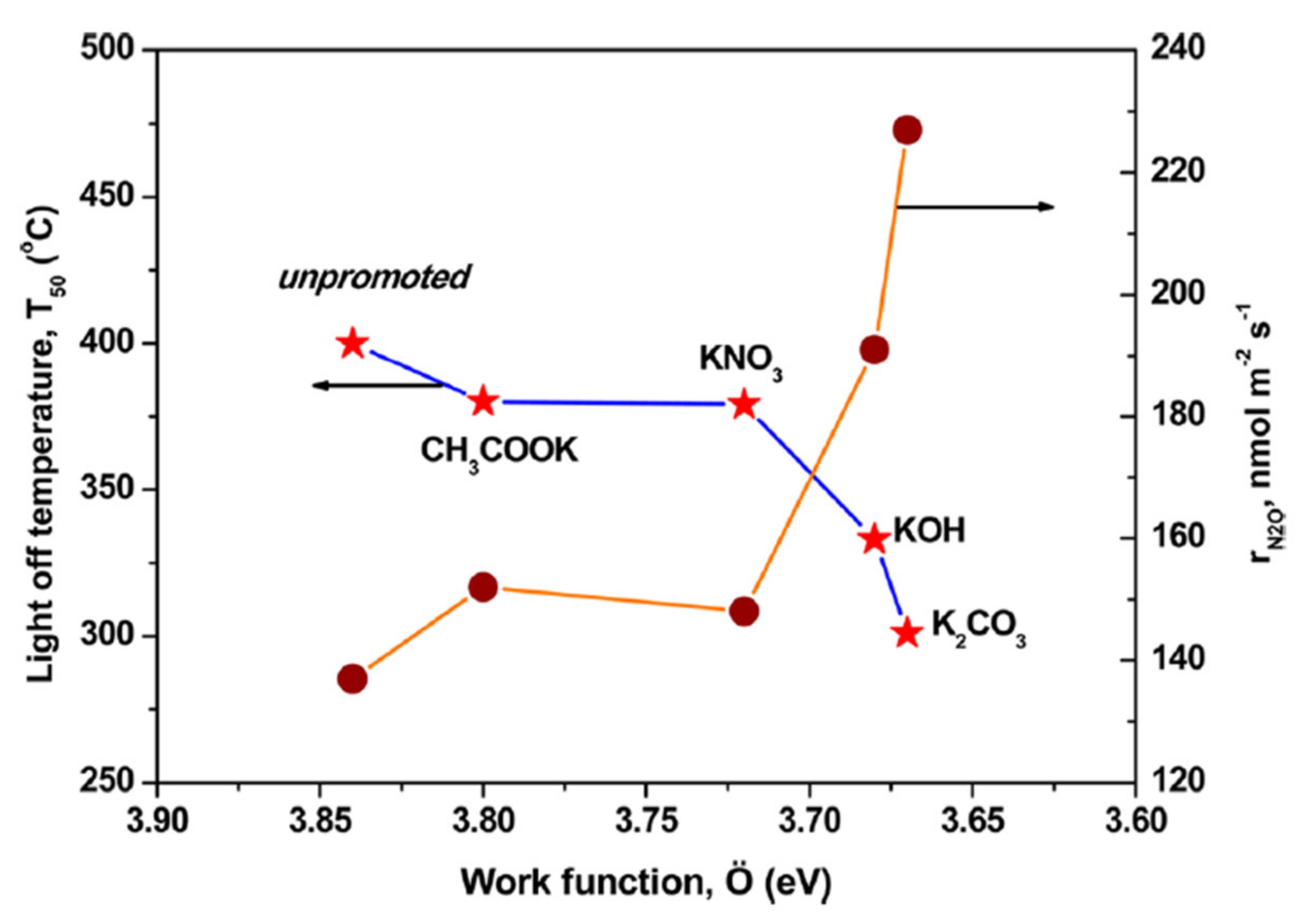
2.1.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50 a (°C) | N2O (ppm) | O2 (%) | H2O (%) | GHSV b or WHSV c | ||||
| Co3O4 | Li, Na, K, Cs | 0.2 | 200–600 | 310 | 1500 | 3 | 1 | 7000 h−1 | [69] |
| Co3O4 | K | 0.01–0.1 d | 100–500 | 160 | 5000 | 2 | 2.5 | 0.3 g s mL−1 | [34] |
| Co3O4-CeO2 | K | 2 | 150–400 | 215 | 1000 | \ | \ | 0.2 g s mL−1 | [66] |
| Co3O4-CeO2 | Li, Na, K, Rb, Cs | 0.02–0.07 d | 150–400 | 225 | 1000 | \ | \ | 0.2 g s mL−1 | [41] |
| CoxCu3-xO4 | Na, K, | 0.005–0.05 d | 250–650 | 280 | 1000 | \ | \ | 0.2 g s mL−1 | [44] |
| CoAl2O4 | Li, Na, K | 0.04 d | 200–500 | 290 | 500 | 4 | 2.6 | 0.12 g s mL−1 | [45] |
| Co3O4 | Li, Na, K, Cs | 0.015 d | 450–550 | 540 | 0.976% | \ | \ | 2 g s mL−1 | [49] |
| Co3O4 | Li, Na, K, Rb, Cs | 0.035 d | 450–700 | 570 | 1000 | \ | \ | 0.5 g s mL−1 | [52] |
| Co-Mn-Al | Li, Na, K | 0.3–1.8 | 300–450 | 330 | 0.1% | \ | \ | 0.06 g s mL−1 | [50] |
| Co-Mn-Al | K | 0.2–3 | 300–450 | 350 | 0.1% | \ | \ | 40,380 h−1 | [51] |
| Co4MnAlOx | Li, Na, K, Rb, Cs | 0.2–3.4 | 300–450 | 325 | 0.1% | \ | \ | 0.06 g s mL−1 | [29] |
| Co3O4 | K | 0.04–0.1 d | 200–500 | 260 | 5000 | 2 | 2.5 | 0.3 g s mL−1 | [56] |
| Co-Mn-Al | Na, K | 0.5–2.5 | 300–450 | 330 | 0.1% | \ | \ | 0.06 g s mL−1 | [70] |
| Co-Mn-Al | K | 0.3–3.1 | 300–450 | 340 | 0.1% | \ | \ | 0.06 g s mL−1 | [67] |
| Co3O4 | Cs | 0.4–13.7 | 50–600 | 110 | 5% | \ | \ | 7000 h−1 | [68] |
| Co2.6Zn0.4O4/α-Al2O3 | K | 0.15 | 100–600 | 280 | 5% | \ | \ | 7000 h−1 | [71] |
| CuO-CeO2 | Cs | 0.6–4.8 | 350–600 | 425 | 1000 | 2 | \ | 40,000 h−1 | [23] |
| Cu0.8Co0.2Co2O4 | Na, K, Cs | 0.05 d | 300–500 | 340 | 2% | 4 | \ | 0.43 g s mL−1 | [42] |
| Y2O3-Co3O4 | K | 0.32 | 200–400 | 285 | 1% | 2 | 8.2 | 12,700 h−1 | [24] |
| Zn-Ce-Co3O4 | Na, K | 2 | 200–600 | 450 | 3000 | \ | \ | 24,000 h−1 | [43] |
| NiAl mixed oxides | K | 0.1 | 300–500 | 355 | 2% | 4 | 8.8 | 0.43 g s mL−1 | [47] |
| AC | Na, K | 5–20 | 200–600 | 280 | 3000 | \ | \ | 0.16 g s mL−1 | [30] |
| AC | K | 5–20 | 200–600 | 285 | 3000 | \ | \ | 0.16 g s mL−1 | [64] |
2.2. NO Decomposition
2.2.1. Promotion Effects of K on Reaction Process
2.2.2. Promotion Effects of K on Catalyst Properties
2.2.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||
|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50 a (°C) | N2O (ppm) | GHSV b or WHSV c | ||||
| Co-Mn-Al | K | 0.6–18.9 | 650–700 | 650 | 1000 | 0.6 g s mL−1 | [72] |
| Co-Mn-Al | K | 1–4 | 560–650 | 650 | 1000 | 0.6 g s mL−1 | [73] |
| Co3O4 | Li, Na, K, Rb, Cs | 0–0.1 d | 450–700 | 580 | 1000 | 0.5 g s mL−1 | [52] |
| Co3O4 | Na, K, Rb, Cs | 0.035 d | 450–600 | 600 | 1000 | 0.1 g s mL−1 | [76] |
| Co3O4 | K | 0.9–3 | 400–650 | <650 | 9700 | 2100 h−1 | [83] |
| Co3O4 | Na | 0–0.091 | 450–700 | 620 | 0.976 | 2 g s mL−1 | [49] |
| Co-Mg-Mn-Al | K | 2 | 55–700 | 660 | 1000 | 0.6 g s mL−1 | [84] |
| Co-Mn-Al | K, Cs | 1.5–4 | 612–650 | \ | 1000 | 0.6 g s mL−1 | [80] |
| Ce-Mn | Li, Na, K, Cs | 0–10 | 800 | >800 | 6000 | 1 g s mL−1 | [85] |
2.3. NO Reduction
2.3.1. Promotion Effects of K on Reaction Process and Catalyst Properties
2.3.2. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50 a (°C) | NO (ppm) | Reductant | Others | GHSV b or WHSV c | ||||
| Pt/γ-Al2O3 | Li, K, Rb, Cs | 1.9–15.5 | 177–527 | 300 | 1000 | 1000 ppm C3H6 | \ | 0.006 g s mL−1 | [103] |
| Pt/γ-Al2O3 | Na | 2.6–10.4 | 200–500 | 300 | 1000 | 1000 ppm C3H6 | \ | 0.006 g s mL−1 | [92] |
| Pt/γ-Al2O3 | K, Rb, Cs | 1.9–15.5 | 100–500 | 320 | 1000 | 1000 ppm CO | \ | 0.006 g s mL−1 | [94] |
| Pd/K2O-6TiO2 | K | \ | 75–275 | 95 | 1000 | 5000 ppm H2 | 5% O2 | 60000 h−1 | [95] |
| Pd/Y2O3-ZrO2 | Na | 0.017–0.102 | 300–450 | 362 | 8000 | 8000 ppm C3H6 | \ | 0.00375 g smL−1 | [104] |
| Pd/γ-Al2O3 | Na | 3.5–7 | 180–350 | 275 | 1000 | 1067 ppm C3H6 7000 ppm CO | 7800 ppm O2 | 0.015 g s mL−1 | [105] |
| Ag/Al2O3 | Li, Na, K, Cs | 0.5–1 | 200–500 | 350 | 1000 | 1000 ppm C3H6 | 5% O2 | 0.09 g s mL−1 | [96] |
| Ag/Al2O3 | Na, K, Cs | 2 | 450–700 | 545 | 500 | 4000 ppm CH4 | 80 ppm SO2 | 9000 h−1 | [101] |
| CuO/AC | K | 0–10 | 150–450 | 317 | 2000 | AC | \ | 20,000 h−1 | [97] |
2.4. NO Oxidation
2.4.1. Promotion Effects of K on Reaction Process
2.4.2. Promotion Effects of K on Catalyst Properties
2.4.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50a (°C) | NO (ppm) | O2 (%) | GHSV b or WHSV c | ||||
| Co/KxTi2O5 | K | 2.15–15.28 | 200–420 | 275 | 700 | 10 | 120,000 h−1 | [103] |
| Co3O4 | K | 0.05–0.2 | 200–450 | 240 | 500 | 8 | 0.27 g s mL−1 | [111] |
| Mn-CoOx | K | 5 | 50–250 | 85 | 500 | 5 | 30,000 h−1 | [110] |
| Ru/K-OMS-2 | K | \ | 27–527 | 270 | 1000 | 8 | 35,000 h−1 | [112] |
3. The Removal and Reuse of COx
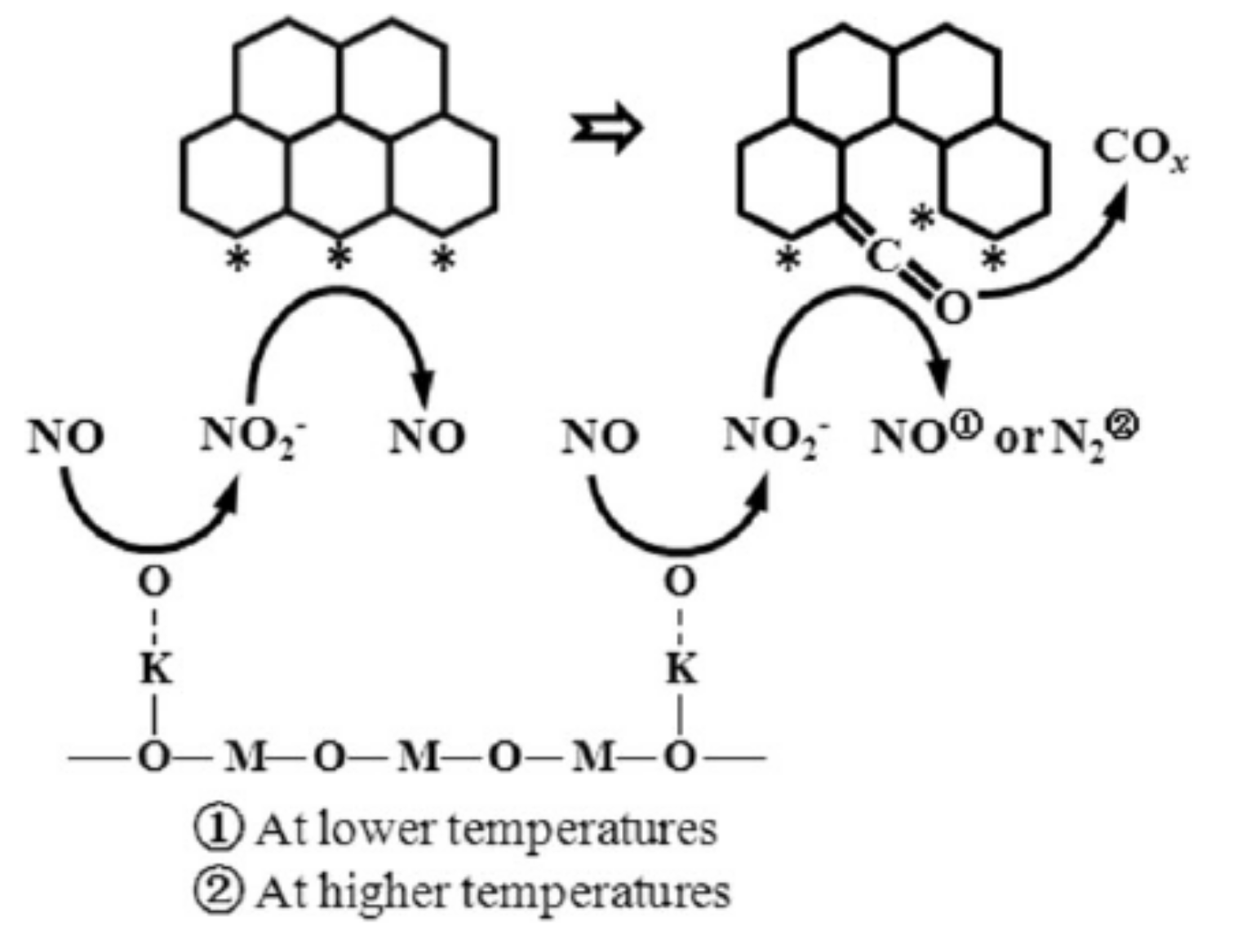
3.1. Soot Oxidation
3.1.1. Promotion Effects of K on Reaction Process
3.1.2. Promotion Effects of K on Catalyst Properties
3.1.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | Tm a (°C) | Soot (g Soot/g Catalyst) | O2 (%) | H2O (%) | NO (ppm) | Others | GHSV b or WHSV c | ||||
| MnOx-CeO2 | K | 0.08–0.67 d | 300–700 | 440 | 0.05 | 10 | 3 | 1000 | \ | 0.043 g s mL−1 | [129] |
| CeO2 | K | 4–14 | 300–650 | 350 | 0.05 | 6 | \ | \ | \ | 0.024 g s mL−1 | [118] |
| CeO2 | K | 3–13.5 | 300–500 | 360 | 0.05 | 6 | \ | \ | \ | 0.00375 g s mL−1 | [114] |
| CeO2-ZrO2 | K | 8 | 400–650 | 430 | 0.1 | 10 | \ | \ | 1000ppm NO | 0.0132 g s mL−1 | [137] |
| Cu/CeO2 | K | 2–5 | 300–500 | 315 | 0.1 | 10 | \ | 1000 | \ | 0.0132 g s mL−1 | [117] |
| Cu/Al Co/Al V/Al | K | 0.5–1 d | 250–650 | 550 | 0.2 | 5 | \ | 500 | \ | ≈1270 h−1 | [113] |
| Co-MgAlO | K | 1.5–10 | 50–300 | 348 | 0.05 | air | \ | \ | [128] | ||
| CaO-MgO | Li, Na, K | 5.4 | 250–750 | 430 | 0.25 | air | 0.0025 g s mL−1 | [116] | |||
| MgO | K | 2.3 | 350–700 | 421 | 0.25 | air | 0.0025 g s mL−1 | [127] | |||
| MgAlO | K | 0.14 | 50–500 | 217e | \ | 10 | \ | 0.1% | 0.5% CO, 0.05% C3H6 | 20,000 h−1 | [120] |
| MgAlO | K | ≈1.9–3.9 | 376–750 | 410 | 0.11 | air | \ | \ | [138] | ||
| Alkaline salts | Li, Na, K | \ | 250–500 | 350 | 0.5 | 10 | \ | \ | \ | 0.138 g s mL−1 | [119] |
| alkaline niobates | Li, Na, K, Rb | \ | 400–700 | 470 | 0.25 | 12 | \ | \ | \ | 0.0025 g s mL−1 | [125] |
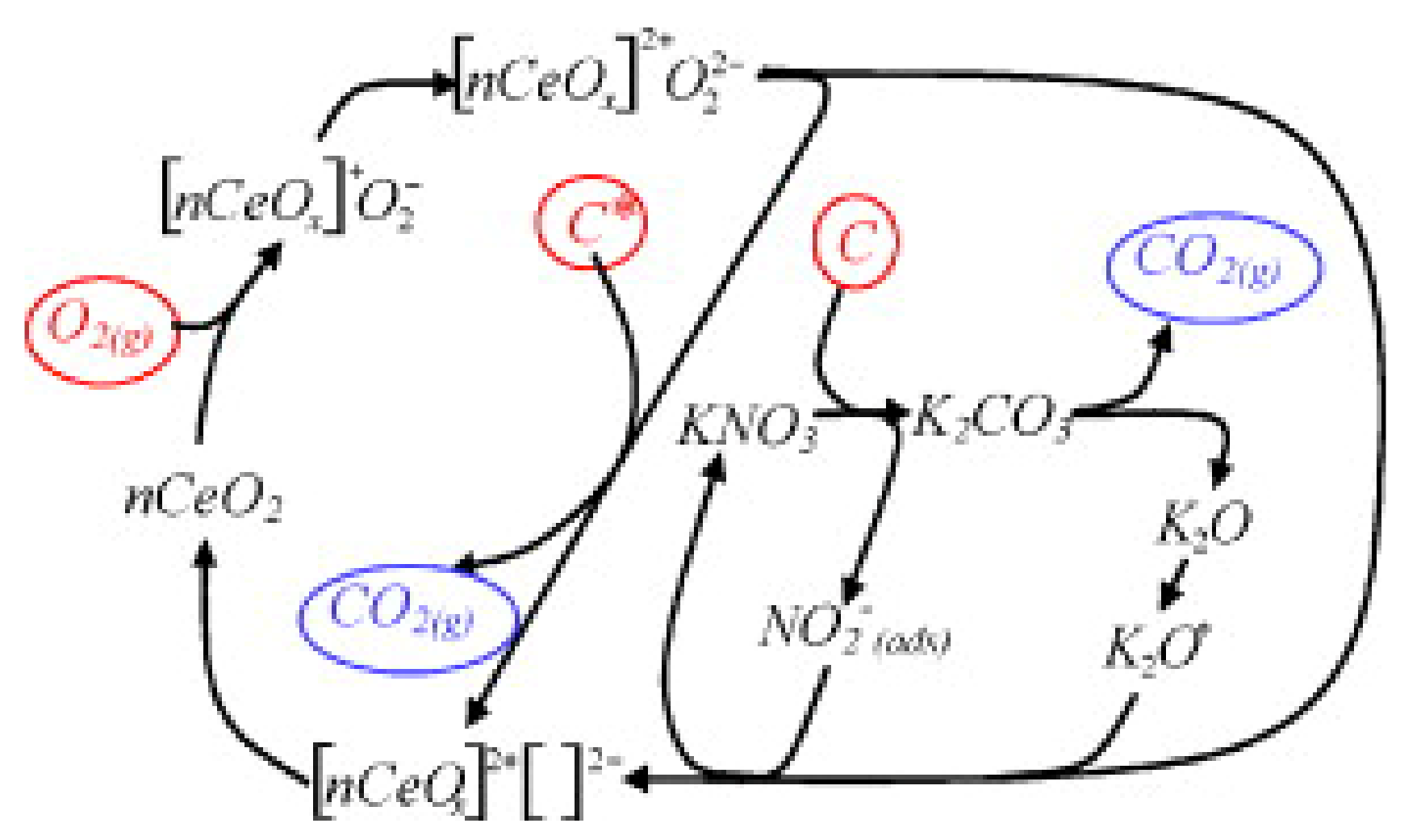
3.2. CO Oxidation
3.2.1. Promotion Effects of K on Reaction Process
3.2.2. Promotion Effects of K on Catalyst Properties
3.2.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50 a (°C) | CO (%) | O2 (%) | H2 (%) | Others | GHSV b or WHSV c | ||||
| Pt/Al2O3 | K | 0.02–0.42 | 120–280 | 165 | 1 | 1 | \ | \ | 9600 h−1 | [140] |
| Pt/Al2O3 | K | 10 d | 50–200 | 80 | 0.2 | 0.2 | 75 | \ | 30,000 h−1 | [142] |
| Pt/Al2O3 | K | 10–20 d | 100–200 | <100 | 0.45 | 0.45 | 50.25 | 0.34% CH4, 16.55% CO2 | 12,000 h−1 | [147] |
| Pt/Al2O3 | K | 1–20 d | 100 | <100 | 0.2 | 0.2 | 75 | \ | 30,000 h−1 | [162] |
| Pt/Al2O3 | K | 5–20 d | 50–180 | 70 | 0.2 | 0.2 | 75 | \ | 30,000 h−1 | [153] |
| Rh/SiO2 Rh/USY | K | 3 | 50–150 | 100 | 0.2 | 0.2 | 75 | \ | 0.015 g s mL−1 | [154] |
| Rh/USY | K | 1–10 d | 80–150 | 100 | 0.2 | 0.2 | 75 | \ | 0.015 g s mL−1 | [157] |
| Au/Al2O3 | Li, Rb | 1–30 e | 50–300 | <50 | 2.67 | 1.33 | \ | \ | 2500 h−1 | [156] |
| Rh/SiO2 | K | 1–10 d | 50–200 | 120 | 0.2 | 0.2 | 75 | \ | 0.015 g s mL−1 | [164] |
| CuO-CeO2/CNT CuO-CeO2/graphene | K | 0.5–2 | 50–200 | 110 | 1 | 1 | 50 | \ | 0.03 g s mL−1 | [160] |
| Ru/SiO2 | K | 0.17–0.71 d | 80–220 | <80 | 1 | 1 | 50 | \ | 0.15 g s mL−1 | [144] |
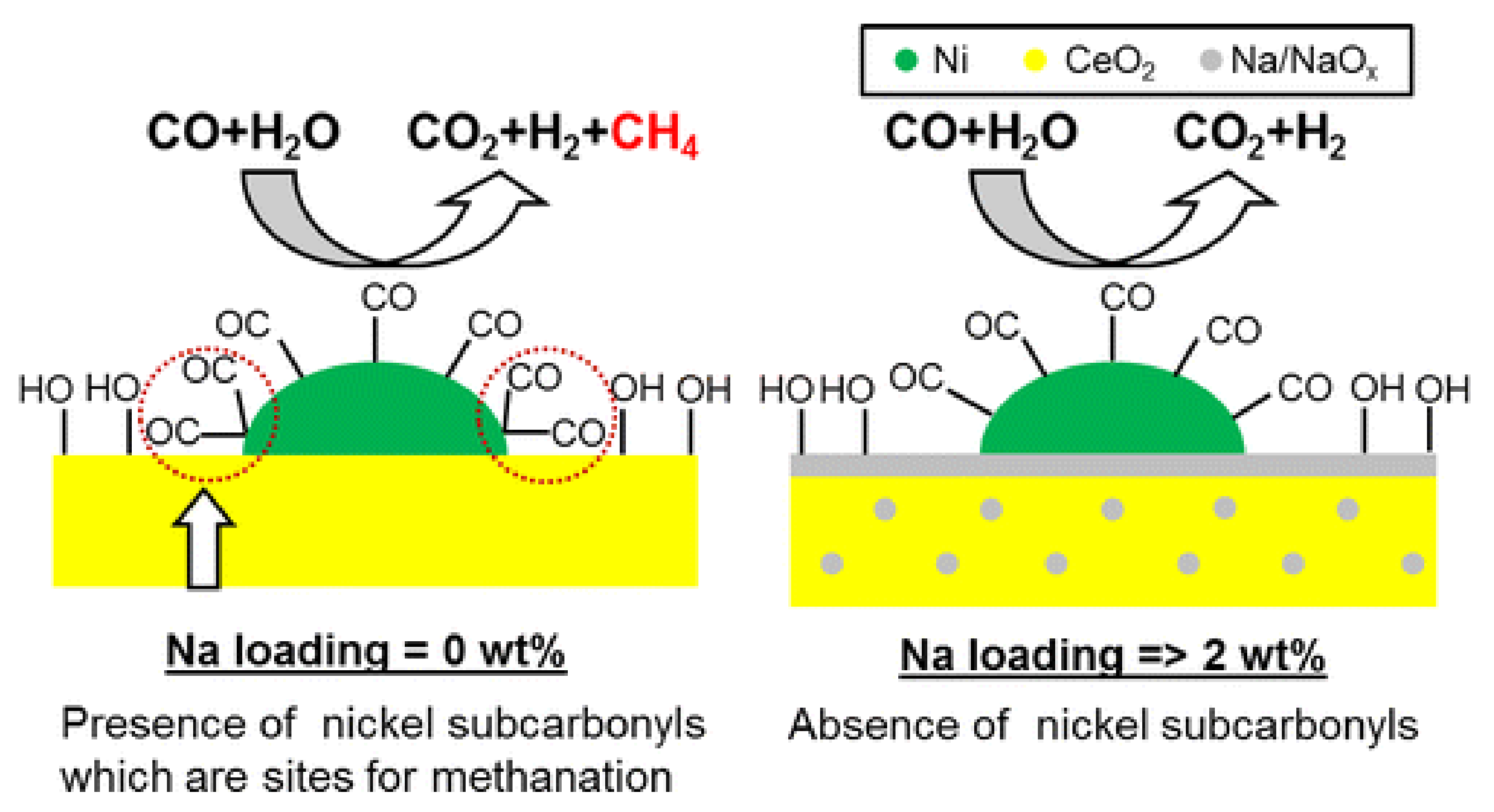
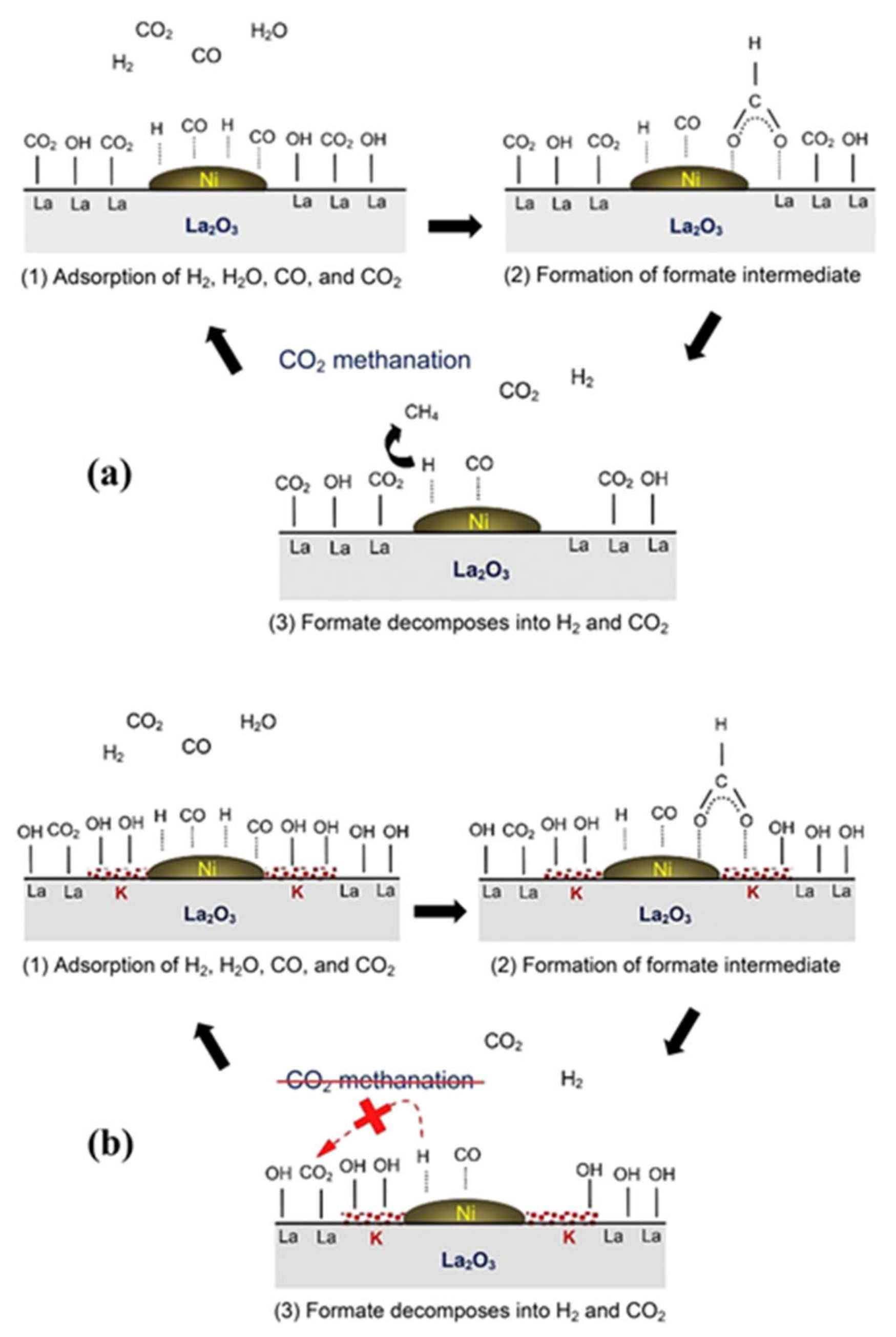
3.3. WGS
3.3.1. Promotion Effects of K on Reaction Process
3.3.2. Promotion Effects of K on Catalyst Properties
3.3.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50a (°C) | CO (%) | H2O (%) | Others | GHSV b or WHSV c | ||||
| Co3O4 | K | 0.2–5.89 | 300 | <300 | 3.22 | 16.13 | 22.58% H2 | 0.0077 g s mL−1 | [178] |
| Co3O4 | Li, Na, K, Rb, Cs | 0.02–0.05 d | 180–300 | 215 | 3 | 26.1 | 29.9% H2 | 2.4 g s mL−1 | [14] |
| cobalt molybdenum carbide | K | 2 | 180 | <180 | 10.5 | 21 | 20% H2 | 0.2 g s mL−1 | [168] |
| Pt/ceria | Li, Na, K, Rb, Cs | 0.15–2.9 | 225–275 | 250 | 2.8 | 46.7 | 50.5% H2 | 0.018 g s mL−1 | [170] |
| Pt/SiO2 | Na | 1–3 | 150–350 | 220 | 2 | 10 | \ | 0.09 g s mL−1 | [173] |
| Pt/Al2O3 | Li, Na, K | 7–125 d | 230–250 | \ | 6.8 | 21.9 | 8.5% CO2, 37.4% H2 | 0.796 g s mL−1 | [176] |
| Pt/TiO2 | Na | 1–10 | 200–300 | \ | 2.83 | 5.66 | 37.74% H2 | 0.012–0.2 g s mL−1 | [180] |
| Ni/CeO2 | K | 1–10 | 300–600 | 320 | 5 mol.% | 25 mol.% | \ | 68,000 h−1 | [18] |
| LaNiO3 | K | 2.5–10 | 350–550 | <350 | 5 | 25 | \ | 0.06 g s mL−1 | [171] |
| Ru/C | K | 2–10 | 200–325 | 295 | 10 | 20 | \ | 0.3 g s mL−1 | [167] |
3.4. RWGS
3.4.1. Promotion Effects of K on Reaction Process
3.4.2. Promotion Effects of K on Catalyst Properties
3.4.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T20a (°C) | CO2 (%) | H2 (%) | GHSV b or WHSV c | ||||
| Fe/Al2O3 | K | 1–4 | 480 | \ | 15/60 | 60/15 | 0.018–0.036 g s mL−1 | [185] |
| Fe/Al2O3 | Cs | 0–5 | 400–800 | <400 | CO2:H2 = 1:4 | 0.3 g s mL−1 | [191] | |
| Ni/Al2O3 | K | 2 | 400–700 | 400 | 50 | 50 | 0.24 g s mL−1 | [181] |
| Pt/zeolite | K | 5–200 d | 200–500 | 450 | 45 | 45 | 0.12 g s mL−1 | [190] |
| Mo2C/γ-Al2O3 | K | 1–3 | 300 | <300 | CO2:H2 = 1:3 | 0.24–1.2 g s mL−1 | [182] | |
| WC/γ-Al2O3 | Na, K | 0.25d | 350 | 350 | CO2:H2 = 1:3 | 0.4–4 g s mL−1 | [187] | |
| Co-CeO2 | K | 1 | 400–600 | 425 | CO2:H2 = 1:1 | 0.012 g s mL−1 | [192] | |
| Cu/SiO2 | K | 0.52–5.2 | 200–600 | >600 | 50 | 50 | \ | [189] |
4. Removal and Reuse of VOCs
4.1. VOCs Oxidation
4.1.1. Promotion Effects of K on Reaction Process
4.1.2. Promotion Effects of K on Catalyst Properties
4.1.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature Range (°C) | T50a (°C) | VOCs | O2 (%) | Others | GHSV b or WHSV c | ||||
| Pt/Al2O3 | K | 0.02–0.05 | 225–500 | 275 | 3000 ppm Dichloromethane | \ | air | 15,000 h−1 | [196] |
| Pt/TiO2 | Na | 1–2 | 15–200 | <15 | 600 ppm Formaldehyde | 20 | 50% Relative humidity | 120,000 h−1 | [198] |
| Pt/Al2O3 | K | 0.02–0.1f | 100–350 | 200 | 500 ppm ethanol | \ | air | 0.072 g s mL−1 | [208] |
| Co/NaY Co/SiO2 | Li, Na, K, Rb, Cs | 2–16 | 350 | >350 | 2.78% benzyl alcohol | 8.33 | N2 | 520 g s mol−1 | [207] |
| Co/NaY Co/NaUSY | K | 0–25 d | 350 | >350 | 2.78% benzyl alcohol | 8.33 | N2 | 520 g s mol−1 | [206] |
| Co-Mn-Al | K | 0–3 | 100–400 | 145 | 1 g/m3 toluene and ethanol | \ | air | 0.36 g s mL−1 | [203] |
| Co0.1/ZrO2 | Cs | 0.15 | \ | 296 | 1000 ppm toluene | 10 | N2 | 10,500 h−1e | [213] |
| NiCo2O4 | K | 2 | 220–400 | 330(T98) | Toluene; acetone; alcohol; acetic ether | \ | air | 5000 h−1 | [205] |
| Cu-Mg-Al | K | 0.9 | 250–600 | 415 | 0.5% Methane | 4.5 | He | \ | [214] |
| Cu/ZrO2 | Cs | 0.15 | 250–500 | 480 | 1000 ppm toluene | 10 | N2 | 10,500 h−1e | [215] |
| Fe/SBA-15 | Li, Na, K, Rb, Cs | 5d | 320 | >320 | 2.5% Propylene | \ | 25% N2O | 0.2 g s mL−1 | [202] |
| Natural manganese ore | K | 0.07 | 200–330 | 225 | 550ppm o-xylene | 20% | N2 | 0.6 g s mL−1 | [216] |
4.2. Reforming Reaction
4.2.1. Promotion Effects of K on Reaction Process
4.2.2. Promotion Effects of K on Catalyst Properties
4.2.3. Influencing Factors on Promotion Effect
| Catalysts | Promoter | Content (wt%) | Reaction Conditions | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature range (°C) | T90 a (°C) | VOCs | CO2 (%) | H2O (%) | GHSV b or WHSV c | ||||||
| Ni/Al2O3 | K | 0.04–0.69 d | 700 | >700 | 50% Methane | 50 | \ | 22,500 h−1 | [223] | ||
| Ni/Al2O3 | Na, K | 0–18 | 350–600 | 400 | 6–7.5 f Acetic acid, ethanol, 1-propanol, propanoic acid | 12.1 h−1 g | [224] | ||||
| Ni/Al2O3 | K | 0.5–2.9 | 800 | <800 | Methane: CO2 = 1:1 | 1.26 g s mL−1 | [231] | ||||
| Ni/MgO | Li, Na, K | 1 | 650 | <650 | 8% Ethanol | \ | 8 | 40,000 h−1 | [232] | ||
| Ni-La/cordierite | K | 5 e | 500–700 | 650 | 3.5 f kerosene | 2300 h−1 | [228] | ||||
| Ni incorporated mesoporous smectite | Li, Na, K, Rb, Cs | 1 | 450 | <450 | 3.3 f Acetic acid | \ | 27 | 0.005 g s mL−1 | [230] | ||
| Pt/Al2O3 | K | 0.04–0.4 | 450 | <450 | Ethanol : H2O = 1:3 | 0.05 g s mL−1 | [219] | ||||
5. Summary
6. Potential
7. Conclusions
Funding
Conflicts of Interest
References
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Strong Enhancement of de Soot Activity of Transition Metal Oxides by Alkali Doping: Additive Effects of Potassium and Nitric Oxide. Top. Catal. 2017, 60, 162–170. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic Oxidation of Volatile Organic Compounds (VOCs)—A Review. Atmospheric Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, J.; Huang, N.; Yang, S.; Peng, Y.; Li, J. Balance between Reducibility and N2O Adsorption Capacity for the N2O Decomposition: CuxCoy Catalysts as an Example. Environ. Sci. Technol. 2019, 53, 10379–10386. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kaercher, B.; Koch, D.; et al. Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, Y.; Wang, Y. Novel Simultaneous Removal Technology of NO and SO2 Using a Semi-Dry Microwave Activation Persulfate System. Environ. Sci. Technol. 2019, 54, 2031–2042. [Google Scholar] [CrossRef]
- Gálvez, M.; Ascaso, S.; Stelmachowski, P.; Legutko, P.; Kotarba, A.; Moliner, R.; Lázaro, M. Influence of the Surface Potassium Species in Fe–K/Al2O3 Catalysts on the Soot Oxidation Activity in the Presence of NOx. Appl. Catal. B Environ. 2014, 152–153, 88–98. [Google Scholar] [CrossRef]
- Lyu, X.; Guo, H.; Wang, Y.; Zhang, F.; Nie, K.; Dang, J.; Liang, Z.; Dong, S.; Zeren, Y.; Zhou, B.; et al. Hazardous Volatile Organic Compounds in Ambient Air of China. Chemosphere 2020, 246, 125731. [Google Scholar] [CrossRef]
- National Bureau of Statistics China. Annual Report on Environmental Statistics; National Bureau of Statistics China: Beijing, China, 2015.
- Sun, W.; Shao, M.; Granier, C.; Liu, Y.; Ye, C.S.; Zheng, J.Y. Long-Term Trends of Anthropogenic SO2, NOx, CO, and NMVOCs Emissions in China. Earth’s Futur. 2018, 6, 1112–1133. [Google Scholar] [CrossRef]
- Atlas, C.C. Annual CO2 Emissions. 2020. Available online: http://www.globalcarbonatlas.org/ (accessed on 1 July 2020).
- Mo, S.; Zhang, Q.; Sun, Y.; Zhang, M.; Li, J.; Ren, Q.; Fu, M.; Wu, J.; Chen, L.; Ye, D. Gaseous CO and toluene co-oxidation over monolithic core–shell Co3O4-based hetero-structured catalysts. J. Mater. Chem. A 2019, 7, 16197–16210. [Google Scholar] [CrossRef]
- Neha; Prasad, R.; Singh, S.V. A review on catalytic oxidation of soot emitted from diesel fuelled engines. J. Environ. Chem. Eng. 2020, 8, 324–331. [Google Scholar] [CrossRef]
- Dongil, A.; Bachiller-Baeza, B.; Castillejos, E.; Escalona, N.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Promoter Effect of Alkalis on CuO/CeO2/Carbon Nanotubes Systems for the PROx Reaction. Catal. Today 2018, 301, 141–146. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Sparks, D.E.; Hopps, S.; Thomas, G.A.; Davis, B.H. Low Temperature Water–Gas Shift Reaction Over Alkali Metal Promoted Cobalt Carbide Catalysts. Top. Catal. 2014, 57, 612–618. [Google Scholar] [CrossRef]
- Zhao, K.; Bkour, Q.; Hou, X.; Kang, S.W.; Park, J.C.; Norton, M.G.; Yang, J.-I.; Ha, S. Reverse Water Gas Shift Reaction over CuFe/Al2O3 Catalyst in Solid Oxide Electrolysis Cell. Chem. Eng. J. 2018, 336, 20–27. [Google Scholar] [CrossRef]
- Teržan, J.; Djinović, P.; Zavašnik, J.; Arčon, I.; Žerjav, G.; Spreitzer, M.; Pintar, A. Alkali and Earth Alkali Modified CuOx/SiO2 Catalysts for Propylene Partial Oxidation: What Determines the Selectivity? Appl. Catal. B Environ. 2018, 237, 214–227. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Ang, M.; Oemar, U.; Kathiraser, Y.; Saw, E.T.; Lew, C.; Du, Y.; Borgna, A.; Kawi, S. High-Temperature Water–Gas Shift Reaction over Ni/xK/CeO2 Catalysts: Suppression of Methanation via Formation of Bridging Carbonyls. J. Catal. 2015, 329, 130–143. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. K (Na)-Promoted Ni, Al Layered Double Hydroxide Catalysts for the Steam Reforming of Methanol. J. Power Sources 2007, 171, 842–849. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. SCR Catalyst Doped with Copper for Synergistic Removal of Slip Ammonia and Elemental Mercury. Fuel Process. Technol. 2018, 181, 268–278. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xu, W.; Yang, W.; Pan, Z.; Wang, Q. Simultaneous Absorption–Oxidation of Nitric Oxide and Sulfur Dioxide Using Ammonium Persulfate Synergistically Activated by UV-Light and Heat. Chem. Eng. Res. Des. 2018, 130, 321–333. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Kapteijn, F.; Schöffel, K.; Moulijn, J. Formation and Control of N2O in Nitric Acid Production. Appl. Catal. B Environ. 2003, 44, 117–151. [Google Scholar] [CrossRef]
- Lykaki, M.; Papista, E.; Carabineiro, S.A.C.; Tavares, P.B.; Konsolakis, M. Optimization of N2O Decomposition Activity of CuO–CeO2 Mixed Oxides by Means of Synthesis Procedure and Alkali (Cs) Promotion. Catal. Sci. Technol. 2018, 8, 2312–2322. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Gao, Q.; Liu, Z.; Xu, X. Potassium Promoted Y2O3-Co3O4 Catalysts for N2O Decomposition. Catal. Commun. 2020, 137, 105948. [Google Scholar] [CrossRef]
- Koukiou, S.; Konsolakis, M.; Lambert, R.; Yentekakis, I.V. Spectroscopic Evidence for the Mode of Action of Alkali Promoters in Pt-Catalyzed Denox Chemistry. Appl. Catal. B Environ. 2007, 76, 101–106. [Google Scholar] [CrossRef]
- Liu, j.; Ding, T.; Tian, Y.; Li, X. Enhanced CO Oxidation Performance over Potassium-promoted Pt/TiO2 Catalysts. Chem. J. Chin. Univ. Chin. 2018, 39, 1467–1474. [Google Scholar]
- Wang, Y.-X.; Wang, G.-C. A Systematic Theoretical Study of Water Gas Shift Reaction on Cu(111) and Cu(110): Potassium Effect. ACS Catal. 2019, 9, 2261–2274. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, Z.; Sun, C.; Qin, F.; Xiong, D.; Shen, W.; Xu, H. Catalytic Decomposition of N2O Over CuxCe1−xOy Mixed Oxides. Appl. Catal. B Environ. 2012, 125, 492–498. [Google Scholar] [CrossRef]
- Obalová, L.; Karásková, K.; Wach, A.; Kustrowski, P.; Mamulová-Kutláková, K.; Michalik, S.; Jirátová, K. Alkali Metals as Promoters in Co–Mn–Al Mixed Oxide for N2O Decomposition. Appl. Catal. A Gen. 2013, 462–463, 227–235. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, G.; Yang, R. New Insights into Alkali-Catalyzed Gasification Reactions of Carbon: Comparison of N2O Reduction with Carbon over Na and K Catalysts. J. Catal. 2000, 192, 77–87. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Guidelines for Optimization of Catalytic Activity of 3d Transition Metal Oxide Catalysts in N2O Decomposition by Potassium Promotion. Catal. Today 2011, 176, 369–372. [Google Scholar] [CrossRef]
- Inger, M.; Kowalik, P.; Saramok, M.; Wilk, M.; Stelmachowski, P.; Maniak, G.; Granger, P.; Kotarba, A.; Sojka, Z. Laboratory and Pilot Scale Synthesis, Characterization and Reactivity of Multicomponent Cobalt Spinel Catalyst for Low Temper-Ature Removal of N2O from Nitric Acid Plant Tail Gases. Catal. Today 2011, 176, 365–368. [Google Scholar] [CrossRef]
- Zasada, F.; Stelmachowski, P.; Maniak, G.; Paul, J.-F.; Kotarba, A.; Sojka, Z. Potassium Promotion of Cobalt Spinel Catalyst for N2O Decomposition—Accounted by Work Function Measurements and DFT Modelling. Catal. Lett. 2009, 127, 126–131. [Google Scholar] [CrossRef]
- Asano, K.; Ohnishi, C.; Iwamoto, S.; Shioya, Y.; Inoue, M. Potassium-doped Co3O4 Catalyst for Direct Decomposition of N2O. Appl. Catal. B Environ. 2008, 78, 242–249. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Kotarba, A.; Sojka, Z.; Rico-Pérez, V.; Bueno-López, A. Rationales for the Selection of the Best Precursor for Potassium Doping of Cobalt Spinel Based de N2O Catalyst. Appl. Catal. B Environ. 2013, 136–137, 302–307. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinović, P.; Erjavec, B.; Dražić, G.; Pintar, A. Small CuO Clusters on CeO2 Nanospheres as Active Species for Catalytic N2O Decomposition. Appl. Catal. B Environ. 2015, 163, 113–122. [Google Scholar] [CrossRef]
- Yakovlev, A.L.; Zhidomirov, G.M.; Van Santen, R.A. N2O Decomposition Catalysed by Transition Metal Ions. Catal. Lett. 2001, 75, 45–48. [Google Scholar] [CrossRef]
- Yuzaki, K.; Yarimizu, T.; Ito, S.-I.; Kunimori, K. Catalytic Decomposition of N2O Over Supported Rhodium Catalysts: High Activities of Rh/USY and Rh/Al2O3 and The Effect of Rh Precursors. Catal. Lett. 1997, 47, 173–175. [Google Scholar] [CrossRef]
- Buenolopez, A.; Suchbasanez, I.; De Lecea, C.S. Stabilization of Active Rh2O3 Species for Catalytic Decomposition of N2O on La-, Pr-doped CeO2. J. Catal. 2006, 244, 102–112. [Google Scholar] [CrossRef]
- Ohnishi, C.; Asano, K.; Iwamoto, S.; Chikama, K.; Inoue, M. Alkali-doped Co3O4 Catalysts for Direct Decomposition of N2O in the Presence of Oxygen. Catal. Today 2007, 120, 145–150. [Google Scholar] [CrossRef]
- Xue, L.; He, H.; Liu, C.; Zhang, C.; Zhang, B. Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth Metals on Cobalt−Cerium Composite Oxide Catalysts for N2O Decomposition. Environ. Sci. Technol. 2009, 43, 890–895. [Google Scholar] [CrossRef]
- Dou, Z.; Zhang, H.-J.; Pan, Y.-F.; Xu, X.-F. Catalytic Decomposition of N2O Over Potassium-modified Cu-Co Spinel Oxides. J. Fuel Chem. Technol. 2014, 42, 238–245. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Xu, J.; Wu, G.; Zeng, Z. N2O Decomposition Over K/Na-promoted Mg/Zn–Ce–cobalt Mixed Oxides Catalysts. J. Environ. Sci. 2014, 26, 1437–1443. [Google Scholar] [CrossRef]
- Franken, T.; Palkovits, R. Investigation of Potassium Doped Mixed Spinels CuxCo3−xO4 as Catalysts for an Efficient N2O Decomposition in Real Reaction Conditions. Appl. Catal. B Environ. 2015, 176–177, 298–305. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Wang, A.; Li, L.; Wang, X.; Zhang, T. N2O Decomposition over K-Promoted Co-Al Catalysts Prepared from Hydrotalcite-like Precursors. Appl. Catal. B Environ. 2009, 89, 391–397. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Zhang, X.; Turxun, M.; Yu, H.; Zhao, J. The Catalytic Activity of NiO for N2O Decomposition Doubly Promoted by Barium and Cerium. Chem. Eng. J. 2014, 256, 365–371. [Google Scholar] [CrossRef]
- Wu, H.-P.; Qian, Z.-Y.; Xu, X.-L.; Xu, X.-F. N2O Decomposition over K-Promoted NiAl Mixed Oxides Derived from Hydrotalcite-like Compounds. J. Fuel Chem. Technol. 2011, 39, 115–121. [Google Scholar] [CrossRef]
- Scagnelli, A.; Di Valentin, C.; Pacchioni, G. Catalytic Dissociation of N2O on Pure and Ni-doped MgO Surfaces. Surf. Sci. 2006, 600, 386–394. [Google Scholar] [CrossRef]
- Park, P.; Kil, J.; Kung, H.; Kung, M. NO Decomposition over Sodium-Promoted Cobalt Oxide. Catal. Today 1998, 42, 51–60. [Google Scholar] [CrossRef]
- Karásková, K.; Obalová, L.; Kovanda, F. N2O Catalytic Decomposition and Temperature Programmed Desorption Tests on Alkali Metals Promoted Co–Mn–Al Mixed Oxide. Catal. Today 2011, 176, 208–211. [Google Scholar] [CrossRef]
- Obalová, L.; Karásková, K.; Jirátová, K.; Kovanda, F. Effect of potassium in calcined Co–Mn–Al layered double hydroxide on the catalytic decomposition of N2O. Appl. Catal. B Environ. 2009, 90, 132–140. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Bion, N.; Hamada, H. Alkali Metal-Doped Cobalt Oxide Catalysts for NO Decomposition. Appl. Catal. B Environ. 2003, 46, 473–482. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, X.; Beaurain, A.; Dujardin, C.; Granger, P. Stoichiometric and Non-stoichiometric Perovskite-Based Catalysts: Consequences on Surface Properties and on Catalytic per-Formances in the Decomposition of N2O from Nitric Acid Plants. Appl. Catal. B Environ. 2012, 125, 149–157. [Google Scholar] [CrossRef]
- Yuan, C.; Bin Wu, H.; Xie, Y.; Lou, X.W. (David) Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Erjavec, B.; Djinović, P.; Pintar, A. Ordered Mesoporous CuO–CeO2 Mixed Oxides as an Effective Catalyst for N2O decomposition. Chem. Eng. J. 2014, 254, 153–162. [Google Scholar] [CrossRef]
- Yoshino, H.; Ohnishi, C.H.; Hosokawa, S.; Wada, K.; Inoue, M. Optimized Synthesis Method for K/Co3O4 Catalyst Towards Direct Decomposition of N2O. J. Mater. Sci. 2010, 46, 797–805. [Google Scholar] [CrossRef]
- Konsolakis, M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Zakirov, V.; Zhang, H.-Y. A Model for the Operation of Nitrous Oxide Monopropellant. Aerosp. Sci. Technol. 2008, 12, 318–323. [Google Scholar] [CrossRef]
- Pasha, N.; Lingaiah, N.; Reddy, P.S.S.; Prasad, P.S.S. Direct Decomposition of N2O over Cesium-doped CuO Catalysts. Catal. Lett. 2008, 127, 101–106. [Google Scholar] [CrossRef]
- Polizzi, S.; Bucella, S.; Speghini, A.; Vetrone, F.; Naccache, R.; Boyer, A.J.C.; Capobianco, J.A. Nanostructured Lanthanide-Doped Lu2O3 Obtained by Propellant Synthesis. Chem. Mater. 2004, 16, 1330–1335. [Google Scholar] [CrossRef]
- Satsuma, A.; Maeshima, H.; Watanabe, K.; Suzuki, K.; Hattori, T. Effects of Methane and Oxygen on Decomposition of Nitrous Oxide over Metal Oxide Catalysts. Catal. Today 2000, 63, 347–353. [Google Scholar] [CrossRef]
- Zhu, J.; Albertsma, S.; Van Ommen, A.J.G.; Lefferts, L. Role of Surface Defects in Activation of O2 and N2O on ZrO2and Yttrium-Stabilized ZrO2. J. Phys. Chem. B 2005, 109, 9550–9555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-X.; Liu, C.-G. New Insight into the Catalytic Cycle about Epoxidation of Alkenes by N2O over a Mn–Substituted Keggin-Type Polyoxometalate. J. Mol. Graph. Model. 2017, 73, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lu, G. Catalytic Conversion of N2O to N2 over Potassium Catalyst Supported on Activated Carbon. J. Catal. 1999, 187, 262–274. [Google Scholar] [CrossRef]
- Papista, E.; Pachatouridou, E.; Goula, M.A.; Marnellos, G.Ε.; Iliopoulou, E.; Konsolakis, M.; Yentekakis, I.V. Effect of Alkali Promoters (K) on Nitrous Oxide Abatement Over Ir/Al2O3 Catalysts. Top. Catal. 2016, 59, 1020–1027. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Promotion Effect of Residual K on the Decomposition of N2O over Cobalt–cerium Mixed Oxide Catalyst. Catal. Today 2007, 126, 449–455. [Google Scholar] [CrossRef]
- Obalová, L.; Maniak, G.; Karásková, K.; Kovanda, F.; Kotarba, A. Electronic Nature of Potassium Promotion Effect in Co–Mn–Al Mixed Oxide on the Catalytic Decomposition of N2O. Catal. Commun. 2011, 12, 1055–1058. [Google Scholar] [CrossRef]
- Grzybek, G.; Stelmachowski, P.; Gudyka, S.; Duch, J.; Ćmil, K.; Kotarba, A.; Sojka, Z. Insights into the Twofold Role of CS Doping on De N2O Activity of Cobalt Spinel Catalyst—Towards Rational Optimization of the Precursor and Loading. Appl. Catal. B Environ. 2015, 168–169, 509–514. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Maniak, G.; Kotarba, A.; Sojka, Z. Strong Electronic Promotion of Co3O4 Towards N2O Decomposition by Surface Alkali Dopants. Catal. Commun. 2009, 10, 1062–1065. [Google Scholar] [CrossRef]
- Karásková, K.; Obalová, L.; Jirátová, K.; Kovanda, F. Effect of Promoters in Co–Mn–Al mixed Oxide Catalyst on N2O Decomposition. Chem. Eng. J. 2010, 160, 480–487. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Legutko, P.; Gryboś, J.; Indyka, P.; Leszczyński, B.; Kotarba, A.; Sojka, Z. Thermal Stability and Repartition of Potassium Promoter between the Support and Active Phase in the K-Co2.6Zn0.4O4|α-Al2O3 Catalyst for N2O Decomposition: Crucial Role of Activation Temperature on Catalytic Performance. Appl. Catal. B Environ. 2017, 205, 597–604. [Google Scholar] [CrossRef]
- Jirátová, K.; Pacultová, K.; Balabánová, J.; Karásková, K.; Klegová, A.; Bílková, T.; Jandová, V.; Koštejn, M.; Martaus, A.; Kotarba, A.; et al. Precipitated K-Promoted Co–Mn–Al Mixed Oxides for Direct NO Decomposition: Preparation and Properties. Catalysts 2019, 9, 592. [Google Scholar] [CrossRef]
- Pacultová, K.; Bílková, T.; Klegova, A.; Karásková, K.; Fridrichová, D.; Jirátová, K.; Kiška, T.; Balabánová, J.; Koštejn, M.; Kotarba, A.; et al. Co-Mn-Al Mixed Oxides Promoted by K for Direct NO Decomposition: Effect of Preparation Parameters. Catalysts 2019, 9, 593. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Masui, T.; Imanaka, N. Fundamental Aspects of Rare Earth Oxides Affecting Direct NO Decomposition Catalysis. Eur. J. Inorg. Chem. 2015, 2015, 1524–1528. [Google Scholar] [CrossRef]
- Hong, W.-J.; Iwamoto, S.; Hosokawa, S.; Wada, K.; Kanai, H.; Inoue, M. Effect of Mn Content on Physical Properties of CeOx–MnOy Support and BaO–CeOx–MnOy Catalysts for Direct NO Decomposition. J. Catal. 2011, 277, 208–216. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Hamada, H. Reaction Mechanism of NO Decomposition over Alkali Metal-Doped Cobalt Oxide Catalysts. Appl. Catal. B Environ. 2005, 55, 169–175. [Google Scholar] [CrossRef]
- Haneda, M.; Nakamura, I.; Fujitani, T.; Hamada, H. Catalytic Active Site for NO Decomposition Elucidated by Surface Science and Real Catalyst. Catal. Surv. Asia 2005, 9, 207–215. [Google Scholar] [CrossRef]
- Jirátová, K.; Pacultová, K.; Karásková, K.; Balabánová, J.; Koštejn, M.; Obalová, L. Direct Decomposition of NO over Co-Mn-Al Mixed Oxides: Effect of Ce and/or K Promoters. Catalysts 2020, 10, 808. [Google Scholar] [CrossRef]
- Brown, W.A.; King, D.A. NO Chemisorption and Reactions on Metal Surfaces: A New Perspective. J. Phys. Chem. B 2000, 104, 2578–2595. [Google Scholar] [CrossRef]
- Pacultová, K.; Draštíková, V.; Chromčáková, Ž.; Bílková, T.; Kutláková, K.M.; Kotarba, A.; Obalová, L. On The Stability of Alkali Metal Promoters in Co Mixed Oxides during Direct NO Catalytic Decomposition. Mol. Catal. 2017, 428, 33–40. [Google Scholar] [CrossRef]
- Kotarba, A.; Kruk, I.; Sojka, Z. Energetics of Potassium Loss from Styrene Catalyst Model Components: Reassignment of K Storage and Release Phases. J. Catal. 2002, 211, 265–272. [Google Scholar] [CrossRef]
- Bieniasz, W.; Trębala, M.; Sojka, Z.; Kotarba, A. Irreversible Deactivation of Styrene Catalyst Due to Potassium Loss—Development of Antidote via Mechanism Pinning. Catal. Today 2010, 154, 224–228. [Google Scholar] [CrossRef]
- Peck, T.C.; Roberts, C.A.; Reddy, G.K. Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis. Catalysts 2020, 10, 561. [Google Scholar] [CrossRef]
- Karásková, K.; Pacultová, K.; Klegova, A.; Fridrichová, D.; Valášková, M.; Jirátová, K.; Stelmachowski, P.; Kotarba, A.; Obalová, L. Magnesium Effect in K/Co-Mg-Mn-Al Mixed Oxide Catalyst for Direct NO Decomposition. Catalysts 2020, 10, 931. [Google Scholar] [CrossRef]
- Hong, W.-J.; Iwamoto, S.; Inoue, M. Direct NO Decomposition over a Ce–Mn Mixed Oxide Modified with Alkali and Alkaline Earth Species and CO2-TPD Behavior of the Catalysts. Catal. Today 2011, 164, 489–494. [Google Scholar] [CrossRef]
- Xiang, J.; Du, X.; Wan, Y.; Chen, Y.; Ran, J.; Zhang, L. Alkali-driven Active Site Shift of fast SCR with NH3 on V2O5–WO3/TiO2 Catalyst via a Novel Eley–Rideal Mechanism. Catal. Sci. Technol. 2019, 9, 6085–6091. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Guo, J.; Xiong, J.; Lin, Y.; Zhu, T. An Efficient and Sulfur Resistant K-Modified Activated Carbon for SCR Denitrification Compared with Acid-and CU-Modified Activated Carbon. Chem. Eng. J. 2020, 395, 125047. [Google Scholar] [CrossRef]
- Hao, Z.; Shen, Z.; Li, Y.; Wang, H.; Zheng, L.; Wang, R.; Liu, G.; Zhan, S. The Role of Alkali Metal in α-MnO 2 Catalyzed Ammonia-Selective Catalysis. Angew. Chem. Int. Ed. 2019, 58, 6351–6356. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Highly Selective Conversion of CO2 to Light Olefins via Fischer-Tropsch Synthesis over Stable Layered K–Fe–Ti Catalysts. Appl. Catal. A Gen. 2019, 573, 32–40. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.; Wang, Y.; Herron, J.A.; Xu, Y.; Allard, L.F.; Lee, S.; Huang, J.; Mavrikakis, M.; Flytzani-Stephanopoulos, M. Catalytically Active Au-O(OH)x-Species Stabilized by Alkali Ions on Zeolites and Mesoporous Oxides. Science 2014, 346, 1498–1501. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.; Shen, M. Promotional Effect of Ion-Exchanged K on the Low-Temperature Hydrothermal Stability of Cu/SAPO-34 And Its Synergic Application with Fe/Beta Catalysts. Front. Environ. Sci. Eng. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Konsolakis, M.; Lambert, R.; MacLeod, N.; Nalbantian, L. Extraordinarily Effective Promotion by Sodium in Emission Control Catalysis: NO Reduction by Propene over Na-Promoted Pt/γ-Al2O3. Appl. Catal. B Environ. 1999, 22, 123–133. [Google Scholar] [CrossRef]
- Konsolakis, M.; Yentekakis, I.V. Strong Promotional Effects of Li, K, Rb and Cs on the Pt-Catalysed Reduction of NO by Propene. Appl. Catal. B Environ. 2001, 29, 103–113. [Google Scholar] [CrossRef]
- Konsolakis, M.; Yentekakis, I.V.; Palermo, A.; Lambert, R. Optimal Promotion by Rubidium of the CO + NO Reaction Over Pt/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2001, 33, 293–302. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Guan, N.; Schreier, E.; Richter, M. NO Selective Reduction by Hydrogen on Potassium Titanate Supported Palladium Catalyst. Catal. Commun. 2008, 9, 1827–1832. [Google Scholar] [CrossRef]
- Son, I.H.; Kim, M.C.; Koh, H.L.; Kim, K.-L. On the Promotion of Ag/γ-Al2O3 by Cs for the SCR of NO by C3H6. Catal. Lett. 2001, 75, 191–197. [Google Scholar] [CrossRef]
- Feng, B.; Lu, G.; Wang, Y.; Guo, Y.; Guo, Y. Promoting Role of Potassium on the Catalytic Performance of Copper Oxide for the Reduction of NO by Activated Carbon. Chin. J. Catal. 2011, 32, 853–861. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, N.; Meier, D.M.; Baiker, A. Potassium Titanate Nanobelts: A Unique Support for Au and AuRh Nanoparticles in the Catalytic Reduction of NO with CO. ChemCatChem 2021, 13, 438–444. [Google Scholar] [CrossRef]
- Gholami, Z.; Luo, G.; Gholami, F.; Yang, F. Recent Advances in Selective Catalytic Reduction of Nox by Carbon Monoxide for Flue Gas Cleaning Process: A Review. Catal. Rev. 2021, 63, 68–119. [Google Scholar] [CrossRef]
- Konsolakis, M.; Yentekakis, I.V. NO Reduction by Propene or CO Over Alkali-promoted Pd/YSZ Catalysts. J. Hazard. Mater. 2007, 149, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Ha, H.P. SO2 Promoted Alkali Metal Doped Ag/Al2O3 Catalysts for CH4-SCR of NOx. Appl. Catal. A Gen. 2012, 433–434, 162–169. [Google Scholar] [CrossRef]
- Morales, C.; López, N.; Aguila, G.; Araya, P.; Scott, F.; Vergara-Fernández, A.; Guerrero, S. Simultaneous use of CO and Naphthalene for the Reduction of NO on Potassium Promoted Copper Catalyst Supported on Ce/TiO2-SiO2 and in the Presence of Oxygen. Mater. Chem. Phys. 2019, 222, 294–299. [Google Scholar] [CrossRef]
- Wang, Q.; Park, S.Y.; Choi, J.S.; Chung, J.S. Co/KxTi2O5 Catalysts Prepared by Ion Exchange Method for NO Oxidation to NO2. Appl. Catal. B Environ. 2008, 79, 101–107. [Google Scholar] [CrossRef]
- Yentekakis, I.; Lambert, R.; Tikhov, M.; Konsolakis, M.; Kiousis, V. Promotion by Sodium in Emission Control Catalysis: A Kinetic and Spectroscopic Study of the Pd-Catalyzed Reduction of NO by Propene. J. Catal. 1998, 176, 82–92. [Google Scholar] [CrossRef]
- MacLeod, N.; Isaac, J.; Lambert, R.M. Sodium Promotion of Pd/γ-Al2O3 Catalysts Operated under Simulated “Three-Way” Conditions. J. Catal. 2001, 198, 128–135. [Google Scholar] [CrossRef]
- Yentekakis, I.; Tellou, V.; Botzolaki, G.; Rapakousios, I. A Comparative Study of the C3H6+NO+O2, C3H6+O2 and NO+O2 Reactions in Excess Oxygen Over Na-Modified Pt/γ-Al2O3 Catalysts. Appl. Catal. B Environ. 2005, 56, 229–239. [Google Scholar] [CrossRef]
- Xie, Y.; Galvez, M.E.; Matynia, A.; Da Costa, P. Experimental Investigation on the Influence of the Presence of Alkali Compounds on the Performance of a Commercial Pt–Pd/Al2O3 Diesel Oxidation Catalyst. Clean Technol. Environ. Policy 2017, 20, 715–725. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, F.; Wei, S.; Yang, W.; Zhou, Y. The Role of Potassium in the Activation of Oxygen to Promote Nitric Oxide Oxidation on Honeycomb-like h-BN(001) Surfaces. Phys. Chem. Chem. Phys. 2018, 20, 26777–26785. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, J.; Yu, X. Density Functional Theory Studies on the Adsorption, Diffusion and Dissociation of O2 on Pt(111). Phys. Lett. A 2010, 374, 4713–4717. [Google Scholar] [CrossRef]
- Tang, X.; Gao, F.; Xiang, Y.; Yi, H.; Zhao, S.; Liu, X.; Li, Y. Effect of Potassium-Precursor Promoters on Catalytic Oxidation Activity of Mn-CoOx Catalysts for NO Removal. Ind. Eng. Chem. Res. 2015, 54, 9116–9123. [Google Scholar] [CrossRef]
- Shang, Z.; Sun, M.; Che, X.; Wang, W.; Wang, L.; Cao, X.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. The Existing States of Potassium Species in K-Doped Co3O4 Catalysts and Their Influence on the Activities for NO and Soot Oxidation. Catal. Sci. Technol. 2017, 7, 4710–4719. [Google Scholar] [CrossRef]
- Adjimi, S.; García-Vargas, J.; Díaz, J.; Retailleau, L.; Gil, S.; Pera-Titus, M.; Guo, Y.; Giroir-Fendler, A. Highly Efficient and Stable Ru/K-OMS-2 Catalyst for NO Oxidation. Appl. Catal. B Environ. 2017, 219, 459–466. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Ascaso, S.; Moliner, R.; Lázaro, M.J. Me (Cu, Co, V)-K/Al2O3 Supported Catalysts for the Simultaneous Removal of Soot and Nitrogen Oxides from Diesel Exhausts. Chem. Eng. Sci. 2013, 87, 75–90. [Google Scholar] [CrossRef]
- Aneggi, E.; De Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Diesel Soot Combustion Activity of Ceria Promoted with Alkali Metals. Catal. Today 2008, 136, 3–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Q.; Li, Q.; Wang, Z.; Gao, X.; Zhang, Z. Determination of Mechanism for Soot Oxidation with NO on Potassium Supported Mg-Al Hydrotalcite Mixed Oxides. Chem. Eng. Technol. 2011, 34, 1864–1868. [Google Scholar] [CrossRef]
- Jimenez, R.; García, X.; Lopez, T.; Gordon, A. Catalytic Combustion of Soot. Effects of Added Alkali Metals on CaO–MgO Physical Mixtures. Fuel Process. Technol. 2008, 89, 1160–1168. [Google Scholar] [CrossRef]
- Weng, D.; Li, J.; Wu, X.; Lin, F. Promotional Effect of Potassium on Soot Oxidation Activity and SO2-Poisoning Resistance of Cu/CeO2 catalyst. Catal. Commun. 2008, 9, 1898–1901. [Google Scholar] [CrossRef]
- Gross, M.S.; Ulla, M.A.; Querini, C.A. Catalytic Oxidation of Diesel Soot: New Characterization and Kinetic Evidence Related to the Reaction Mechanism on K/CeO2 Catalyst. Appl. Catal. A Gen. 2009, 360, 81–88. [Google Scholar] [CrossRef]
- Hirabayashi, D.; Hashikawa, H.; Suzuki, K. Low Temperature Soot Oxidation via the Melting Process of Alkaline Salts. Waste Manag. Environ. IV 2008, 109, 847–856. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, C.; Shu, X.; Yue, T.; Wang, S.; Deng, Z. The Mechanism of Pd, K Co-doping on Mg–Al Hydrotalcite for Simultaneous Removal of Diesel Soot and NOx in SO2-Containing Atmosphere. Fuel 2019, 240, 244–251. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. Diesel Soot Elimination over Potassium-Promoted Co3O4 Nanowires Monolithic Catalysts under Gravitation Contact Mode. Appl. Catal. B Environ. 2017, 218, 32–45. [Google Scholar] [CrossRef]
- Rinkenburger, A.; Toriyama, T.; Yasuda, K.; Niessner, R. Catalytic Effect of Potassium Compounds in Soot Oxidation. ChemCatChem 2017, 9, 3513–3525. [Google Scholar] [CrossRef]
- Jiménez, R.; García, X.; Gordon, A.L. CaO-MgO Catalysts for Soot Combustion: KnO3 as Source for Doping with Potassium. J. Chil. Chem. Soc. 2005, 50, 651–665. [Google Scholar] [CrossRef]
- Jiménez, R.; García, X.; Cellier, C.; Ruiz, P.; Gordon, A.L. Soot Combustion with K/MgO as Catalyst. Appl. Catal. A Gen. 2006, 297, 125–134. [Google Scholar] [CrossRef]
- Pecchi, G.; Cabrera, B.; Buljan, A.; Delgado, E.; Gordon, A.; Jimenez, R. Catalytic Oxidation of Soot over Alkaline Niobates. J. Alloy. Compd. 2013, 551, 255–261. [Google Scholar] [CrossRef]
- McKee, D.W. Mechanisms of the Alkali Metal Catalysed Gasification of Carbon. Fuel 1983, 62, 170–175. [Google Scholar] [CrossRef]
- Jiménez, R.; García, X.; Cellier, C.; Ruiz, P.; Gordon, A.L. Soot Combustion with K/MgO as Catalyst II. Effect of K-precursor. Appl. Catal. A Gen. 2006, 314, 81–88. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Zou, Z.-Q.; Li, X.-G.; Zha, Y.-Q. Simultaneous Soot Combustion and Nitrogen Oxides Storage on Potassium-Promoted Hydrotalcite-Based CoMgAlO Catalysts. J. Hazard. Mater. 2009, 161, 366–372. [Google Scholar] [CrossRef]
- Tikhomirov, K.; Kröcher, O.; Wokaun, A. Influence of Potassium Doping on the Activity and the Sulfur Poisoning Resistance of Soot Oxidation Catalysts. Catal. Lett. 2006, 109, 49–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X. The Catalytic Activities and Thermal Stabilities of Li/Na/K Carbonates for Diesel Soot Oxidation. Catal. Commun. 2007, 8, 760–764. [Google Scholar] [CrossRef]
- Li, C.; Soh, K.C.K.; Wu, P. Formability of ABO3 Perovskites. J. Alloy. Compd. 2004, 372, 40–48. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. The Role of Suprafacial Oxygen in Some Perovskites for the Catalytic Combustion of Soot. J. Catal. 2003, 217, 367–375. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Badini, C.; Saracco, G.; Specchia, V. Effect of Active Species Mobility on Soot-Combustion over CS-V Catalysts. AIChE J. 2003, 49, 2173–2180. [Google Scholar] [CrossRef]
- Neri, G. K- and Cs-FeV/Al2O3 Soot Combustion Catalysts for Diesel Exhaust Treatment. Appl. Catal. B Environ. 2003, 42, 381–391. [Google Scholar] [CrossRef]
- Stanmore, B.; Brilhac, J.; Gilot, P. The Oxidation of Soot: A Review of Experiments, Mechanisms and Models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Badini, C.; Saracco, G.; Russo, N.; Specchia, V. A Screening Study on the Activation Energy of Vanadate-Based Catalysts for Diesel Soot Combustion. Catal. Lett. 2000, 69, 207–215. [Google Scholar] [CrossRef]
- Weng, D.; Li, J.; Wu, X.; Si, Z. Modification of CeO2-ZrO2 Catalyst by Potassium for NOx-Assisted Soot Oxidation. J. Environ. Sci. 2011, 23, 145–150. [Google Scholar] [CrossRef]
- Zhang, Z.; Mou, Z.; Yu, P.; Zhang, Y.; Ni, X. Diesel Soot Combustion on Potassium Promoted Hydrotalcite-Based Mixed Oxide Catalysts. Catal. Commun. 2007, 8, 1621–1624. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.; Zhang, Z.; Chen, M.; Shangguan, W. Promotion Effect of Potassium Carbonate on Catalytic Activity of Co3 O4 for Formaldehyde Removal. J. Chem. Technol. Biotechnol. 2018, 93, 3562–3568. [Google Scholar] [CrossRef]
- Liu, H.; Jia, A.; Yuwang; Luo, M.; Lu, J. Enhanced CO Oxidation over Potassium-Promoted Pt/Al2O3 Catalysts: Kinetic and Infrared Spectroscopic Study. Chin. J. Catal. 2015, 36, 1976–1986. [Google Scholar] [CrossRef]
- Tanaka, H.; Kuriyama, M.; Ishida, Y.; Ito, S.-I.; Tomishige, K.; Kunimori, K. Preferential CO Oxidation in Hydrogen-Rich Stream over Pt Catalysts Modified with Alkali Metals Part I. Catalytic performance. Appl. Catal. A Gen. 2008, 343, 117–124. [Google Scholar] [CrossRef]
- Kuriyama, M.; Tanaka, H.; Ito, S.-I.; Kubota, T.; Miyao, T.; Naito, S.; Tomishige, K.; Kunimori, K. Promoting Mechanism of Potassium in Preferential CO Oxidation on Pt/Al2O3. J. Catal. 2007, 252, 39–48. [Google Scholar] [CrossRef]
- Derrouiche, S.; Gravejat, P.; Bassou, B.; Bianchi, D. Impact of Potassium on the Heats of Adsorption of Adsorbed CO Species on Supported Pt Particles by Using the AEIR Method. Appl. Surf. Sci. 2007, 253, 5894–5898. [Google Scholar] [CrossRef]
- Niu, T.; Wang, C.; Zhang, L.; Liu, Y. Potassium Promoted Ru/Meso-Macroporous SiO2 Catalyst for the Preferential Oxidation of CO in H2-Rich Gases. Int. J. Hydrog. Energy 2013, 38, 7801–7810. [Google Scholar] [CrossRef]
- Pavlenko, N.; Kostrobij, P.; Suchorski, Y.; Imbihl, R. Alkali Metal Effect on Catalytic Co Oxidation on a Transition Metal Surface: A Lattice-Gas Model. Surf. Sci. 2001, 489, 29–36. [Google Scholar] [CrossRef]
- Yakovkin, I.; Chernyi, V.; Naumovets, A. Oxidation of CO on Li-precovered Pt. Surf. Sci. 1999, 442, 81–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Jin, L.; Xie, D. Development and Performance of a K–Pt/γ-Al 2 O 3 Catalyst for the Preferential Oxidation of CO in Hydrogen-Rich Synthesis Gas. Int. J. Hydrog. Energy 2014, 39, 18668–18674. [Google Scholar] [CrossRef]
- Liotta, L.F.; Martin, G.A.; Deganello, G. The Influence of Alkali Metal Ions in the Chemisorption of CO and CO2on Supported Palladium Catalysts: A Fourier Transform Infrared Spectroscopic Study. J. Catal. 1996, 164, 322–333. [Google Scholar] [CrossRef]
- Solymosi, F.; Pásztor, M.; Rákhely, G. Infrared Studies of the Effects of Promoters on CO-induced Structural Changes in Rh. J. Catal. 1988, 110, 413–415. [Google Scholar] [CrossRef]
- Dai, C.H.; Worley, S.D. Effects of Potassium on Carbon Monoxide Methanation over Supported Rhodium Films. J. Phys. Chem. 1986, 90, 4219–4221. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Grinter, D.C.; Ramírez, P.J.; Stacchiola, D.J.; Senanayake, S.D. High Activity of Au/K/TiO2(110) for CO Oxidation: Alkali-Metal-Enhanced Dispersion of Au and Bonding of CO. J. Phys. Chem. C 2018, 122, 4324–4330. [Google Scholar] [CrossRef]
- Erikat, I.A. First Principle Study on Alkali Metals Promotion of CO Oxidation over Ir(100). Phys. Status Solidi 2016, 253, 983–989. [Google Scholar] [CrossRef]
- Minemura, Y.; Ito, S.-I.; Miyao, T.; Naito, S.; Tomishige, K.; Kunimori, K. Preferential CO Oxidation Promoted by the Presence of H2 over K–Pt/Al2O3. Chem. Commun. 2005, 1429–1431. [Google Scholar] [CrossRef]
- Tanaka, H.; Ito, S.-I.; Kameoka, S.; Tomishige, K.; Kunimori, K. Promoting Effect of Potassium in Selective Oxidation of CO in Hydrogen-Rich Stream on Rh Catalysts. Catal. Commun. 2003, 4, 1–4. [Google Scholar] [CrossRef]
- Ang, M.L.; Oemar, U.; Saw, E.T.; Mo, L.; Kathiraser, Y.; Chia, B.H.; Kawi, S. Highly Active Ni/xNa/CeO2 Catalyst for the Water–Gas Shift Reaction: Effect of Sodium on Methane Suppression. ACS Catal. 2014, 4, 3237–3248. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Tang, X.; Marginean, P.; Nieuwenhuys, B.E. Characterization and Catalytic Activity of Unpromoted and Alkali (earth)-Promoted Au/Al2O3 Catalysts for Low-Temperature CO Oxidation. Top. Catal. 2006, 39, 101–110. [Google Scholar] [CrossRef]
- Tanaka, H.; Ito, S.-I.; Kameoka, S.; Tomishige, K.; Kunimori, K. Catalytic Performance of K-promoted Rh/USY Catalysts in Preferential Oxidation of CO in Rich Hydrogen. Appl. Catal. A Gen. 2003, 250, 255–263. [Google Scholar] [CrossRef]
- Sheffer, G. Potassium’s Promotional Effect of Unsupported Copper Catalysts for Methanol Synthesis. J. Catal. 1989, 115, 376–387. [Google Scholar] [CrossRef]
- Farkas, A.P.; Solymosi, F. Activation and Reactions of CO2 on a K-Promoted Au(111) Surface. J. Phys. Chem. C 2009, 113, 19930–19936. [Google Scholar] [CrossRef]
- Dongil, A.B.; Bachiller-Baeza, B.; Castillejos, E.; Escalona, N.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. The Promoter Effect of Potassium in CuO/CeO2 Systems Supported on Carbon Nanotubes and Graphene for the CO-PROX Reaction. Catal. Sci. Technol. 2016, 6, 6118–6127. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Maslakov, K.I.; Chernyak, S.A.; Lunin, V.V. Promoting Effect of Potassium and Calcium Additives to Cerium–Zirconium Oxide Catalysts for the Complete Oxidation of Carbon Monoxide. Kinet. Catal. 2017, 58, 585–592. [Google Scholar] [CrossRef]
- Minemura, Y.; Kuriyama, M.; Ito, S.-I.; Tomishige, K.; Kunimori, K. Additive Effect of Alkali Metal Ions on Preferential CO Oxidation Over Pt/Al2O3. Catal. Commun. 2006, 7, 623–626. [Google Scholar] [CrossRef]
- Pedrero, C.; Waku, T.; Iglesia, E. Oxidation of CO in H2–CO Mixtures Catalyzed by Platinum: Alkali Effects on Rates and Selectivity. J. Catal. 2005, 233, 242–255. [Google Scholar] [CrossRef]
- Ito, S.-I.; Tanaka, H.; Minemura, Y.; Kameoka, S.; Tomishige, K.; Kunimori, K. Selective CO Oxidation in H2-Rich Gas over K2CO3-promoted Rh/SiO2 Catalysts: Effects of Preparation Method. Appl. Catal. A Gen. 2004, 273, 295–302. [Google Scholar] [CrossRef]
- Tanaka, H.; Kuriyama, M.; Ishida, Y.; Ito, S.-I.; Kubota, T.; Miyao, T.; Naito, S.; Tomishige, K.; Kunimori, K. Preferential CO Oxidation in Hydrogen-Rich Stream over PT Catalysts Modified with Alkali Metals Part II. Catalyst characterization and role of alkali metals. Appl. Catal. A Gen. 2008, 343, 125–133. [Google Scholar] [CrossRef]
- Bugyi, L.; Solymosi, F. Effects of Potassium on the Chemisorption of CO on the Mo2C/Mo(100) Surface. J. Phys. Chem. B 2001, 105, 4337–4342. [Google Scholar] [CrossRef]
- Liu, B.; Huang, T.; Zhang, Z.; Wang, Z.; Zhang, Y.; Li, J. The Effect of the Alkali Additive on the Highly Active Ru/C Catalyst for Water Gas Shift Reaction. Catal. Sci. Technol. 2014, 4, 1286–1292. [Google Scholar] [CrossRef]
- Nagai, M.; Zahidul, A.M.; Kunisaki, Y.; Aoki, Y. Water–Gas Shift Reactions on Potassium- and Zirconium-Promoted Cobalt Molybdenum Carbide Catalysts. Appl. Catal. A Gen. 2010, 383, 58–65. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Remensal, E.R.; Ramírez, P.J.; Orozco, I.; Liu, Z.; Graciani, J.; Senanayake, S.D.; Sanz, J.F. Water–Gas Shift Reaction on K/Cu(111) and Cu/K/TiO2(110) Surfaces: Alkali Promotion of Water Dissociation and Production of H2. ACS Catal. 2019, 9, 10751–10760. [Google Scholar] [CrossRef]
- Evin, H.N.; Jacobs, G.; Ruiz-Martinez, J.; Graham, U.M.; Dozier, A.; Thomas, G.; Davis, B.H. Low Temperature Water–Gas Shift/Methanol Steam Reforming: Alkali Doping to Facilitate the Scission of Formate and Methoxy C–H Bonds over Pt/ceria Catalyst. Catal. Lett. 2008, 122, 9–19. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. K-doped LaNiO3 Perovskite for High-Temperature Water-Gas Shift of Reformate Gas: Role of Potassium on Suppressing Methanation. Int. J. Hydrog. Energy 2017, 42, 9840–9857. [Google Scholar] [CrossRef]
- Kaftan, A.; Kusche, M.; Laurin, M.; Wasserscheid, P.; Libuda, J. KOH-promoted Pt/Al2O3 Catalysts for Water Gas Shift and Methanol Steam Reforming: An Operando DRIFTS-MS Study. Appl. Catal. B Environ. 2017, 201, 169–181. [Google Scholar] [CrossRef]
- Zhai, Y.; Pierre, D.; Si, R.; Deng, W.; Ferrin, P.; Nilekar, A.U.; Peng, G.; Herron, J.A.; Bell, D.C.; Saltsburg, H.; et al. Alkali-Stabilized Pt-OHx Species Catalyze Low-Temperature Water-Gas Shift Reactions. Science 2010, 329, 1633–1636. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Pierre, D.; Deng, W.; Flytzani-Stephanopoulos, M. The Importance of Strongly Bound Pt–CeOx Species for the Water-gas Shift Reaction: Catalyst Activity and Stability Evaluation. Top. Catal. 2007, 46, 363–373. [Google Scholar] [CrossRef]
- Pazmiño, J.H.; Shekhar, M.; Williams, W.D.; Akatay, M.C.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Metallic Pt as Active Sites for the Water–Gas Shift Reaction on Alkali-Promoted Supported Catalysts. J. Catal. 2012, 286, 279–286. [Google Scholar] [CrossRef]
- Kantschewa, M. Nature and Properties of a Potassium-Promoted NiMo/Al2O3 Water Gas Shift Catalyst. J. Catal. 1984, 87, 482–496. [Google Scholar] [CrossRef]
- Majima, T.; Kono, E.; Ogo, S.; Sekine, Y. Pre-Reduction and K Loading Effects on Noble Metal Free Co-system Catalyst for Water Gas Shift Reaction. Appl. Catal. A Gen. 2016, 523, 92–96. [Google Scholar] [CrossRef]
- Kordulis, C.; Voliotis, S.; Lycourghiotis, A. Molybdena Catalysts Prepared on Modified Carriers: Regulation of the Symmetry and Valence of the Molybdenum Species Formed on γ-Al2O3 Modified with Alkali Cations. J. Less Common Met. 1982, 84, 187–200. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, M.; Lobban, L.L.; Mallinson, R.G. Structural Effects of Na Promotion for High Water Gas Shift Activity on Pt–Na/TiO2. J. Catal. 2011, 278, 123–132. [Google Scholar] [CrossRef]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Catalytic Activity of Rare Earth and Alkali Metal Promoted (Ce, La, Mg, K) Ni/Al2O3 Nanocatalysts in Reverse Water Gas Shift Reaction. Res. Chem. Intermed. 2019, 45, 5125–5141. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Baldwin, J.W.; Peng, X.; Mpourmpakis, G.; Willauer, H.D. Potassium-Promoted Molybdenum Carbide as a Highly Active and Selective Catalyst for CO2 Conversion to CO. ChemSusChem 2017, 10, 2408–2415. [Google Scholar] [CrossRef]
- Prasad, P.S.S.; Bae, J.W.; Jun, K.-W.; Lee, K.-W. Fischer–Tropsch Synthesis by Carbon Dioxide Hydrogenation on Fe-Based Catalysts. Catal. Surv. Asia 2008, 12, 170–183. [Google Scholar] [CrossRef]
- Kotarba, A.; Barański, A.; Hodorowicz, S.; Sokołowski, J.; Szytuła, A.; Holmlid, L. Stability and Excitation of Potassium Promoter in Iron Catalysts—the Role of KFeO2 and KAlO2 Phases. Catal. Lett. 2000, 67, 129–134. [Google Scholar] [CrossRef]
- Loiland, J.A.; Wulfers, M.J.; Marinkovic, N.S.; Lobo, R.F. Fe/γ-Al2O3 and Fe–K/γ-Al2O3 as Reverse Water-Gas Shift Catalysts. Catal. Sci. Technol. 2016, 6, 5267–5279. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Delgado, J.J.; Zhang, T. Promoting Role of Potassium in the Reverse Water Gas Shift Reaction on Pt/Mullite Catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]
- Morse, J.R.; Juneau, M.; Baldwin, J.W.; Porosoff, M.D.; Willauer, H.D. Alkali Promoted Tungsten Carbide as a Selective Catalyst for the Reverse Water Gas Shift Reaction. J. CO2 Util. 2020, 35, 38–46. [Google Scholar] [CrossRef]
- Pistonesi, C.; Pronsato, M.E.; Bugyi, L.; Juan, A. The Adsorption of CO on Potassium Doped Molybdenum Carbide Surface: An Ab-Initio Study. Catal. Today 2012, 181, 102–107. [Google Scholar] [CrossRef]
- Chen, C.-S.; Cheng, W.-H.; Lin, S.-S. Study of Reverse Water Gas Shift Reaction by TPD, TPR and CO2 Hydrogenation over Potassium-promoted Cu/SiO2 Catalyst. Appl. Catal. A Gen. 2003, 238, 55–67. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Chen, X.; Duan, H.; Liang, B.; Liu, Q.; Liu, X.; Ren, Y.; Huang, Y.; Zhang, T. Promotion Effects of Potassium on the Activity and Selectivity of Pt/Zeolite Catalysts for Reverse Water Gas Shift Reaction. Appl. Catal. B Environ. 2017, 216, 95–105. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Shah, M.; Le Saché, E.; Reina, T.R. Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts 2018, 8, 608. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Chen, Y.; Zhang, R.; Yang, S. K-Promoted Co–CeO2 Catalyst for the Reverse Water–Gas Shift Reaction. Chem. Lett. 2013, 42, 682–683. [Google Scholar] [CrossRef]
- Williams, F.J.; Bird, D.P.C.; Palermo, A.; Santra, A.K.; Lambert, R.M. Mechanism, Selectivity Promotion, and New Ultraselective Pathways in Ag-Catalyzed Heterogeneous Epoxidation. J. Am. Chem. Soc. 2004, 126, 8509–8514. [Google Scholar] [CrossRef]
- Linic, S.; Barteau, M.A. On the Mechanism of Cs Promotion in Ethylene Epoxidation on Ag. J. Am. Chem. Soc. 2004, 126, 8086–8087. [Google Scholar] [CrossRef] [PubMed]
- Mross, W.D. Alkali Doping in Heterogeneous Catalysis. Catal. Rev. 1983, 25, 591–637. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.-H.; Wang, S.-Y.; Luo, M.-F.; Lu, J.-Q. Remarkable Enhancement of Dichloromethane Oxidation over Potassium-Promoted Pt/Al2O3 Catalysts. J. Catal. 2014, 311, 314–324. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K.-I. Catalytic Performance and Mechanism of a Pt/TiO2 Catalyst for the Oxidation of Formaldehyde at Room Temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Zhai, Y.; Ariga, H.; Yi, N.; Liu, Y.; Asakura, K.; Flytzani-Stephanopoulos, M.; He, H. Alkali-Metal-Promoted Pt/TiO2 Opens a More Efficient Pathway to Formaldehyde Oxidation at Ambient Temperatures. Angew. Chem. Int. Ed. 2012, 51, 9628–9632. [Google Scholar] [CrossRef]
- Xu, J.; Ekblad, M.; Nishiyama, S.; Tsuruya, S.; Masai, M. Effect of Alkali Metals Added to Cu Ion-Exchanged Y-Type Zeolite Catalysts in the Gas-Phase Catalytic Oxidation of Benzyl Alcohol. J. Chem. Soc. Faraday Trans. 1998, 94, 473–479. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, K.H.; Hong, S.C. A Study on HCHO Oxidation Characteristics at Room Temperature Using a Pt/TiO2 Catalyst. Appl. Catal. A Gen. 2011, 398, 96–103. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.; Fan, C.; Yang, L.; Wu, Z.; Qin, Z.; Zhu, H.; Fan, W.; Wang, J. Promoting Effect of Alkali Metal Cations on the Catalytic Performance of Pd/H-ZSM-5 in the Combustion of Lean Methane. Appl. Catal. A Gen. 2020, 602, 117678. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Yang, S.; Wang, Y. Iron-Catalyzed Propylene Epoxidation by Nitrous Oxide: Studies on the Effects of Alkali Metal Salts. J. Phys. Chem. B 2005, 109, 23500–23508. [Google Scholar] [CrossRef]
- Jirátová, K.; Mikulová, J.; Klempa, J.; Grygar, T.; Bastl, Z.; Kovanda, F. Modification of Co–Mn–Al Mixed Oxide with Potassium and Its Effect on Deep Oxidation of VOC. Appl. Catal. A Gen. 2009, 361, 106–116. [Google Scholar] [CrossRef]
- Sobczak, I.; Rydz, M.; Ziolek, M. The Effect of Alkali Metal on the Surface Properties of Potassium Doped Au-Beta Zeolites. Mater. Res. Bull. 2013, 48, 795–801. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, X.-M. The Effect of K and Al over NiCo2O4 Catalyst on Its Character and Catalytic Oxidation of VOCs. J. Mol. Catal. A Chem. 2004, 221, 77–80. [Google Scholar] [CrossRef]
- Nakashima, D.; Ichihashi, Y.; Nishiyama, S.; Tsuruya, S. Promoted Partial Oxidation Activity of Alkali Metal Added-Co Catalysts Supported on NaY and NaUSY Zeolites in the Gas-Phase Catalytic Oxidation of Benzyl Alcohol. J. Mol. Catal. A Chem. 2006, 259, 108–115. [Google Scholar] [CrossRef]
- Li, Y.; Nakashima, D.; Ichihashi, Y.; Nishiyama, S.; Tsuruya, S. Promotion Effect of Alkali Metal Added to Impregnated Cobalt Catalysts in the Gas-Phase Catalytic Oxidation of Benzyl Alcohol. Ind. Eng. Chem. Res. 2004, 43, 6021–6026. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Oikonomopoulos, E.; Kanistras, D.; Ioannides, T. Complete Oxidation of Ethanol over Alkali-Promoted Pt/Al2O3 Catalysts. Appl. Catal. B Environ. 2006, 65, 62–69. [Google Scholar] [CrossRef]
- Filkin, N.C.; Tikhov, M.S.; Palermo, A.; Lambert, R.M. A Kinetic and Spectroscopic Study of thein SituElectrochemical Promotion by Sodium of the Platinum-Catalyzed Combustion of Propene. J. Phys. Chem. A 1999, 103, 2680–2687. [Google Scholar] [CrossRef]
- Vernoux, P. In-Situ Electrochemical Control of the Catalytic Activity of Platinum for the Propene Oxidation. Solid State Ionics 2004, 175, 609–613. [Google Scholar] [CrossRef]
- Petrolekas, P.D.; Brosda, S.; Vayenas, C.G. Electrochemical Promotion of Pt Catalyst Electrodes Deposited on Na3Zr2Si2 PO 12 during Ethylene Oxidation. J. Electrochem. Soc. 1998, 145, 1469–1477. [Google Scholar] [CrossRef]
- De Lucasconsuegra, A.; Dorado, F.; Valverde, J.L.; Karoum, R.; Vernoux, P. Low-Temperature Propene Combustion over Pt/K-β-Al2O3 Electrochemical Catalyst: Characterization, Catalytic Activity Measurements, and Investigation of the NEMCA Effect. J. Catal. 2007, 251, 474–484. [Google Scholar] [CrossRef]
- Aissat, A.; Siffert, S.; Courcot, D. Preparation of Alkali-M/ZrO2 (M = Co or Cu) for VOCs Oxidation in the Presence of NOx or carbonaceous particles. Stud. Surf. Sci. Catal. 2010, 175, 747–750. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Rutkowska, M.; Wojciechowska, M.; Dudek, B.; Witkowski, S.; Michalik, M. Total Oxidation of Selected Mono-Carbon VOCs over Hydrotalcite Originated Metal Oxide Catalysts. Catal. Commun. 2012, 17, 118–125. [Google Scholar] [CrossRef]
- Aissat, A.; Siffert, S.; Courcot, D.; Cousin, R.; Aboukaïs, A. VOCs and Carbonaceous Particles Removal Assisted by NOx on Alkali0.15/ZrO2 and Csx–M0.1/ZrO2 Catalysts (M = Cu or Co). Comptes Rendus Chim. 2010, 13, 515–526. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, M.; Ma, Z.; Xing, S.T. Effect of Alkali Metal Promoters on Natural Manganese Ore Catalysts for the Complete Catalytic Oxidation of O-Xylene. Catal. Today 2011, 175, 196–201. [Google Scholar] [CrossRef]
- Lee, J.H.; Koo, K.Y.; Jung, U.H.; Park, J.E.; Yoon, W.L. The Promotional Effect of K on the Catalytic Activity of Ni/MgAl2O4 for the Combined H2O and CO2 Reforming of Coke Oven Gas for Syngas Production. Korean J. Chem. Eng. 2016, 33, 3115–3120. [Google Scholar] [CrossRef]
- Barthos, R.; Széchenyi, A.; Koós, Á.; Solymosi, F. The Decomposition of Ethanol over Mo2C/Carbon Catalysts. Appl. Catal. A Gen. 2007, 327, 95–105. [Google Scholar] [CrossRef]
- Dömök, M.; Baán, K.; Kecskés, T.; Erdőhelyi, A. Promoting Mechanism of Potassium in the Reforming of Ethanol on Pt/Al2O3 Catalyst. Catal. Lett. 2008, 126, 49–57. [Google Scholar] [CrossRef]
- Ogo, S.; Shimizu, T.; Nakazawa, Y.; Mukawa, K.; Mukai, D.; Sekine, Y. Steam Reforming of Ethanol over K Promoted Co Catalyst. Appl. Catal. A Gen. 2015, 495, 30–38. [Google Scholar] [CrossRef]
- Pendem, C.; Sarkar, B.; Siddiqui, N.; Konathala, L.N.S.K.; Baskar, C.; Bal, R. K-Promoted Pt-Hydrotalcite Catalyst for Production of H2 by Aqueous Phase Reforming of Glycerol. ACS Sustain. Chem. Eng. 2017, 6, 2122–2131. [Google Scholar] [CrossRef]
- Luna, A.E.C.; Iriarte, M.E. Carbon Dioxide Reforming of Methane over a Metal Modified Ni-Al2O3 Catalyst. Appl. Catal. A Gen. 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.; Illán-Gómez, M. Effect of Potassium Content in the Activity of K-Promoted Ni/Al2O3 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2006, 301, 9–15. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Inhibition of Methane Formation in Steam Reforming Reactions through Modification of Ni Catalyst and the Reactants. Green Chem. 2009, 11, 724–732. [Google Scholar] [CrossRef]
- Darujati, A.R.; Thomson, W.J. Stability of Supported and Promoted-Molybdenum Carbide Catalysts in Dry-Methane Reforming. Appl. Catal. A Gen. 2005, 296, 139–147. [Google Scholar] [CrossRef]
- Osaki, T.; Mori, T. Role of Potassium in Carbon-Free CO2 Reforming of Methane on K-Promoted Ni/Al2O3 Catalysts. J. Catal. 2001, 204, 89–97. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Calogero, G.; Torre, T.; Parmaliana, A. Potassium-enhanced Stability of Ni/MgO Catalysts in the Dry-reforming of Methane. Catal. Commun. 2001, 2, 49–56. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, S.; Wang, L.; Jiang, Q.; Li, S.; Tao, Z. Hydrogen Production from Steam Reforming of Kerosene Over Ni–La and Ni–La–K/Cordierite Catalysts. Fuel 2006, 85, 1708–1713. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Spadaro, L.; Bonura, G.; Cavallaro, S. Potassium Improved Stability of Ni/MgO in the Steam Reforming of Ethanol for the Production of Hydrogen for MCFC. J. Power Sources 2004, 132, 139–144. [Google Scholar] [CrossRef]
- Iwasa, N.; Yamane, T.; Arai, M. Influence of Alkali Metal Modification and Reaction Conditions on the Catalytic Activity and Stability of Ni Containing Smec-Tite-Type Material for Steam Reforming of Acetic Acid. Int. J. Hydrog. Energy 2011, 36, 5904–5911. [Google Scholar] [CrossRef]
- Nandini, A.; Pant, K.; Dhingra, S. K-, CeO2-and Mn-promoted Ni/Al2O3 Catalysts for Stable CO2 Reforming of Methane. Appl. Catal. A Gen. 2005, 290, 166–174. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Spadaro, L.; Di Blasi, O.; Bonura, G.; Cavallaro, S. Steam Reforming of Bio-Ethanol on Alkali-Doped Ni/MgO Catalysts: Hydrogen Production for MC Fuel Cell. Appl. Catal. A Gen. 2004, 270, 1–7. [Google Scholar] [CrossRef]
- Shi, H.; Ni, J.; Zheng, T.; Wang, X.; Wu, C.; Wang, Q. Remediation of Wastewater Contaminated by Antibiotics. A review. Environ. Chem. Lett. 2020, 18, 345–360. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Zhu, T.; Ding, S. Adsorption and Desorption of SO2, NO and Chlorobenzene on Activated Carbon. J. Environ. Sci. 2016, 43, 128–135. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Zhu, T.; Wang, J.; Ye, M. Modeling of Dioxin Adsorption on Activated Carbon. Chem. Eng. J. 2016, 283, 1210–1215. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhu, Y.; Du, X.; Lyu, Y.; Li, S.; Zhai, Y. Insight into the Enhanced Performance of Toluene Removal from Simulated Flue Gas over Mn-Cu Oxides Modified Activated Coke. Fuel 2020, 276, 118099. [Google Scholar] [CrossRef]








| Components | Sintering Flue Gas | Pelletizing Flue Gas | Coke Oven Flue Gas | Motor Vehicle Exhaust |
|---|---|---|---|---|
| NOx (mg/m3) | 200–400 | 200–300 | 300–1000 | 500–4000 |
| Soot (g/m3) | 1–5 | 1–10 | 0.02–0.1 | 2–6 * |
| CO (%) | 0.3–1 | 0.6–1 | 0.04–0.8 | 1–8 |
| CO2 (%) | 3–7 | 1–1.5 | 4–5 | 6–16 |
| NMVOCs (g/m3) | 0.5–5 | 6–18 | 15–20 | 1–50 |
| Type | Content | Preparation | Precursor | Calcination Temperature | Calcination time | Carrier | Reaction Temperature | Atmosphere | |
|---|---|---|---|---|---|---|---|---|---|
| N2O decomposition | ↑ | ▲ | Impregnation | K2CO3 | - | - | - | - | - |
| NO decomposition | - | ▲ | co-precipitation | - | ▲ | ↓ | - | - | - |
| Soot oxidation | K | ↑ | - | - | ▲ | - | - | - | - |
| CO oxidation | K | ▲ | Sequential impregnation | - | - | - | electron transfer effect | - | - |
| WGS | K | ▲ | - | K2CO3 | - | - | - | Not too high | - |
| RWGS | - | ▲ | - | - | - | - | - | - | - |
| VOCs oxidaiton | K | ▲ | - | KCl | - | - | - | - | stoichiometric ratio |
| Reforming | - | ↓ | - | -- | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Li, Y.; Shi, H.; Lin, Y.; Wang, Y.; Wang, Q.; Zhu, T. Application Prospect of K Used for Catalytic Removal of NOx, COx, and VOCs from Industrial Flue Gas: A Review. Catalysts 2021, 11, 419. https://doi.org/10.3390/catal11040419
Xu Z, Li Y, Shi H, Lin Y, Wang Y, Wang Q, Zhu T. Application Prospect of K Used for Catalytic Removal of NOx, COx, and VOCs from Industrial Flue Gas: A Review. Catalysts. 2021; 11(4):419. https://doi.org/10.3390/catal11040419
Chicago/Turabian StyleXu, Zhicheng, Yuran Li, Huimin Shi, Yuting Lin, Yan Wang, Qiang Wang, and Tingyu Zhu. 2021. "Application Prospect of K Used for Catalytic Removal of NOx, COx, and VOCs from Industrial Flue Gas: A Review" Catalysts 11, no. 4: 419. https://doi.org/10.3390/catal11040419
APA StyleXu, Z., Li, Y., Shi, H., Lin, Y., Wang, Y., Wang, Q., & Zhu, T. (2021). Application Prospect of K Used for Catalytic Removal of NOx, COx, and VOCs from Industrial Flue Gas: A Review. Catalysts, 11(4), 419. https://doi.org/10.3390/catal11040419







