Visible-Light Photocatalyst to Remove Indoor Ozone under Ambient Condition
Abstract
1. Introduction
2. Results and Discussion
2.1. Batch System
2.1.1. Background Test
2.1.2. Batch System—UV Irradiation
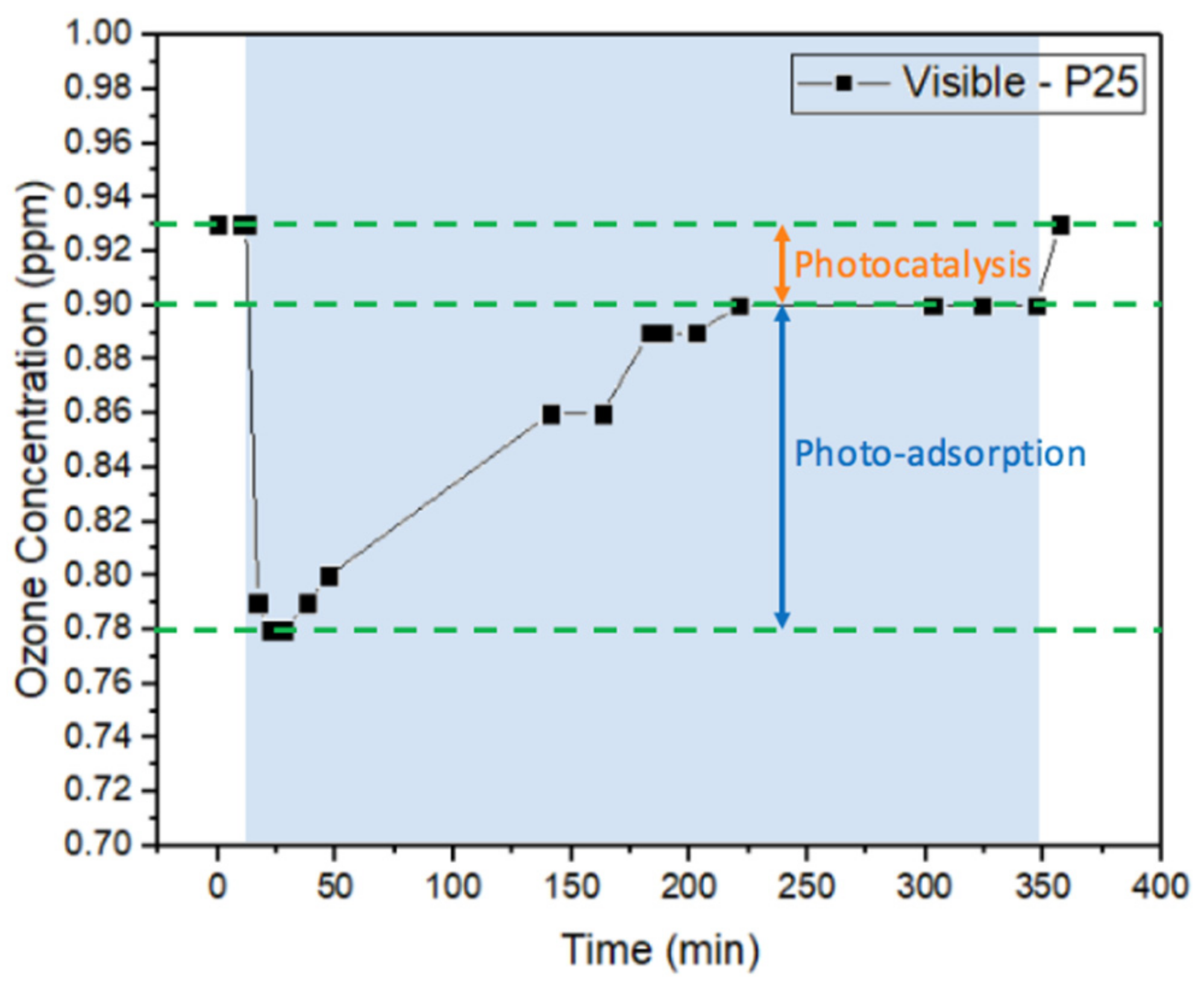
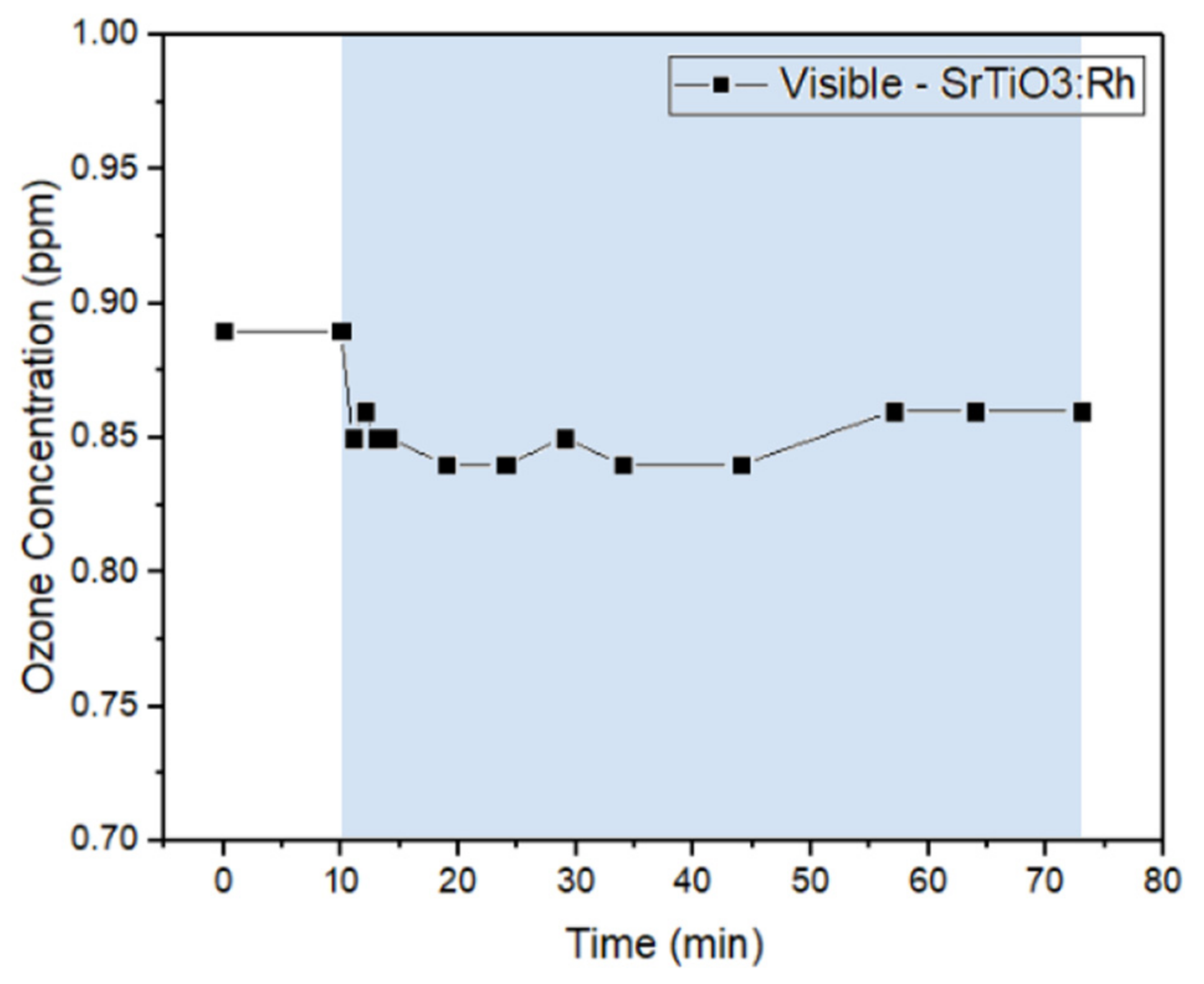
2.1.3. Batch System—Visible Light Irradiation
2.1.4. Ozone Removal Rate in the Batch System
2.2. Continuous Flow System
2.2.1. Pre-Experiment Setup
2.2.2. Continuous Flow System—UV irradiation
2.2.3. Continuous Flow System—Visible Light Irradiation
2.2.4. Conversion of Ozone in the Continuous Flow System
- : initial ozone concentration when light is off (ppm);
- : the lowest ozone concentration when the light is on (ppm).
3. Materials and Methods
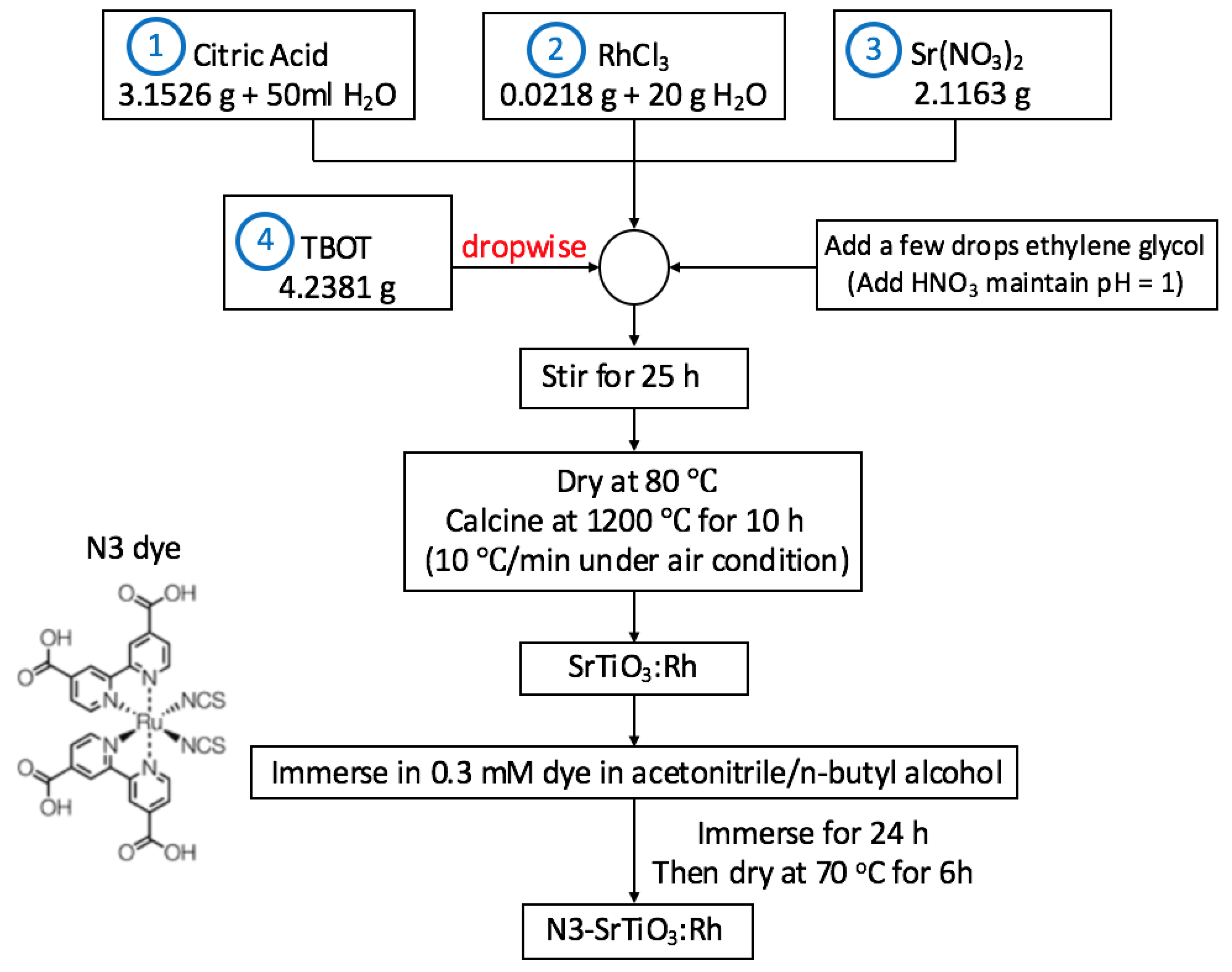
3.1. Catalyst Synthesis
3.2. Characterization
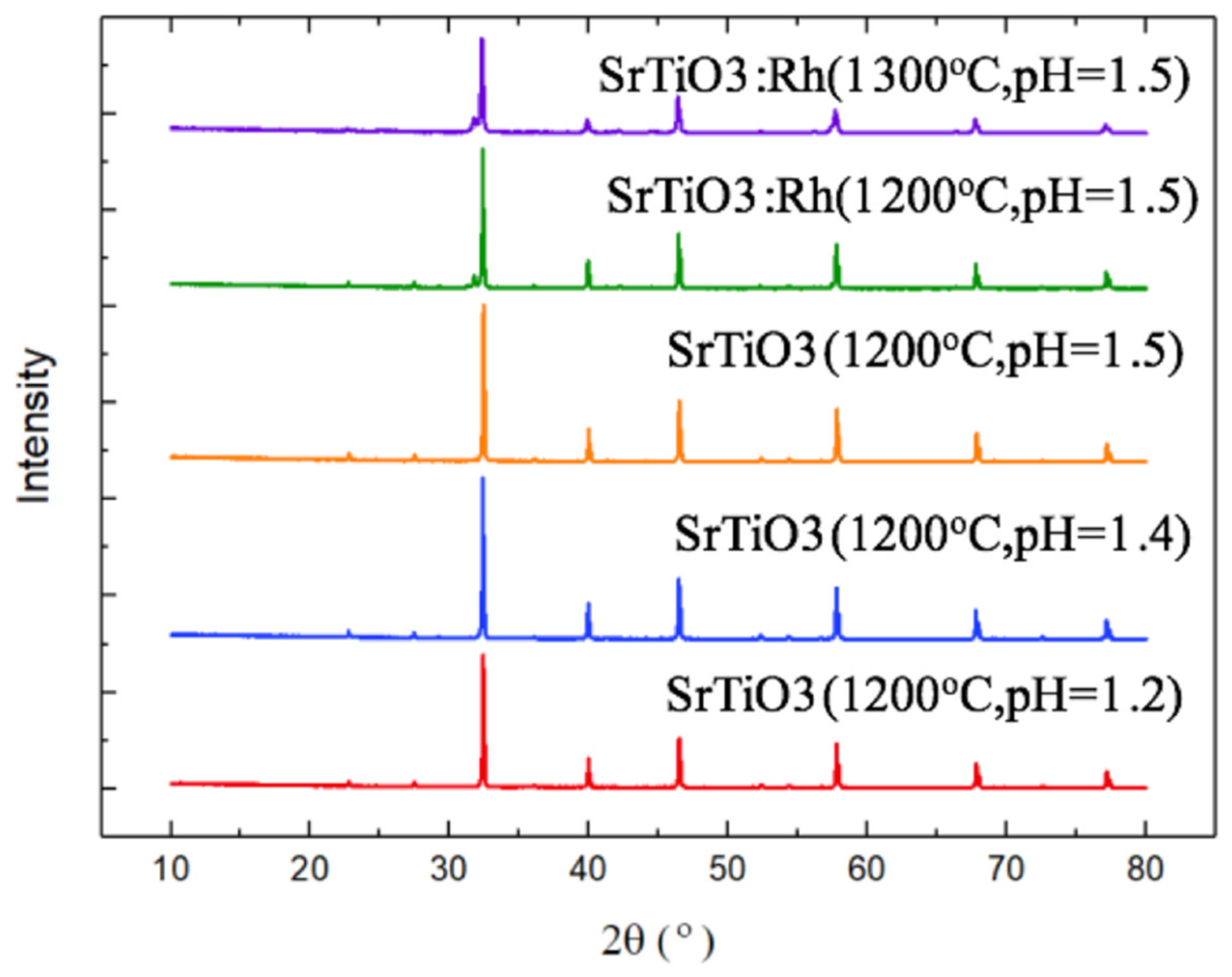
3.2.1. X-ray Diffraction (XRD)
3.2.2. UV–Vis Spectrometry
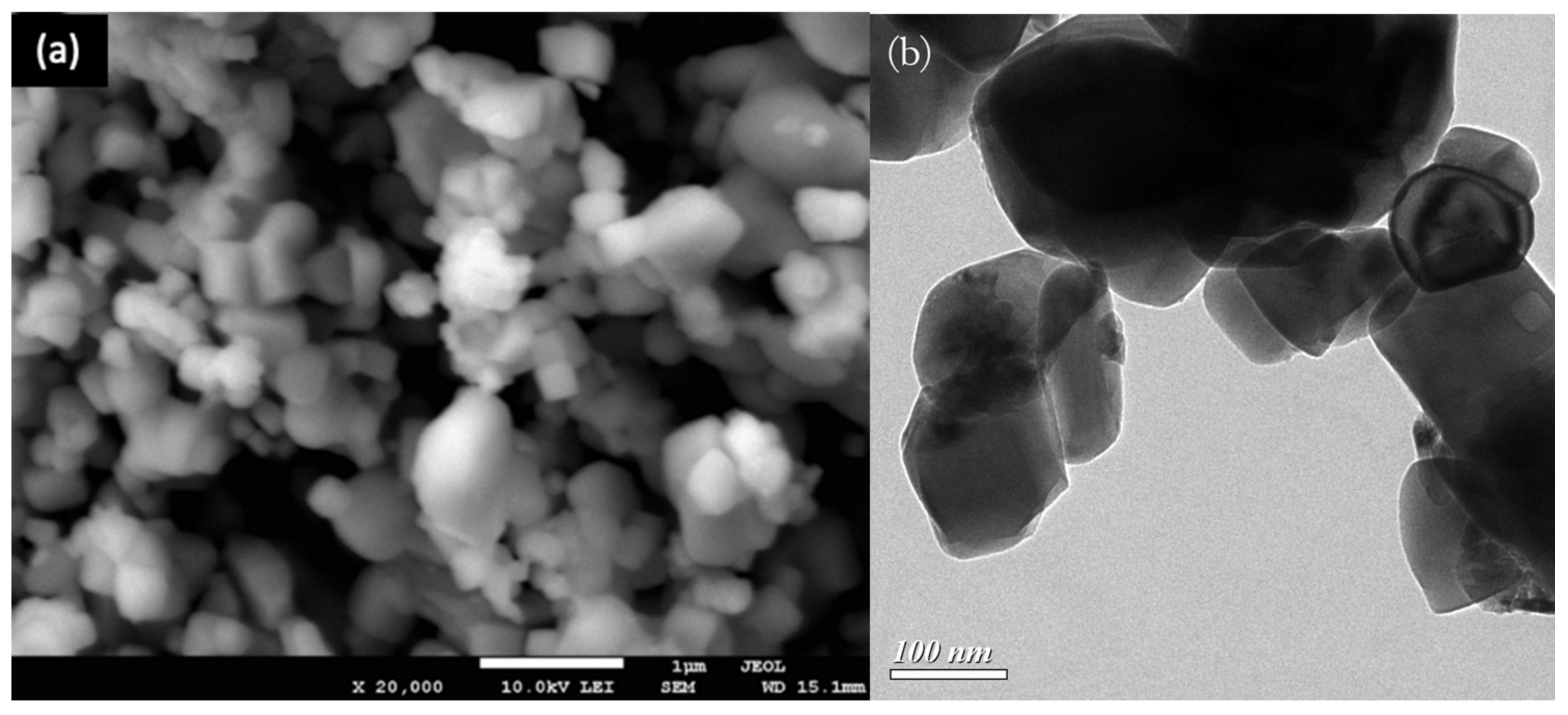
3.2.3. SEM/TEM and EDS Analysis
3.2.4. BET Surface Area Analysis
3.3. Experiment Setup
3.3.1. Batch System
3.3.2. Continuous Flow System
3.4. Data Analysis
Quantitative Analysis of Ozone Removal Rate
- Cf: Final ozone concentration (ppm);
- Ci: Initial ozone concentration (ppm);
- dm: mass of ozone removal amount (mg);
- dt: time required to remove all ozone in batch reactor (s);
- mcat.: catalyst amount used (g)
- V: reactor volume (mL);
- : gas density (kg/m3).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, O.R.; Parrish, D.D.; Ziemke, J.R.; Balashov, N.V.; Cupeiro, M.; Galbally, I.E.; Gilge, S.; Horowitz, L.W.; Jensen, N.R.; Lamarque, J.-F.; et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elem. Sci. Anth. 2014, 2, 000029. [Google Scholar] [CrossRef]
- Cheng, H.; Guo, H.; Wang, X.; Saunders, S.M.; Lam, S.H.M.; Jiang, F.; Wang, T.; Ding, A.; Lee, S.; Ho, K.F. On the relationship between ozone and its precursors in the Pearl River Delta: Application of an observation-based model (OBM). Environ. Sci. Pollut. Res. 2009, 17, 547–560. [Google Scholar] [CrossRef]
- Duncan, B.N.; Yoshida, Y.; Olson, J.R.; Sillman, S.; Martin, R.V.; Lamsal, L.; Hu, Y.; Pickering, K.E.; Retscher, C.; Allen, D.J.; et al. Application of OMI observations to a space-based indicator of NOx and VOC controls on surface ozone formation. Atmospheric Environ. 2010, 44, 2213–2223. [Google Scholar] [CrossRef]
- Kurtenbach, R.; Kleffmann, J.; Niedojadlo, A.; Wiesen, P. Primary NO2 emissions and their impact on air quality in traffic environments in Germany. Environ. Sci. Eur. 2012, 24, 21. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nat. Cell Biol. 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Fowler, D.; Granier, C.; Law, K.S.; Mills, G.E.; et al. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmospheric Chem. Phys. Discuss. 2015, 15, 8889–8973. [Google Scholar] [CrossRef]
- Parrish, D.D.; Lamarque, J.-F.; Naik, V.; Horowitz, L.; Shindell, D.T.; Staehelin, J.; Derwent, R.; Cooper, O.R.; Tanimoto, H.; Volz-Thomas, A.; et al. Long-term changes in lower tropospheric baseline ozone concentrations: Comparing chemistry-climate models and observations at northern midlatitudes. J. Geophys. Res. Atmos. 2014, 119, 5719–5736. [Google Scholar] [CrossRef]
- Sadanaga, Y.; Matsumoto, J.; Kajii, Y. Photochemical reactions in the urban air: Recent understandings of radical chemistry. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 85–104. [Google Scholar] [CrossRef]
- Goudarzi, G.; Geravandi, S.; Foruozandeh, H.; Babaei, A.A.; Alavi, N.; Niri, M.V.; Khodayar, M.J.; Salmanzadeh, S.; Mohammadi, M.J. Cardiovascular and respiratory mortality attributed to ground-level ozone in Ahvaz, Iran. Environ. Monit. Assess. 2015, 187, 1–9. [Google Scholar] [CrossRef]
- Færden, K.; Randem, B.G.; Lier, P.A.; Granhus, B.; Søstrand, P.; Kongerud, J. 736 Acute irritant-induced asthma caused by ozone. Respir. Disord. 2018, 75, A444. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Shavrina, A.; Mikulskaya, I.; Kiforenko, S.; Sheminova, V.; Veles, A.; Blum, O.J. The study of ground-level ozone in Kiev and its impact on public health. arXiv 2012, arXiv:1204.1902. [Google Scholar]
- Shi, Z.; Han, Y.; Bai, J. Evaluation of ozone risk under different air-supply mode in aircraft cabins. Aircr. Eng. Aerosp. Technol. 2018, 90, 270–279. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; He, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B Environ. 2017, 201, 503–510. [Google Scholar] [CrossRef]
- Wu, M.-C.; A Kelly, N. Clean-air catalyst system for on-road applications: II. Mechanistic studies of pollutant removal. Appl. Catal. B Environ. 1998, 18, 93–104. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; von Gunten, U.; Rivera-Utrilla, J. Efficiency of activated carbon to transform ozone into OH radicals: Influence of operational parameters. Water Res. 2005, 39, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, C.; Bulushev, D.A.; Kiwi-Minsker, L. Dynamic behaviour of activated carbon catalysts during ozone decomposition at room temperature. Appl. Catal. B Environ. 2005, 61, 98–106. [Google Scholar] [CrossRef]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of Ozone Treatment on Surface Properties of Activated Carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Benson, S.W.; Axworthy, A.E. Reconsideration of the Rate Constants from the Thermal Decomposition of Ozone. J. Chem. Phys. 1965, 42, 2614. [Google Scholar] [CrossRef]
- Benson, S.W.; Axworthy, A.E. Mechanism of the Gas Phase, Thermal Decomposition of Ozone. J. Chem. Phys. 1957, 26, 1718–1726. [Google Scholar] [CrossRef]
- Michael, J.V. Thermal Decomposition of Ozone. J. Chem. Phys. 1971, 54, 4455. [Google Scholar] [CrossRef]
- Castellano, E.; Schumacher, H. Photochemical Decomposition of Ozone in Yellow-Red Light and the Mechanism of Its Thermal Decomposition. J. Chem. Phys. 1962, 36, 2238. [Google Scholar] [CrossRef]
- Inn, E.C.Y.; Tanaka, Y. Absorption Coefficient of Ozone in the Ultraviolet and Visible Regions. J. Opt. Soc. Am. 1953, 43, 870–872. [Google Scholar] [CrossRef]
- Murphy, A.; McAllister, T. Destruction of ozone-depleting substances in a thermal plasma reactor. Appl. Phys. Lett. 1998, 73, 459–461. [Google Scholar] [CrossRef]
- Batakliev, T.; Tyuliev, G.; Georgiev, V.; Anachkov, M.; Eliyas, A.; Rakovsky, S. Ozone Decomposition Reaction over α-Alumina-Supported Silver Catalyst: Comparative Study of Catalytic Surface Reactivity. Ozone Sci. Eng. 2015, 37, 216–220. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Z.; Jiang, Z.; Jiang, Z.; Zhang, Y.; Zhang, Z.; Shangguan, W. Trifunctional C@ MnO catalyst for enhanced stable simultaneously catalytic removal of formaldehyde and ozone. ACS Catal. 2018, 8, 3164–3180. [Google Scholar] [CrossRef]
- Lian, Z.; Ma, J.; He, H. Decomposition of high-level ozone under high humidity over Mn–Fe catalyst: The influence of iron precursors. Catal. Commun. 2015, 59, 156–160. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone. Catal. Sci. Technol. 2016, 6, 5841–5847. [Google Scholar] [CrossRef]
- Asgari, G.; Mohammadi, A.S.; Mortazavi, S.B.; Ramavandi, B. Investigation on the pyrolysis of cow bone as a catalyst for ozone aqueous decomposition: Kinetic approach. J. Anal. Appl. Pyrolysis 2013, 99, 149–154. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Milenova, K.I.; Nikolov, P.M.; Kasabova, N.A.; Avramova, I.A. Ozone decomposition on ZnO catalysts obtained from different precursors. Pol. J. Chem. Technol. 2014, 16, 55–59. [Google Scholar] [CrossRef]
- Spasova, I.; Nikolov, P.; Mehandjiev, D. Ozone Decomposition over Alumina-Supported Copper, Manganese and Copper-Manganese Catalysts. Ozone Sci. Eng. 2007, 29, 41–45. [Google Scholar] [CrossRef]
- Wang, C.; Ma, J.; Liu, F.; He, H.; Zhang, R. The Effects of Mn2+ Precursors on the Structure and Ozone Decomposition Activity of Cryptomelane-Type Manganese Oxide (OMS-2) Catalysts. J. Phys. Chem. C 2015, 119, 23119–23126. [Google Scholar] [CrossRef]
- Dhandapani, B.; Oyama, S.T. Kinetics and Mechanism of Ozone Decomposition on a Manganese Oxide Catalyst. Chem. Lett. 1995, 24, 413–414. [Google Scholar] [CrossRef]
- Li, W.; Oyama, S.T. Mechanism of Ozone Decomposition on a Manganese Oxide Catalyst. 2. Steady-State and Transient Kinetic Studies. J. Am. Chem. Soc. 1998, 120, 9047–9052. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, P.; Li, J.; Jiang, C. The effects of Mn loading on the structure and ozone decomposition activity of MnOx supported on activated carbon. Chin. J. Catal. 2014, 35, 335–341. [Google Scholar] [CrossRef]
- Gopi, T.; Swetha, G.; Shekar, S.C.; Ramakrishna, C.; Saini, B.; Krishna, R.; Rao, P. Catalytic decomposition of ozone on nanostructured potassium and proton containing δ-MnO2 catalysts. Catal. Commun. 2017, 92, 51–55. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P. Catalytic decomposition of gaseous ozone over todorokite-type manganese dioxides at room temperature: Effects of cerium modification. Appl. Catal. A Gen. 2017, 530, 102–110. [Google Scholar] [CrossRef]
- Mills, A.; Lee, S.-K.; Lepre, A.J.J.; Chemistry, P.A. Photodecomposition of ozone sensitised by a film of titanium dioxide on glass. J. Photochem. Photobiol. A 2003, 155, 199–205. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Oyama, S. Ozone Decomposition over Manganese Oxide Supported on ZrO2 and TiO2: A Kinetic Study Using in Situ Laser Raman Spectroscopy. J. Catal. 2001, 199, 282–290. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, L.; Rahimi, M.G.; Gong, S.; Nie, L.; Han, N.; Chen, Y. Defect engineering of ZnO for electron transfer in O3 catalytic decomposition. Appl. Catal. B Environ. 2020, 277, 119223. [Google Scholar] [CrossRef]
- Cho, K.-C.; Hwang, K.-C.; Sano, T.; Takeuchi, K.; Matsuzawa, S. Photocatalytic performance of Pt-loaded TiO2 in the decomposition of gaseous ozone. J. Photochem. Photobiol. A Chem. 2004, 161, 155–161. [Google Scholar] [CrossRef]
- Patzsch, J.; Bloh, J.Z. Improved photocatalytic ozone abatement over transition metal-grafted titanium dioxide. Catal. Today 2018, 300, 2–11. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Wang, M.; Yang, Z.; Zhang, X.; Yang, C. Feasibility analysis on photocatalytic removal of gaseous ozone in aircraft cabins. Build. Environ. 2014, 81, 42–50. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, P.; Li, J. Photocatalytic degradation of low concentration formaldehyde and simultaneous elimination of ozone by-product using palladium modified TiO2 films under UV254+185nm irradiation. Appl. Catal. B Environ. 2011, 105, 220–228. [Google Scholar] [CrossRef]
- Gong, J.-Y.; Chen, Y.-C.; Yu, K.-P. Photocatalytic decomposition of indoor ozone motivated by the white-light-emitting diode. Clean Technol. Environ. Policy 2017, 19, 2393–2404. [Google Scholar] [CrossRef]
- He, P.; Yang, J.; Yang, D.; Wang, X.; Zhang, M.J.C.J.C. Photocatalytic Decomposition of Gaseous Ozone on Au/TiO2. Chin. J. Catal. 2006, 27, 71. [Google Scholar]
- Hunger, M.; Hüsken, G.G.; Brouwers, H.J.H. Photocatalytic degradation of air pollutants—From modeling to large scale application. Cem. Concr. Res. 2010, 40, 313–320. [Google Scholar] [CrossRef]
- Ye, S.-Y.; Li, M.-B.; Song, X.-L.; Luo, S.-C.; Fang, Y.-C. Enhanced photocatalytic decomposition of gaseous ozone in cold storage environments using a TiO2/ACF film. Chem. Eng. J. 2011, 167, 28–34. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, P.; Li, J.; Wang, J.; Fu, P. Photocatalytic degradation of gaseous toluene and ozone under UV254+185 nm irradiation using a Pd-deposited TiO2 film. Chem. Eng. J. 2014, 252, 337–345. [Google Scholar] [CrossRef]
- Dvoranová, D.; Brezová, V.; Mazúr, M.; Malati, M.A. Investigations of metal-doped titanium dioxide photocatalysts. Appl. Catal. B Environ. 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. Phys. Inorg. Chem. 2004, 35, 8992–8995. [Google Scholar] [CrossRef]
- Chen, H.-C.; Huang, C.-W.; Wu, J.C.S.; Lin, S.-T. Theoretical Investigation of the Metal-Doped SrTiO3 Photocatalysts for Water Splitting. J. Phys. Chem. C 2012, 116, 7897–7903. [Google Scholar] [CrossRef]
- Boonstra, A.H.; Mutsaers, C.A.H.A. Relation between the photoadsorption of oxygen and the number of hydroxyl groups on a titanium dioxide surface. J. Phys. Chem. 1975, 79, 1694–1698. [Google Scholar] [CrossRef]
- Bowering, N.; Walker, G.S.; Harrison, P.G. Photocatalytic decomposition and reduction reactions of nitric oxide over Degussa P25. Appl. Catal. B Environ. 2006, 62, 208–216. [Google Scholar] [CrossRef]
- Emeline, A.V.; Smirnova, L.G.; Kuzmin, G.N.; Basov, L.L.; Serpone, N. Spectral dependence of quantum yields in gas-solid heterogeneous photosystems—Influence of anatase/rutile content on the photostimulated adsorption of dioxygen and dihydrogen on titania. J. Photochem. Photobiol. A 2002, 148, 97–102. [Google Scholar] [CrossRef]
- Nakajima, H.; Mori, T.; Kobayashi, E.; Watanabe, M. Influence of pH on Photoadsorption of Dissolved Oxygen in TiO2 Suspensions. Jpn. J. Appl. Phys. 2003, 42, 6623–6624. [Google Scholar] [CrossRef]
- Saladin, F.; Alxneit, I. Temperature dependence of the photochemical reduction of CO2 in the presence of H2O at the solid/gas interface of TiO2. J. Chem. Soc. Faraday Trans. 1997, 93, 4159–4163. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Namdari, M.; Lee, C.-S.; Haghighat, F. Active ozone removal technologies for a safe indoor environment: A comprehensive review. Build. Environ. 2021, 187, 107370. [Google Scholar] [CrossRef]
- Xuewen, W.; Zhiyong, Z.; Shuixian, Z. Preparation of nano-crystalline SrTiO3 powder in sol-gel process. Mater. Sci. Eng. B 2001, 86, 29–33. [Google Scholar] [CrossRef]
- Yu, S.-C.; Huang, C.-W.; Liao, C.-H.; Wu, J.C.; Chang, S.-T.; Chen, K.-H. A novel membrane reactor for separating hydrogen and oxygen in photocatalytic water splitting. J. Membr. Sci. 2011, 382, 291–299. [Google Scholar] [CrossRef]
- Lo, C.-C.; Huang, C.-W.; Liao, C.-H.; Wu, J.C. Novel twin reactor for separate evolution of hydrogen and oxygen in photocatalytic water splitting. Int. J. Hydrogen Energy 2010, 35, 1523–1529. [Google Scholar] [CrossRef]
- Chang, C.-W. Photocatalytic Water Splitting Using Sol-Gel Perpared Pt/SrTiO3:Rh in Twin Membrane Reactor via Ce ions Mediator by Visible Light Irradiation. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2011. [Google Scholar]
- Vehkamäki, M.; Hatanpää, T.; Hänninen, T.; Ritala, M.; Leskelä, M.J.E. Growth of SrTiO3 and BaTiO3 thin films by atomic layer deposition. Electrochem. Solid-State Lett. 1999, 2, 504–506. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Brabec, L.; Rathousky, J. Photocatalytic performance of porous TiO2 layers prepared by quantitative electrophoretic deposition from organic solvents. Appl. Catal. B Environ. 2018, 227, 70–78. [Google Scholar] [CrossRef]
- He, X.; Wu, M.; Ao, Z.; Lai, B.; Zhou, Y.; An, T.; Wang, S. Metal-organic frameworks derived C/TiO2 for visible light photocatalysis: Simple synthesis and contribution of carbon species. J. Hazard. Mater. 2021, 403, 124048. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Liu, X.; Wang, J.; Liu, Y.; Liu, Z.; Tang, L.; Shao, B.; Zhang, W.; Gong, S.; Cheng, M.; et al. In-situ self-assembly construction of hollow tubular g-C3N4 isotype heterojunction for enhanced visible-light photocatalysis: Experiments and theories. J. Hazard. Mater. 2021, 401, 123355. [Google Scholar] [CrossRef]





| Light Source | Catalyst (Type-Cycle) | |
|---|---|---|
| Visible light | SrTiO3:Rh-1 | |
| SrTiO3:Rh 2 | ||
| SrTiO3:Rh-3 | ||
| SrTiO3-1 | ||
| SrTiO3-2 | ||
| SrTiO3-3 | ||
| P25-1 | ||
| P25-2 | ||
| P25-3 | ||
| UV light | SrTiO3:Rh-1 | |
| SrTiO3:Rh-2 | ||
| SrTiO3:Rh-3 | 0 | |
| SrTiO3-1 | ||
| SrTiO3-2 | ||
| SrTiO3-3 | ||
| P25-1 | ||
| P25-2 | ||
| P25-3 |
| Light Source | Catalyst | Ozone Conversion (%) |
|---|---|---|
| Visible light | N3-SrTiO3:Rh | 7.3 |
| SrTiO3:Rh | 5.6 | |
| P25 | 3.2 | |
| UV light | SrTiO3:Rh | 0.0 |
| SrTiO3 | 0.0 | |
| P25 | 6.3 |
| Element | Weight (%) | Atomic (%) |
|---|---|---|
| O | 24.6 | 57.8 |
| Ti | 27.5 | 21.6 |
| Sr | 47.9 | 20.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.Q.; Chang, Y.-C.; Wu, J.C.S. Visible-Light Photocatalyst to Remove Indoor Ozone under Ambient Condition. Catalysts 2021, 11, 383. https://doi.org/10.3390/catal11030383
Su JQ, Chang Y-C, Wu JCS. Visible-Light Photocatalyst to Remove Indoor Ozone under Ambient Condition. Catalysts. 2021; 11(3):383. https://doi.org/10.3390/catal11030383
Chicago/Turabian StyleSu, Jia Quan, Yi-Chun Chang, and Jeffrey C. S. Wu. 2021. "Visible-Light Photocatalyst to Remove Indoor Ozone under Ambient Condition" Catalysts 11, no. 3: 383. https://doi.org/10.3390/catal11030383
APA StyleSu, J. Q., Chang, Y.-C., & Wu, J. C. S. (2021). Visible-Light Photocatalyst to Remove Indoor Ozone under Ambient Condition. Catalysts, 11(3), 383. https://doi.org/10.3390/catal11030383








