Effects of Lower Temperature on Expression and Biochemical Characteristics of HCV NS3 Antigen Recombinant Protein
Abstract
:1. Introduction
2. Results and Discussions
2.1. Preparation of Recombinant HCV NS3
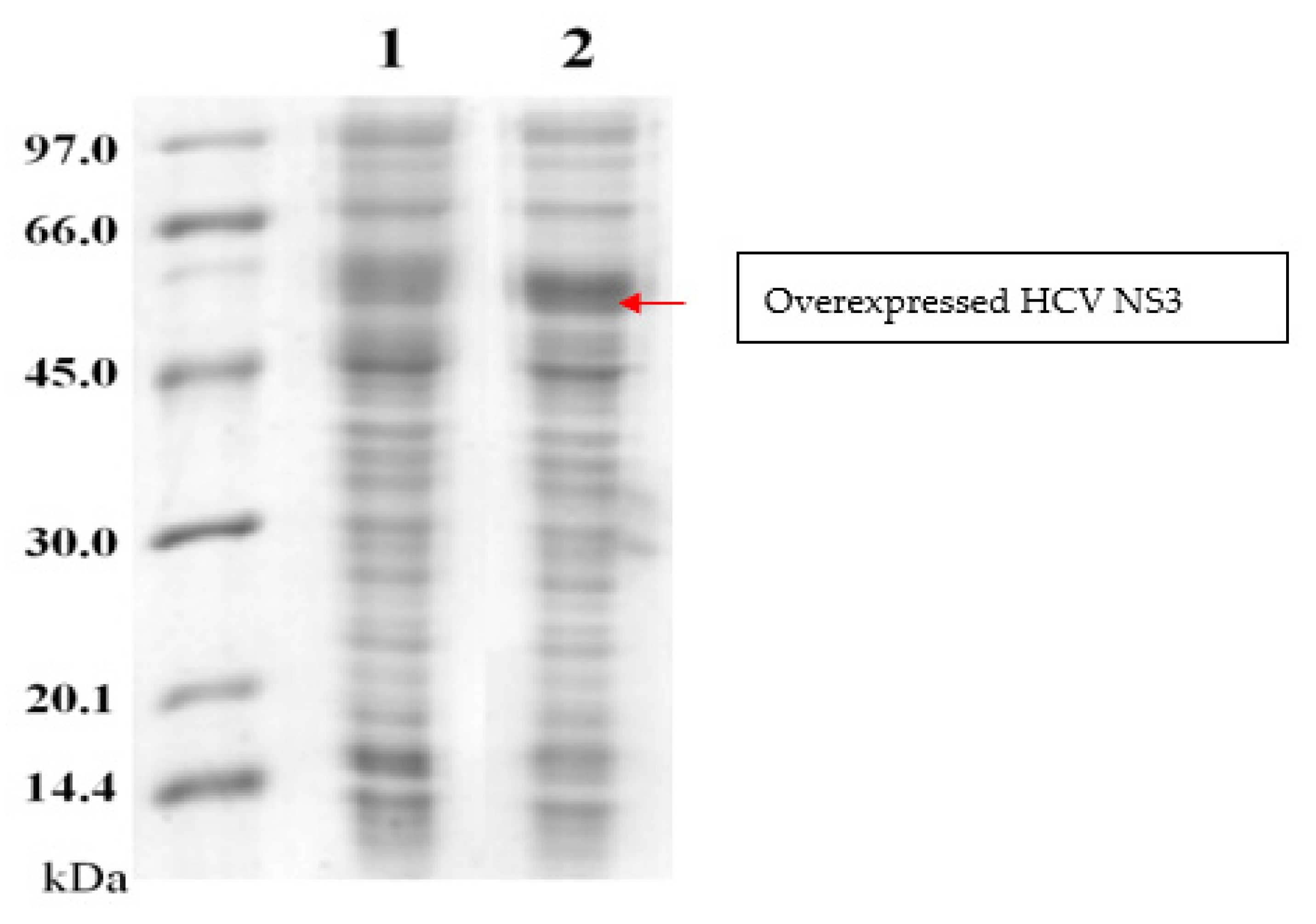
2.1.1. Expression and Purification of the Recombinant NS3 Proteins
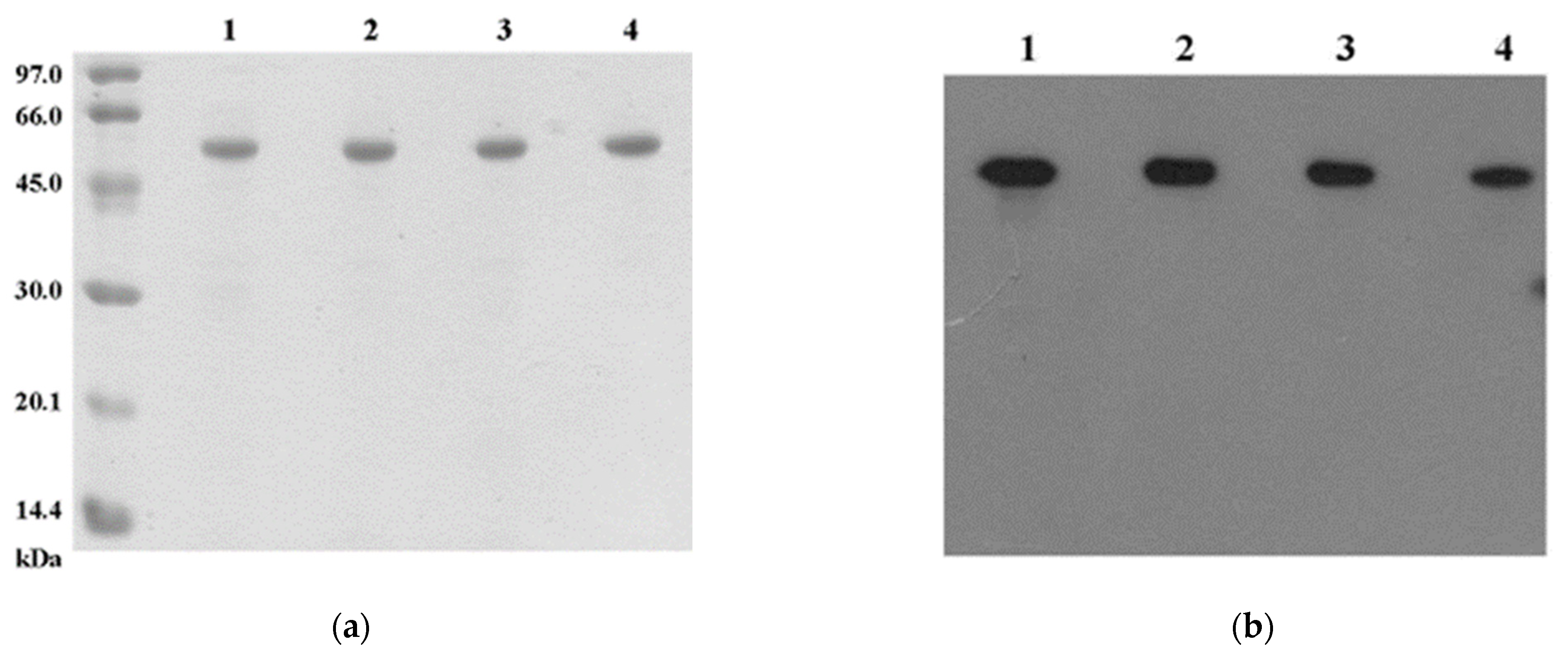
2.1.2. Property and Stability of Fresh and Stored NS3 Proteins
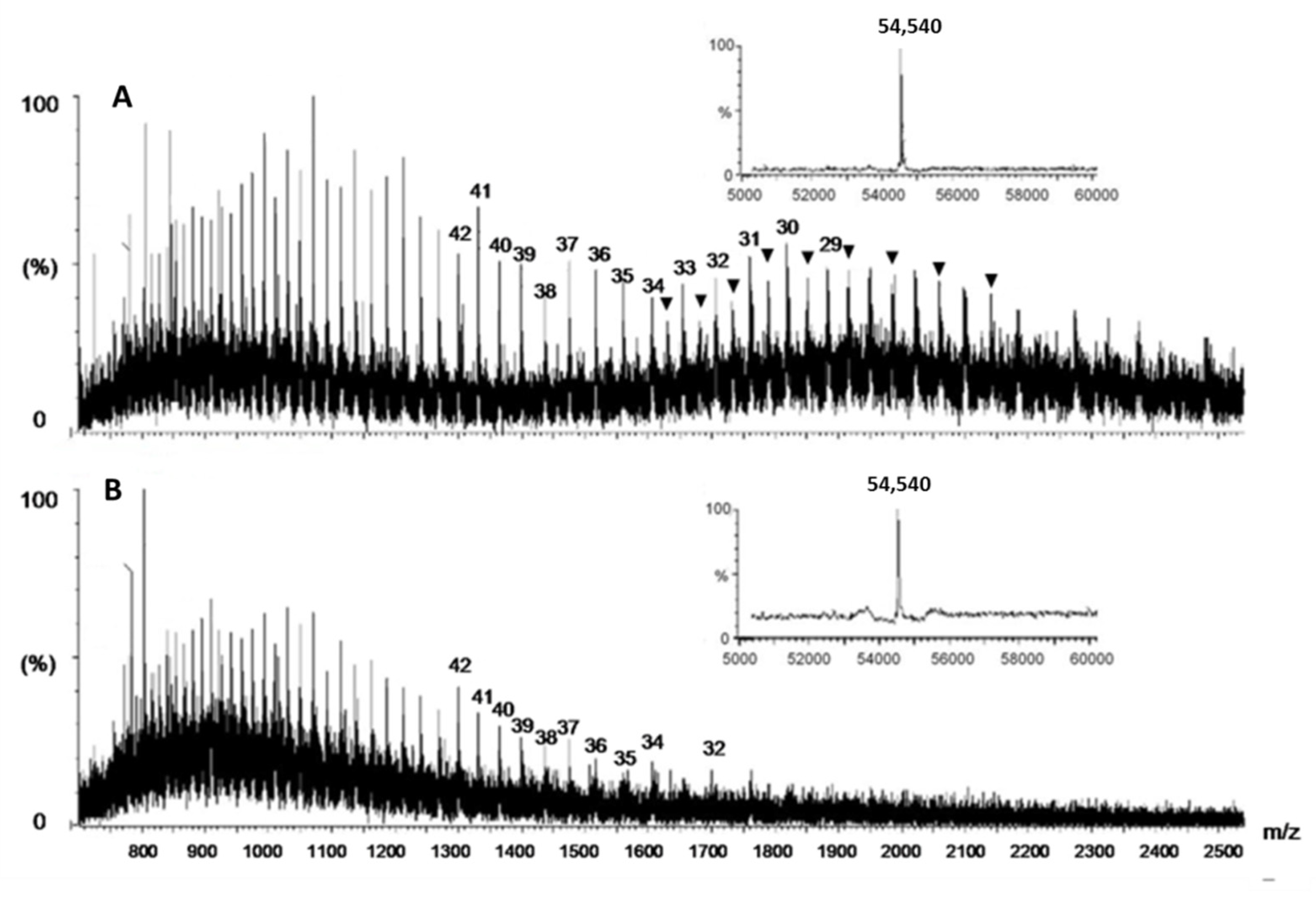
2.1.3. Electrospray Ionization Mass Spectrometric (ESI-MS) Analysis
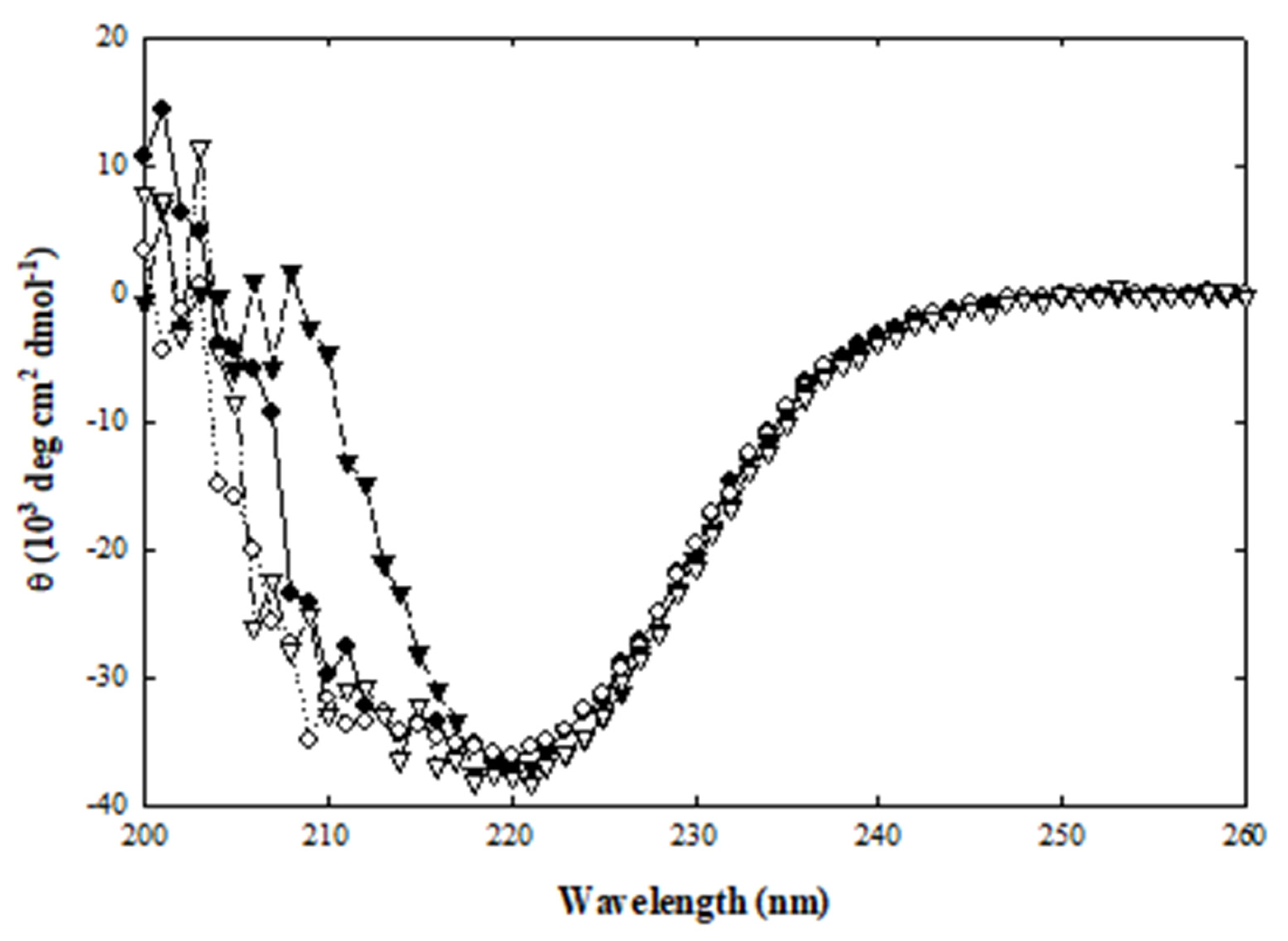
2.1.4. Protein Structure Stability
2.1.5. Thermal Stability of NS3 Proteins
2.1.6. ELISA Specificity of NS3 Proteins
3. Materials and Methods
3.1. Materials
3.2. Expression of the Recombinant NS3 Proteins
3.3. Purification of the Recombinant NS3 Proteins
3.4. HPLC Performance
3.5. Western Blot Analysis and Protein Estimation
3.6. Electrospray Ionization Mass Spectrometric (ESI-MS) Analysis
3.7. CD Measurements
3.8. Thermal Unfolding Experiments
3.9. ELISA Analysis of the Recombinant NS3 Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moriishi, K.; Mori, Y.; Matsuura, Y. Processing and pathogenicity of HCV core protein. Uirusu 2008, 58, 183–190. [Google Scholar] [CrossRef]
- Dash, S.; Haque, S.; Joshi, V.; Prabhu, R.; Hazari, S.; Fermin, C. HCV-Hepatocellular carcinoma: New findings and hope for effective treatment. Microsc. Res. Tech. 2005, 68, 130–148. [Google Scholar] [CrossRef]
- Muerhoff, A.S.; Jiang, L.; Shah, D.O.; Gutierrez, R.A.; Patel, J.; Garolis, C. Detection of HCV core antigen in human serum and plasma with an automated chemiluminescent immunoassay. Transfusion 2002, 42, 349–356. [Google Scholar] [CrossRef]
- Lindsay, K. Therapy of chronic hepatitis C: Overview. Hepatology 1997, 26, 71S. [Google Scholar] [CrossRef]
- Kolykhalov, A.A.; Agapov, E.V.; Blight, K.J.; Mihalik, K.; Feinstone, S.M.; Rice, C.M. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 1997, 277, 570. [Google Scholar] [CrossRef]
- Encke, J.; Geissler, M.; Stremmel, W.; Wands, J.R. DNA-based immunization breaks tolerance in a hepatitis C virus transgenic mouse model. Hum. Vaccin. 2006, 2, 78–83. [Google Scholar] [CrossRef]
- Rodrigue-Gervais, I.G.; Rigsby, H.; Jouan, L.; Sauvé, D.; Sékaly, R.P.; Willems, B. Dendritic cell inhibition is connected to exhaustion of CD8+ T cell polyfunctionality during chronic hepatitis C virus infection. J. Immunol. 2010, 184, 3134. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Wood, C.L.; Lane, M.J.; Francis, B.; Gust, C.; Higgs, C.M. Increased detection of hepatitis C virus infection in commercial plasma donors by a third-generation screening assay. Transfusion 1995, 35, 845–849. [Google Scholar] [CrossRef]
- Hidajat, R.; Nagano-Fujii, M.; Deng, L.; Tanaka, M.; Takigawa, Y.; Kitazawa, S. Hepatitis C virus NS3 protein interacts with ELKS-d and ELKS-a, members of a novel protein family involved in intracellular transport and secretory pathways. J. Gen. Virol. 2005, 86, 2197–2208. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.L.; Chi, W.K.; Chen, D.S.; Hwang, L.H. The helicase activity associated with hepatitis C virus nonstructural protein 3 (NS3). J. Virol. 1996, 70, 8477–8484. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Shi, C.; Li, P.; Tong, Y.; Ma, Q. Diagnosis of hepatitis C virus (HCV) infection by antigen-capturing ELISA. Clin. Diagn. Virol. 1996, 6, 137. [Google Scholar] [CrossRef]
- Ferrer, F.; Candela, M.J.; Garcia, C.; Martinez, L.; Rivera, J.; Vicente, V. A Comparative Study of Two Third-Generation Anti-Hepatitis C Virus ELISAs. Haematologica 1997, 82, 690–691. [Google Scholar]
- Martin, P.; Fabrizi, F.; Dixit, V.; Quan, S.; Brezina, M.; Kaufman, E. Automated RIBA hepatitis C virus (HCV) strip immunoblot assay for reproducible HCV diagnosis. J. Clin. Microbiol. 1998, 36, 387–390. [Google Scholar] [CrossRef] [Green Version]
- Poliakov, A.; Hubatsch, I.; Shuman, C.F.; Stenberg, G.; Danielson, U.H. Expression and purification of recombinant full-length NS3 protease-helicase from a new variant of Hepatitis C virus. Protein Expr. Purif. 2002, 25, 363. [Google Scholar] [CrossRef]
- Poliakov, A.; Danielson, U.H. Refolding of the full-length non-structural protein 3 of hepatitis C virus. Protein Expr. Purif. 2005, 41, 298. [Google Scholar] [CrossRef]
- Urbani, A.; Bazzo, R.; Nardi, M.C.; Cicero, D.O.; De Francesco, R.; Steinkühler, C. The metal binding site of the hepatitis C virus NS3 protease: A spectroscopic investigation. J. Biol. Chem. 1998, 273, 18760. [Google Scholar] [CrossRef] [Green Version]
- Wardell, A.D.; Errington, W.; Ciaramella, G.; Merson, J.; McGarvey, M.J. Characterization and mutational analysis of the helicase and NTPase activities of hepatitis C virus full-length NS3 protein. J. Gen. Virol. 1999, 80, 701. [Google Scholar] [CrossRef]
- Beran, R.K.; Pyle, A.M. Hepatitis C viral NS3-4A protease activity is enhanced by the NS3 helicase. J. Biol. Chem. 2008, 283, 29929–29937. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, N.J. Applications of circular dichroism in protein and peptide analysis. Trends Anal. Chem. 1999, 18, 236–244. [Google Scholar] [CrossRef]
- De Moraes, L.N.; Grotto, R.M.T.; Valente, G.T. A novel molecular mechanism to explain mutations of the HCV protease associated with resistance against covalently bound inhibitors. Virus Gene. 2019, 274, 197778. [Google Scholar]
- Khu, Y.L.; Koh, E.; Lim, S.P.; Tan, Y.H.; Brenner, S.; Lim, S.G. Mutations that affect dimer formation and helicase activity of the hepatitis C virus helicase. J. Virol. 2001, 75, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marecki, J.C.; Aarattuthodiyil, S.; Byrd, A.K.; Penthala, N.R.; Crooks, P.A.; Paney, K.D. N-Naphthoyl-substituted indole thio-barbituric acid analogs inhibit the helicase activity of the hepatitis C virus NS3. Bioorg. Med. Chem. Lett. 2019, 29, 430–434. [Google Scholar] [CrossRef]
- Hernández, S.; Díaz, A.; Loyola, A.; Villanueva, R.A. Recombinant HCV NS3 and NS5B enzymes exhibit multiple posttranslational modifications for potential regulation. Virus Gene. 2019, 55, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Konermann, L.; Pan, J.; Wilson, D.J. Protein folding mechanisms studied by time-resolved electrospray mass spectrometry. Biotechniques 2006, 40, 135–141. [Google Scholar] [CrossRef]
- Wilcox, W.C.; Long, D.; Sodora, D.L.; Eisenberg, R.J.; Cohen, G.H. The contribution of cysteine residues to antigenicity and extent of processing of herpes simplex virus type 1 glycoprotein D. J. Virol. 1988, 62, 1941–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elleuche, S.; Krull, A.; Lorenz, U.; Antranikian, G. Parallel N- and C-Terminal Truncations Facilitate Purification and Analysis of a 155-kDa Cold-Adapted Type-I Pullulanase. Protein J. 2017, 36, 56–63. [Google Scholar] [CrossRef]
- Lawyer, F.C.; Stoffel, S.; Saiki, R.K.; Chang, S.H.; Landre, P.A.; Abrarnson, R.D.; Gelfand, D.H. High-level expression, purification, and enzymatic characterization of full-length thermus aquatlcus DNA polymerase and a truncated form deficient in 5’ to 3’ exonuclease activity. Genome Res. 1993, 2, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Mühlmann, M.; Forsten, E.; Noack, S.; Büchs, J. Optimizing recombinant protein expression via automated induction profling in microtiter plates at diferent temperatures. Microb. Cell Fact. 2017, 16, 220. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Liu, H. Enhanced recombinant protein production under special environmental stress. Front. Microbiol. 2021, 12, 630814. [Google Scholar] [CrossRef]
- Sandomenico, A.; Sivaccumar, J.P.; Ruvo, M. Evolution of Escherichia coli expression system in producing antibody recombinant fragments. Int. J. Mol. Sci. 2020, 1, 6324. [Google Scholar] [CrossRef]
- Chen, P.J.; Lin, M.H.; Tai, K.F.; Liu, P.C.; Lin, C.J.; Chen, D.S. The Taiwanese hepatitis C virus genome: Sequence determination and mapping the 5’ termini of viral genomic and antigenomic RNA. Virology 1992, 188, 102. [Google Scholar] [CrossRef]
- Xue, H.; Wang, J.; Xie, J. Isolation, purification, and structure identification of antioxidant peptides from embryonated eggs. Poult. Sci. 2019, 98, 2360–2370. [Google Scholar] [CrossRef]
- Kiely, P.R.; Eliades, L.A.; Kebede, M.; Stephenson, M.D.; Jardine, D.K. Anti-HCV confirmatory testing of voluntary blood donors: Comparison of the sensitivity of two immunoblot assays. Transfusion 2002, 42, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.D.; Breidinger, S.A.; Woolf, E.J. Effect of disease state on ionization during bioanalysis of MK-7009, a selective HCV NS3/NS4 protease inhibitor, in human plasma and human Tween-treated urine by high-performance liquid chromatography with tandem mass spectrometric detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 1047–1056. [Google Scholar] [CrossRef]
- Honda, Y.; Fukamizo, T.; Okajima, T.; Goto, S.; Boucher, I.; Brzezinski, R. Thermal unfolding of chitosanase from Streptomyces sp. N174: Role of tryptophan residues in the protein structure stabilization. Biochim. Biophys. Acta 1999, 1429, 365. [Google Scholar] [CrossRef]



| Temperature | Crude Extract | HCV NS3 | Production Yield |
|---|---|---|---|
| (°C) | (mg) | (mg) | (mg/L) |
| 37 | 1030 (±5%) | 166 (±3%) | 4.15 (±3%) |
| 25 | 910 (±4%) | 445 (±1%) | 11.1 (±1%) |
| 50% Unfolding | First Transition Phase | Second Transition Phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample a | Tm | Tm | ΔTm | ΔHm | ΔSm | ΔΔGm | Tm | ΔTm | ΔHm | ΔSm | ΔΔGm |
| °C | °C | °C | kcal/mol | kcal/mol/K | kcal/mol | °C | °C | kcal/mol | kcal/mol/K | kcal/mol | |
| F37 | 59 | 65.2 | - | 85 | 0.3 | - | 59.7 | - | 365 | 1.1 | - |
| F25 | 59 | 64.0 | −1.2 | 96 | 0.3 | −0.3 | 59.1 | −0.6 | 332 | 1.0 | −0.7 |
| L37 | 55 | 63.7 | −1.5 | 81 | 0. | −0.4 | 55.5 | −4.2 | 338 | 1.0 | −4.6 |
| L25 | 57 | 62.0 | −3.2 | 89 | 0.3 | −0.8 | 57.8 | −1.9 | 387 | 1.2 | −2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-J.; Peng, H.-L.; Patel, A.K.; Singhania, R.R.; Dong, C.-D.; Cheng, C.-Y. Effects of Lower Temperature on Expression and Biochemical Characteristics of HCV NS3 Antigen Recombinant Protein. Catalysts 2021, 11, 1297. https://doi.org/10.3390/catal11111297
Huang C-J, Peng H-L, Patel AK, Singhania RR, Dong C-D, Cheng C-Y. Effects of Lower Temperature on Expression and Biochemical Characteristics of HCV NS3 Antigen Recombinant Protein. Catalysts. 2021; 11(11):1297. https://doi.org/10.3390/catal11111297
Chicago/Turabian StyleHuang, Chen-Ji, Hwei-Ling Peng, Anil Kumar Patel, Reeta Rani Singhania, Cheng-Di Dong, and Chih-Yu Cheng. 2021. "Effects of Lower Temperature on Expression and Biochemical Characteristics of HCV NS3 Antigen Recombinant Protein" Catalysts 11, no. 11: 1297. https://doi.org/10.3390/catal11111297
APA StyleHuang, C.-J., Peng, H.-L., Patel, A. K., Singhania, R. R., Dong, C.-D., & Cheng, C.-Y. (2021). Effects of Lower Temperature on Expression and Biochemical Characteristics of HCV NS3 Antigen Recombinant Protein. Catalysts, 11(11), 1297. https://doi.org/10.3390/catal11111297










