Abstract
CO2 is a compound of high stability which proves useful in some organic syntheses as a solvent or component decreasing explosivity of gases. It is also a good carbonylating agent for aliphatic amines although not for aromatic ones, the latter being carbonylated with phosgene or, as in our previous works, with CO/O2 in the presence of Pd(II) complexes. In this work we have used the mixture of CO/O2 and CO2 for carbonylation of aniline to N,N’-diphenylurea. After optimization of the reaction conditions (56% of CO2 in CO2/CO mixture) we studied the activity of three kinds of pre-catalysts: (a) Pd(II) complexes, (b) Pdblack, and (c) palladium nanoparticles (PdNPs) in the presence of derivatives of pyridine (XnPy). The highest conversion of aniline (with selectivity towards N,N-diphenylurea ca. 90%) was observed for PdNPs. The results show that catalytic cycle involves Pd(0) stabilized by pyridine ligand as active species. Basing on this observation, we put the hypothesis that application of PdNPs instead of Pd(II) complex can efficiently reduce the reaction time.
1. Introduction
Environment-friendly organic synthesis is the main challenge of modern science in order to meet the objectives of sustainable development. Among many types of functionalization, insertion of a single carbonyl group into an organic molecule plays a special role due to large-scale demand for carbonyl compounds [1,2]. Excellent examples of products (both commodities and specialty chemicals) with carbonyl groups are urea derivatives, widely used in modern chemical industry— they find application in the production of pesticides (herbicides, fungicides), resin precursors, and fiber dyes [3,4], as well as antiviral and anticancer agents and other pharmaceuticals [4,5,6,7,8]. Numerous derivatives of ureas—including isocyanates and carbamates—are employed in syntheses of adhesives, varnishes, rubbers, paints, and polyurethane foams [9]. Unfortunately, dominating technologies of production of diphenylureas from aromatic amines are based on the phosgene method [9,10], and the real challenge in this sector of industry is to replace them by phosgene-free methods. The most common approaches involve less environmentally harmful carbonylating agents such as CO or alkyl carbonates [1,3,5,11,12,13], including carbonylation performed in beneficial nonconventional solvents such as ionic liquids [12,13]. The main limitation of CO-based methods is the high pressure applied.
Over the past few years, our studies have been focused on the carbonylation of aromatic nitrocompounds and amines by CO in the presence of the PdCl2(XnPy)2/Fe/I2/XnPy catalytic system, where Py = pyridine, X = Cl or CH3, n = 0–2 [14,15,16,17]. We have successfully optimized the reaction conditions and proposed detailed mechanisms for carbonylation of aniline (AN) to N,N′-diphenylurea (DPU, equation 1), or to ethyl N-phenylcarbamate (EPC, Equation (2)), by CO/O2.
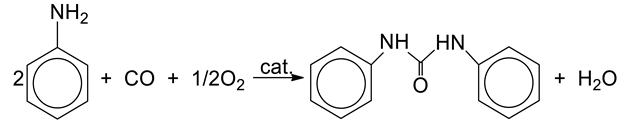
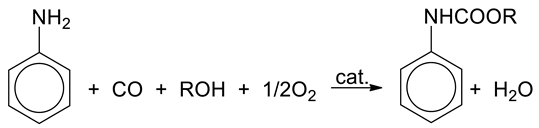
Processes presented by Equations (1) and (2) involve mixture of CO (a common source of carbonyl group) and O2 (oxidizing agent), which is potentially explosive in a wide range of concentrations: 16.7–93.5% (under atmospheric pressure and at 18 °C) [18,19]. Therefore, strategies of eliminating or replacing hazardous substrates with safer and less expensive compounds are actively researched [20], and introduction of carbon dioxide to gaseous components is one of such approaches following the promising trends of green chemistry. CO2 is already used as a reagent in relatively few industrial processes such as production of urea, salicylic acid, and some carbonates. Despite limited number of applications of CO2 in organic synthesis caused mainly by high kinetic inertness and thermodynamic stability of CO2 [21,22,23,24], every year new approaches involving CO2 have been investigated [25,26,27,28]. For many years, carbonylation of aromatic amines by CO2 was poorly represented in the literature, in contrast to carbonylation of ammonia and aliphatic amines by CO2 [29,30,31,32,33,34]. Perhaps, lower nucleophilicity of nitrogen atom in the aromatic ring of aromatic amines decreases their reactivity towards CO2 [5]. However, in recent years significant progress has been made toward the synthesis of isocyanates, ureas, carbamates, and other compounds using CO2 and aromatic amines [35,36,37,38,39,40,41]. Despite promising results, many of these methods suffer from some limitations such as long reaction times required, harsh reaction conditions, low yields, and other difficulties in application of CO2 as a carbonylating agent [42,43]. Therefore, some reports are focused on the use of CO2 as an additive for CO, or as a reaction medium (liquid CO2) [5,27,44]. Gabriele et al. [44] observed that in carbonylation of amines performed in the presence of CO/air and PdI2, the addition of CO2 significantly increased yield of the reaction for aliphatic amines, whereas less satisfactory results were obtained for carbonylation of aromatic amines. Surprisingly, good performance was observed for both aliphatic and aromatic amines when carbonylation was conducted in pure CO2 as a non-polar aprotic solvent. Using an appropriate amount of CO2 resulted in nearly three times higher catalytic activity of PdI2 catalyst [44]. Based on the results obtained by Gabriele, although in many processes it is very difficult to replace CO by CO2 as carbonylating agent, addition of CO2 may increase the yield of the carbonylation of amines by CO. Moreover, carbon dioxide is a byproduct formed during industrial production of CO and its complete removal makes an additional complication in the production process. Furthermore, CO2 exhibits a much stronger suppression effect on the explosion of flammable gases than nitrogen [45], and thus it decreases explosiveness of gases employed in the synthesis (CO and O2) leading to enhanced safety of the process [46]. Last but not least, presence of CO2 in a traditional liquid phase under mild pressures (tens of bar) results in generation of a gas-expanded liquid (GXL) phase. GXL retains the beneficial attributes of a conventional solvent (polarity, catalyst/reactant solubility) with some additional advantages: higher miscibility of permanent gases (O2, CO, etc.) and enhanced transport rates compared to organic solvents at ambient conditions. The enhanced gas solubilities in GXLs may result in reaction rates greater than those achieved in neat organic solvent or supercritical carbon dioxide (sCO2) [47]. In the case of our process, even if CO2 cannot serve as a carbonylating agent, it is interesting to explore other potential benefits of replacing CO with CO2, i.e., decreasing the amount of CO used and introducing CO2 without any preconceived notion regarding its exact function.
Inspired by the promising properties of CO2 and results reported by Gabriele et al. for carbonylation of aniline in the presence of CO2, K2PdI4 as catalyst and without any additives [44], we decided to study the effect of CO2 as one of the components of CO/O2/CO2 mixture on the carbonylation of aniline (model aromatic amine) in the presence of our original catalytic system PdCl2(XnPy)2/Fe/I2/XnPy, at shorter reaction time and under lower total pressure. Also, our goal is to develop our previous catalytic system based on PdCl2(XnPy)2 complexes into nanocatalysts, characterized by unique catalytic properties. Based on the results of our recent studies, we turned our attention to derivatives of pyridine as ligands that optimally stabilize palladium NPs [48,49,50,51] i.e., the access to the catalyst surface is not restricted, in contrast to bulky ligands [52,53,54]. Choosing 4-methylpyridine (model derivative of pyridine) as stabilizing ligand allows nanoparticles (NPs) to effectively interact with ligands and the reacting compound(s). In our previous works, we developed a reduction of aromatic nitrocompounds to aromatic amines in the presence of palladium nanoparticles stabilized by 4-methylpyridine (PdNPs/4MePy) [55,56]. Obtained results encourage us investigation of the catalytic activity of PdNPs/4-MePy in another process i.e., oxidative carbonylation of aniline in the presence of mixture CO2/CO/O2. For the first time, carbonylation of aniline is carried out in the presence of CO2 and palladium nanoparticles stabilized by 4-methylpyridine.
2. Results and Discussion
Referring to our previous work [56], monodentate N-heterocyclic compounds are potentially a new family of stabilizing agents that could be a starting point for design new catalytically active nanoparticles with higher catalytic efficiency. In this work, the catalytic activity of PdNPs is compared with effectiveness of catalytic system based on Pd(II) complexes in the carbonylation of aniline (AN) to N,N′-diphenylurea (DPU) by CO2/CO/O2 mixture. Based on our previous results, we proposed the mechanism of AN carbonylation by CO/O2 in the presence PdCl2(XnPy)2 complexes (where: Py = pyridine, X = -Cl or -CH3, n = 0–2), with Pd(II) reduced to Pd(0) in situ in the catalytic cycle, see Scheme 1. Partial precipitation of inactive Pdblack reported by Ragaini [57] is one of the proofs of the Pd0 presence in the system, further supported by our isolation of palladium black precipitated during the reaction of PdCl2(PhNH2)2 complex with carbon monoxide [15]. The next step is reoxidation of Pd(0) to Pd(II) and in this cycle both molecular oxygen and iodine are supposed to be potential oxidants responsible for recycling Pd(II) from Pd(0), step 1a–1b. Although oxidation of Pd(0) by oxygen is possible, it is very slow [58]. Alternatively, Pd(0) in 1a may be oxidized during the oxidative addition of I2 to Pd(0), according to the equation: Pd(0) + I2 → PdI2. Then, HI (instead of water) is released and this HI is oxidized by molecular oxygen: 4HI + O2 → 2I2 + 2H2O [1]. The intermediate 1b is able to coordinate aniline with subsequent insertion of CO to NH-Pd bond in 1c, creating a new carbon-nitrogen bond, intermediate 1d reductive elimination gives N,N′-diphenylurea, generating Pd(0) species [15]. According to the proposed mechanism presented in Scheme 1, Pd(0) stabilized by pyridine ligands plays a crucial role as an acceptor of oxidizing agent (O2 or I2). In order to verify the hypothesis on the participation of Pd(0) in the catalytic cycle, we planned experiments with Pd(0) nanoparticles (PdNPs), and the results (conversion, selectivity, and yield of carbonylation) were compared with the same parameters for process catalyzed by Pd(II) complexes. Prior to that, we searched for the optimal conditions to make both processes, catalyzed by PdNPs and Pd(II) complexes, comparable.
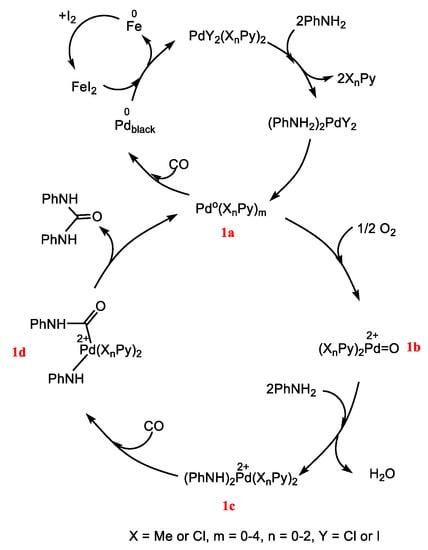
Scheme 1.
Proposed path of the carbonylation of aniline by CO/O2 catalyzed by PdCl2(XnPy)2 complexes.
2.1. Optimization of Reaction Conditions of AN Carbonylation with CO2/CO/O2 Mixture
Conversion of aniline, selectivity towards DPU, and TOF for DPU are presented in Table 1, indicating a strong correlation between the rate of reaction and the amount of CO2 used. Relatively high conversion and selectivity are observed when no CO2 is loaded into the system (entry 5, Table 1). However, even a moderate addition of CO2 is associated with the increasing rate of reaction, and its optimal amount in the gaseous mixture is ca. 50% (entry 4). High yield of DPU (96%) was also observed by Gabriele et al. for carbonylation of aniline performed in the presence of CO2 and a different Pd-based catalytic system. Authors applied a very simple catalyst (K2PdI4), without any co-catalyst and additives; however, a long reaction time (24–72 h) was required [44]. Figure 1 shows that conversion of aniline and selectivity towards DPU decrease drastically when more than 50% of CO2 is introduced. Eventually, no formation of DPU is observed at 100% fraction of CO2 (entry 1, Table 1). Investigation of the impact of CO/CO2 ratio was complemented by additional experiment in which carbonylation of aniline under conditions from entry 4 in Table 1 was conducted in the presence of Ar/CO/O2 instead of CO2/CO/O2 mixture, and no difference in DPU yield between the two cases was observed. Obtained results suggest that replacing CO with certain amount of CO2 allows for achieving higher yields of DPU, although, as indicated by the experiment with argon, CO2 itself does not seem to act as a carbonylating agent. We propose two possible explanations for the beneficial effect of CO2. First, it is likely that the observed optimal CO/CO2 ratio is a result of the balance between enhancement of mass transfer by CO2 (as commonly observed in GXLs) and minimal amount of CO necessary for efficient carbonylation of aniline. In the presence of 88% of CO2 content, the amount of CO (12%) is lower than required according to stoichiometry, and therefore, unsurprisingly, is associated with lower conversion and TOF values. On the other hand, the presence of CO2 might prevent (inhibit) possible side reactions, e.g., oxidation of CO to CO2 (which to some extent may occur in the presence of palladium catalyst). Unless the necessary amount of CO is provided in the system, no DPU is produced and such results confirm that CO2 is not a source of carbonyl group in this reaction. However, we performed further processes in the presence of CO2 as an additional gas due to its economic benefits (CO2 is a natural waste in CO production).

Table 1.
Parameters obtained for the carbonylation a of aniline with CO/CO2/O2 mixture, catalyzed by Pd(II) complexes: conversion (CAN), selectivity (SDPU) and turnover frequency (TOFDPU) of the catalyst, depending on the contents of CO2 in CO2/CO, iodine, and iron.
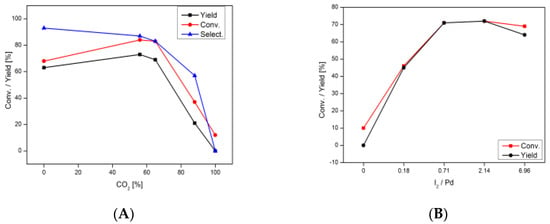
Figure 1.
Effect of percentage of CO2 in CO2/CO mixture (A) and amount of iodine (B) on the conversion (Conv.) and yield and selectivity (Select.) of the catalyst. For the reaction conditions see footnotes in Table 1.
The substituent effect in the pyridine ring was investigated for the optimized content of CO2 in CO2/CO mixture (i.e., 56%). On the basis of obtained results, there is no consistent trend in the effect of derivatives of pyridine in PdCl2 complexes on the rate of reaction (Figure S1 in Supplementary Material). The slightly higher yield of DPU (comparing to other Pd-based complexes) is noticed for PdCl2(2,4-Cl2Py)2 complex, and most further studies in this area are performed in the presence of this complex.
The effect of iodine on the rate of carbonylation was also investigated. As shown in Table 1, when no iodine is used, the desired reaction does not proceed, regardless of the amount of iron present in the system (entries 6–7, Table 1). Increasing amount of iodine results in higher conversion, selectivity and TOF values, with the maximum at 0.04–0.12 mmoL of I2 (entry 9 and 10, Table 1). This observation, in agreement with previous reports [1,59,60,61,62], indicates that iodine might play various crucial roles such as: (i) recovery of the catalytic system, perhaps by oxidation of iron powder to iron(II), (ii) generation of palladium complexes [Pd(CO)3I]−, considered to be the catalytically active species, (iii) reoxidation of Pd(0) to Pd(II). However, if a large excess of iodine is used, the yield of DPU decreases, and the TOF value decreases (entry 11, Table 1). This effect may be attributed to undesirable side reactions, such as formation of insoluble anilinium iodide, which limits the amount of free aniline in the system [60,63]. Moreover, excess of iodide ions may get coordinated to Pd(II), effectively competing with other reagents, which is commonly referred to as catalyst poisoning [10,60,64].
The effect of iron on the rate of reaction is minor (entries 12–16, Table 1). Although a small addition of iron to the mixture seems to increase the conversion of aniline and selectivity towards DPU (see entries 12 and 13, Table 1), it is not a significant change. As more iron is introduced to the system, conversion of aniline decreases slightly (entry 14, Table 1). These observations indicate that a certain amount of iron is beneficial and it slightly increases TOF values during DPU formation. In agreement with our report [15], Fe(0) is oxidized by I2 to Fe(II) and the possible role of Fe(II) is to react with Pdblack in order to return Pdblack to the catalytic cycle as shown in Scheme 1. In the literature, we can find reports for [1] and against [65] the suggested role of iron. Lower conversion of aniline, when excess of iron is used, can be attributed to the reaction between iron and oxygen, which in turn decreases the amount of necessary oxidizing agent (O2). Both reactions of metallic iron, with iodine and with O2, occur easily [66]. Our previous research indicates that even traces of iron (from stainless steel reactor and stirring element) are kinetically significant.
The reaction rate seems to be strongly dependent on the temperature settings selected (see Table 2): at 80 °C the reaction proceeds only to some extent (entry 1). Optimal value for the synthesis of DPU is 100–120 °C (entry 2 and 3) with even higher temperature (140 °C) leading to formation of EPC (entry 4, Table 2, selectivity and TOF values for EPC are placed in parentheses). These results suggest that, after achieving the activation parameters suitable for the formation of DPU, further increase of temperature does not enhance the catalyst activity and may even have a slightly negative impact on the conversion of aniline, possibly due to occurrence of side reactions such as formation of N-ethylaniline, 2-methylquinoline, polyaniline, and EPC (entry 4, Table 2). Further studies were conducted at 100 °C because one of our aims was to operate at desirable energy-saving conditions, i.e., at the lowest temperature allowing formation of satisfactory amount of DPU. The temperature of 100 °C was also chosen for other practical reasons—it was the most appropriate temperature for comparative tests (high conversion and selectivity of Pd(II) complex were observed at this temperature during our previous studies reported in [15]). Although NPs might be more active at 120 °C than at 100 °C, the goal of this work was not to achieve the highest possible activity of catalyst but to study and compare activity of various types of pre-catalysts at the same temperature.

Table 2.
Parameters obtained for the carbonylation of aniline by CO/CO2/O2 catalyzed by PdCl2(2,4-Cl2Py)2: conversion (CAN), selectivity (SDPU) and turnover frequency (TOFDPU), depending on the temperature a.
2.2. Synthesis of PdNPs
PdNPs stabilized by 4MePy were synthesized in water, following the procedure [43,50]. Before NPs are used as catalysts they are dried because the presence of excess H2O in the reaction mixture might diminish the yield of diphenylurea formed during the carbonylation of aniline by CO/O2 (partial hydrolysis of urea may occur at high temperature) [1]. The TEM images presented in Figure 2 indicate agglomeration after drying (left panel versus middle panel), therefore, we also decided to synthesize NPs in ethanol. Right panel in Figure 2 demonstrates that palladium nanoparticles are not stable in ethanol and bigger aggregates are formed. A more appropriate name for these aggregates would be a palladium-based nanostructural material (PdNM) rather than nanoparticles.
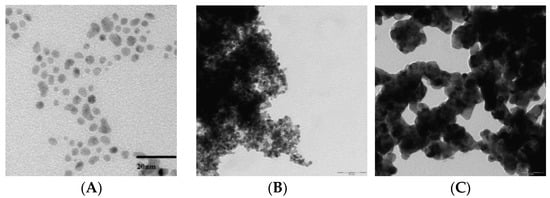
Figure 2.
(A) TEM image of the PdNPs stabilized by 4-methylpyridine (PdNPs/4MePy) from raw aqueous solution [51]. Pd: NaBH4 molar ratio = 1:2, concentration of NaBH4 solution = 1%. For synthesis conditions see Experimental Section. (B) TEM image of the PdNPs stabilized by 4-methylpyridine (PdNPs/4MePy) dried and re-suspended in distilled-deionized water. (C) TEM image of the PdNM (palladium-based nanostructural material) stabilized by 4-methylpyridine (PdNM/4MePy) obtained in ethanol.
2.3. Catalytic Activity of NPs Compared with Other Pd Species
Conversion of aniline, selectivity towards DPU, and TOF for DPU formed in the presence of PdNPs/4MePy are presented in Table 3. Results obtained for PdNPs are compared with results obtained for commercially available Pdblack and two Pd(II) complexes: with 4MePy (the ligand that forms the most stable PdNPs) and with 2,4-Cl2Py (the ligand forming the most catalytically active complex of Pd(II)). TOF values of PdNPs, Pdblack, and Pd(II) complex, measured after 60 min demonstrate that all studied substances are effective pre-catalysts for carbonylation of aniline during the standard time of reaction. The highest yield observed for PdNPs indicates that PdNPs are either catalytically active species or the most efficient source of other catalytically active species e.g., [Pd(CO)3I]− [10]. Perhaps, when Pd(II) complex is applied as a pre-catalyst, it takes longer time to generate in situ catalytically active Pd(0) species from Pd(II), which might explain TOF for Pd(II) complexes being lower than for PdNPs. Our hypothesis, that active species responsible for catalytic activity are easily formed from PdNPs, is confirmed by results obtained for initial stages of the process (i.e., reaction carried out within first 15 min)-significant difference between TOF of PdNPs and TOF of Pd(II) complex is noticed. It is possible that, when carbonylation is carried out in the presence of PdNPs, catalytically active Pd(0) species are immediately present in the reaction from the beginning of the process. Differences in TOF values noticed for Pd(0) in the form of Pdblack (entry 4) and Pd(0) in the form of PdNM (entry 3) indicate that higher catalytic activity of PdNM might have an origin in the nanostructure of investigated material (image in the right panel of Figure 2 displays the PdNM formed from PdNPs with diameter ca. 10 nm). The lowest TOF value observed for Pdblack suggests that catalytically active species are not in the form of a heterogeneous bulk metal, but rather homogeneous complexes (with Pd0), as suggested in the literature [60]. Such a hypothesis that homogeneous complexes (with Pd0) are the real catalysts can explain why TOF for Pd(II) is higher than for Pd(0) –formation of homogeneous Pd(0) species from bulky Pdblack is more difficult than from Pd(II).

Table 3.
Parameters obtained for the carbonylation of aniline by CO/CO2/O2: conversion (CAN), selectivity (SDPU) and turnover frequency (TOFDPU), depending on the catalyst a.
3. Materials and Methods
3.1. Materials
Palladium chloride, sodium chloride, and sodium borohydride were used as received. Pyridine (Py), 2-methylpyridine (2-MePy), 3-methylpyridine (3-MePy), 4-methylpyridine (4-MePy), 2,6-dimethylpyridine (2,6-Me2Py), 2,4-dimethylpyridine (2,4-Me2Py), 3,5-dimethylpyridine (3,5-Me2Py), 2-chloropyridine (2-ClPy), 3-chloropyridine (3-ClPy), 2,4-dichloropyridine (2,4-Cl2Py), aniline, and ethanol were distilled (or fractionally distilled) over drying agent and stored under argon. 2,6-dichloropyridine (2,6-Cl2Py), 3,5-dichloropyridine (3,5-Cl2Py), iron powder, and iodine were used as received. Ultrapure (Milli-Q, 18.2 MΩ·cm resistivity at 25 °C) water was used in all experiments.
3.2. Synthesis of Palladium Nanoparticles
PdNPs were prepared according to the method described elsewhere [48,55]. NaCl (0.188 mmoL; 0.11 g) and PdCl2 (0.084 mmoL; 0.015 g) were dissolved in 6 mL of ultrapure water and stirred at room temperature to form water soluble PdCl42− species. Then, a freshly prepared solution of ligand (derivative of pyridine; 0.628 mmoL in 9 mL of water) was added, stirred for 20 min, and reduced by NaBH4 (1% w/v, 1.1 mL) added in 20 μL portions. The progress of reaction was observed as an immediate darkening of the mixture from light orange to dark brown (almost black). The resulting PdNPs were stirred for 30 min. In some experiments, for comparison purposes, nanoparticles were synthesized in ethanol instead of water. The size of centrifuged NPs as well as centrifuged and dried NPs was measured by transmission electron microscopy (TEM). The composition of Pd/ligand expressed as a percentage of the organic ligand and the metal in the centrifuged and dried palladium nanoparticles stabilized by 4-methylpyridine (PdNPs/4MePy) was determined by thermogravimetry (TG) under nitrogen atmosphere, with the heating rate = 10 K/min (see Figure S1 in Supplementary Information).
3.3. Techniques
Transmission electron microscopy (TEM) observations were carried out using JEM 1400 JEOL Co. microscope, at 120 kV acceleration voltage. The samples were obtained by casting aqueous (or ethanol) solution of palladium nanoparticles onto a carbon coated nickel microgrid (200 mesh) and air-dried overnight. The thermogravimetric (TG) measurements of PdNPs/4-MePy were performed with thermogravimeter Q50-1261 TA Instruments (USA) under nitrogen flow (6 dm3/h), heating rate = 10 K/min. Weight loss during thermal decomposition of PdNPs/4-MePy was determined in the temperature range 40–600 °C. TG measurements were performed in platinum pan, and the weight of the sample was around 1–2 mg. Results presented in this paper are the arithmetic mean of three repetitions, and the difference of results in a series of determinations of the sample was up to 2%.
3.4. Carbonylation of Aniline by CO/CO2/O2 to N,N’-Diphenylurea
The procedure described elsewhere [15] was applied with some modifications. Briefly, the reaction was carried out in a 200 mL stainless-steel autoclave equipped with magnetic stirrer. Before the experiment, the autoclave was heated at 120 °C for 3 h (evaporation of water in order to avoid shifting the balance to the left, which may occur in the presence of an excess of water at high temperature) and cooled down to room temperature. Subsequently, one of the following catalysts: PdCl2(XnPy)2, PdCl2, Pdblack, or PdNPs (0.056 mmoL), and Fe powder (0–2.7 mmoL) were placed in the autoclave, the air was evacuated, and the system was filled with purified argon. Then, under a gentle stream of argon, other reagents and solvents were added: I2 (0–0.39 mmoL), aniline (54 mmoL), ethanol (20 mL), and optionally Py or XnPy (6.2 mmoL). After getting its cover closed, the autoclave was directly filled with molecular oxygen (0.6 MPa), a mixture of carbon dioxide and carbon monoxide (pressure of CO2/CO = 3.4 MPa), then placed in a hot oil bath, and kept at 80–140 °C for 15 or 60 min, depending on the reaction. After 15 or 60 min (depending on the reaction), the autoclave was cooled in a water bath, and then vented. The solid phase obtained after centrifugation (15,000 rpm for 15 min) in the form of white needles (with traces of precipitated palladium black) was re-crystallized from methanol and analyzed by elemental analysis, IR and 1H NMR. N,N-diphenylurea was identified on the basis of elemental analysis % (exp./calc.): C(73.60/73.57), H (5.75/5.70), N (13,22/13.20); FT-IR (KBr): 3326, 3283(s) νNH; 3000–3100 (s) νC-H aromat; 1648 (s) νC = O; 1595, 1555 (m) νC = Caromat; 1496, 1444 (m) νN-H; 1313, 1233 (m) νC-N; 755, 697 (s) νC-Haromat cm−1. M.P. = 235–237 °C; 1H NMR (300 MHz, DMSO): δ (ppm): 8.65 (s, 2 H), 7.46 (d, 4 H), 7.28 (t, 4 H), 6.97 (t, 2 H), and obtained values are in agreement with literature data [67]. Analysis of the liquid phase was performed using gas chromatography (GC-FID, GC-MS). Calculation of conversion of aniline was based on GC-FID analysis with n-decyl alcohol as standard.
4. Conclusions
In this work, the effect of CO2 on the rate of carbonylation of aniline to N,N’-diphenylurea was investigated. Taking into account results obtained for various types of Pd-based pre-catalysts—namely, PdNPs, PdNM, Pd(0) and Pd(II) complexes—the highest TOF is observed for PdNPs. Thus, we suggest that PdNPs act as active species or as the most efficient source of other catalytically active Pd(0) species. High yields observed for Pd(II) complexes support proposed mechanism where Pd(II) is easily reduced to catalytically active Pd(0) during catalytic cycle. We conclude that introduction of PdNPs to the reaction mixture instead of Pdblack or Pd(II) results in higher conversion and turnover frequency. We believe that obtained results may be helpful in elucidating the role of metallic nanoparticles in other organic processes.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/8/877/s1, Figure S1: TG curve for centrifuged and dried PdNPs/4MePy (obtained in water), Figure S2: Effect of derivatives of pyridine on the conversion (Conv.), yield and selectivity (Select.) of the catalyst. Reaction conditions: 54 mmol AN, PdCl2(XnPy)2/Fe/I2 = 0.056/2.68/0.118 mmol, 15 atm CO, 19 atm CO2, 6 atm O2, 20 ml EtOH, 100°C, 60 min.
Author Contributions
D.M.: Investigation, Visualization, Writing—original draft, Writing—review & editing. A.K.: Visualization, Formal analysis, Writing—review & editing. P.P.: Methodology, Data curation, Writing—original draft, Writing—review & editing. A.K.-S.: Conceptualization, Investigation, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing—original draft, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Centre for Research and Development-Poland, grant LIDER no. LIDER/34/0102/L-7/15/NCBR/2016.
Conflicts of Interest
This manuscript has not been submitted elsewhere for consideration, and the authors declare no competing financial interests.
References
- Ferretti, F.; Barraco, E.; Gatti, C.; Ramadan, D.R.; Ragaini, F. Palladium/iodide catalyzed oxidative carbonylation of aniline to diphenylurea: Effect of ppm amounts of iron salts. J. Catal. 2019, 369, 257–266. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-Catalyzed Oxidative Carbonylation Reactions. ChemSusChem 2013, 6, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Didgikar, M.R.; Roy, D.; Gupte, S.P.; Joshi, S.S.; Chaudhari, R.V. Immobilized Palladium Nanoparticles Catalyzed Oxidative Carbonylation of Amines. Ind. Eng. Chem. Res. 2010, 49, 1027–1032. [Google Scholar] [CrossRef]
- Guan, Z.H.; Lei, H.; Chen, M.; Ren, Z.H.; Bai, Y.; Wang, Y.Y. Palladium-Catalyzed Carbonylation of Amines: Switchable Approaches to Carbamates and N,N’-Disubstituted Ureas. Adv. Synth. Catal. 2012, 354, 489–496. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Oliferenko, A.; Lomaka, A.; Karelson, M. Six-Membered cyclic ureas as HIV-1 protease inhibitors: A QSAR study based on CODESSA PRO approach. Bioorg. Med. Chem. Lett. 2002, 12, 3453–3457. [Google Scholar] [CrossRef]
- Patel, M.; Rodgers, J.D.; McHugh, R.J., Jr.; Johnson, B.L.; Cordova, B.C.; Klabe, R.M.; Bacheler, L.T.; Erickson-Viitanen, S. Ko SS Unsymmetrical cyclic ureas as HIV-1 protease inhibitors: Novel biaryl indazoles as P2/P2’ substituents. Bioorg. Med. Chem. Lett. 1999, 9, 3217–3220. [Google Scholar] [CrossRef]
- Inaloo, I.D.; Majnooni, S.A. Fe3O4@SiO2/Schiff Base/Pd Complex as an Efficient Heterogeneous and Recyclable Nanocatalyst for One-Pot Domino Synthesis of Carbamates and Unsymmetrical Ureas. Eur. J. Org. Chem. 2019, 37, 6359–6368. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Mulla, S.A.R.; Rode, C.V.; Kelkar, A.A.; Gupte, S.P. Activity of homogeneous transition metal catalysts for oxidative carbonylation of aniline to N,N’diphenyl urea. J. Mol. Catal. A Chem. 1997, 122, 103–109. [Google Scholar] [CrossRef]
- Bigi, F.; Maggi, R.; Sartori, G. Selected syntheses of ureas throuth phosgene substitutes. Green Chem. 2000, 2, 140–148. [Google Scholar] [CrossRef]
- Zahrtmann, N.; Claver, C.; Godard, C.; Riisager, A.; Garcia-Suarez, E. Selective Oxidative Carbonylation of Aniline to Diphenylurea with Ionic Liquids. J. ChemCatChem 2018, 10, 2450–2457. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.S.; Della Ca’, N.; Fini, F.; Carfagna, C.; Gabriele, B. Catalytic Oxidative Carbonylation of Amino Moieties to Ureas, Oxamides, 2-Oxazolidinones, and Benzoxazolones. ChemSusChem 2015, 13, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Krogul, A.; Litwinienko, G. Application of Pd(II) Complexes with Pyridines as Catalysts for the Reduction of Aromatic Nitro Compounds by CO/H2O. Org. Process. Res. Dev. 2015, 19, 2017–2021. [Google Scholar] [CrossRef]
- Krogul, A.; Litwinienko, G. One pot synthesis of ureas and carbamates via oxidative carbonylation of aniline-type substrates by CO/O2 mixture catalyzed by Pd-complexes. J. Mol. Catal. A Chem. 2015, 407, 204–211. [Google Scholar] [CrossRef]
- Krogul, A.; Skupinska, J.; Litwinienko, G. Tuning of the catalytic properties of PdCl2(XnPy)2 complexes by variation of the basicity of aromatic ligands. J. Mol. Catal. A Chem. 2014, 385, 141–148. [Google Scholar] [CrossRef]
- Krogul, A.; Skupinska, J.; Litwinienko, G. Catalytic activity of PdCl2 complexes with pyridines in nitrobenzene carbonylation. J. Mol. Catal. A Chem. 2011, 337, 9–16. [Google Scholar] [CrossRef]
- Bartish, C.M.; Drissel, G.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1978; Volume 4, p. 774. [Google Scholar]
- Cheng, J.; Zhou, F. Revised Explosibility Diagram to Judge Best Practice of Controlling an Explosive Gas-Mixture. Fire Technol. 2015, 51, 293–308. [Google Scholar] [CrossRef]
- Chen, Y.; Hone, C.A.; Gutmann, B.; Kappe, C.O. Continuous Flow Synthesis of Carbonylated Heterocycles via Pd-Catalyzed Oxidative Carbonylation Using CO and O2 at Elevated Temperatures and Pressures. Org. Process Res. Dev. 2017, 21, 1080–1087. [Google Scholar] [CrossRef]
- Liu, A.H.; Li, Y.N.; He, L.N. Organic synthesis using carbon dioxide as phosgene-free carbonyl reagent. Pure Appl. Chem. 2012, 84, 581–602. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, P.; Matt, D.; Nobel, D. Reactions of Carbon Dioxide with Carbon-Carbon Bond Formation Catalyzed by Transition-Metal Complexes. Chem. Rev. 1988, 88, 747–764. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Shen, Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 2014, 4, 49672–49722. [Google Scholar] [CrossRef]
- Leclaire, J.; Heldebrant, D.J. A call to (green) arms: A rallying cry for green chemistry and engineering for CO2 capture, utilisation and storage. Green Chem. 2018, 20, 5058–5081. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, B. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Chang, P.; Cheng, H.; Yu, Y.; Wu, Z.; Donga, D.; Zhao, F. Polyureas from diamines and carbon dioxide: Synthesis, structures and properties. Phys. Chem. Chem. Phys. 2012, 14, 464–468. [Google Scholar] [CrossRef]
- Franz, M.; Stalling, T.; Steinert, H.; Martens, J. First catalyst-free CO2 trapping of N-acyliminium ions under ambient conditions: Sustainable multicomponent synthesis of thia- and oxazolidinyl carbamates. Org. Biomol. Chem. 2018, 16, 8292–8304. [Google Scholar] [CrossRef]
- Krawczyk, T.; Jasiak, K.; Kokolus, A.; Baj, S. Polymer- and Carbon Nanotube-Supported Heterogeneous Catalysts for the Synthesis of Carbamates from Halides, Amines, and CO2. Catal. Lett. 2016, 146, 1163–1168. [Google Scholar] [CrossRef][Green Version]
- Nomura, R.; Hasegawa, Y.; Ishimoto, M.; Toyasaki, T.; Matauda, H. Carbonylation of Amines by Carbon Dioxide in the Presence of an Organoantimony Catalyst. J. Org. Chem. 1992, 57, 7339–7342. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Narracci, M. Carbon Dioxide Capture by Amines: Increasing the Efficiency by Amine Structure Modification. Fuel Chem. Div. Prep. 2002, 47, 53. [Google Scholar]
- Abla, M.; Choi, J.C.; Sakakura, T. Halogen-free process for the conversion of carbon dioxide to urethanes by homogeneous catalysis. Chem. Commun. 2001, 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Heyn, R.H.; Jacobs, I.; Carr, R.H. Synthesis of Aromatic Carbamates from CO2: Implications for the Polyurethane Industry. Adv. Inorg. Chem. 2014, 66, 83–115. [Google Scholar]
- Choi, J.C.; Yuan, H.Y.; Fukaya, N.; Onozawa, S.; Zhang, Q.; Choi, S.J.; Yasuda, H. Halogen-Free Synthesis of Carbamates from CO2 and Amines Using Titanium Alkoxides. Chem. Asian J. 2017, 12, 1297–1300. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.Y.; Fukaya, N.; Choi, J.C. Alkali Metal Salt as Catalyst for Direct Synthesis of Carbamate from Carbon Dioxide. ACS Sustain. Chem. Eng. 2018, 6, 6675–6681. [Google Scholar] [CrossRef]
- Ren, Y.; Rousseaux, S.A.L. Metal-Free Synthesis of Unsymmetrical Ureas and Carbamates from CO2 and Amines via Isocyanate Intermediates. J. Org. Chem. 2018, 83, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Inaloo, I.D.; Majnoonib, S. Carbon dioxide utilization in the efficient synthesis of carbamates by deep eutectic solvents (DES) as green and attractive solvent/catalyst Systems. New J. Chem. 2019, 43, 11275–11281. [Google Scholar] [CrossRef]
- Lam, R.H.; McQueen, C.M.A.; Pernik, I.; McBurney, R.T.; Hill, A.F.; Messerle, B.A. Selective formylation or methylation of amines using carbon dioxide catalysed by a rhodium perimidine-based NHC complex. Green Chem. 2019, 21, 538–549. [Google Scholar] [CrossRef]
- Riemer, D.; Hirapara, P.; Das, S. Chemoselective Synthesis of Carbamates Using CO2 as Carbon Source. ChemSusChem 2016, 9, 1916–1920. [Google Scholar] [CrossRef]
- Wang, P.; Fei, Y.; Deng, Y. Transformation of CO2 into polyureas with 3-amino-1,2,4-triazole potassium as a solid base catalyst. New J. Chem. 2018, 42, 1202–1207. [Google Scholar] [CrossRef]
- Honda, M.; Sonehara, S.; Yasuda, H.; Nakagawaa, Y.; Tomishige, K. Heterogeneous CeO2 catalyst for the one-pot synthesis of organic carbamates from amines, CO2 and alcohols. Green Chem. 2011, 13, 3406–3413. [Google Scholar] [CrossRef]
- Ca’, N.D.; Bottarelli, P.; Dibenedetto, A.; Aresta, M.; Gabriele, B.; Salerno, G.; Costa, M. Palladium-catalyzed synthesis of symmetrical urea derivatives by oxidative carbonylation of primary amines in carbon dioxide medium. J. Catal. 2011, 282, 120–127. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, Y.; Deng, J.; Wang, Q. Effect of CO2 on explosion limits of flammable gases in goafs. Min. Sci. Technol. 2010, 20, 193–197. [Google Scholar] [CrossRef]
- Leitner, W. Supercritical Carbon Dioxide as a Green Reaction Medium for Catalysis. Acc. Chem. Res. 2002, 35, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B. Gas Expanded Liquids for Sustainable Catalysis. Encycl. Sustain. Sci. Technol. 2012, 3933. [Google Scholar]
- Flanagan, K.A.; Sullivan, J.A.; Mueller-Bunz, H. Preparation and Characterization of 4-Dimethylaminopyridine-Stabilized Palladium Nanoparticles. Langmuir 2007, 23, 12508–12520. [Google Scholar] [CrossRef][Green Version]
- Shaughnessy, K.H.; DeVasher, R.B. Palladium-Catalyzed Cross-Coupling in Aqueous Media: Recent Progress and Current Applications. Curr. Org. Chem. 2005, 9, 585–604. [Google Scholar] [CrossRef]
- Vasylyev, M.V.; Maayan, G.; Hovav, Y.; Haimov, A.; Neumann, R. Palladium Nanoparticles Stabilized by Alkylated Polyethyleneimine as Aqueous Biphasic Catalysts for the Chemoselective Stereocontrolled Hydrogenation of Alkenes. Org. Lett. 2006, 8, 5445–5448. [Google Scholar] [CrossRef]
- Rucareanu, S.; Gandubert, V.J.; Lennox, R.B. 4-(N,N-Dimethylamino)pyridine-Protected Au Nanoparticles: Versatile Precursors for Water- and Organic-Soluble Gold Nanoparticles. Chem. Mater. 2006, 18, 4674–4680. [Google Scholar] [CrossRef]
- Kaim, A.; Szydłowska, J.; Piotrowski, P.; Megiel, E. One-pot synthesis of gold nanoparticles densely coated with nitroxide spins. Polyhedron 2012, 46, 119–123. [Google Scholar] [CrossRef]
- Oh, S.K.; Niu, Y.; Crooks, R.M. Size-Selective Catalytic Activity of Pd Nanoparticles Encapsulated within End-Group Functionalized Dendrimers. Langmuir 2005, 21, 10209–10213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Crooks, R.M. Homogeneous Hydrogenation Catalysis with Monodisperse, Dendrimer-Encapsulated Pd and Pt Nanoparticles. Angew. Chem. Int. Ed. 1999, 38, 364–366. [Google Scholar] [CrossRef]
- Krogul-Sobczak, A.; Kasperska, P.; Litwinienko, G. N-heterocyclic monodentate ligands as stabilizing agents for catalytically active Pd-nanoparticles. Catal. Commun. 2018, 104, 86–90. [Google Scholar] [CrossRef]
- Krogul-Sobczak, A.; Cedrowski, J.; Kasperska, P.; Litwinienko, G. Reduction of Nitrobenzene to Aniline by CO/H2O in the Presence of Palladium Nanoparticles. Catalysts 2019, 9, 404. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S. Mechanistic studies of palladium-catalysed carbonylation reactions of nitro compounds to isocyanates, carbamates and ureas. J. Mol. Catal. A Chem. 1996, 109, 1–25. [Google Scholar] [CrossRef]
- Stahl, S.S. Palladium Oxidase Catalysis: Selective Oxidation of Organic Chemicals by Direct Dioxygen-Coupled Turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef]
- Fukuoka, S.; Chono, M.; Kohno, M. Isocyanate without phosgene. Chemtech 1984, 14, 670–676. [Google Scholar]
- Gupte, S.P.; Chaudhari, R.V. Oxidative carbonylation of aniline over PdC catalyst: Effect of promoters, solvents, and reaction conditions. J. Catal. 1988, 114, 246–258. [Google Scholar] [CrossRef]
- Pri-Bar, I.; Schwartz, J. I2-Promoted Palladium-Catalyzed Carbonylation of Amines. J. Org. Chem. 1995, 60, 8124–8125. [Google Scholar] [CrossRef]
- Shi, F.; Deng, Y.; SiMa, T.; Yang, H. A novel ZrO2-SO42− supported palladium catalyst for syntheses of disubstituted ureas from amines by oxidative carbonylation. Tetrahedron Lett. 2001, 42, 2161–2163. [Google Scholar] [CrossRef]
- McCusker, J.E.; Qian, F.; McElwee-White, L. Catalytic oxidative carbonylation of aliphatic secondary amines to tetrasubstituted ureas. J. Mol. Catal. A Chem. 2000, 159, 11–17. [Google Scholar] [CrossRef]
- Maitlis, P.M.; Haynes, A.; James, B.R.; Catellani, M.; Chiusoli, G.P. Iodide effects in transition metal catalyzed reactions. Dalton Trans. 2004, 3409–3419. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Mancuso, R.; Veltri, L.; Della Ca’, N. Polemic against conclusions drawn in “Palladium/iodide catalyzed oxidative carbonylation of aniline to diphenylurea: Effect of ppm amounts of iron salts”. J. Catal. 2019, 380, 387–390. [Google Scholar] [CrossRef]
- Benesovsky, F. Gmelin Handbuch der Anorganischen Chemie; Springer: New York, NY, USA, 1979; Volume 59, p. 337. [Google Scholar]
- Kwiatkowski, A.; Jędrzejewska, B.; Józefowicz, M.; Grela, I.; Ośmiałowski, B. The trans/cis photoisomerization in hydrogen bonded complexes with stability controlled by substituent effects: 3-(6-aminopyridin-3-yl) acrylate case study. RSC Adv. 2018, 8, 23698–23710. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).