Fe(III) Complexes in Cyclohexane Oxidation: Comparison of Catalytic Activities under Different Energy Stimuli
Abstract
1. Introduction
2. Results and Discussion
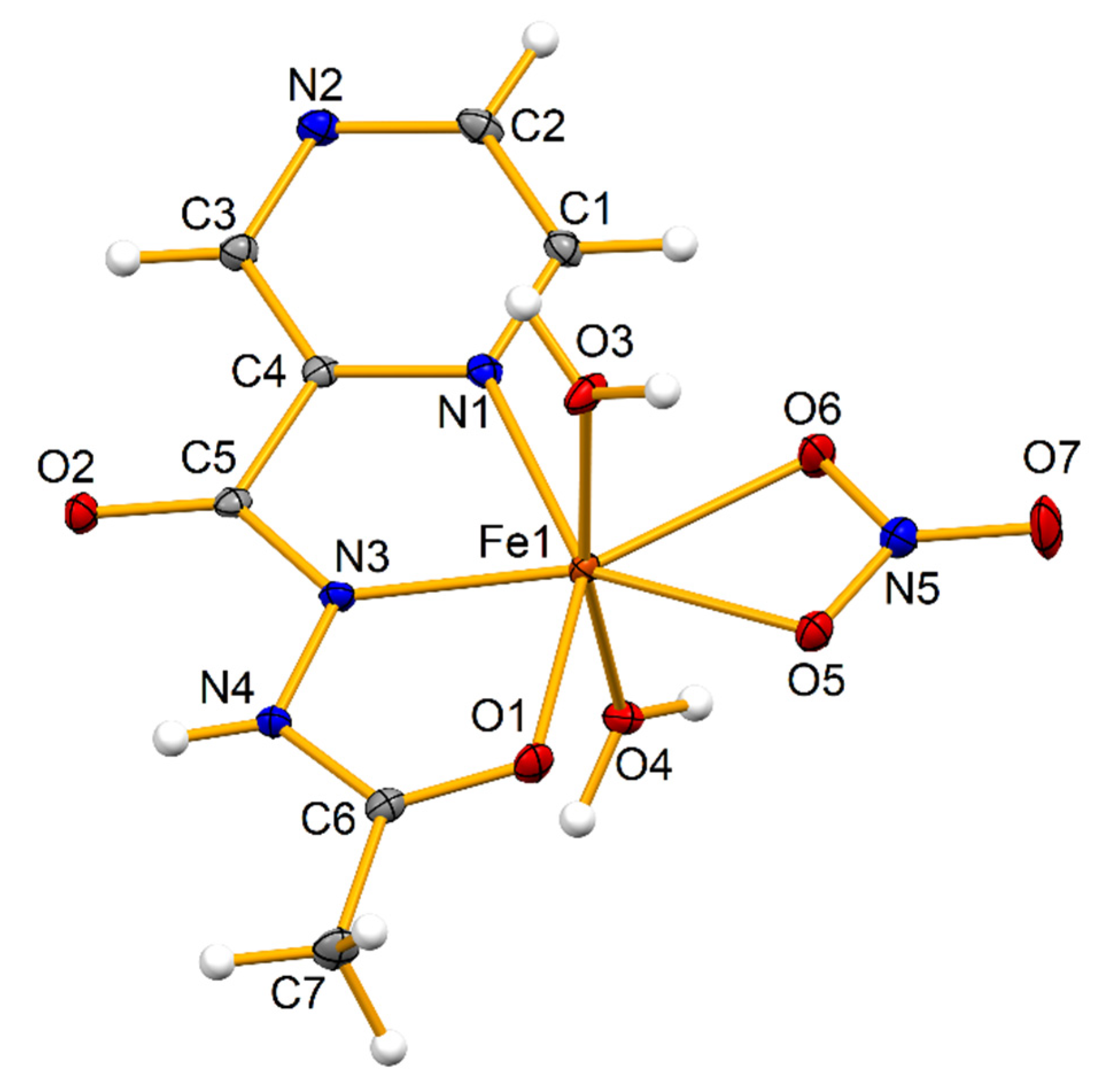
2.1. Crystal Structure
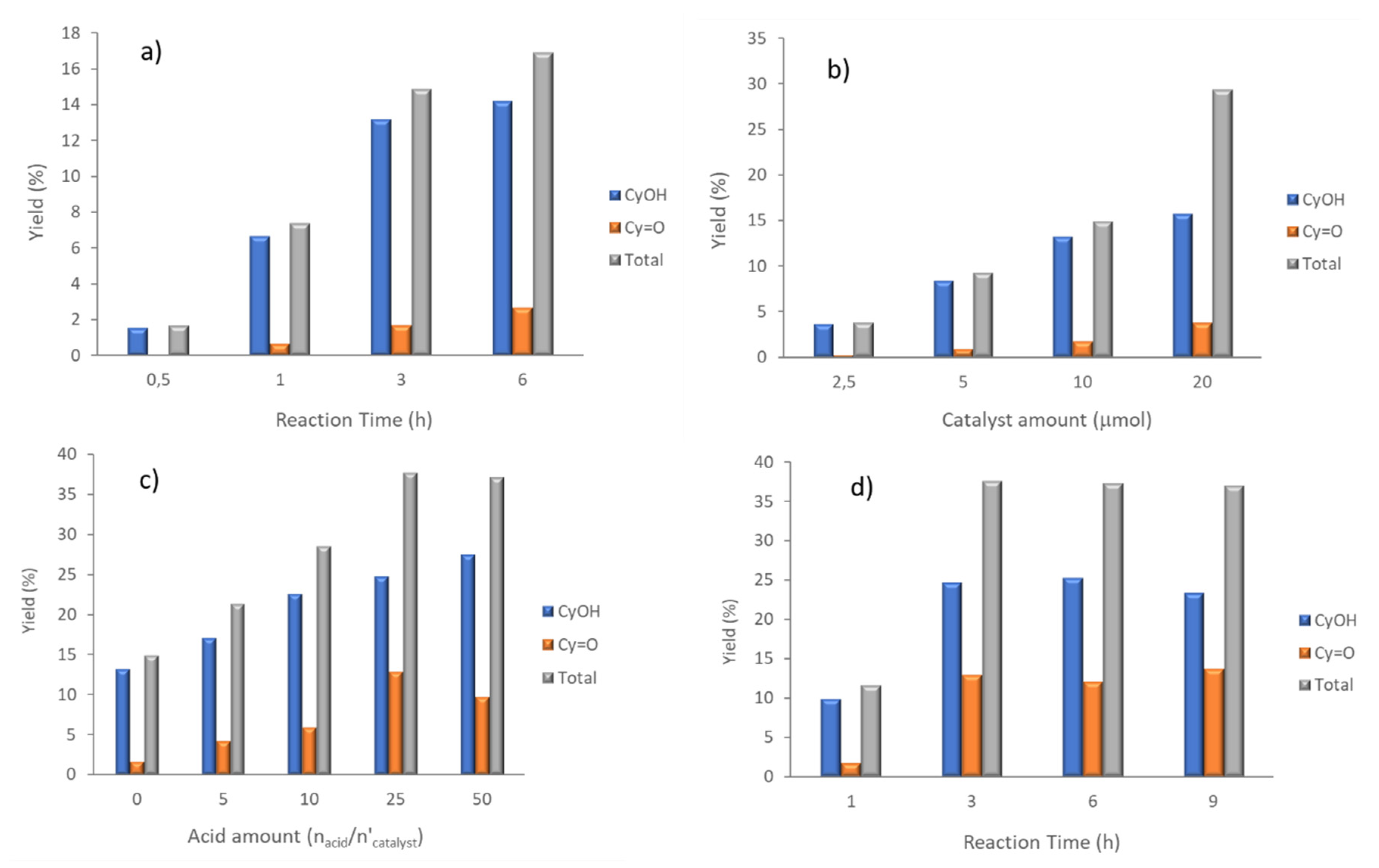
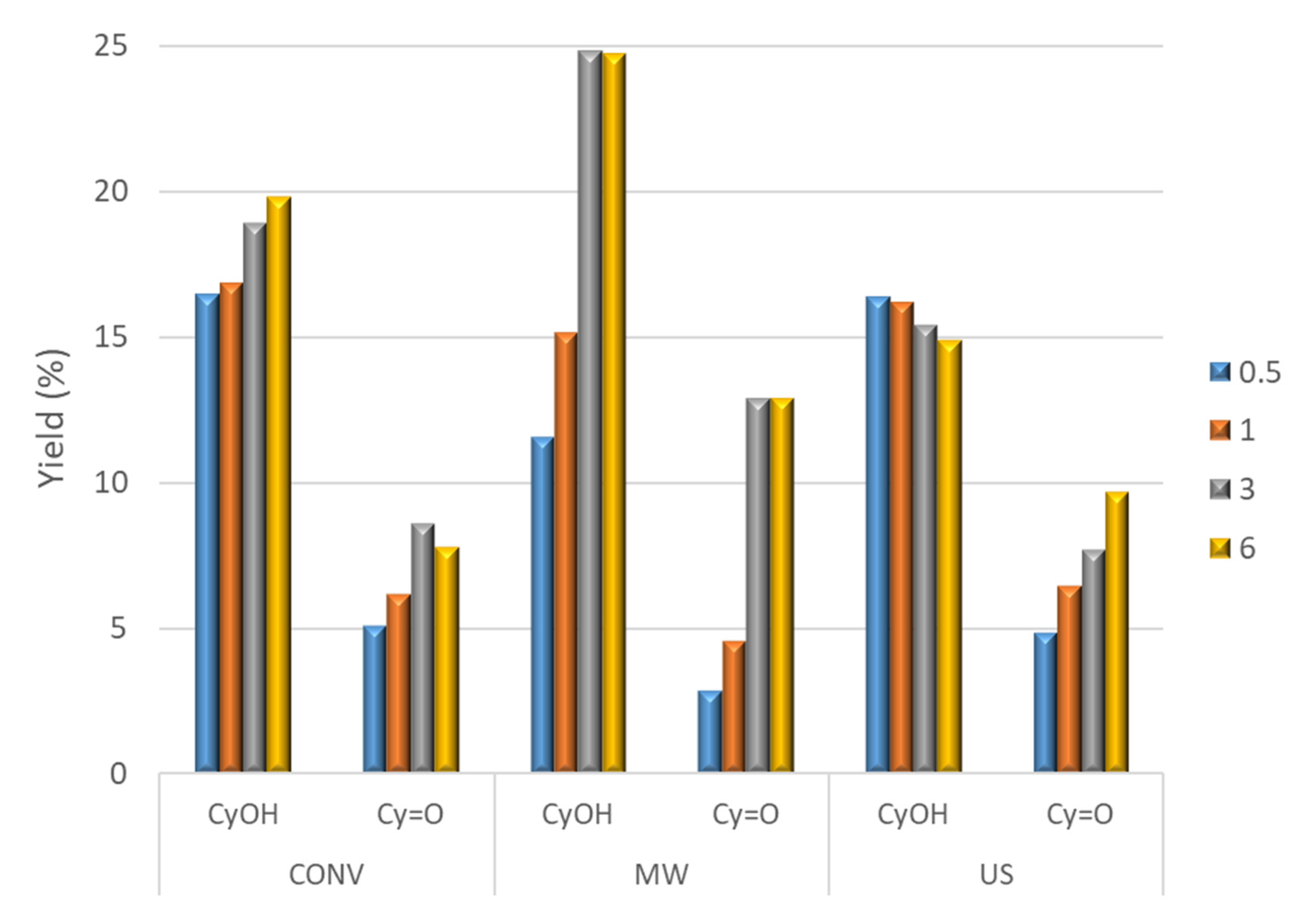
2.2. Peroxidative Oxidation of Cyclohexane
3. Experimental
3.1. General Materials and Procedures
3.2. Synthesis of the Pro-Ligand H2L
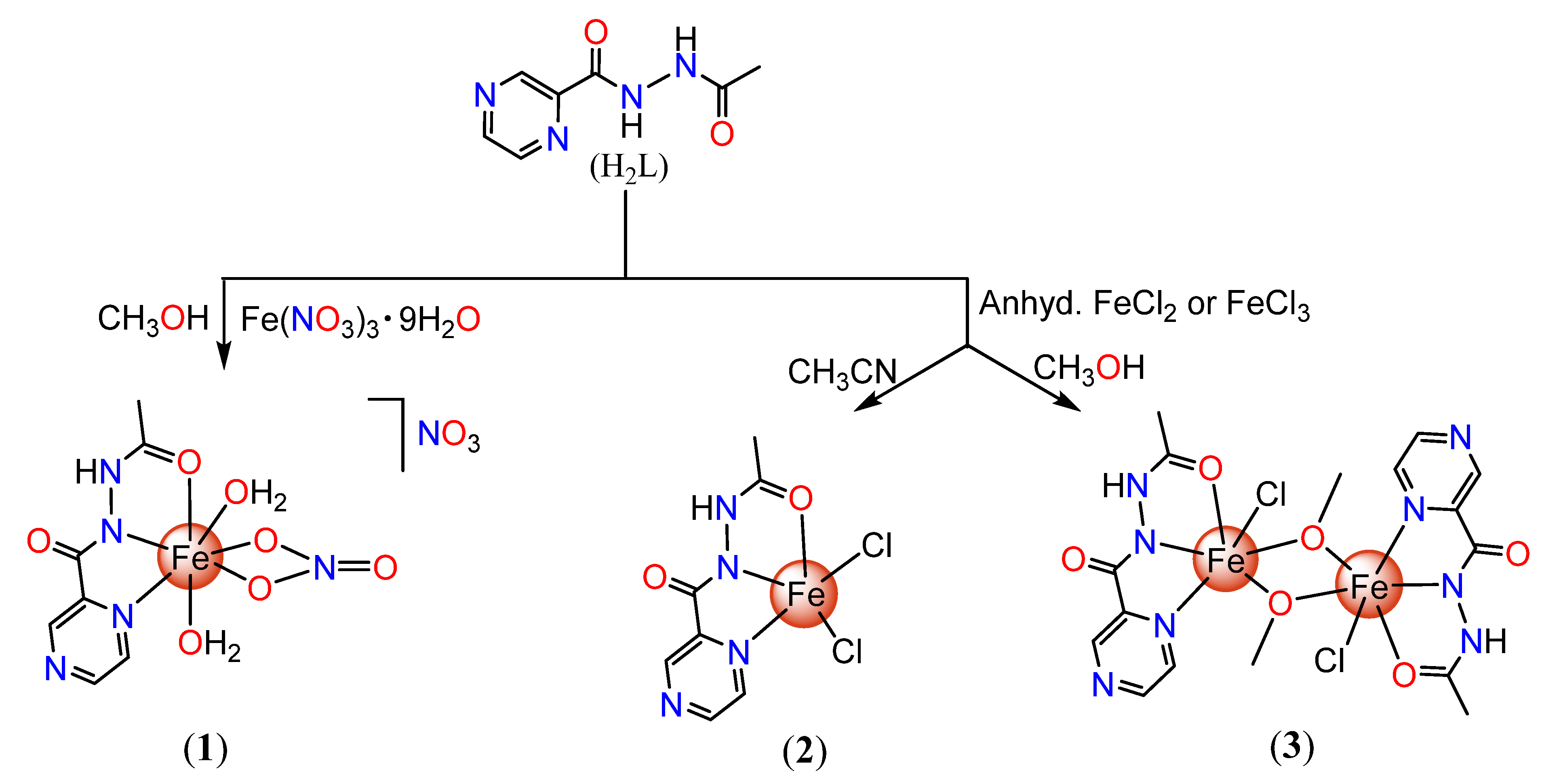
3.3. Syntheses of Fe(III) Complexes of Nʹ-acetylpyrazine-2-carbohydrazide
3.3.1. [Fe(HL)(H2O)2(NO3)]NO3 (1)
3.3.2. [Fe(HL)Cl2] (2) and [Fe(HL)Cl(µ-OMe)]2 (3)
3.4. X-ray Measurements
3.5. Catalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pombeiro, A.J.L.; Guedes da Silva, M.F.C. (Eds.) Alkane Functionalization; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Pombeiro, A.J.L. Alkane Functionalization: Introduction and overview. In Alkane Functionalization; Pombeiro, A.J.L., Guedes da Silva, M.F.C., Eds.; Wiley: Hoboken, NJ, USA, 2019; Chapter 1; pp. 1–15. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation of C-H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C-H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301, 200–239. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCH: New York, NY, USA, 2004; Volume 2, Chapter 2; pp. 215–242. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Crabtree, R.H. Alkane C-H activation and functionalization with homogeneous transition metal catalysts: A century of progress—A new millennium in prospect. J. Chem. Soc. Dalton Trans. 2001, 17, 2437–2450. [Google Scholar] [CrossRef]
- Derouane, E.G.; Haber, J.; Lemos, F.; Ribeiro, F.R.; Guinet, M. (Eds.) Catalytic Activation and Functionalization of Light Alkanes; NATO ASI Series; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1998; Volume 44. [Google Scholar]
- Retcher, B.; Sánchez Costa, J.; Tang, J.; Hage, R.; Gamez, P.; Reedijk, J. Unexpected high oxidation of cyclohexane by Fe salts and dihydrogen peroxide in acetonitrile. J. Mol. Catal. A Chem. 2008, 286, 1–5. [Google Scholar] [CrossRef]
- Antony, R.; Manickam, T.S.; Kollu, P.; Chandrasekar, P.V.; Karuppasamy, K.; Balakumar, S. Highly dispersed Cu(II), Co(II) and Ni(II) catalysts covalently immobilized on imine-modified silica for cyclohexane oxidation with hydrogen peroxide. RSC Adv. 2014, 4, 24820–24830. [Google Scholar] [CrossRef]
- Rahman, A.; Mupa, M.; Mahamadi, C. A mini review on new emerging trends for the synthesis of adipic acid from metal-nano heterogeneous catalysts. Catal. Lett. 2016, 146, 788–799. [Google Scholar] [CrossRef]
- Guo, X.; Xu, M.; She, M.; Zhu, Y.; Shi, T.; Chen, Z.; Peng, L.; Guo, X.; Lin, M.; Ding, W. Morphology-reserved synthesis of discrete nanosheets of CuO@SAPO-34 and pore mouth catalysis for one-pot oxidation of cyclohexane. Angew. Chem. Int. Ed. Engl. 2020, 59, 2606–2611. [Google Scholar] [CrossRef]
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; Cruz, R.S.; Guerreiro, M.C.; Pires, E.L. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A Gen. 2001, 211, 1–17. [Google Scholar] [CrossRef]
- Pokutsa, A.; Le Bras, J. Muzart, Glyoxal-promoted homogeneous catalytic oxygenation of cyclohexane with hydrogen peroxide in the presence of V and Co compounds. J. Russ. Chem. Bull. Int. Ed. 2005, 54, 312–315. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. (Ed.) Advances in Organometallic Chemistry and Catalysis, The Silver/Gold Jubilee ICOMC Celebratory Book; J.Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Shul’pin, G.B. Hydrocarbon oxygenations with peroxides catalyzed by metal compounds. Mini Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Li, J.J. (Ed.) C-H Bond Activation in Organic Synthesis; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Pérez, P.J. (Ed.) Alkane C-H Activation by Single-Site Metal Catalysis; Springer: Berlin, Germany, 2012. [Google Scholar]
- Bäckvall, J.-E. Modern Oxidation Methods; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- White, M.C. Chemistry. Adding aliphatic C-H bond oxidations to synthesis. Science 2012, 335, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, T.; Baran, P.S. If C-H bonds could talk: Selective C-H bond oxidation. Angew. Chem. Int. Ed. Engl. 2011, 123, 3422–3435. [Google Scholar] [CrossRef]
- Olah, G.A.; Molnar, A.; Surya Prakash, G.K. Hydrocarbon Chemistry, 3rd ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear Cu(II) structural isomers: Coordination, magnetism, electrochemistry and catalytic activity toward oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 2015, 3959–3969. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Liu, C.-M.; Kou, H.-Z.; Pombeiro, A.J.L. Cu(II) complexes of N-rich aroylhydrazone: Magnetism and catalytic activity towards microwave-assisted oxidation of xylenes. Dalton Trans. 2019, 48, 12839–12849. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Aroylhydrazone Cu(II) complexes in keto form: Structural characterization and catalytic activity towards cyclohexane oxidation. Molecules 2016, 21, 425. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Peroxidative oxidation of alkanes and alcohols under mild conditions by di- and tetranuclear copper(II) complexes of bis(2-hydroxybenzylidene)isophthalohydrazide. Molecules 2018, 23, 2699. [Google Scholar] [CrossRef]
- Bonchio, M.; Carraro, M.; Scorrano, G.; Kortz, U. Microwave-assisted fast cyclohexane oxygenation catalyzed by iron-substituted polyoxotungstates. Adv. Synth. Catal. 2005, 347, 1909–1912. [Google Scholar] [CrossRef]
- Carvalho, N.M.; Alvarez, H.M.; Horn, A., Jr.; Antunes, O.A. Influence of microwave irradiation in the cyclohexane oxidation catalyzed by Fe(III) complexes. Catal. Today 2008, 133, 689–694. [Google Scholar] [CrossRef]
- Fernandes, R.; Lasri, J.; Guedes da Silva, M.F.C.; da Silva, J.A.L.; Pombeiro, A.J.L. Bis- and tris-pyridyl amino and imino thioether Cu and Fe complexes. Thermal and microwave-assisted peroxidative oxidations of 1-phenylethanol and cyclohexane in the presence of various N-based additives. J. Mol. Catal. A Chem. 2011, 351, 100–111. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning cyclohexane oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organometallics 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenised C-scorpionate iron(II) complex on nanostructured carbon materials as catalysts for microwave-assisted oxidation reactions. ChemCatChem 2018, 10, 1821–1828. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Matias, I.A.S.; Alegria, E.C.B.A.; Ferraria, A.M.; Botelho do Rego, A.M.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. New trendy magnetic C-scorpionate iron catalyst and its performance towards cyclohexane oxidation. Catalysts 2018, 8, 69. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. A hexanuclear mixed-valence oxovanadium(IV,V) complex as a highly efficient alkane oxidation catalyst. Inorg. Chem. 2012, 51, 11229–11231. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.; Roy, B.T.; Kuznetsov, M.L.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes of different nuclearities in the catalytic oxidation of cyclohexane and cyclohexanol—An experimental and theoretical investigation. New. J. Chem. 2019, 43, 17557–17570. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Guedes da Silva, M.F.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Oxidovanadium(V) complexes anchored on carbon materials as catalysts for the oxidation of 1-phenylethanol. ChemCatChem 2016, 8, 2254–2266. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Alegria, E.C.B.A.; Liu, C.-M.; Pombeiro, A.J.L. Mn(II,II) complexes: Magnetic properties and microwave assisted oxidation of alcohols. Dalton Trans. 2014, 43, 3966–3977. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Catalytic activity of polynuclear vs. dinuclear aroylhydrazone Cu(II) complexes in microwave-assisted oxidation of neat aliphatic and aromatic hydrocarbons. Molecules 2019, 24, 47. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Iron(III) and cobalt(III) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: Catalysts for the microwave assisted oxidation of alcohols. RSC Adv. 2016, 6, 8079–8088. [Google Scholar] [CrossRef]
- Zaltariov, M.-F.; Alexandru, M.; Cazacu, M.; Shova, S.; Novitchi, G.; Train, C.; Dobrov, A.; Kirillova, M.V.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; et al. Tetranuclear copper(II) complexes with macrocyclic and open-chain disiloxane ligands as catalyst precursors for hydrocarboxylation and oxidation of alkanes and 1-phenylethanol. Eur. J. Inorg. Chem. 2014, 29, 4946–4956. [Google Scholar] [CrossRef]
- Dobrov, A.; Darvasiová, D.; Zalibera, M.; Bučinský, L.; Puškárová, I.; Rapta, P.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Arion, V.B. Nickel(II) complexes with redox noninnocent octaazamacrocycles as catalysts in oxidation reactions. Inorg. Chem. 2019, 58, 11133–11145. [Google Scholar] [CrossRef]
- Dragancea, D.; Talmaci, N.; Shova, S.; Novitchi, G.; Darvasiová, D.; Rapta, P.; Breza, M.; Galanski, M.; Kožıšek, J.; Martins, N.M.R.; et al. Vanadium(V) complexes with substituted 1,5-bis(2-hydroxybenzaldehyde)carbohydrazones and their use as catalyst precursors in oxidation of cyclohexane. Inorg. Chem. 2016, 55, 9187–9203. [Google Scholar] [CrossRef] [PubMed]
- Arion, V.B.; Platzer, S.; Rapta, P.; Machata, P.; Breza, M.; Vegh, D.; Dunsch, L.; Telser, J.; Shova, S.; Mac Leod, T.; et al. Marked stabilization of redox states and enhanced catalytic activity in galactose oxidase models based on transition metal S-methylisothiosemicarbazonates with—SR group in ortho-position to the phenolic oxygen. Inorg. Chem. 2013, 52, 7524–7540. [Google Scholar] [CrossRef] [PubMed]
- Dobrov, A.; Fesenko, A.; Yankov, A.; Stepanenko, I.; Darvasiová, D.; Breza, M.; Rapta, P.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Shutalev, A.; et al. Nickel(II), Copper(II) and Palladium(II) complexes with Bis-Semicarbazide hexaazamacrocycles: Redox-noninnocent behavior and catalytic activity in oxidation and C-C coupling reactions. Inorg. Chem. 2020, 59, 10650–10664. [Google Scholar] [CrossRef] [PubMed]
- Roy Barman, T.; Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Kuznetsov, M.L.; Pombeiro, A.J.L. Efficient Solvent-Free Friedel-Crafts Benzoylation and Acylation of m-Xylene Catalyzed by N-acetylpyrazine-2-carb hydrazide-Fe(III)-chloro Complexes. Chem. Select 2018, 3, 8349–8355. [Google Scholar]
- Dudley, G.B.; Richert, R.; Stiegman, A.E. On the existence of and mechanism for microwave-specific reaction rate enhancement. Chem. Sci. 2015, 6, 2144–2152. [Google Scholar] [CrossRef]
- Varma, R.S. Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Alegria, E.C.B.A.; Palavra, A.; Pombeiro, A.J.L. Alkane functionalization under unconventional conditions: In ionic liquid, in supercritical CO2 and microwave assisted. In Alkane Functionalization; Pombeiro, A.J.L., Guedes da Silva, M.F.C., Eds.; Wiley: Hoboken, NJ, USA, 2019; Chapter 24; pp. 523–537. [Google Scholar]
- Ribeiro, A.P.C.; Alegria, E.C.B.A.; Kopylovich, M.N.; Ferraria, A.M.; Botelho do Rego, A.M.; Pombeiro, A.J.L. On the comparison of microwave and mechanochemical energy inputs in catalytic oxidation of cyclohexane. Dalton Trans. 2018, 47, 8193–8198. [Google Scholar] [CrossRef]
- Perkas, N.; Wang, Y.; Koltypin, Y.; Gedanken, A.; Chandrasekaran, S. Mesoporous iron–titania catalyst for cyclohexane oxidation. Chem. Commun. 2001, 988–989. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Cu(II) and Fe(III) complexes derived from N-acetylpyrazine-2-carbohydrazide as efficient catalysts towards solvent-free microwave assisted oxidation of alcohols. Catalysts 2019, 9, 1053. [Google Scholar] [CrossRef]
- Sutradhar, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis and chemical reactivity of an Fe(III) metallacrown-6 towards N-donor Lewis bases. Inorg. Chem. Commun. 2013, 30, 42–45. [Google Scholar] [CrossRef]
- Sutradhar, M.; Guedes da Silva, M.F.C.; Nesterov, D.S.; Jezierska, J.; Pombeiro, A.J.L. 1D coordination polymer with octahedral and square-planar nickel(II) centers. Inorg. Chem. Commun. 2013, 29, 82–84. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Pokutsa, A.; Pawel Bloniarz, P.; Fliunt, O.; Kubaj, Y.; Zaborovskyia, A.; Paczeŝniakc, T. Sustainable oxidation of cyclohexane catalyzed by a VO(acac)2-oxalic acid tandem: The electrochemical motive of the process efficiency. RSC Adv. 2020, 10, 10959–10971. [Google Scholar] [CrossRef]
- Shul’pin, G.S.; Mishra, L.S.; Shul’pina, T.V.; Strelkova, A.J.L. Pombeiro. Oxidation of hydrocarbons with hydrogen peroxide catalysed by maltolato vanadium complexes covalently bonded to silica gel. Catal. Commun. 2007, 8, 1516–1520. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Barman, T.R.; Scorcelletti, F.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enol aroylhydrazone Cu(II) complexes. Mol. Catal. 2017, 439, 224–232. [Google Scholar] [CrossRef]
- Días-Ortiz, Á.; Prieto, P.; de la Hoz, A. A critical overview on the effect of microwave irradiation in organic synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal microwave effects revisited: On the importance of internal temperature monitoring and agitation in microwave chemistry. J. Organomet. Chem. 2007, 73, 36–47. [Google Scholar] [CrossRef]
- Obermayer, D.; Kappe, C.O. On the importance of simultaneous infrared/fiber-optic temperature monitoring in the microwave-assisted synthesis of ionic liquids. Org. Biomol. Chem. 2010, 8, 114–121. [Google Scholar] [CrossRef]
- Piermattei, A.; Karthikeyan, S.; Sijbesma, R.P. Activating catalysts with mechanical force. Nat. Chem. 2009, 1, 133–137. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Bruker, APEX2; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS. In Program for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 2000. [Google Scholar]
- Sheldrick, G.M. SHELX97. In Programs for Crystal Structure Analysis (Release 97-2); University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. wingx and ortep for windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]


| 1 | |
|---|---|
| Empirical formula | C7H11FeN6O11.25 |
| Formula weight | 415.04 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Temperature/K | 150(2) |
| a/Å | 7.2541(4) |
| b/Å | 14.4633(8) |
| c/Å | 14.7415(9) |
| β/° | 93.275(2) |
| V (Å3) | 1544.13(15) |
| Z | 4 |
| Dcalc (g cm–3) | 1.785 |
| F000 | 844 |
| μ(Mo Kα) (mm–1) | 1.052 |
| Rfls. collected/unique/observed | 22957/2837/2385 |
| Rint | 0.0478 |
| Final R1a, wR2b (I ≥ 2σ) | 0.0319, 0.0711 |
| Goodness-of-fit on F2 | 1.051 |
| Entry | Catalyst | Catalyst Amount (mol) | Reaction Time (h) | Yield (%) b | Selectivity to Cyclohexanol (%) d | ||
|---|---|---|---|---|---|---|---|
| CyOH | Cy=O | TOTAL c | |||||
| 1 | 1 | 10 | 0.5 | 1.6 | 0.1 | 1.7 | 94 |
| 2 | 10 | 1 | 6.7 | 0.7 | 7.4 | 91 | |
| 3 e | 10 | 1 | 0.7 | 0.04 | 0.74 | 95 | |
| 4 f | 10 | 3 | 7.5 | 3.1 | 10.6 | 71 | |
| 5 | 10 | 3 | 13.2 | 1.7 | 14.9 | 86 | |
| 6 | 10 | 6 | 14.2 | 2.7 | 16.9 | 84 | |
| 7 g | 10 | 3 | 7.6 | 1.1 | 8.7 | 87 | |
| 8 h | 10 | 3 | 16.7 | 4.8 | 21.5 | 78 | |
| 9 | 2.5 | 3 | 3.6 | 0.2 | 3.8 | 95 | |
| 10 | 5 | 3 | 8.4 | 0.9 | 9.3 | 90 | |
| 11 | 20 | 3 | 15.7 | 3.7 | 19.4 | 80 | |
| 12 i | 10 | 3 | 17.1 | 4.3 | 21.4 | 80 | |
| 13 j | 10 | 3 | 22.6 | 6.1 | 28.7 | 79 | |
| 14 k | 10 | 3 | 24.8 | 12.9 | 37.7 | 66 | |
| 15 l | 10 | 3 | 27.5 | 9.7 | 37.2 | 74 | |
| 16 k | 10 | 1 | 9.9 | 1.7 | 11.6 | 85 | |
| 17 k | 10 | 6 | 24.7 | 12.9 | 37.6 | 66 | |
| 18 k | 10 | 9 | 23.5 | 13.6 | 37.1 | 63 | |
| 19 m | 10 | 3 | 7.7 | 1.1 | 8.8 | 88 | |
| 20 n | 10 | 3 | 21.8 | 6.5 | 28.3 | 77 | |
| 21 o | 10 | 3 | 1.3 | 0.4 | 1.7 | 76 | |
| 22 | 2 | 10 | 3 | 9.1 | 1.3 | 10.4 | 88 |
| 23 k | 10 | 3 | 9.8 | 0.9 | 10.7 | 92 | |
| 24 o | 10 | 3 | 2.5 | 0.2 | 2.7 | 93 | |
| 25 | 3 | 10 | 3 | 12.3 | 2.1 | 14.4 | 85 |
| 26 k | 10 | 3 | 16.3 | 1.9 | 18.2 | 90 | |
| 27 o | 10 | 3 | 1.9 | 0.4 | 2.3 | 83 | |
| 28 | Fe(NO3)3.9H2O | 10 | 3 | 3.8 | 1.5 | 5.3 | 72 |
| 29 | Anhy. FeCl2 | 10 | 3 | 3.3 | 0.9 | 4.2 | 79 |
| Entry | Catalyst | Method | Reaction Time (h) | Yield (%) b | Selectivity to Cyclohexanol (%) d | ||
|---|---|---|---|---|---|---|---|
| CyOH | Cy=O | TOTAL c | |||||
| 1 | 1 | CONV | 0.5 | 16.5 | 5.1 | 21.6 | 76 |
| 2 | 1 | 16.9 | 6.2 | 23.1 | 73 | ||
| 3 | 3 | 18.9 | 8.6 | 27.5 | 69 | ||
| 4 | 6 | 19.8 | 7.8 | 27.6 | 72 | ||
| 5 | 24 | 28.8 | 11.8 | 40.6 | 71 | ||
| 6 | MW | 0.5 | 11.6 | 2.9 | 14.5 | 82 | |
| 7 e | 1 | 15.2 | 4.6 | 19.8 | 77 | ||
| 8 e | 3 | 24.8 | 12.9 | 37.7 | 66 | ||
| 9 e | 6 | 24.7 | 12.9 | 37.6 | 66 | ||
| 10 | US | 0.5 | 16.4 | 4.9 | 21.3 | 77 | |
| 11 | 1 | 16.2 | 6.5 | 22.7 | 71 | ||
| 12 | 3 | 15.4 | 7.7 | 23.1 | 67 | ||
| 13 | 6 | 14.9 | 9.7 | 24.6 | 61 | ||
| 14 | 2 | CONV | 3 | 15.2 | 6.9 | 22.1 | 69 |
| 15 | MW | 3 | 9.8 | 0.9 | 10.7 | 92 | |
| 16 | US | 3 | 7.3 | 4.0 | 11.3 | 65 | |
| 17 | 3 | CONV | 3 | 13.3 | 4.4 | 17.7 | 75 |
| 18 | MW | 3 | 16.3 | 1.9 | 18.2 | 90 | |
| 19 | US | 3 | 20.9 | 9.1 | 29.9 | 70 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy Barman, T.; Sutradhar, M.; C. B. A. Alegria, E.; C. Guedes da Silva, M.d.F.; Pombeiro, A.J.L. Fe(III) Complexes in Cyclohexane Oxidation: Comparison of Catalytic Activities under Different Energy Stimuli. Catalysts 2020, 10, 1175. https://doi.org/10.3390/catal10101175
Roy Barman T, Sutradhar M, C. B. A. Alegria E, C. Guedes da Silva MdF, Pombeiro AJL. Fe(III) Complexes in Cyclohexane Oxidation: Comparison of Catalytic Activities under Different Energy Stimuli. Catalysts. 2020; 10(10):1175. https://doi.org/10.3390/catal10101175
Chicago/Turabian StyleRoy Barman, Tannistha, Manas Sutradhar, Elisabete C. B. A. Alegria, Maria de Fátima C. Guedes da Silva, and Armando J. L. Pombeiro. 2020. "Fe(III) Complexes in Cyclohexane Oxidation: Comparison of Catalytic Activities under Different Energy Stimuli" Catalysts 10, no. 10: 1175. https://doi.org/10.3390/catal10101175
APA StyleRoy Barman, T., Sutradhar, M., C. B. A. Alegria, E., C. Guedes da Silva, M. d. F., & Pombeiro, A. J. L. (2020). Fe(III) Complexes in Cyclohexane Oxidation: Comparison of Catalytic Activities under Different Energy Stimuli. Catalysts, 10(10), 1175. https://doi.org/10.3390/catal10101175










