Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica
Abstract
1. Introduction
2. Results and Discussion
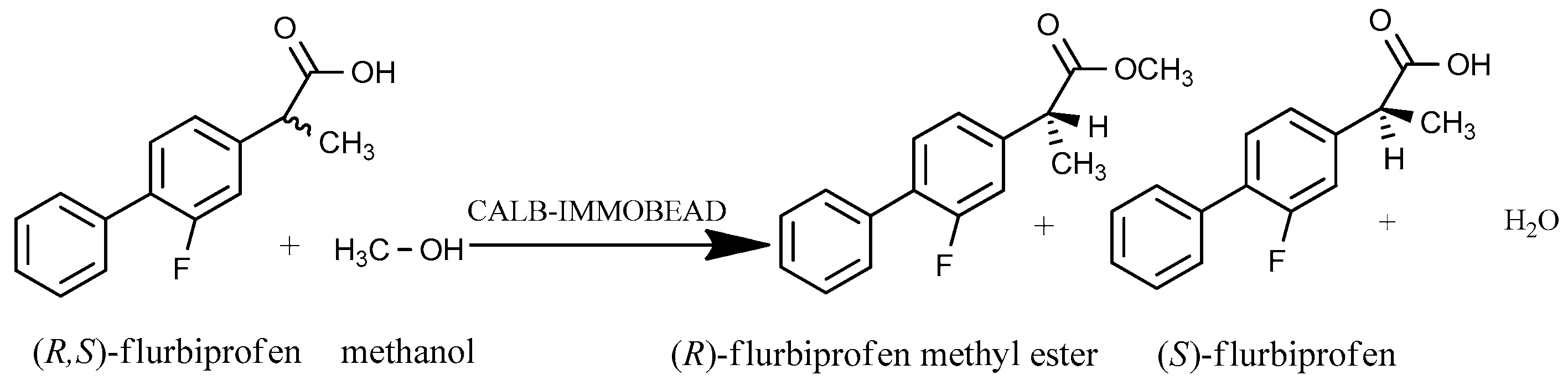
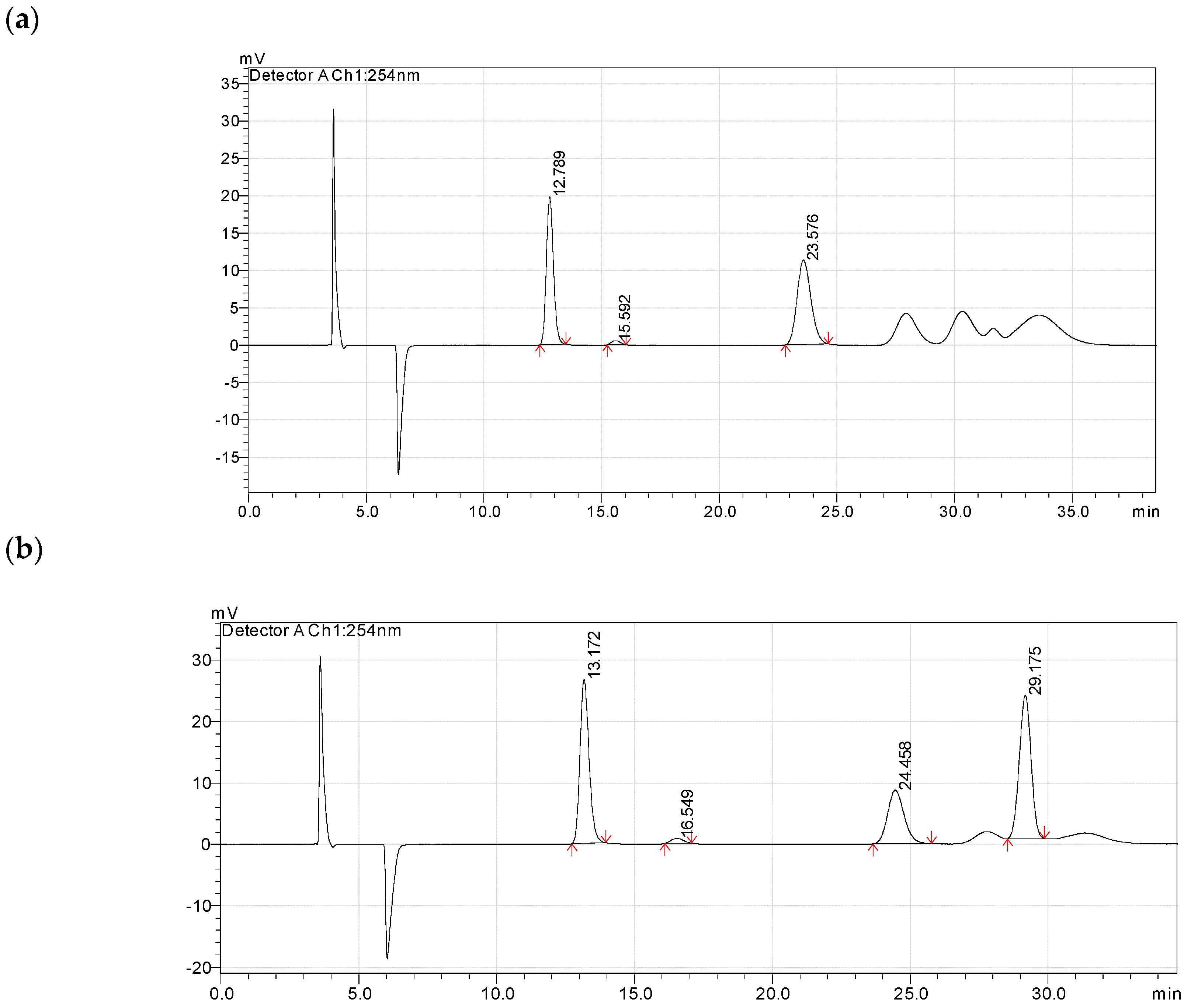
2.1. Enantioselectivity
Screening of Enzyme Carriers, Types of Binding, Reaction Media, and Acyl Acceptors
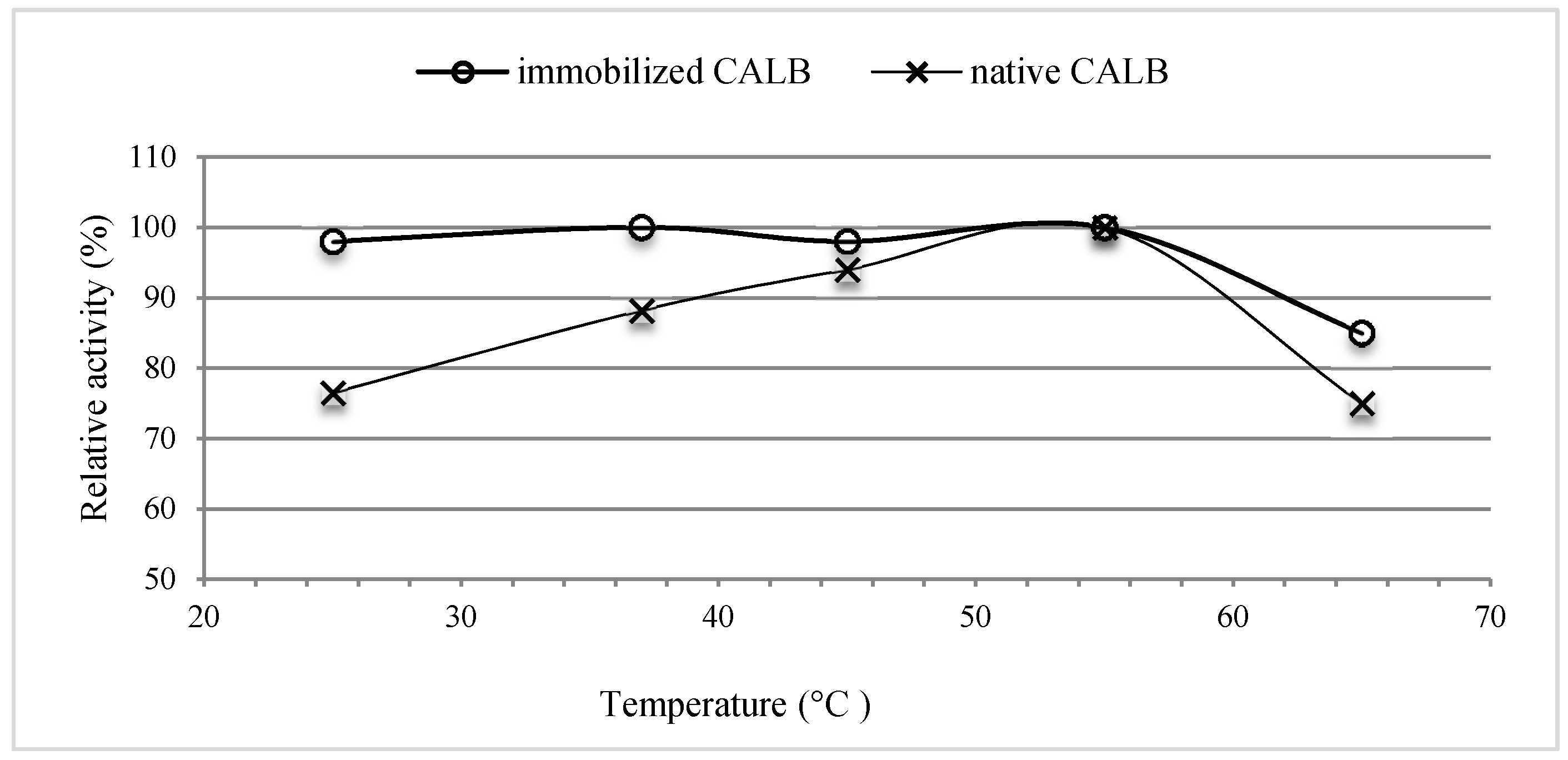
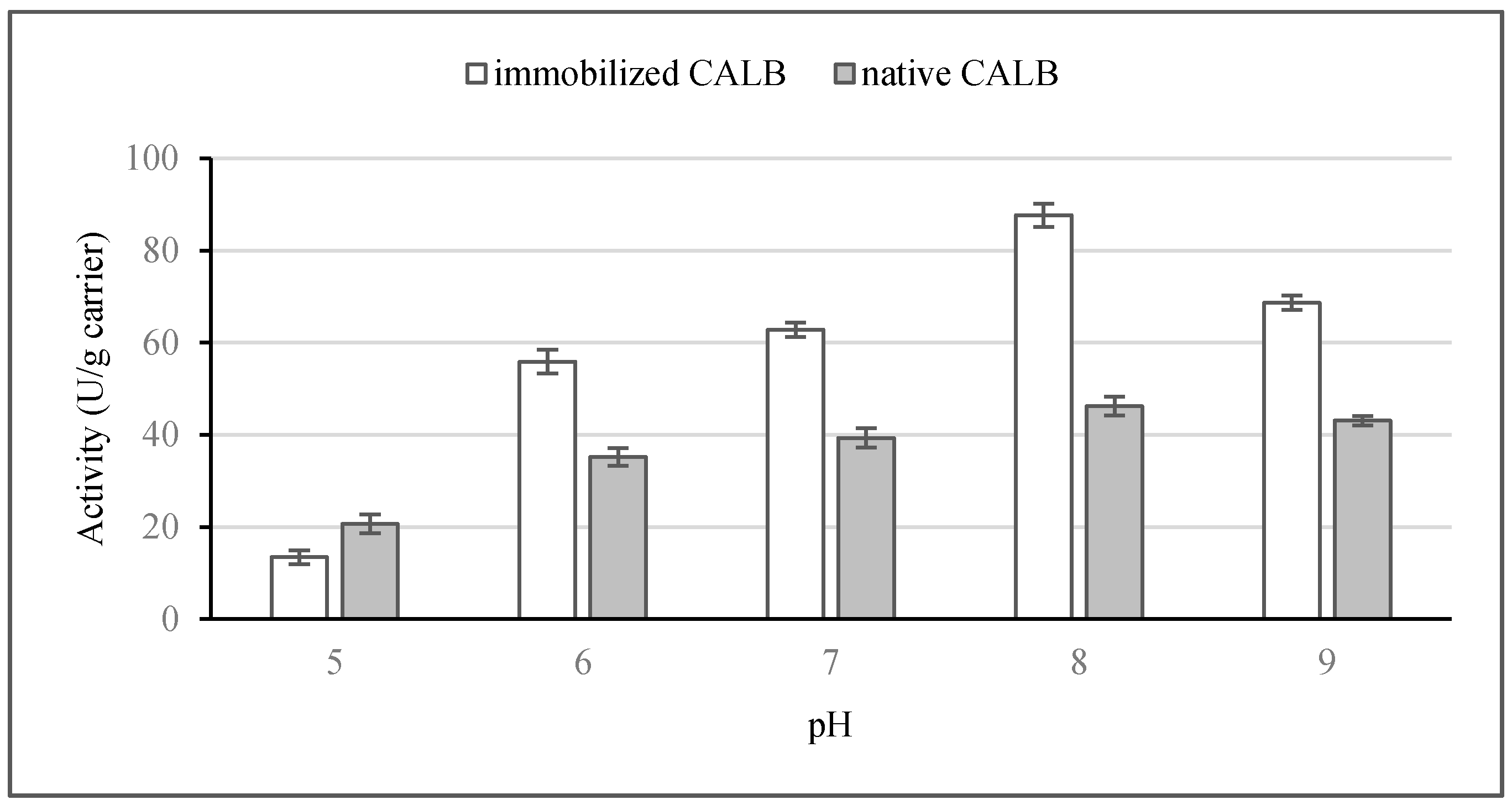
2.2. Lipolytic Activity
2.2.1. Effect of Temperature
2.2.2. Effect of pH
3. Materials and Methods
3.1. Materials
3.2. Immobilization of CALB onto Immobeads
3.3. Determination of the Amount of Immobilized CALB
3.4. Enantioselectivity
3.5. Lipolytic Activity
3.6. Effect of pH and Temperature on CALB Activity
3.7. Chromatographic Conditions
3.8. Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muñoz, S.D.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R,S)-ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Gudmundsson, H.G.; Linderborg, K.M.; Kallio, H.; Yang, B.; Haraldsson, G.G. Synthesis of enantiopure ABC-type triacylglycerols. Tetrahedron 2020, 76, 130813. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Effect of the immobilization protocol on the properties of lipase B from Candida antarctica in organic media: Enantiospecific production of atenolol acetate. J. Mol. Catal. B Enzym. 2011, 71, 124–132. [Google Scholar] [CrossRef]
- Masomian, M.; Rahman, R.N.Z.R.A.; Salleh, A.B. A Novel Method of Affinity Tag Cleavage in the Purification of a Recombinant Thermostable Lipase from Aneurinibacillus thermoaerophilus Strain HZ. Catalysts 2018, 8, 479. [Google Scholar] [CrossRef]
- Cunha, A.G.; Besteti, M.D.; Manoel, E.A.; da Silva, A.A.T.; Almeida, R.V.; Simas, A.B.C.; Fernandez-Lafuente, R.; Pinto, J.C.; Freire, D.M.G. Preparation of core-shell polymer supports to immobilize lipase B from Candida antarctica: Effect of the support nature on catalytic properties properties. J. Mol. Catal. B Enzym. 2014, 100, 59–67. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseling, M.C.F.; Rowan, A.E.; Blank, K.G. Interfacial Activation of Candida antarctica Lipase B: Combined Evidence from Experiment and Simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar]
- Gruber, C.C.; Pleiss, J. Lipase B from Candida antarctica binds to hydrophobic substrate–water interfaces via hydrophobic anchors surrounding the active site entrance. J. Mol. Catal. B Enzym. 2012, 84, 48–54. [Google Scholar] [CrossRef]
- Rognvaldsdottir, E.K.; Magnusson, C.D.; Haraldsson, G.G. Lipids from the marine world: Perspectives of an organic chemist. Eur. J. Lipid Sci. Technol. 2017, 119, 1700166. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzym. Microb. Technol. 2017, 96, 30–35. [Google Scholar]
- Marszałł, M.P.; Siódmiak, T. Immobilization of Candida rugosa lipase onto magnetic beads for kinetic resolution of (R,S)-ibuprofen. Catal. Commun. 2012, 24, 80–84. [Google Scholar] [CrossRef]
- Peirce, S.; Tacias-Pascacio, V.G.; Russo, M.E.; Marzocchella, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI). Molecules 2016, 21, 751. [Google Scholar] [CrossRef]
- An, J.; Li, G.; Zhang, Y.; Zhang, T.; Liu, X.; Gao, F.; Peng, M.; He, Y.; Fan, H. Recent Advances in Enzyme-Nanostructure Biocatalysts with Enhanced Activity. Catalysts 2020, 10, 338. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wiśniewska, J.; Kaczmarek, H.; Marszałł, M.P. Chitosan-Collagen Coated Magnetic Nanoparticles for Lipase Immobilization-New Type of “Enzyme Friendly” Polymer Shell Crosslinking with Squaric Acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B-Thermal Stabilization by Bisepoxide-Activated Aminoalkyl Resins in Continuous-Flow Reactors. Molecules 2016, 21, 767. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Idris, A.; Bukhari, A. Immobilized Candida antarctica lipase B: Hydration, stripping off and application in ring opening polyester synthesis. Biotechnol. Adv. 2012, 30, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.C.; Pfromm, P.H.; Rezac, M.E. Immobilization of Candida antarctica Lipase B on fumed silica. Process Biochem. 2009, 44, 62–69. [Google Scholar] [CrossRef]
- Öztürk, H.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Nanoclays for Lipase Immobilization: Biocatalyst Characterization and Activity in Polyester Synthesis. Polymers 2016, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; dos Santos, C.S.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2015, 259, 107–118. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzym. Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.P.; de Almeida, R.A.D.; Garcia, G.G.; Leão, R.A.C.; Bassut, J.; de Souza, R.O.M.A.; Itabaiana, I., Jr. Immobilization of lipase B from Candida antarctica on epoxy-functionalized silica: Characterization and improving biocatalytic parameters. J. Chem. Technol. Biotechnol. 2018, 93, 105–111. [Google Scholar] [CrossRef]
- De Souza, T.C.; Fonseca, T.D.S.; da Costa, J.A.; Rocha, M.V.P.; de Mattos, M.C.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: Application to the chemoenzymatic production of (R)-Indanol. J. Mol. Catal. B Enzym. 2016, 130, 58–69. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Norra, G.F.; Stamatis, H.; Voutsas, E. Prediction of solvent effect on enzyme enantioselectivity. Fluid Phase Equilibria 2017, 450, 126–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Liu, Z. Enhanced Activity of Immobilized or Chemically Modified Enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Cai, C.; Gao, Y.; Liu, Y.; Zhong, N.; Liu, N. Immobilization of Candida antarctica Lipase B onto SBA-15 and their application in glycerolysis for diacylglycerols synthesis. Food Chem. 2016, 212, 205–212. [Google Scholar] [CrossRef]
- Kamble, M.P.; Shinde, S.D.; Yadav, G.D. Kinetic resolution of (R,S)-α-tetralol catalyzed by crosslinked Candida antarctica lipase B enzyme supported on mesocellular foam: A nanoscale enzyme reactor approach. J. Mol. Catal. B Enzym. 2016, 132, 61–66. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, X.J.; Chen, P.C.; Xu, Z.K. Polymer materials for enzyme immobilization and their application in bioreactors. BMB Rep. 2011, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Ćorović, M.; Mihailović, M.; Banjanac, K.; Carević, M.; Milivojević, A.; Milosavić, N.; Bezbradica, D. Immobilization of Candida antarctica lipase B onto Purolite® MN102 and its application in solvent-free and organic media esterification. Bioprocess Biosyst. Eng. 2017, 40, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.S.; Pinheiro, M.P.; dos Santos, J.C.S.; de S. Fonseca, T.; Lima, L.D.; de Mattos, M.C.; Freire, D.M.G.; da Silva, I.J.; Rodríguez-Aguado, E.; Gonçalves, L.R.B. Strategies of Covalent Immobilization of a Recombinant Candida antarctica Lipase B on Pore-Expanded SBA-15 and Its Application in the Kinetic Resolution of (R,S)-Phenylethyl Acetate. J. Mol. Catal. B 2016, 133, 246–258. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System. Catalysts 2017, 7, 14. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Siódmiak, T.; Mangelings, D.; Heyden, Y.V.; Ziegler-Borowska, M.; Marszałł, M.P. High Enantioselective Novozym 435-Catalyzed Esterification of (R,S)-Flurbiprofen Monitored with a Chiral Stationary Phase. Appl. Biochem. Biotechnol. 2015, 175, 2769–2785. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Dulęba, J.; Siódmiak, T.; Marszałł, M.P. Amano Lipase PS from Burkholderia cepacia—Evaluation of the Effect of Substrates and Reaction Media on the Catalytic Activity. Curr. Org. Chem. 2020, 24, 798–807. [Google Scholar] [CrossRef]
- Tsai, S.-W. Enantiopreference of Candida antarctica lipase B toward carboxylic acids: Substrate models and enantioselectivity thereof. J. Mol. Catal. B Enzym. 2016, 127, 98–116. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Methods to increase enantioselectivity of lipases and esterases. Curr. Opin. Biotech. 2002, 13, 543–547. [Google Scholar] [CrossRef]
- Kahar, U.M.; Chan, K.G.; Sani, M.H.; Mohd Noh, N.I.; Goh, K.M. Effects of single and co-immobilization on the product specificity of type I pullulanase from Anoxybacillus sp. SK3-4. Int. J. Biol. Macromol. 2017, 104, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Coronel, L.A.; Cobas, M.; Rostro-Alanis, M.J.; Parra-Saldívar, R.; Hernandez-Luna, C.; Pazos, M.; Sanromán, M.Á. Immobilization of laccase of Pycnoporus sanguineus CS43. New Biotechnol. 2017, 39, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kahar, U.M.; Sani, M.H.; Chan, K.G.; Goh, K.M. Immobilization of α-Amylase from Anoxybacillus sp. SK3-4 on ReliZyme and Immobead Supports. Molecules 2016, 21, 1196. [Google Scholar] [CrossRef] [PubMed]
- Matte, C.R.; Bordinhão, C.; Poppe, J.K.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Synthesis of butyl butyrate in batch and continuous enzymatic reactors using Thermomyces lanuginosus lipase immobilized in Immobead 150. J. Mol. Catal. B Enzym. 2016, 127, 67–75. [Google Scholar] [CrossRef]
- Matte, C.R.; Bussamara, R.; Dupont, J.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A. Immobilization of Thermomyces lanuginosus lipase by different techniques on Immobead 150 support: Characterization and applications. Appl. Biochem. Biotechnol. 2014, 172, 2507–2520. [Google Scholar] [CrossRef]
- Poppe, J.K.; Costa, A.P.O.; Brasil, M.C.; Rodrigues, R.C.; Ayub, M.A.Z. Multipoint covalent immobilization of lipases on aldehyde-activated support: Characterization and application in transesterification reaction. J. Mol. Catal. B Enzym. 2013, 94, 57–62. [Google Scholar] [CrossRef]
- Gupta, S.M.; Kamble, M.P.; Yadav, G.D. Insight into microwave assisted enzyme catalysis in process intensification of reaction and selectivity: Kinetic resolution of (R,S)-flurbiprofen with alcohols. Mol. Catal. 2017, 440, 50–56. [Google Scholar] [CrossRef]
- Ghanem, A. Direct Enantioselective HPLC Monitoring of Lipase-Catalyzed Kinetic Resolution of Flurbiprofen. Chirality 2010, 22, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, L.; Romano, D.; Pinto, A.; Bertolani, A.; Molinari, F.; Conti, P. An efficient method for the lipase-catalysed resolution and in-line purification of racemic flurbiprofen in a continuous-flow reactor. J. Mol. Catal. B Enzym. 2012, 84, 78–82. [Google Scholar] [CrossRef]
- Mitsuya, D.; Yamamoto, M.; Okai, M.; Inoue, A.; Suzuki, T.; Ojima, T.; Urano, N. Continuous Saccharification of Laminarin by Immobilized Laminarinase ULam111 Followed by Ethanol Fermentation with a Marine-Derived Yeast. Adv. Microbiol. 2017, 7, 387–403. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, M.J.; Rodríguez-Vico, F.; Las Heras-Vázquez, F.J.; Clemente-Jiménez, J.M. Immobilization of a multi-enzyme system for L-amino acids production. J. Chem. Technol. Biotechnol. 2016, 91, 1972–1981. [Google Scholar] [CrossRef]
- Soriano-Maldonado, P.; Las Heras-Vazquez, F.J.; Clemente-Jimenez, J.M.; Rodriguez-Vico, F.; Martínez-Rodríguez, S. Enzymatic dynamic kinetic resolution of racemic N-formyl-and N-carbamoyl-amino acids using immobilized L-N-carbamoylase and N-succinyl-amino acid racemase. Appl. Microbiol. Biotechnol. 2015, 99, 283–291. [Google Scholar] [CrossRef]
- De Lima, L.N.; Mendes, A.A.; Fernandez-Lafuente, R.; Tardioli, P.W.; de Lima Camargo Giordano, R. Performance of Different Immobilized Lipases in the Syntheses of Short- and Long-Chain Carboxylic Acid Esters by Esterification Reactions in Organic Media. Molecules 2018, 23, 766. [Google Scholar] [CrossRef]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barcelo, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of Endocrine Disrupting Chemicals in Wastewater by Enzymatic Treatment with Fungal Laccases. Org. Process Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Borges, D.G.; Baraldo, A., Jr.; Farinas, C.S.; de L. Giordano, R.; Tardioli, P.W. Enhanced saccharification of sugarcane bagasse using soluble cellulase supplemented with immobilized β-glucosidase. Bioresour. Technol. 2014, 167, 206–213. [Google Scholar] [CrossRef]
- Madalozzo, A.D.; Martini, V.P.; Kuniyoshi, K.K.; de Souza, E.M.; Pedrosa, F.O.; Glogauer, A.; Zanin, G.M.; Mitchell, D.A.; Krieger, N. Immobilization of LipC12, a new lipase obtained by metagenomics, and its application in the synthesis of biodiesel esters. J. Mol. Catal. B Enzym. 2015, 116, 45–51. [Google Scholar] [CrossRef]
- Alagöz, D.; Çelik, A.; Yildirim, D.; Tükel, S.S.; Binay, B. Covalent immobilization of Candida methylica formate dehydrogenase on short spacer arm aldehyde group containing supports. J. Mol. Catal. B Enzym. 2016, 130, 40–47. [Google Scholar] [CrossRef]
- SreeHarsha, N.; Ghorpade, R.V.; Alzahrani, A.M.; Al-Dhubiab, B.E.; Venugopala, K.N. Immobilization studies of Candida antarctica lipase B on gallic acid resin-grafted magnetic iron oxide nanoparticles. Int. J. Nanomed. 2019, 14, 3235–3244. [Google Scholar]
- Urrutia, P.; Arrieta, R.; Alvarez, L.; Cardenas, C.; Mesa, M.; Wilson, L. Immobilization of lipases in hydrophobic chitosan for selective hydrolysis of fish oil: The impact of support functionalization on lipase activity, selectivity and stability. Int. J. Biol. Macromol. 2018, 108, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization of Eversa Lipase on Octyl Agarose Beads and Preliminary Characterization of Stability and Activity Features. Catalysts 2018, 8, 511. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization on Octyl-Agarose Beads and Some Catalytic Features of Commercial Preparations of Lipase A from Candida antarctica (Novocor ADL): Comparison with Immobilized Lipase B from Candida antarctica. Biotechnol. Prog. 2019, 35, 2735. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Watanabe, N.; Ota, Y.; Minoda, Y.; Yamada, K. Isolation and Identification of Alkaline Lipase Producing Microorganisms, Cultural Conditions and Some Properties of Crude Enzymes. Agric. Biol. Chem. 1977, 41, 1353–1358. [Google Scholar] [CrossRef]
- Yong, Y.; Bai, Y.; Li, Y.; Lin, L.; Cui, Y.; Xia, C. Characterization of Candida rugosa lipase immobilized onto magnetic microspheres with hydrophilicity. Process Biochem. 2008, 43, 1179–1185. [Google Scholar] [CrossRef]
- Zhang, D.; Yuwen, L.; Xie, Y.; Li, W.; Li, X. Improving immobilization of lipase onto magnetic microspheres with moderate hydrophobicity/hydrophilicity. Colloids Surf. B 2012, 89, 73–78. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Application of Lipases in Kinetic Resolution of Racemates. Chirality 2005, 17, 1–15. [Google Scholar] [CrossRef]


| Support | Type of Bond | Matrix | Functional Group | Particle Size (μm) |
|---|---|---|---|---|
| IB-150P | covalent | polyacrylic | epoxide, polar | 150–300 |
| IB-150A | covalent | polyacrylic | epoxide, apolar | 150–300 |
| IB-D152 | cationic | polyacrylic | carboxylic acid | 350–700 |
| IB-C435 | cationic | polyacrylic | carboxylic acid | 350–700 |
| IB-A161 | anionic, strong | polystyrene | quaternary ammonium type | 350–700 |
| IB-A171 | anionic, strong | polystyrene | quaternary ammonium type | 350–700 |
| IB-A369 | anionic, weak | polystyrene | quaternary ammonium type | 350–700 |
| IB-EC1 | non-ionic | polyacrylic | carboxylic ester | 350–700 |
| IB-S861 | non-ionic | polystyrene | aromatic | 350–700 |
| IB-S500 | non-ionic | polypropylene | alkyl | 150–1500 |
| IB-S60P | non-ionic | silica, porous | hydroxyl | 60–200 |
| IB-S60S | non-ionic | silica, super porous | hydroxyl | 60–200 |
| Support | Lipase Loading (mg/50 mg Support) | Reaction Medium | Stereopreference | ees (%) | eep (%) | C (%) |
|---|---|---|---|---|---|---|
| IB-150A | DCM | 60 | 91 | 39 | ||
| 7.37 | DCE | (R) | 95 | 71 | 57 | |
| DCP | 91 | 40 | 69 | |||
| IB-150P | DCM | 26 | 96 | 21 | ||
| 6.87 | DCE | (R) | 62 | 90 | 41 | |
| DCP | 91 | 83 | 52 | |||
| IB-D152 | DCM | - | - | - | ||
| 1.26 | DCE | (R) | - | - | - | |
| DCP | - | - | - | |||
| IB-C435 | DCM | 2 | 96 | 2 | ||
| 1.06 | DCE | (R) | 2 | 93 | 2 | |
| DCP | 2 | 89 | 2 | |||
| IB-A161 | DCM | * | * | * | ||
| 1.89 | DCE | (R) | * | * | * | |
| DCP | 26 | 89 | 23 | |||
| IB-A171 | DCM | * | * | * | ||
| 1.78 | DCE | (R) | * | * | * | |
| DCP | 15 | 89 | 14 | |||
| IB-A369 | DCM | - | - | - | ||
| 1.39 | DCE | (R) | * | * | * | |
| DCP | 26 | 88 | 22 | |||
| IB-EC1 | DCM | 87 | 89 | 49 | ||
| 6.69 | DCE | (R) | 70 | 60 | 54 | |
| DCP | 91 | 39 | 70 | |||
| IB-S861 | DCM | 61 | 95 | 39 | ||
| 4.15 | DCE | (R) | 83 | 87 | 49 | |
| DCP | 88 | 79 | 53 | |||
| IB-S500 | DCM | 50 | 92 | 35 | ||
| 3.73 | DCE | (R) | 93 | 80 | 54 | |
| DCP | 48 | 22 | 69 | |||
| IB-S60S | DCM | 15 | 96 | 14 | ||
| 6.49 | DCE | (R) | 26 | 92 | 22 | |
| DCP | 40 | 82 | 33 | |||
| IB-S60P | DCM | 6 | 97 | 6 | ||
| 5.04 | DCE | (R) | 16 | 92 | 15 | |
| DCP | 21 | 84 | 20 |
| Medium, Time | Form of CALB | ees (%) | eep (%) | C (%) |
|---|---|---|---|---|
| DCE, 46 h | free form (7.37 mg) | 5 | 96 | 5 |
| DCE, 46 h | immobilized on IB-150A (7.37 mg of lipase) | 95 | 71 | 57 |
| DCM, 94 h | free form (4.15 mg) | 0.8 | 91 | 0.9 |
| DCM, 94 h | immobilized on IB-S861 (4.15mg of lipase) | 61 | 95 | 39 |
| Alcohol | ees (%) | eep (%) | C (%) |
|---|---|---|---|
| methanol | 82 | 87 | 49 |
| ethanol | 74 | 83 | 47 |
| n-propanol | 59 | 85 | 41 |
| n-butanol | 76 | 92 | 45 |
| Alcohol | ees (%) | eep (%) | C (%) |
|---|---|---|---|
| methanol | 61 | 95 | 39 |
| ethanol | 65 | 94 | 41 |
| n-propanol | 77 | 90 | 46 |
| —n-butanol | 70 | 92 | 43 |
| Catalyst | Lipase Loading (mg/g Carrier) | Activity Retention (%) | Activity [U] (μmol/mL/min) | Specific Activity (U/mg Lipase) | Activity (U/g Carrier) |
|---|---|---|---|---|---|
| Free CALB | n/a | n/a | 2.69 * | 1.010 * | n/a |
| IB-150A | 147.4 | 41.2 | 1.17 | 0.16 | 23.4 |
| IB-150P | 137.4 | 17.6 | 0.5 | 0.07 | 10.0 |
| IB-D152 | 25.2 | 178.0 | 4.17 | 3.31 | 83.4 |
| IB-C435 | 21.2 | 80.0 | 2.0 | 1.89 | 40.0 |
| IB-A161 | 37.8 | 21.4 | 0.5 | 0.26 | 10.0 |
| IB-A171 | 35.6 | 28.6 | 0.67 | 0.37 | 13.4 |
| IB-A369 | 27.8 | 42.9 | 1.0 | 0.72 | 20.0 |
| IB-EC1 | 133.8 | 17.6 | 0.5 | 0.07 | 10.0 |
| IB-S861 | 83.0 | 14.3 | 0.42 | 0.1 | 8.4 |
| IB-S500 | 74.6 | 85.7 | 2.0 | 0.54 | 40.0 |
| IB-S60S | 129.8 | 80.0 | 2.67 | 0.41 | 53.4 |
| IB-S60P | 100.8 | 71.4 | 2.5 | 0.5 | 50.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siódmiak, T.; G. Haraldsson, G.; Dulęba, J.; Ziegler-Borowska, M.; Siódmiak, J.; Marszałł, M.P. Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica. Catalysts 2020, 10, 876. https://doi.org/10.3390/catal10080876
Siódmiak T, G. Haraldsson G, Dulęba J, Ziegler-Borowska M, Siódmiak J, Marszałł MP. Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica. Catalysts. 2020; 10(8):876. https://doi.org/10.3390/catal10080876
Chicago/Turabian StyleSiódmiak, Tomasz, Gudmundur G. Haraldsson, Jacek Dulęba, Marta Ziegler-Borowska, Joanna Siódmiak, and Michał Piotr Marszałł. 2020. "Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica" Catalysts 10, no. 8: 876. https://doi.org/10.3390/catal10080876
APA StyleSiódmiak, T., G. Haraldsson, G., Dulęba, J., Ziegler-Borowska, M., Siódmiak, J., & Marszałł, M. P. (2020). Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica. Catalysts, 10(8), 876. https://doi.org/10.3390/catal10080876






