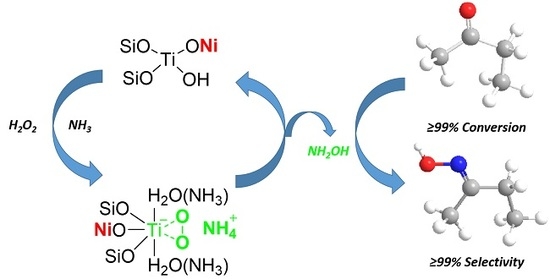
Nickel-Modified TS-1 Catalyzed the Ammoximation of Methyl Ethyl Ketone
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Activity on the Ammoximation of MEK
2.1.1. The Catalytic Activity of M-TS-1
2.1.2. The Catalytic Activity of XNi-TS-1
2.2. Reusability Tests of 3% Ni-TS-1
2.3. Characterization of Catalysts
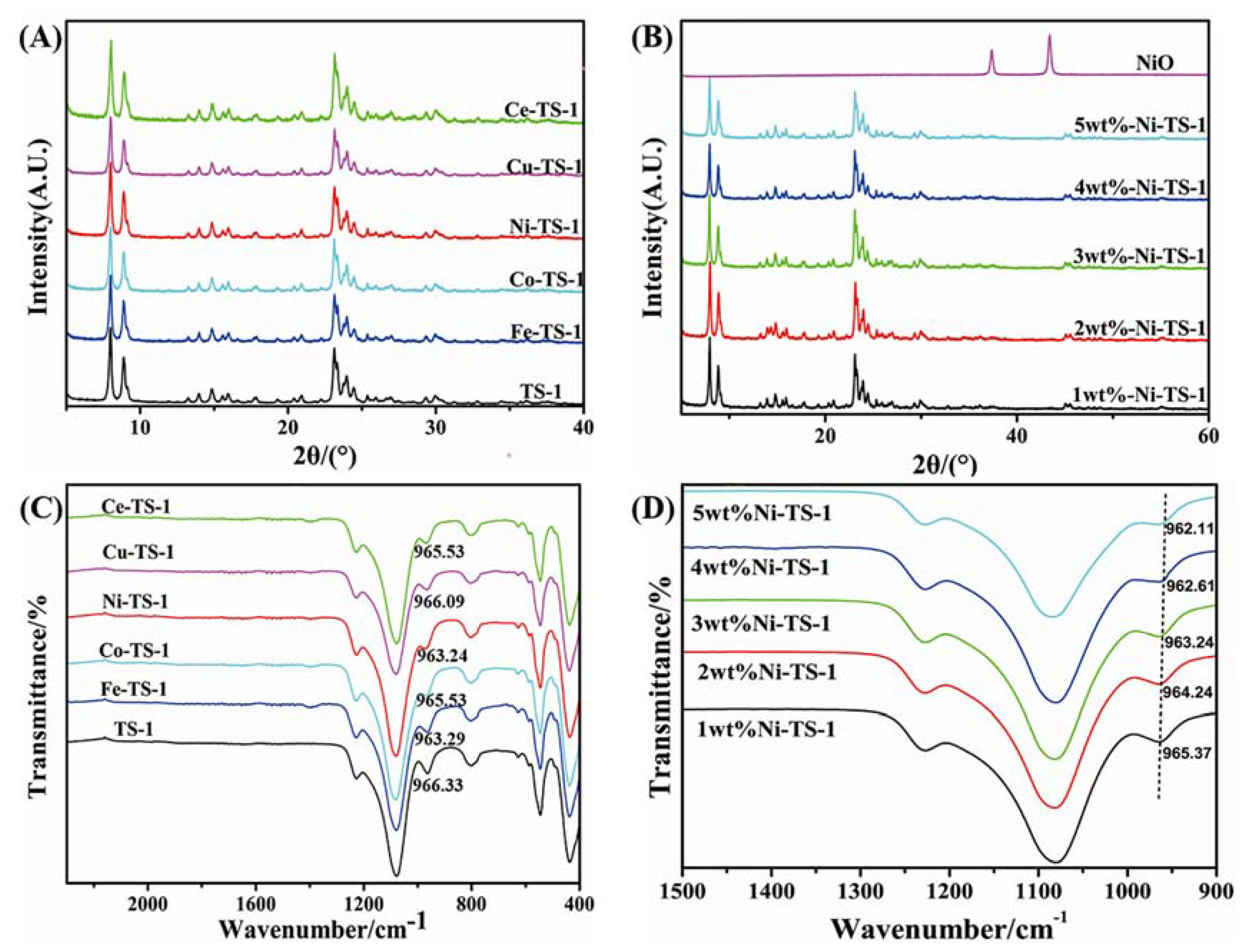
2.3.1. XRD and FT-IR Characterization
2.3.2. SEM Characterization
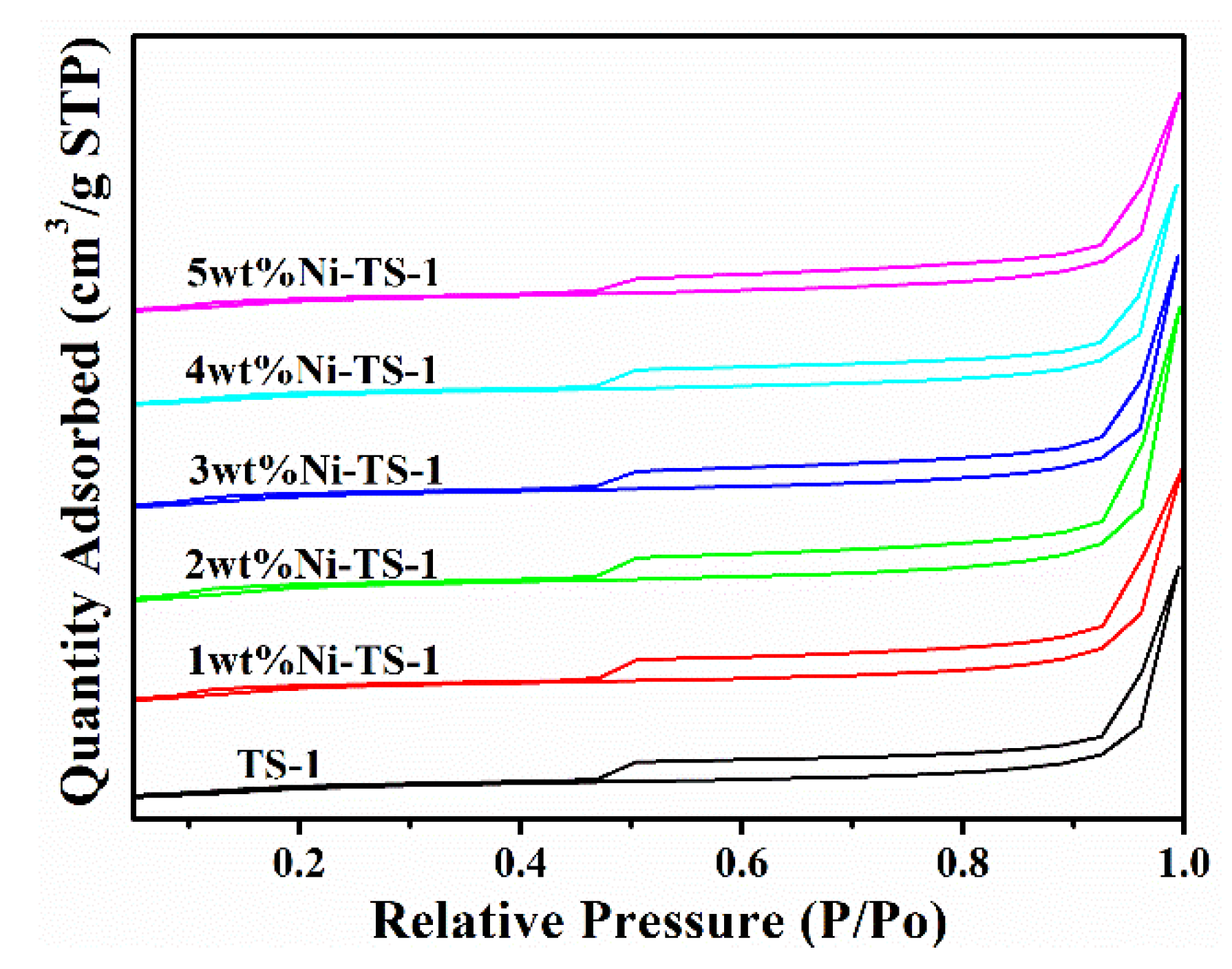
2.3.3. Induced Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) and N2 Adsorption-Desorption Characterization
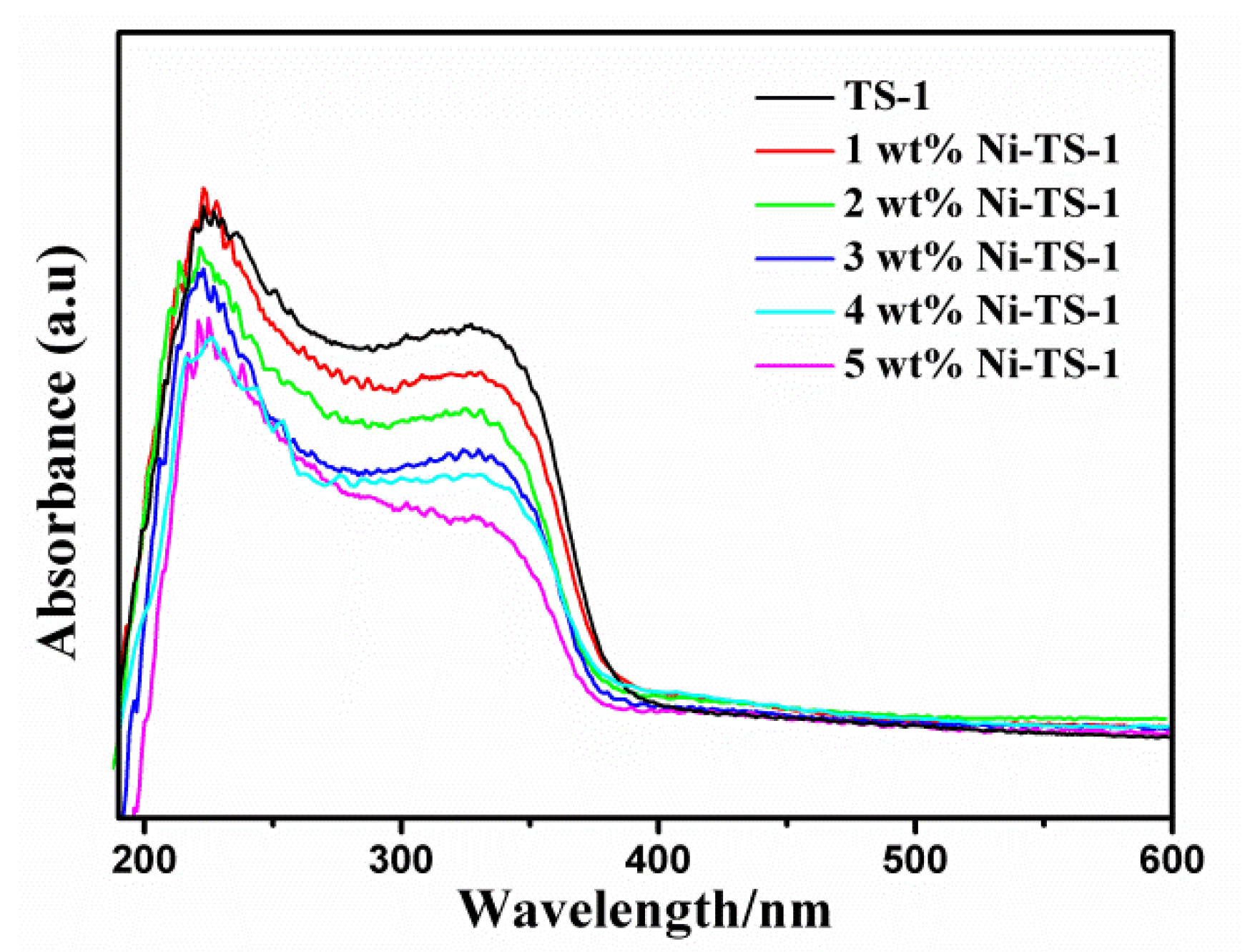
2.3.4. DR UV-Vis Characterization
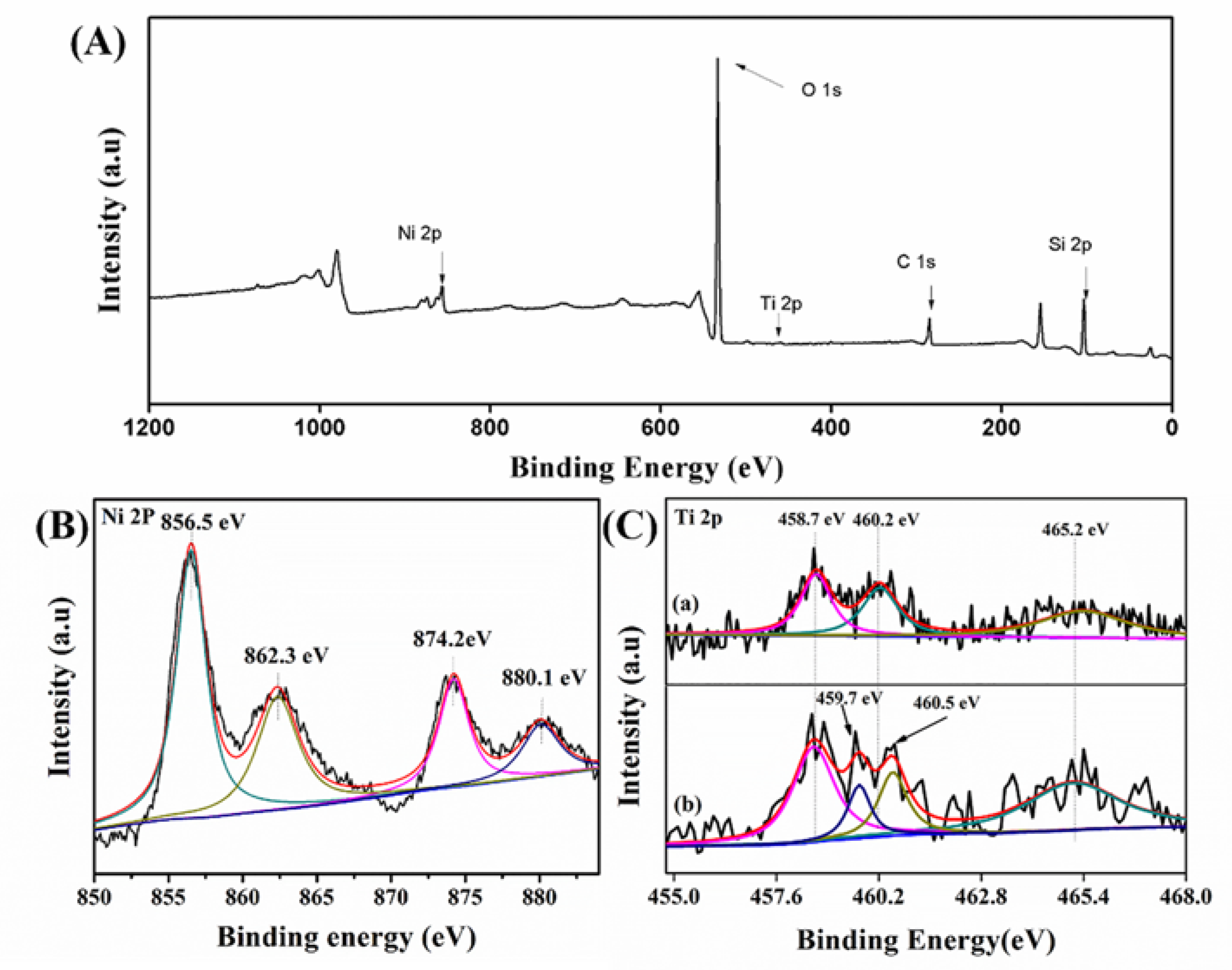
2.3.5. XPS Characterization
2.3.6. The Analysis of Point of Zero Charge
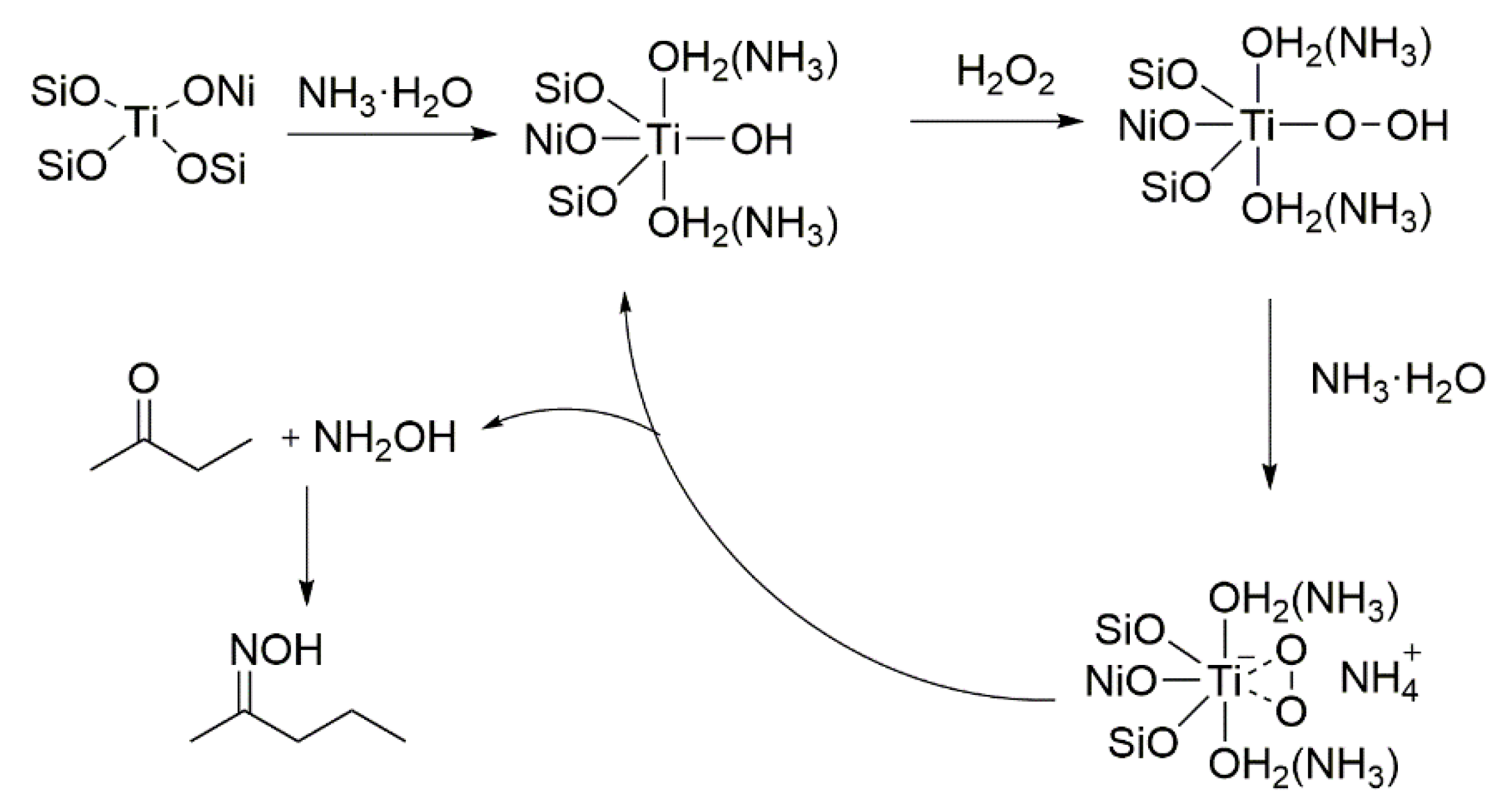
2.4. Mechanism Analysis
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Catalyst Preparation
3.2.2. Catalyst Characterization
3.2.3. Point of Zero Charge (PZC) of Catalyst
3.3. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Dong, C.; Wang, T.; Luo, G. Cyclohexanone ammoximation over TS-1 catalyst without organic solvent in a microreaction system. Chem. Eng. Sci. 2018, 187, 60–66. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, P.; He, G.; Li, M.; Zhu, H.; Liu, R.; Pei, F. Continuous three-phase 2-butanone ammoximation process via spray forming TS-1 microspheres in a highly efficient jet loop reactor. Chem. Eng. J. 2017, 325, 169–175. [Google Scholar] [CrossRef]
- Xue, X.; Song, F.; Ma, B.; Yu, Y.; Li, C.; Ding, Y. Selective ammoximation of ketones and aldehydes catalyzed by a trivanadium-substituted polyoxometalate with H2O2 and ammonia. Catal. Commun. 2013, 33, 61–65. [Google Scholar] [CrossRef]
- Song, F.; Liu, Y.; Wang, L.; Zhang, H.; He, M.; Wu, P. Highly selective synthesis of methyl ethyl ketone oxime through ammoximation over Ti-MWW. Appl. Catal. A Gen. 2007, 327, 22–31. [Google Scholar] [CrossRef]
- Jin, H.; Meng, C.; He, G.; Guo, X.; Yang, S. Green synthesis of acetaldehyde oxime using ammonia oxidation in the TS-1/H2O2 system. React. Kinet. Mech. Catal. 2018, 125, 1113–1125. [Google Scholar] [CrossRef]
- Wang, W.; Fu, Y.; Guo, Y.; Guo, Y.; Gong, X.Q.; Lu, G. Preparation of lamellar-stacked TS-1 and its catalytic performance for the ammoximation of butanone with H2O2. J. Mater. Sci. 2017, 53, 4034–4045. [Google Scholar] [CrossRef]
- Lu, T.; Zou, J.; Zhan, Y.; Yang, X.; Wen, Y.; Wang, X.; Zhou, L.; Xu, J. Highly Efficient Oxidation of Ethyl Lactate to Ethyl Pyruvate Catalyzed by TS-1 Under Mild Conditions. ACS Catal. 2018, 8, 1287–1296. [Google Scholar] [CrossRef]
- Marco, T.; Giovanni, P.; Bruno, N. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent 4,410,501, 18 October 1983. [Google Scholar]
- Pei, X.; Liu, X.; Liu, X.; Shan, J.; Fu, H.; Xie, Y.; Yan, X.; Meng, X.; Zheng, Y.; Li, G.; et al. Synthesis of Hierarchical Titanium Silicalite-1 Using a Carbon-Silica-Titania Composite from Xerogel Mild Carbonization. Catalysts 2019, 9, 672. [Google Scholar] [CrossRef]
- Xue, Y.; Zuo, G.; Wen, Y.; Wei, H.; Liu, M.; Wang, X.; Li, B. Seed-assisted synthesis of TS-1 crystals containing Al with high catalytic performances in cyclohexanone ammoximation. RSC Adv. 2019, 9, 2386–2394. [Google Scholar] [CrossRef]
- Xue, Y.; Wen, Y.; Wei, H.; Liu, M.; Huang, X.; Ye, X.; Wang, X.; Li, B. Hollow TS-1 mesocrystals: Hydrothermal construction and high catalytic performances in cyclohexanone ammoximation. RSC Adv. 2015, 5, 51563–51569. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, L.; Zhang, X.; Wu, L.; Liu, Y.; He, M. Insights into the key to highly selective synthesis of oxime via ammoximation over titanosilicates. J. Catal. 2015, 329, 107–118. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wu, L.; Wang, L.; Ding, Y.; Zhang, X.; Liu, Y.; He, M. Lewis acidic strength controlled highly selective synthesis of oxime via liquid-phase ammoximation over titanosilicates. RSC Adv. 2014, 4, 55685–55688. [Google Scholar] [CrossRef]
- Wei, Y.; Li, G.; Su, R.; Lu, H.; Guo, H. Ti-sites environment-mediated hierarchical TS-1 catalyzing the solvent-free epoxidation: The remarkably promoting role of alcohol modification. Appl. Catal. A Gen. 2019, 582, 117108. [Google Scholar] [CrossRef]
- Meng, C.; Yang, S.; He, G.; Luo, G.; Xu, X.; Jin, H. The Reaction Mechanism of Acetaldehyde Ammoximation to Its Oxime in the TS-1/H2O2 System. Catalysts 2016, 6, 109. [Google Scholar] [CrossRef]
- Kumar, N.; Leino, E.; Mäki-Arvela, P.; Aho, A.; Käldström, M.; Tuominen, M.; Laukkanen, P.; Eränen, K.; Mikkola, J.P.; Salmi, T.; et al. Synthesis and characterization of solid base mesoporous and microporous catalysts: Influence of the support, structure and type of base metal. Microporous Mesoporous Mater. 2012, 152, 71–77. [Google Scholar] [CrossRef]
- Fan, J.; Chen, A.; Chen, S.; Han, X.; Sun, H. Synthesis of Fe,Co,Ni loaded MCM-41 mesoporous molecular sieves and their catalytic oxidation performance. Environ. Chem. 2016, 35, 1116–1124. [Google Scholar]
- Wu, G.; Xiao, J.; Zhang, L.; Wang, W.; Hong, Y.; Huang, H.; Jiang, Y.; Li, L.; Wang, C. Copper-modified TS-1 catalyzed hydroxylation of phenol with hydrogen peroxide as the oxidant. RSC Adv. 2016, 6, 101071–101078. [Google Scholar] [CrossRef]
- Chen, S.; Ciotonea, C.; Ungureanu, A.; Dumitriu, E.; Catrinescu, C.; Wojcieszak, R.; Dumeignil, F.; Royer, S. Preparation of nickel (oxide) nanoparticles confined in the secondary pore network of mesoporous scaffolds using melt infiltration. Catal. Today 2019, 334, 48–58. [Google Scholar] [CrossRef]
- Wu, M.; Chou, L.; Song, H. Effect of metals on titanium silicalite TS-1 for butadiene epoxidation. Chin. J. Catal. 2013, 34, 789–797. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, H.; Yang, J.; Zhao, J.; Song, H.; Chou, L. Precursor effect on the property and catalytic behavior of Fe-TS-1 in butadiene epoxidation. Russ. J. Phys. Chem. A 2017, 91, 2103–2109. [Google Scholar] [CrossRef]
- Capel-Sanchez, M. Impregnation treatments of TS-1 catalysts and their relevance in alkene epoxidation with hydrogen peroxide. Appl. Catal. A Gen. 2003, 246, 69–77. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Y.; Li, J.; Jiang, C. Oxidative degradation of nitrobenzene catalyzed by Cu2+-H2O2 system in copper rinse water. Chin. J. Environ. Eng. 2016, 10, 4775–4782. [Google Scholar]
- Tang, Q.; Zhang, Q.; Wu, H.; Wang, Y. Epoxidation of styrene with molecular oxygen catalyzed by cobalt(II)-containing molecular sieves. J. Catal. 2005, 230, 384–397. [Google Scholar] [CrossRef]
- Vayssilov, G.N. Structural and Physicochemical Features of Titanium Silicalites. Catal. Rev. 1997, 39, 209–251. [Google Scholar] [CrossRef]
- Wu, M.; Chou, L.; Song, H. Epoxidation of Butadiene Over Nickel Modified TS-1 Catalyst. Catal. Lett. 2012, 142, 627–636. [Google Scholar] [CrossRef]
- Bordiga, S.; Damin, A.; Bonino, F.; Lamberti, C. Single Site Catalyst for Partial Oxidation Reaction: TS-1 Case Study. Surf. Interfacial Organomet. Chem. Catal. 2005, 16, 37–68. [Google Scholar]
- Jorda, E.; Tuel, A.; Teissier, R.; Kervennal, J. TiF4: An original and very interesting precursor to the synthesis of titanium containing silicalite-1. Zeolites 1997, 19, 238–245. [Google Scholar] [CrossRef]
- Balducci, L.; Bianchi, D.; Bortolo, R.; D’Aloisio, R.; Ricci, M.; Tassinari, R.; Ungarelli, R. Direct oxidation of benzene to phenol with hydrogen peroxide over a modified titanium silicalite. Angew. Chem. 2003, 42, 4937–4940. [Google Scholar] [CrossRef]
- Singh, H.; Rai, A.; Yadav, R.; Sinha, A.K. Glucose hydrogenation to sorbitol over unsupported mesoporous Ni/NiO catalyst. Mol. Catal. 2018, 451, 186–191. [Google Scholar] [CrossRef]
- Li, J.; Qian, X.; Peng, Y.; Lin, J. Hierarchical structure NiO/CdS for highly performance H2 evolution. Mater. Lett. 2018, 224, 82–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, X.X.; Feng, M.; Luo, Z.H.; Lu, H.; Cao, G.P. In Situ Synthesis of TS-1 on Carbon Nanotube Decorated Nickel Foam with Ultrafine Nanoparticles and High Content of Skeleton Titanium. Ind. Eng. Chem. Res. 2018, 58, 69–78. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Alam, M.A.; Tauer, K.; Hossan, M.S.; Sharafat, M.K.; Rahman, M.M.; Minami, H.; Ahmad, H. Core-shell structured epoxide functional NiO/SiO2 nanocomposite particles and photocatalytic decolorization of congo red aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 783–792. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Channei, D. Photocatalytic activity of the binary composite CeO2/SiO2 for degradation of dye. Appl. Surf. Sci. 2016, 387, 214–220. [Google Scholar] [CrossRef]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A. Comparison of Different Methods for the Point of Zero Charge Determination of NiO. Ind. Eng. Chem. Res. 2011, 50, 10017–10023. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Lamberti, C.; Ricchiardi, G.; Scarano, D.; Petrini, G.; Leofanti, G.; Mantegazza, M. Structural characterization of Ti centres in Ti-silicalite and reaction mechanisms in cyclohexanone ammoximation. Catal. Today 1996, 32, 97–106. [Google Scholar] [CrossRef]
- Chu, Q.; He, G.; Xi, Y.; Wang, P.; Yu, H.; Liu, R.; Zhu, H. Green synthesis of low-carbon chain nitroalkanes via a novel tandem reaction of ketones catalyzed by TS-1. Catal. Commun. 2018, 108, 46–50. [Google Scholar] [CrossRef]



| Catalyst | Conversion (%) | Selectivity (%) |
|---|---|---|
| TS-1 a | 98.9 | 92.8 |
| Fe-TS-1 a | 96.5 | 95.6 |
| Co-TS-1 b | 90.7 | 91.3 |
| Ni-TS-1 a | 99 | 99.3 |
| Cu-TS-1 b | 74.1 | 0 |
| Ce-TS-1 a | 97.3 | 90.6 |
| Catalyst | Conversion (%) | Selectivity (%) |
|---|---|---|
| 1 wt % Ni-TS-1 | 99.0 | 92.4 |
| 2 wt % Ni-TS-1 | 98.8 | 93.9 |
| 3 wt % Ni-TS-1 | 99.0 | 99.3 |
| 4 wt % Ni-TS-1 | 99.1 | 96.7 |
| 5 wt % Ni-TS-1 | 99.1 | 91.4 |
| The Number of Uses | Weight Loss Rate (%) |
|---|---|
| 1 | 5.10661 |
| 2 | 8.21042 |
| 3 | 8.90195 |
| 4 | 9.68479 |
| 5 | 10.20894 |
| Catalyst | w (Ni)(%) | e (Ni)(%) | BET Surface area/m2·g−1 | Micropore Volume/cm3·g−1 |
|---|---|---|---|---|
| Actual | ||||
| TS-1 | - | - | 394.7 | 0.0722 |
| 1 wt % Ni-TS-1 | 0.73 | 73.0 | 388.0 | 0.0719 |
| 2 wt % Ni-TS-1 | 1.39 | 69.5 | 387.9 | 0.0714 |
| 3 wt % Ni-TS-1 | 2.07 | 69.0 | 384.9 | 0.0709 |
| 4 wt % Ni-TS-1 | 2.75 | 68.8 | 377.9 | 0.0708 |
| 5 wt % Ni-TS-1 | 3.43 | 68.6 | 363.3 | 0.0699 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Wang, H.; Wang, W.; Peng, S.; Yang, X.; Xu, X.; Jia, S. Nickel-Modified TS-1 Catalyzed the Ammoximation of Methyl Ethyl Ketone. Catalysts 2019, 9, 1027. https://doi.org/10.3390/catal9121027
Yang D, Wang H, Wang W, Peng S, Yang X, Xu X, Jia S. Nickel-Modified TS-1 Catalyzed the Ammoximation of Methyl Ethyl Ketone. Catalysts. 2019; 9(12):1027. https://doi.org/10.3390/catal9121027
Chicago/Turabian StyleYang, Dandan, Haiyan Wang, Wenhua Wang, Sihua Peng, Xiuzhen Yang, Xingliang Xu, and Shouhua Jia. 2019. "Nickel-Modified TS-1 Catalyzed the Ammoximation of Methyl Ethyl Ketone" Catalysts 9, no. 12: 1027. https://doi.org/10.3390/catal9121027
APA StyleYang, D., Wang, H., Wang, W., Peng, S., Yang, X., Xu, X., & Jia, S. (2019). Nickel-Modified TS-1 Catalyzed the Ammoximation of Methyl Ethyl Ketone. Catalysts, 9(12), 1027. https://doi.org/10.3390/catal9121027