Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural
Abstract
1. Introduction
2. Results and Discussion
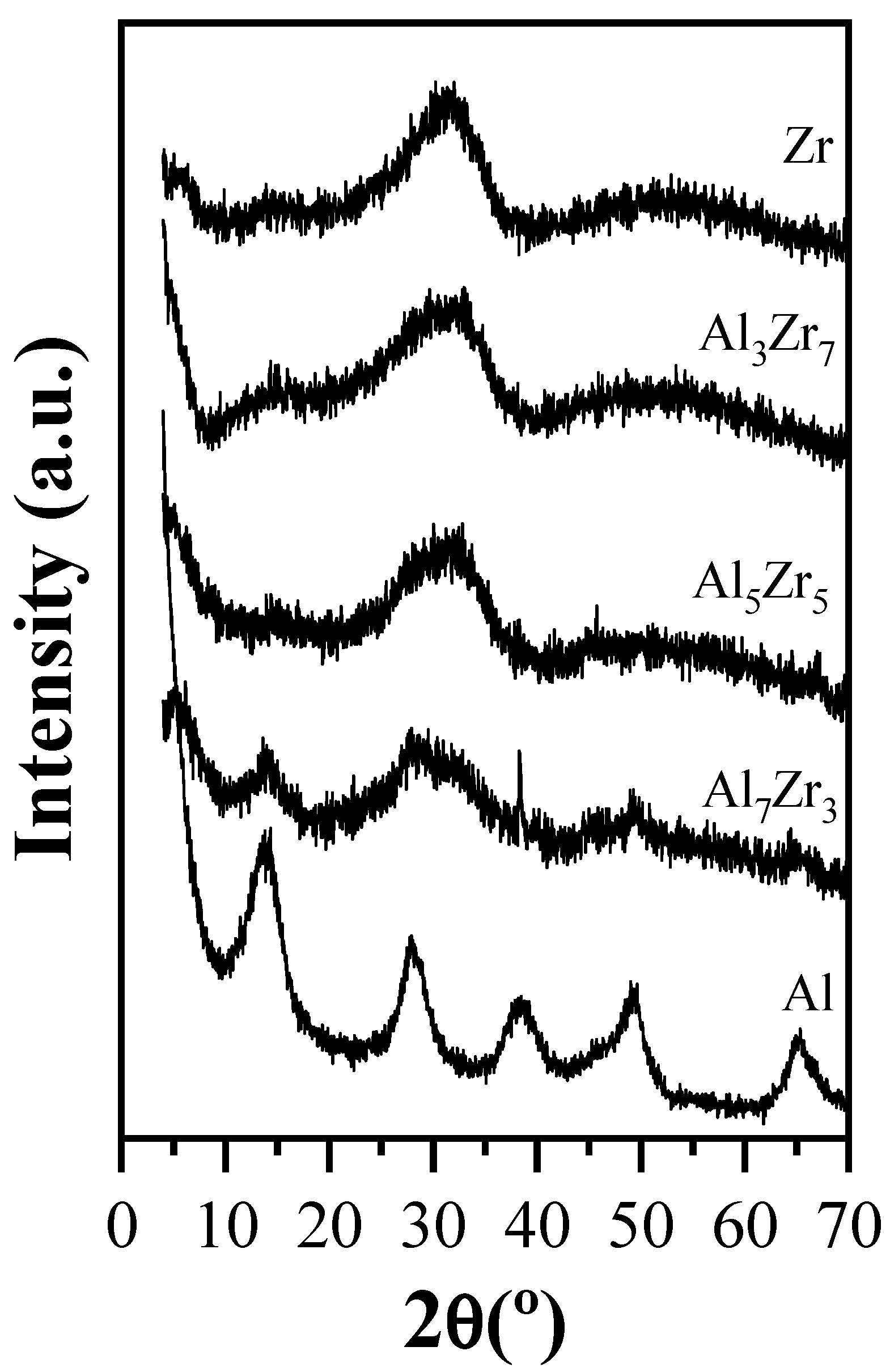
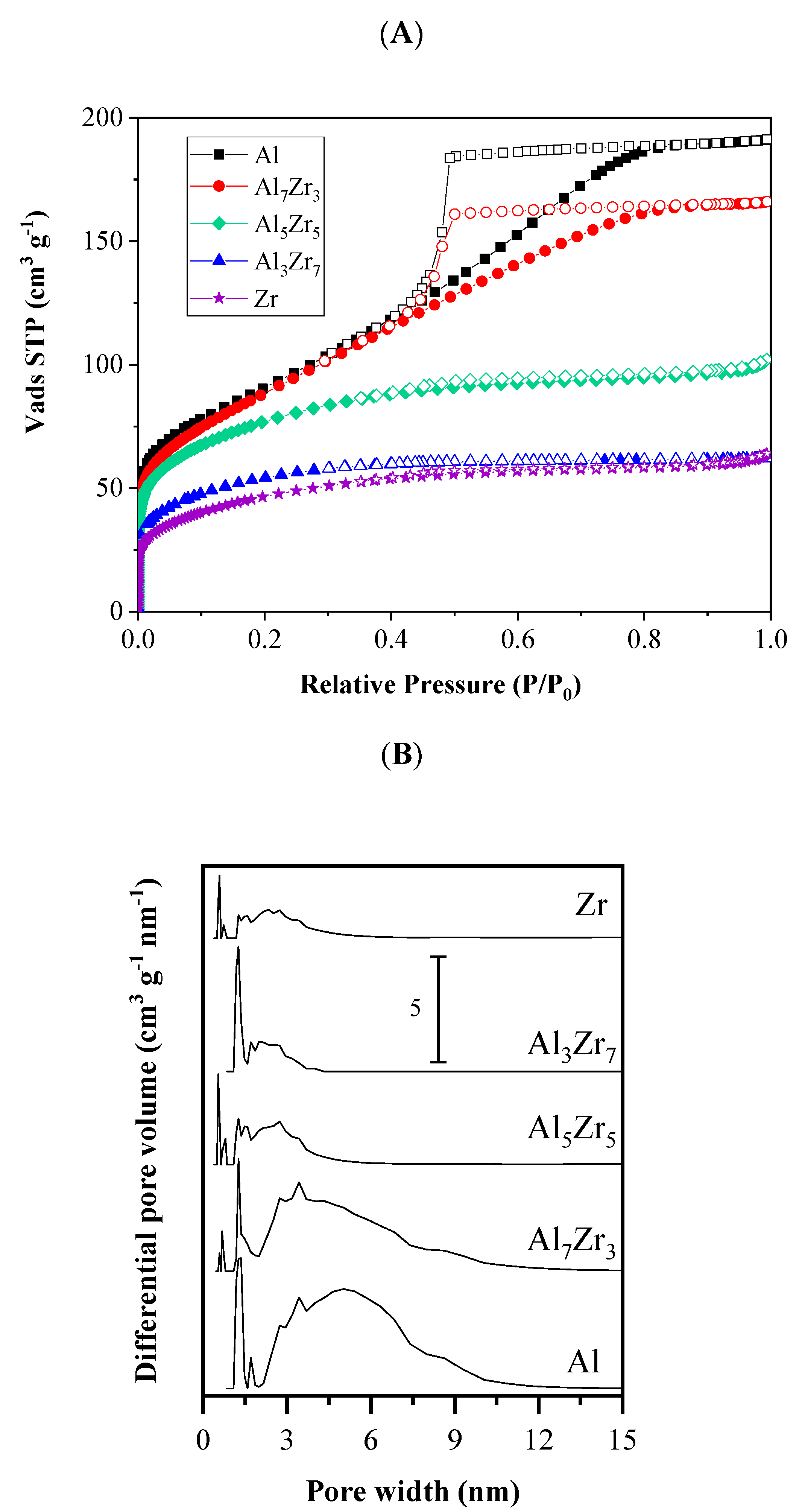
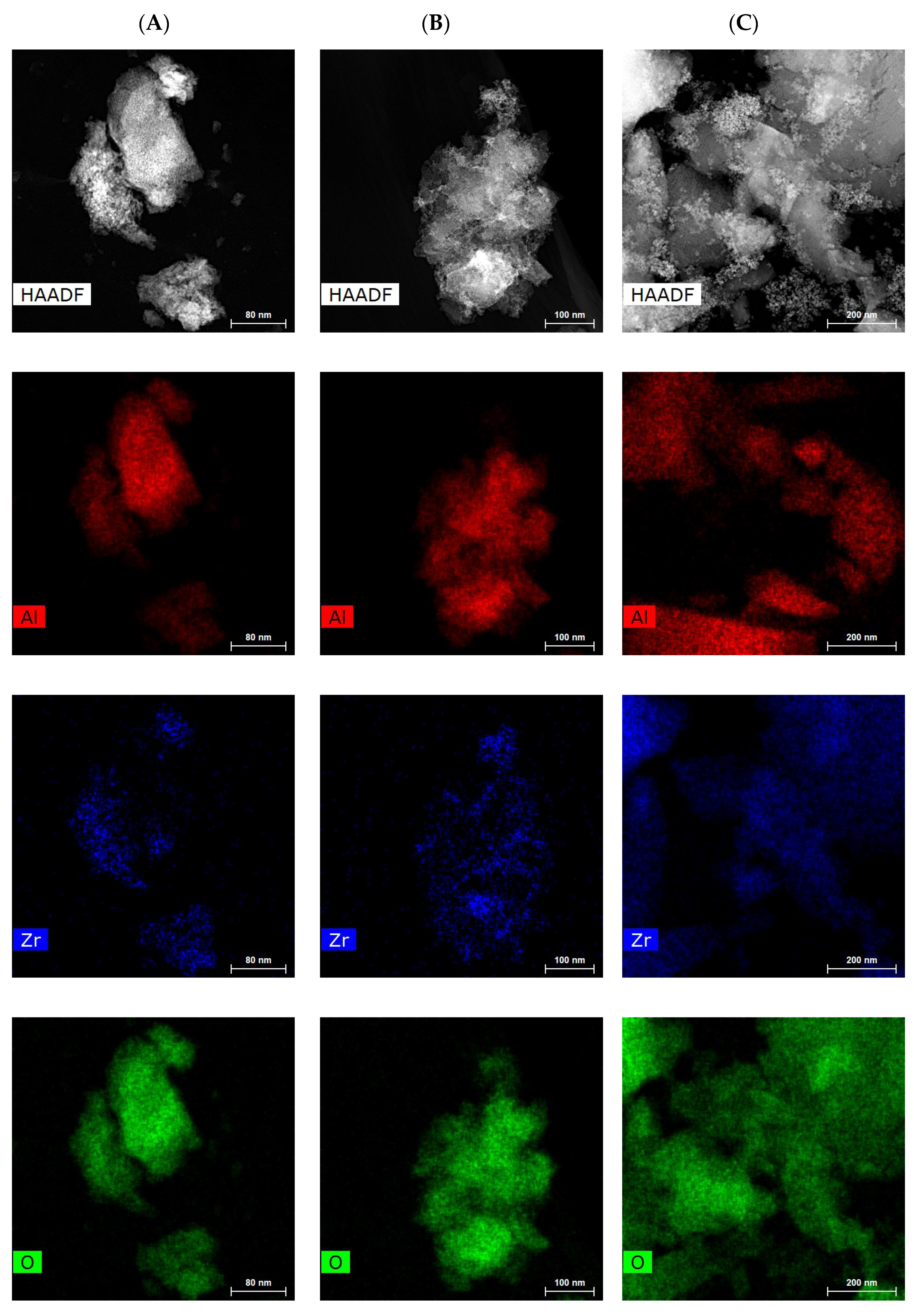
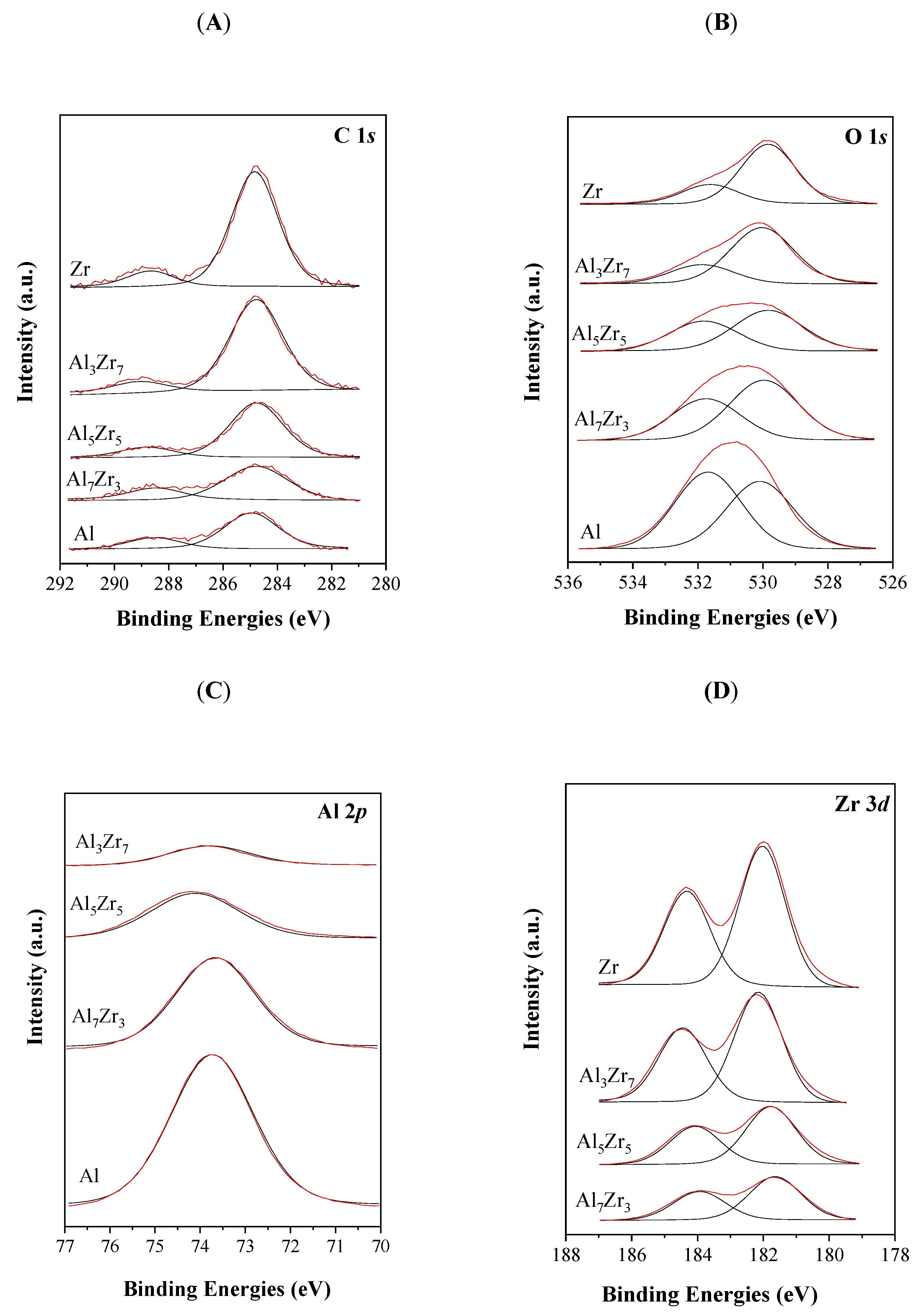
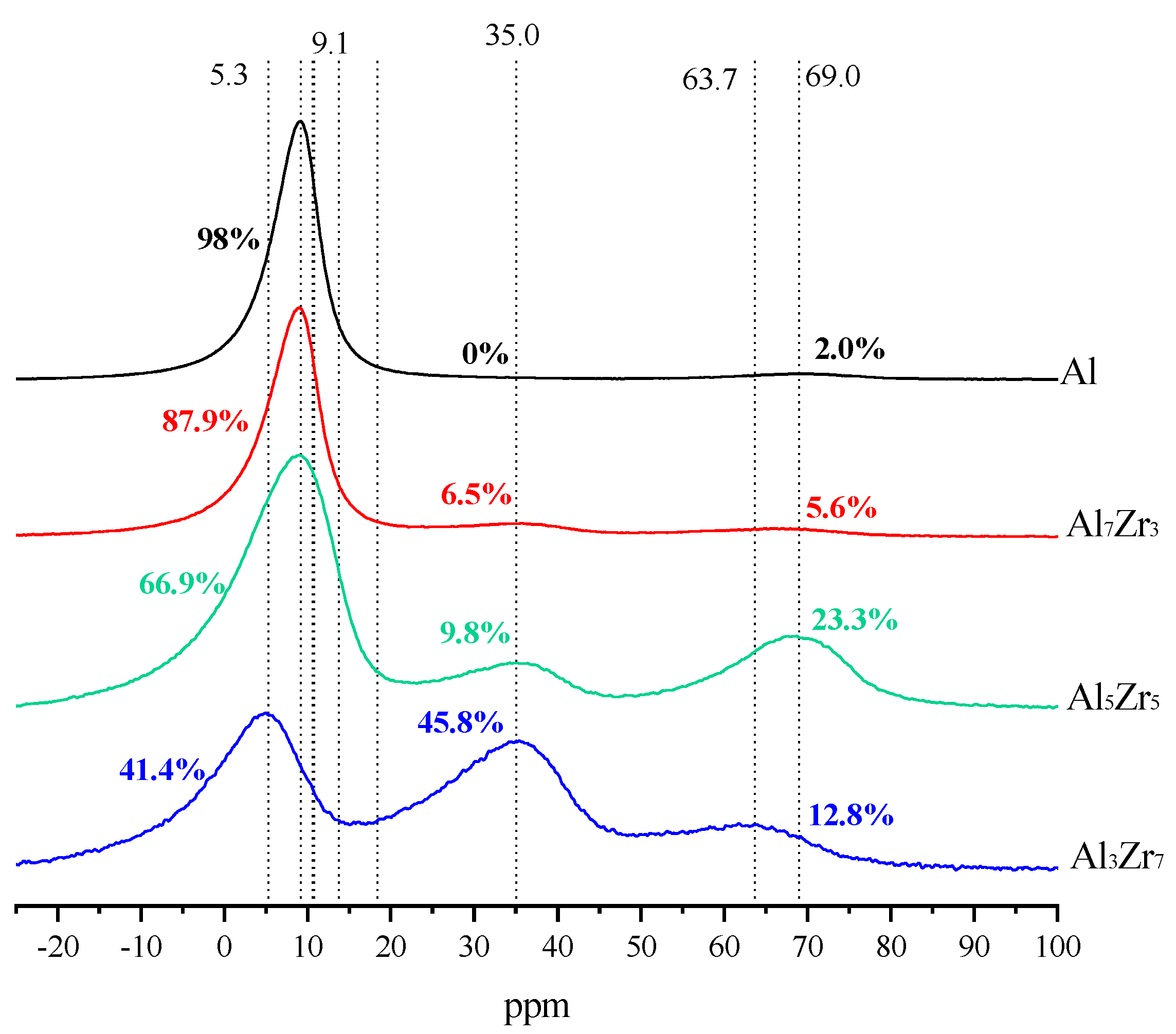
2.1. Catalyst Characterization
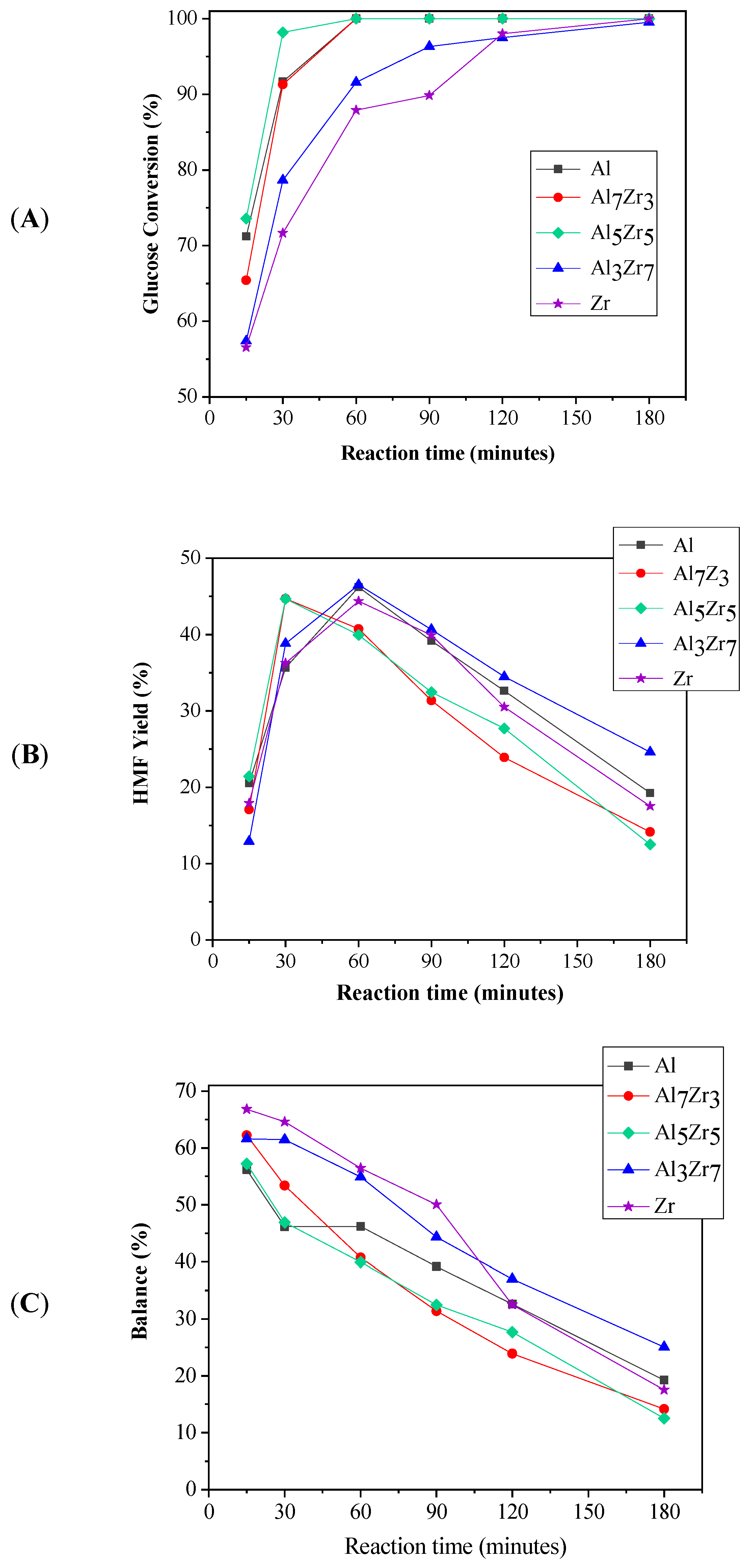
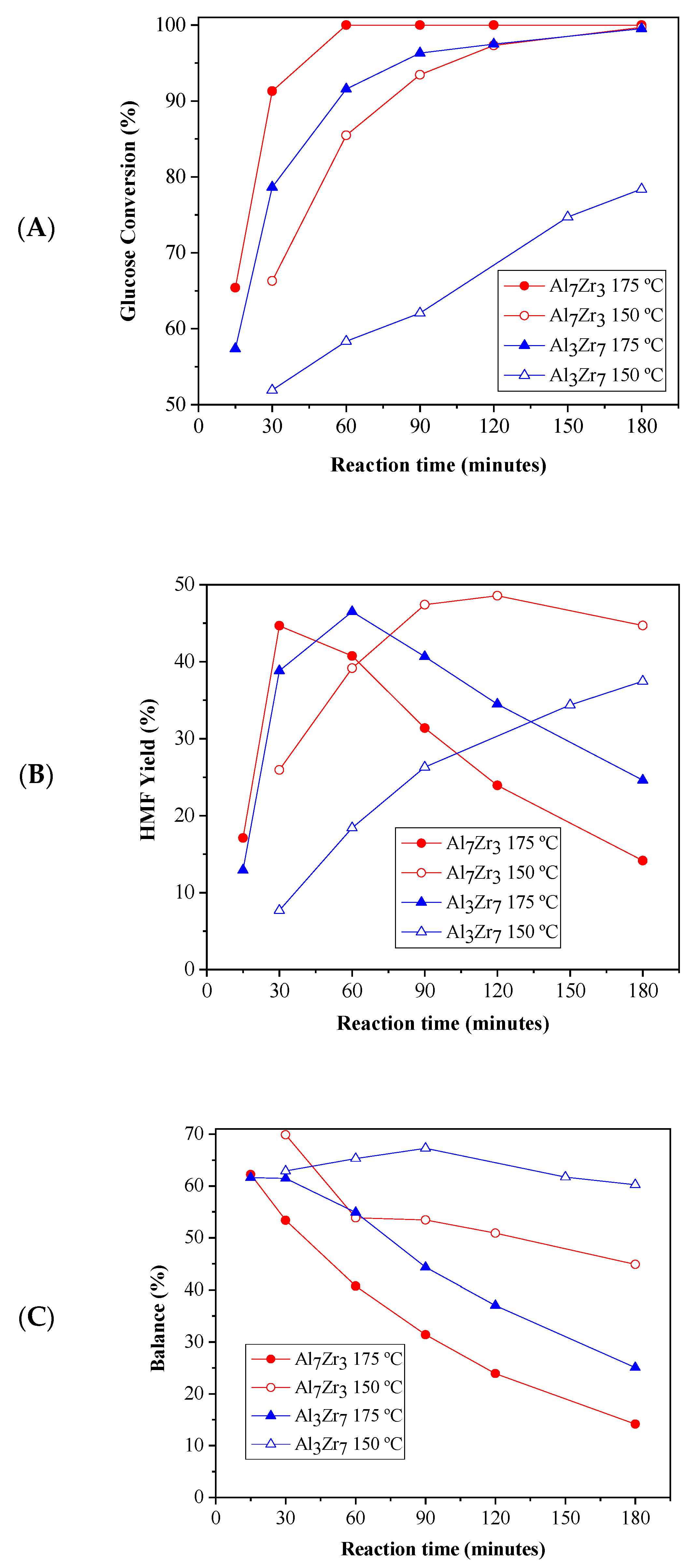
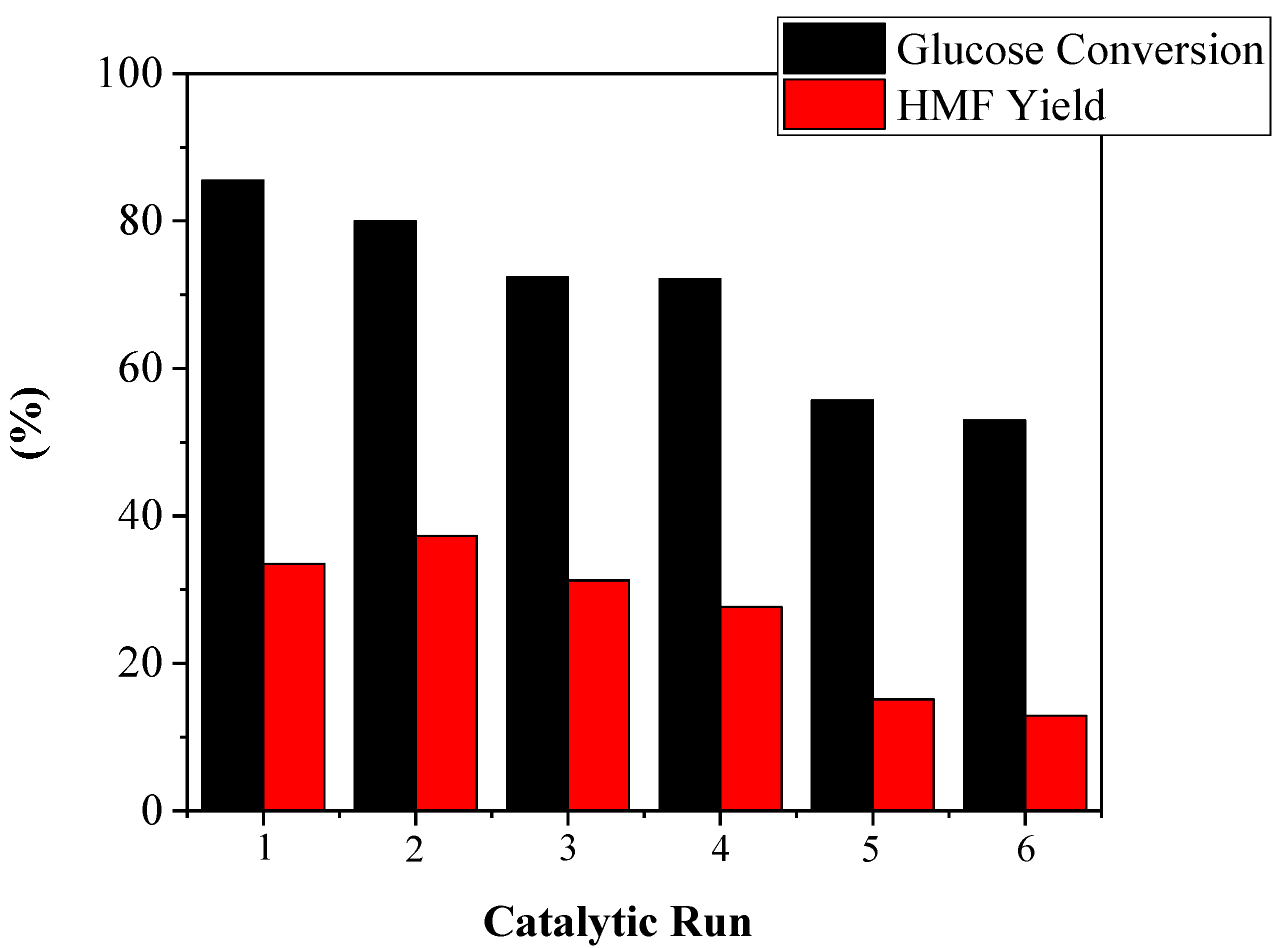
2.2. Catalytic Results
3. Materials and Methods
3.1. Reagents
3.2. Catalyst Synthesis
3.3. Characterization of Catalysts
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining Oxygen in the Global Ocean and Coastal Waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Emissions Gap Report 2019; UNEP: Nairobi, Kenya, 2019. [Google Scholar]
- IPCC. Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Pirani, P.R.S.A., Moufouma-Okia, W., Péan, C., Pidcock, R., Connors, S., Eds.; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. In Lignocellulosic Biomass to Liquid Biofuels; Academic Press: Cambridge, MA, USA, 2020; pp. 1–15. [Google Scholar]
- McGlone, J.; Priecel, P.; da Vià, L.; Majdal, L.; Lopez-Sanchez, J. Desilicated ZSM-5 Zeolites for the Production of Renewable p-Xylene via Diels–Alder Cycloaddition of Dimethylfuran and Ethylene. Catalysts 2018, 8, 253. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective Transformation of 5-Hydroxymethylfurfural into the Liquid Fuel 2,5-Dimethylfuran over Carbon-Supported Ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Bohre, A.; Dutta, S.; Saha, B.; Abu-Omar, M.M. Upgrading Furfurals to Drop-in Biofuels: An Overview. ACS Sustain. Chem. Eng. 2015, 3, 1263–1277. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Zhang, J.; Weitz, E. An in Situ NMR Study of the Mechanism for the Catalytic Conversion of Fructose to 5-Hydroxymethylfurfural and Then to Levulinic Acid Using 13c Labeled d-Fructose. ACS Catal. 2012, 2, 1211–1218. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Uozumi, R.; Satsuma, A. Enhanced Production of Hydroxymethylfurfural from Fructose with Solid Acid Catalysts by Simple Water Removal Methods. Catal. Commun. 2009, 10, 1849–1853. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chem. Int. Ed. 2010, 49, 8954–8957. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of Dimethylfuran for Liquid Fuels from Biomass-Derived Carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Q.; Xie, W.; Peng, L.; He, L.; He, Z.; Chowdhury, S.P.; Christensen, R.; Ni, Y. An Eco-Friendly Method to Get a Bio-Based Dicarboxylic Acid Monomer 2,5-Furandicarboxylic Acid and Its Application in the Synthesis of Poly(Hexylene 2,5-Furandicarboxylate) (PHF). Polymers 2019, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and Fructose Decomposition in Subcritical and Supercritical Water: Detailed Reaction Pathway, Mechanisms, and Kinetics. Ind. Eng. Chem. Res. 1999, 38, 2888–2895. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Orella, M.J.; Lin, Z.; Cheng, Z.; Zheng, W.; Nikolakis, V.; Vlachos, D.G. Molecular Structure, Morphology and Growth Mechanisms and Rates of 5-Hydroxymethyl Furfural (HMF) Derived Humins. Green Chem. 2016, 18, 1983–1993. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Yue, M.; Bai, X.; Du, Y. Tantalum Compounds as Heterogeneous Catalysts for Saccharide Dehydration to 5-Hydroxymethylfurfural. Chem. Commun. 2011, 47, 4469–4471. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Souzanchi, S.; Yuan, Z.; Ray, M.B.; Xu, C. Simple and Green Route for Preparation of Tin Phosphate Catalysts by Solid-State Grinding for Dehydration of Glucose to 5-Hydroxymethylfurfural (HMF). RSC Adv. 2017, 7, 48501–48511. [Google Scholar] [CrossRef]
- He, J.; Liu, M.; Huang, K.; Walker, T.W.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W. Production of Levoglucosenone and 5-Hydroxymethylfurfural from Cellulose in Polar Aprotic Solvent-Water Mixtures. Green Chem. 2017, 19, 3642–3653. [Google Scholar] [CrossRef]
- García-Sancho, C.; Fúnez-Núñez, I.; Moreno-Tost, R.; Santamaría-González, J.; Pérez-Inestrosa, E.; Fierro, J.L.G.; Maireles-Torres, P. Beneficial Effects of Calcium Chloride on Glucose Dehydration to 5-Hydroxymethylfurfural in the Presence of Alumina as Catalyst. Appl. Catal. Environ. 2017, 206, 617–625. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Yoshitaka, K.; Tatsuya, T.; Yukitaka, K. Chemical Conversion and Liquid–Liquid Extractionof 5-Hydroxymethylfurfural from Fructoseby Slug Flow Microreactor. AIcHE J. 2016, 62, 2135–2143. [Google Scholar] [CrossRef]
- Kruger, J.S.; Choudhary, V.; Nikolakis, V.; Vlachos, D.G. Elucidating the Roles of Zeolite H-BEA in Aqueous-Phase Fructose Dehydration and HMF Rehydration. ACS Catal. 2013, 3, 1279–1291. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Production of 5-Hydroxymethylfurfural from Glucose Using Aluminium Doped MCM-41 Silica as Acid Catalyst. Appl. Catal. Environ. 2015, 164, 70–76. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Pallagi, A.; Dudás, C.; Csendes, Z.; Forgó, P.; Pálinkó, I.; Sipos, P. Structure and Equilibria of Ca2+-Complexes of Glucose and Sorbitol from Multinuclear (1H, 13C and 43Ca) NMR Measurements Supplemented with Molecular Modelling Calculations. J. Mol. Struct. 2011, 993, 336–340. [Google Scholar] [CrossRef]
- Blanshard, J.M.V.; Mitchell, J.R. Polysaccharides in Food; Elsvier: Amsterdam, The Netherlands, 2013; Volume 2. [Google Scholar]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)Furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Saravanamurugan, S.; Paniagua, M.; Melero, J.A.; Riisager, A. Efficient Isomerization of Glucose to Fructose over Zeolites in Consecutive Reactions in Alcohol and Aqueous Media. J. Am. Chem. Soc. 2013, 135, 5246–5249. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Teckchandani-Ortiz, A.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Selective Dehydration of Glucose to 5-Hydroxymethylfurfural on Acidic Mesoporous Tantalum Phosphate. Appl. Catal. Environ. 2014, 144, 22–28. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Roux, A.; Tichit, D. Isomerization of Glucose into Fructose in the Presence of Cation-Exchanged Zeolites and Hydrotalcites. Appl. Catal. Gen. 2000, 193, 257–264. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Activity and Stability of Hydrophobic Mg-Al Hydrotalcites in the Continuous Aqueous-Phase Isomerization of Glucose into Fructose. Catal. Sci. Tech. 2014, 4, 4322–4329. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Structure-Performance Correlations of Mg-Al Hydrotalcite Catalysts for the Isomerization of Glucose into Fructose. J. Catal. 2015, 327, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Carraher, J.M.; Swedberg, J.L.; Herndon, C.R.; Fleitman, C.N.; Tessonnier, J.P. Selective Base-Catalyzed Isomerization of Glucose to Fructose. ACS Catal. 2014, 4, 4295–4298. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, B.; Li, N.; Zhong, H. Isomerization of Glucose into Fructose with Homogenous Amine-Type Base Catalysts: Amine Structure, Chain Length, and Kinetics. Bioresour. Bioprocess. 2019, 6, 35. [Google Scholar] [CrossRef]
- Yue, C.; Rigutto, M.S.; Hensen, E.J.M. Glucose Dehydration to 5-Hydroxymethylfurfural by a Combination of a Basic Zirconosilicate and a Solid Acid. Catal. Lett. 2014, 144, 2121–2128. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)Furfural from Carbohydrates Using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Degirmenci, V.; Pidko, E.A.; Magusin, P.C.M.M.; Hensen, E.J.M. Towards a Selective Heterogeneous Catalyst for Glucose Dehydration to 5-Hydroxymethylfurfural in Water: CrCl2 Catalysis in a Thin Immobilized Ionic Liquid Layer. Chem. Cat. Chem. 2011, 3, 969–972. [Google Scholar] [CrossRef]
- Hayashi, S.; Hara, M.; Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5.NH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar]
- Watanabe, M.; Aizawa, Y.; Iida, T.; Aida, T.M.; Levy, C.; Sue, K.; Inomata, H. Glucose Reactions with Acid and Base Catalysts in Hot Compressed Water at 473 K. Carbohydr. Res. 2005, 340, 1925–1930. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Sulfated Zirconia as a Solid Acid Catalyst for the Dehydration of Fructose to 5-Hydroxymethylfurfural. Catal. Commun. 2009, 10, 1771–1775. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Aluminum Oxide in Ionic Liquid. Appl. Catal. Environ. 2019, 253, 1–10. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and Upgrading of 5-Hydroxymethylfurfural Using Heterogeneous Catalysts and Biomass-Derived Solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Xu, H.; Gaborieau, M.; Huang, J.; Jiang, Y. Glucose Conversion to 5-Hydroxymethylfurfural on Zirconia: Tuning Surface Sites by Calcination Temperatures. Catal. Today 2020, 351, 133–140. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glucose Dehydration to 5-Hydroxymethylfurfural on Zirconium Containing Mesoporous MCM-41 Silica Catalysts. Fuel 2014, 118, 265–271. [Google Scholar] [CrossRef]
- Atanda, L.; Silahua, A.; Mukundan, S.; Shrotri, A.; Torres-Torres, G.; Beltramini, J. Catalytic Behaviour of TiO2-ZrO2 Binary Oxide Synthesized by Sol-Gel Process for Glucose Conversion to 5-Hydroxymethylfurfural. RSC Adv. 2015, 5, 80346–80352. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Semeykina, V.S.; Danilova, I.G.; Pestunov, A.V.; Parkhomchuk, E.V.; Parmon, V.N. Solid Acidic NbOx/ZrO2 Catalysts for Transformation of Cellulose to Glucose and 5-Hydroxymethylfurfural in Pure Hot Water. Catal. Lett. 2017, 147, 1485–1495. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, X.; Yan, Y.; Xue, S.; Zhang, Y. Efficient and Selective Conversion of Hexose to 5-Hydroxymethylfurfural with Tin-Zirconium-Containing Heterogeneous Catalysts. Catal. Commun. 2014, 50, 38–43. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Liu, J.; Liu, X.; Chang, F.; Liu, Y.; Xue, W.; Yang, S. Selective Transformation of Carbohydrates into HMF Promoted by Carboxylic Acids Modified ZrMo Mixed Oxides. Biomass Convers. Biorefinery 2014, 4, 59–66. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Tao, S. Mesoporous ZrO2 Nanopowder Catalysts for the Synthesis of 5-Hydroxymethylfurfural. ACS Appl. Nano Mater. 2019, 2, 5125–5131. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Q.; Chen, Y.; Liu, M.; Jin, P.; Yan, Y.; Pan, J. Synthesis of Ceria and Sulfated Zirconia Catalysts Supported on Mesoporous SBA-15 toward Glucose Conversion to 5-Hydroxymethylfurfural in a Green Isopropanol-Mediated System. Ind. Eng. Chem. Res. 2018, 57, 1968–1979. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Lee, A.F.; Granollers, M.; Brown, D.R.; Olivi, L.; Morales, G.; Melero, J.A.; Wilson, K. Hydrothermally Stable, Conformal, Sulfated Zirconia Monolayer Catalysts for Glucose Conversion to 5-HMF. ACS Catal. 2015, 5, 4345–4352. [Google Scholar] [CrossRef]
- O’Dell, L.A.; Savin, S.L.P.; Chadwick, A.V.; Smith, M.E. A 27Al MAS NMR Study of a Sol-Gel Produced Alumina: Identification of the NMR Parameters of the θ-Al2O3 Transition Alumina Phase. Solid State Nucl. Magn. Reson. 2007, 31, 169–173. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- López-Asensio, R.; Cecilia, J.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Selective Production of Furfuryl Alcohol from Furfural by Catalytic Transfer Hydrogenation over Commercial Aluminas. Appl. Catal. Gen. 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. Systematic XPS Study of Gallium-Substituted Boehmite. J. Mater. Sci. 2016, 51, 5436–5444. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS Study of the Major Minerals in Bauxite: Gibbsite, Bayerite and (Pseudo-)Boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Damyanova, S.; Grange, P.; Delmon, B. Surface Characterization of Zirconia-Coated Alumina and Silica Carriers. J. Catal. 1997, 168, 421–430. [Google Scholar] [CrossRef]
- Guerrero-Urbaneja, P.; García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glycerol Valorization by Etherification to Polyglycerols by Using Metal Oxides Derived from MgFe Hydrotalcites. Appl. Catal. Gen. 2014, 470, 199–207. [Google Scholar] [CrossRef]
- Reddy, B.M.; Sreekanth, P.M.; Yamada, Y.; Kobayashi, T. Surface Characterization and Catalytic Activity of Sulfate-, Molybdate- and Tungstate-Promoted Al2O3-ZrO2 Solid Acid Catalysts. J. Mol. Catal. Chem. 2005, 227, 81–89. [Google Scholar] [CrossRef]
- Smith, M.E. Application of 27A1 NMR Techniques to Structure Determination in Solids. Appl. Magn. Reson 1993, 4, 1–64. [Google Scholar] [CrossRef]
- Hill, M.R.; Bastow, T.J.; Celotto, S.; Hill, A.J. Integrated Study of the Calcination Cycle from Gibbsite to Corundum. Chem. Mater. 2007, 19, 2877–2883. [Google Scholar] [CrossRef]
- Chagas, L.H.; de Carvalho, G.S.G.; San Gil, R.A.S.; Chiaro, S.S.X.; Leitão, A.A.; Diniz, R. Obtaining Aluminas from the Thermal Decomposition of Their Different Precursors: An 27Al MAS NMR and X-Ray Powder Diffraction Studies. Mater. Res. Bull. 2014, 49, 216–222. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.Z.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Penta-Coordinated Al3+ Ions as Preferential Nucleation Sites for BaO on γ-Al2O3: An Ultra-High-Magnetic Field 27Al MAS NMR Study. J. Catal. 2007, 251, 189–194. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Bieliński, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevsky, A.; Witońska, I.A. Products of Sugar Beet Processing as Raw Materials for Chemicals and Biodegradable Polymers. RSC Adv. 2018, 8, 3161–3177. [Google Scholar] [CrossRef]
- Ranoux, A.; Djanashvili, K.; Arends, I.W.C.E.; Hanefeld, U. 5-Hydroxymethylfurfural Synthesis from Hexoses Is Autocatalytic. ACS Catal. 2013, 3, 760–763. [Google Scholar] [CrossRef]
- Portillo Perez, G.; Mukherjee, A.; Dumont, M.J. Insights into HMF Catalysis. J. Ind. Eng. Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Yan, L.; Ma, R.; Wei, H.; Li, L.; Zou, B.; Xu, Y. Ruthenium Trichloride Catalyzed Conversion of Cellulose into 5-Hydroxymethylfurfural in Biphasic System. Bioresour. Tech. 2019, 279, 84–91. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef]
- Combs, E.; Cinlar, B.; Pagan-Torres, Y.; Dumesic, J.A.; Shanks, B.H. Influence of Alkali and Alkaline Earth Metal Salts on Glucose Conversion to 5-Hydroxymethylfurfural in an Aqueous System. Catal. Commun. 2013, 30, 1–4. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Pavlović, V.; Rakić, V.; Auroux, A. Tuning the Acidity of Niobia: Characterization and Catalytic Activity of Nb2O5-MeO2 (Me = Ti, Zr, Ce) Mesoporous Mixed Oxides. Mater. Chem. Phys. 2014, 146, 337–345. [Google Scholar] [CrossRef]
- Córdova-Pérez, G.E.; Torres-Torres, G.; Ortíz-Chi, F.; Godavarthi, S.; Silahua-Pavón, A.A.; Izquierdo-Colorado, A.; da Costa, P.; Hernández-Como, N.; Aleman, M.; Espinosa-González, C.G. Effect of Acid-Basic Sites Ratio on the Catalytic Activity to Obtain 5-HMF from Glucose Using Al2O3-TiO2-W Catalysts. Chem. Sel. 2018, 3, 12854–12864. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Yang, H.; Chen, H. Characterization of products from hydrothermal treatments of cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Riisager, A.; Yang, S. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol with Recyclable Al–Zr@Fe Mixed Oxides. Chem. Cat. Chem. 2018, 10, 430–438. [Google Scholar] [CrossRef]



| Catalyst | SBET (m2∙g−1) | Smicro (m2∙g−1) | Vp (cm3·g−1) | Vmicro (cm3∙g−1) | μmol NH3·gcat−1 | μmol NH3·m−2 | μmol CO2 gcat−1 | μmol CO2 m−2 |
|---|---|---|---|---|---|---|---|---|
| Al | 325 | 5 | 0.30 | 0.00 | 279 | 0.86 | 70 | 0.21 |
| Al7Zr3 | 321 | 84 | 0.26 | 0.04 | 188 | 0.59 | 46 | 0.14 |
| Al5Zr5 | 274 | 227 | 0.15 | 0.11 | 270 | 0.98 | 67 | 0.24 |
| Al3Zr7 | 263 | 248 | 0.10 | 0.08 | 185 | 0.70 | 40 | 0.15 |
| Zr | 168 | 135 | 0.09 | 0.06 | 82 | 0.49 | 27 | 0.16 |
| Catalyst | Binding Energy (eV) (Atomic Concentration (%)) | Atomic Ratio by XPS | Nominal Atomic Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C 1s | O 1s | Al 2p | Zr 3d5/2 | Al/Zr | O/(Al + Zr) | Al/Zr | |||
| Al | 284.9 | 288.5 | 530.1 | 531.7 | 73.7 | - | - | 2.03 | - |
| (3.8) | (1.3) | (30.3) | (33.3) | (31.3) | |||||
| Al7Zr3 | 284.7 | 288.4 | 530.0 | 531.7 | 73.7 | 181.6 | 3.78 | 2.04 | 2.33 |
| (5.5) | (1.5) | (37.4) | (24.9) | (24.2) | (6.4) | ||||
| Al5Zr5 | 284.8 | 288.7 | 529.8 | 531.8 | 74.1 | 181.7 | 1.68 | 1.94 | 1.0 |
| (9.3) | (1.8) | (33.5) | (25.2) | (19.0) | (11.3) | ||||
| Al3Zr7 | 284.7 | 289.0 | 530.0 | 531.9 | 73.8 | 182.1 | 0.32 | 2.11 | 0.43 |
| (15.6) | (1.7) | (42.3) | (13.8) | (6.5) | (20.1) | ||||
| Zr | 284.8 | 288.7 | 529.8 | 531.6 | - | 182.0 | - | 2.12 | - |
| (17.8) | (2.5) | (41.6) | (12.6) | (25.5) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Olea, B.; Mérida-Morales, S.; García-Sancho, C.; Cecilia, J.A.; Maireles-Torres, P. Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural. Catalysts 2020, 10, 878. https://doi.org/10.3390/catal10080878
Torres-Olea B, Mérida-Morales S, García-Sancho C, Cecilia JA, Maireles-Torres P. Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural. Catalysts. 2020; 10(8):878. https://doi.org/10.3390/catal10080878
Chicago/Turabian StyleTorres-Olea, Benjamín, Sandra Mérida-Morales, Cristina García-Sancho, Juan Antonio Cecilia, and Pedro Maireles-Torres. 2020. "Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural" Catalysts 10, no. 8: 878. https://doi.org/10.3390/catal10080878
APA StyleTorres-Olea, B., Mérida-Morales, S., García-Sancho, C., Cecilia, J. A., & Maireles-Torres, P. (2020). Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural. Catalysts, 10(8), 878. https://doi.org/10.3390/catal10080878







