Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review
Abstract
1. Introduction
- (1)
- Their higher band gap energy, making the materials suitable for mainly UV light absorption instead of visible light [20];
- (2)
- Only having the ability to catalyze either water oxidation or reduction at a time, leading to their unsuitability to act as dual function overall water splitting catalysts [21];
- (3)
- Their higher charge recombination rates in bulk medium and on the surface, resulting in lower activity [22];
- (4)
- Inaccessibility of active sites lying in bulk material [23].
1.1. Photocatalysis: Theoretical Digest
1.2. Limitations of Photocatalytic Water Splitting
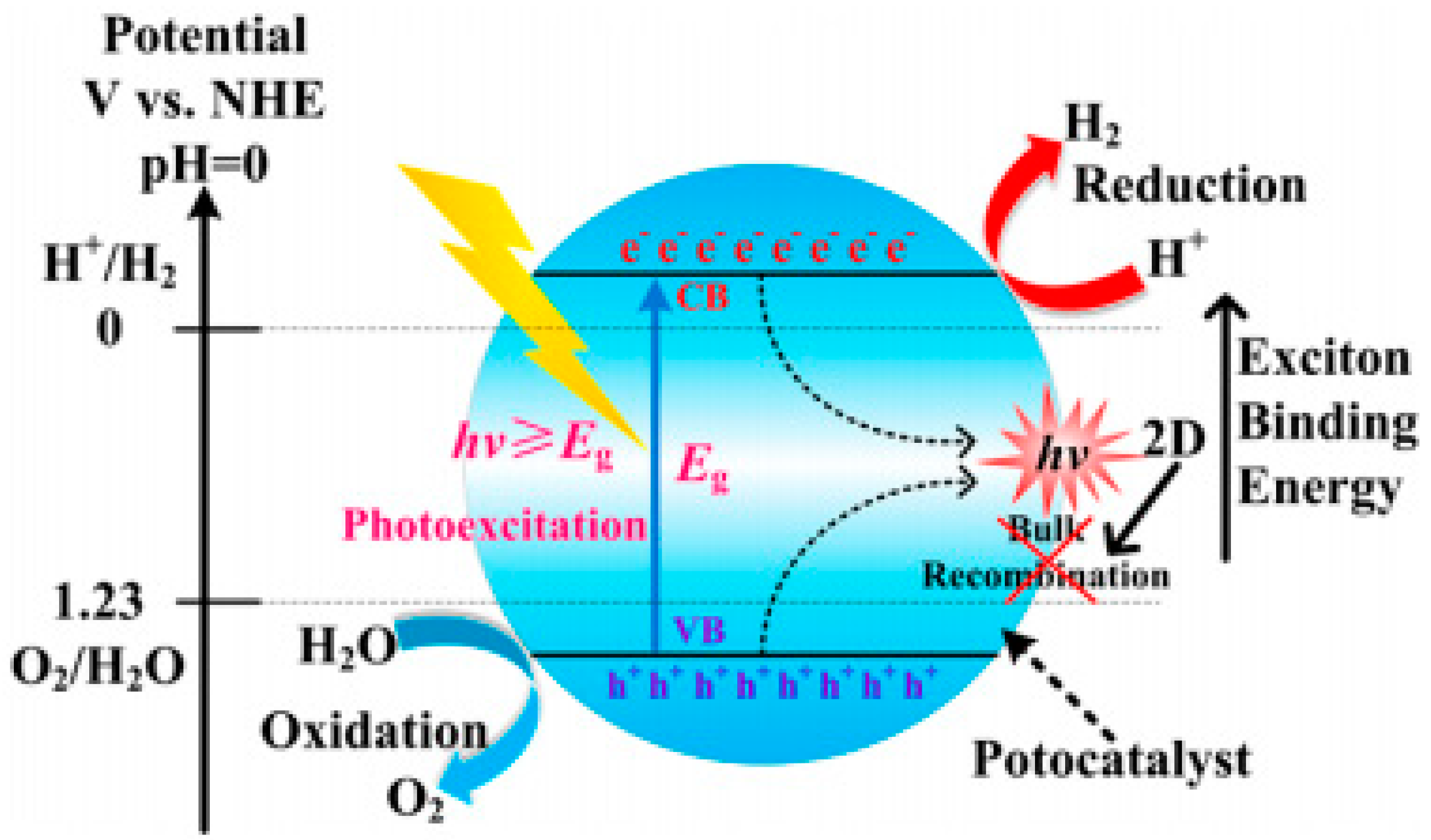
- To catalyze the splitting of water at the interface of electrolyte and electrode, the charge carriers are required to be transferred to catalyst surface once the electron-hole pairs are created. The quick recombination of photo-generated electron-hole pairs (Figure 2) releasing heat or photon energy before they can catalyze the redox reactions is a major challenge in this step, requiring a high degree of crystallinity [32].
- The predilection for semiconductor materials to work under the ultraviolet (UV) light is another major challenge, as only about 4% of solar energy is comprised of UV light. It is advantageous for photocatalysts to work under visible light, which requires the band gap to be in visible range.
- Photo-corrosion and catalyst decay are also among the limitations of the photocatalytic splitting of water. TiO2, ZrO2, KTaO3, SrTiO3 and BiVO4 are among the notable contenders for photocatalytic water splitting because of having band gaps around 1.23 eV. Typical sulfide-based photocatalysts, such as cadmium sulfide (CdS), have a tendency to undergo decay under operating conditions due to oxidation of sulfide into elemental sulfur at the same potentials that are used for water splitting, requiring the use of certain sacrificial reagents, e.g., sodium sulfide, to control any sulfur lost [33].
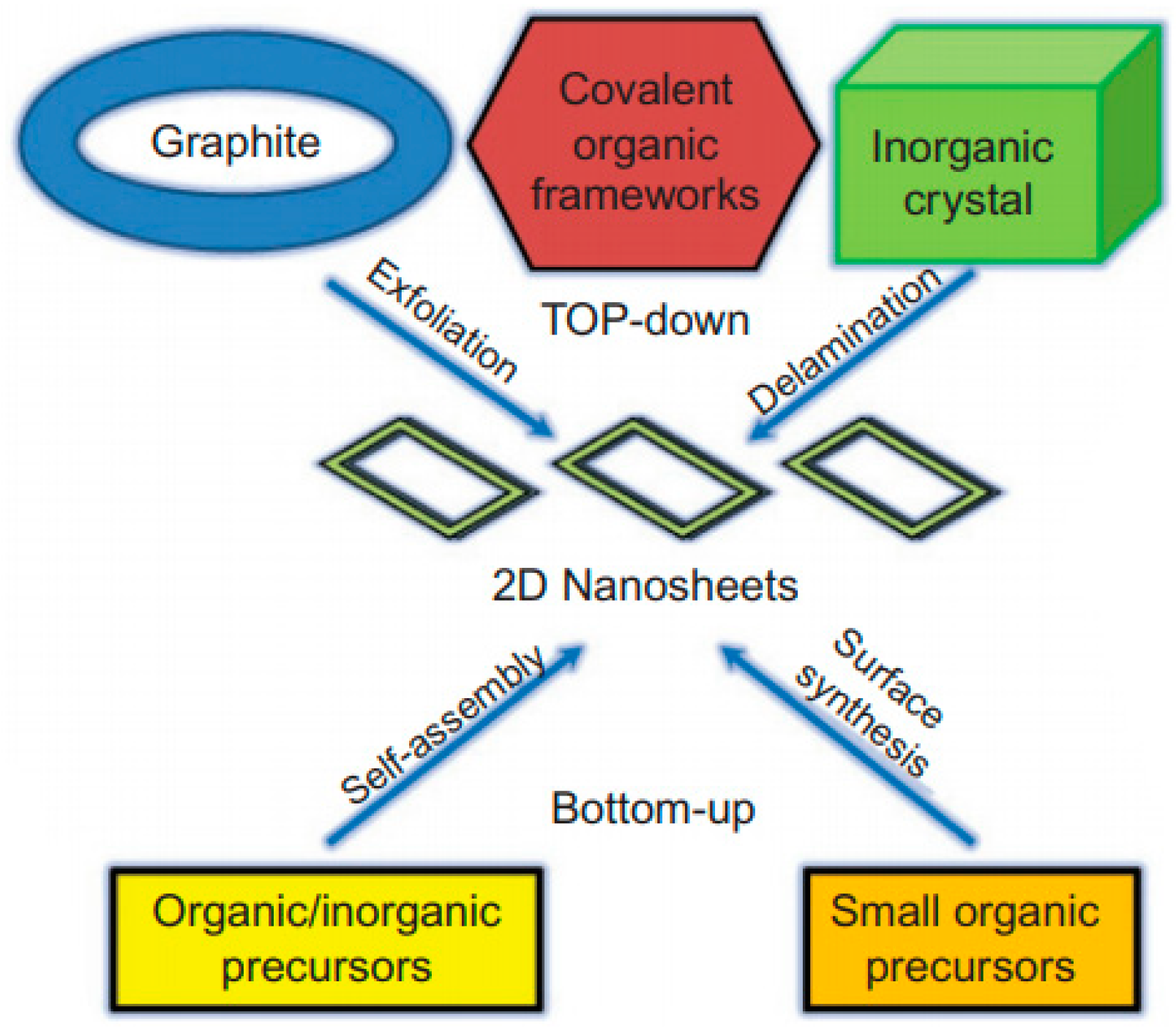
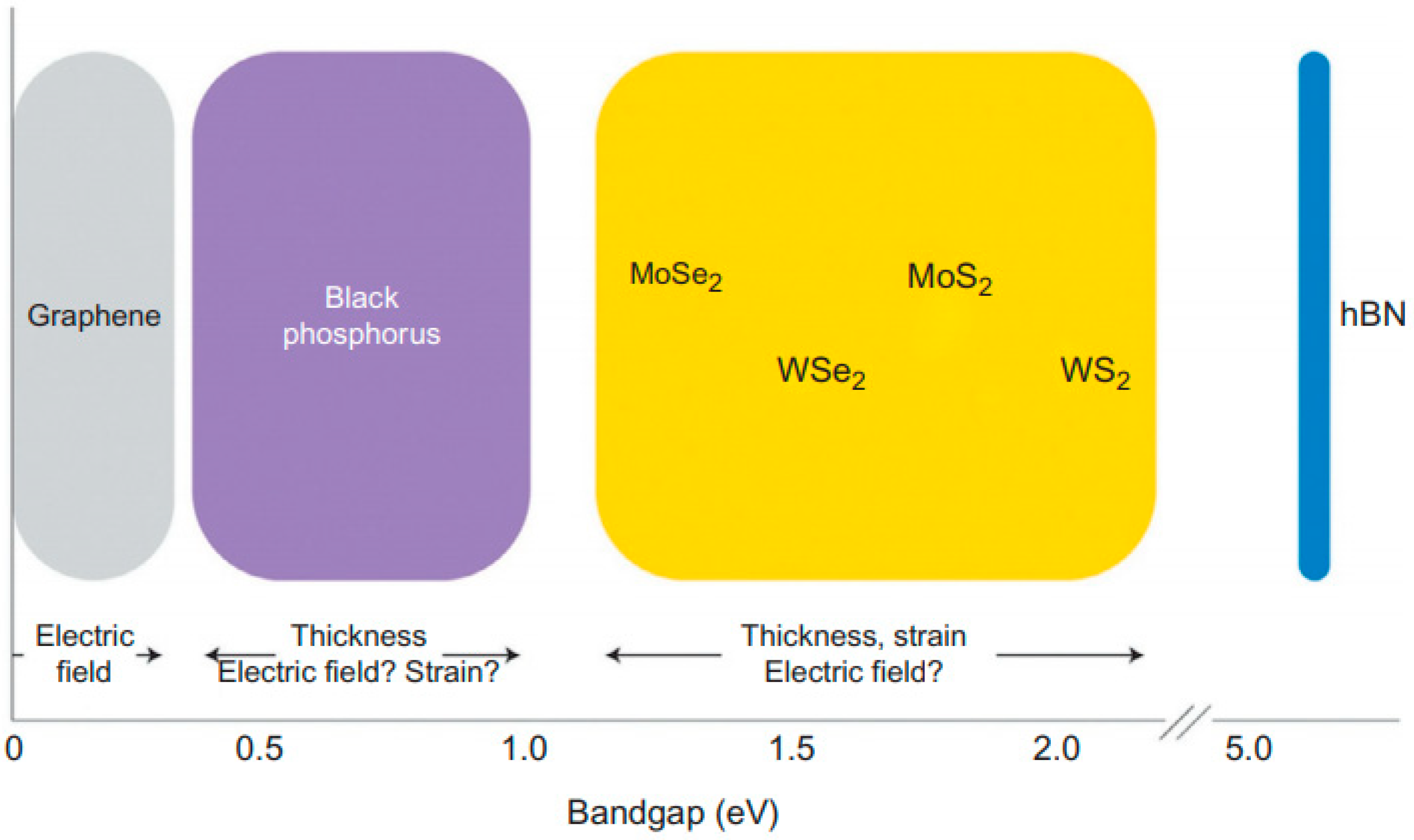
2. Two Dimensional Materials as Photocatalysts for Water Splitting
- (1)
- The adjustable number of layers, so that the band gap and photo-absorption can be tuned [38].
- (2)
- The sheet-like ultra-thin structure facilitating the charge transport towards surface, resulting in lower recombination rate [39].
- (3)
- The enhanced specific surface area leading to greater exposure of active sites on surface and better catalytic performance [40].
2.1. Graphitic Carbon Nitride and Derivatives
2.2. Graphene-Based Photocatalysts for Water Splitting
2.3. Phosphorene
2.4. Metal Phosphides
2.5. Metal Organic Frameworks and Derivatives
3. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhao, Q.; Zhang, G.; Xiong, B. Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Resch, G.; Held, A.; Faber, T.; Panzer, C.; Toro, F.; Haas, R. Potentials and prospects for renewable energies at global scale. Energy Policy 2008, 36, 4048–4056. [Google Scholar] [CrossRef]
- Uyar, T.S.; Beşikci, D. Integration of hydrogen energy systems into renewable energy systems for better design of 100% renewable energy communities. Int. J. Hydrogen Energy 2017, 42, 2453–2456. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Yao, L.; Wei, D.; Ni, Y.; Yan, D.; Hu, C. Surface localization of cdzns quantum dots onto 2D g-C3N4 ultrathin microribbons: Highly efficient visible light-induced H2-generation. Nano Energy 2016, 26, 248–256. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, Y.; Shang, L.; Shi, R.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Facile synthesis of ultrathin snnb2o6 nanosheets towards improved visible-light photocatalytic h2-production activity. Chem. Commun. 2016, 52, 8239–8242. [Google Scholar] [CrossRef]
- Inoue, Y. Photocatalytic water splitting by ruo2-loaded metal oxides and nitrides with d0- and d10 -related electronic configurations. Energy Environ. Sci. 2009, 2, 364. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, N.; Chen, C.; Liu, X.; Ma, R.; Tong, S.; Mei, Z.; Roy, V.A.L.; Wang, H.; Tang, Y. Hierarchical yolk–shell layered potassium niobate for tuned ph-dependent photocatalytic h2 evolution. Catal. Sci. Technol. 2017, 7, 1000–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Pan, Z.; Hisatomi, T.; Wang, Q.; Chen, S.; Nakabayashi, M.; Shibata, N.; Pan, C.; Takata, T.; Katayama, M.; Minegishi, T.; et al. Photocatalyst sheets composed of particulate lamg1/3ta2/3o2n and mo-doped bivo4 for z-scheme water splitting under visible light. ACS Catal. 2016, 6, 7188–7196. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Li, K.; Ao, Z.; Wang, C.; Liu, H.; Sun, K.; Wang, G. Electrospun cobalt embedded porous nitrogen doped carbon nanofibers as an efficient catalyst for water splitting. J. Mater. Chem. A 2016, 4, 12818–12824. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Ida, S.; Ishihara, T. Recent progress in two-dimensional oxide photocatalysts for water splitting. J. Phys. Chem. Lett. 2014, 5, 2533–2542. [Google Scholar] [CrossRef]
- Coronado, J.M. A Historical Introduction to Photocatalysis. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications. In Green Energy and Technology; Springer: London, UK, 2013; pp. 1–4. [Google Scholar]
- Lee, Y.Y.; Jung, H.S.; Kang, Y.T. A review: Effect of nanostructures on photocatalytic co 2 conversion over metal oxides and compound semiconductors. J. CO2 Util. 2017, 20, 163–177. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Suzuki, Y.; Pan, Z.; Seo, J.; Katayama, M.; Minegishi, T.; Nishiyama, H.; Takata, T.; Seki, K.; et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient z-scheme pure-water splitting at ambient pressure. J. Am. Chem. Soc. 2017, 139, 1675–1683. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-L.; Sa, B.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Tewari, V.K.; Zhang, Y. (Eds.) nanostructured two-dimensional materials. In Modeling, Characterization, and Production of Nanomaterials; Woodhead Publishing: Cambridge, UK, 2015; pp. 477–524. [Google Scholar]
- Peng, R.; Ma, Y.; Huang, B.; Dai, Y. Two-dimensional janus ptsse for photocatalytic water splitting under the visible or infrared light. J. Mater. Chem. A 2019, 7, 603–610. [Google Scholar] [CrossRef]
- Wang, J.; Waters, J.L.; Kung, P.; Kim, S.M.; Kelly, J.T.; McNamara, L.E.; Hammer, N.I.; Pemberton, B.C.; Schmehl, R.H.; Gupta, A.; et al. A facile electrochemical reduction method for improving photocatalytic performance of α-fe2o3 photoanode for solar water splitting. ACS Appl. Mater. Interfaces 2017, 9, 381–390. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting-the untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Murashkina, A.A.; Bakiev, T.V.; Artemev, Y.M.; Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Photoelectrochemical behavior of the ternary heterostructured systems cds/wo3/tio2. Catalysts 2019, 9, 999. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Alvarez Galvan, M.C.; del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L. Water splitting on semiconductor catalysts under visible-light irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef]
- Shen, S.; Shi, J.; Guo, P.; Guo, L. Visible-light-driven photocatalytic water splitting on nanostructured semiconducting materials. Int. J. Nanotechnol. 2011, 8, 523–591. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.; Minggu, L.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Agbe, H.; Nyankson, E.; Raza, N.; Dodoo-Arhin, D.; Chauhan, A.; Osei, G.; Kumar, V.; Kim, K.-H. Recent advances in photoinduced catalysis for water splitting and environmental applications. J. Ind. Eng. Chem. 2019, 72, 31–49. [Google Scholar] [CrossRef]
- Yousaf, M.U.; Pervaiz, E.; Minallah, S.; Afzal, M.J.; Honghong, L.; Yang, M. Tin oxide quantum dots decorated graphitic carbon nitride for enhanced removal of organic components from water: Green process. Results Phys. 2019, 2019, 102455. [Google Scholar] [CrossRef]
- Xing, J.; Fang, W.Q.; Zhao, H.J.; Yang, H.G. Inorganic photocatalysts for overall water splitting. Chem. Asian J. 2012, 7, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Hara, M. Heterogeneous photocatalytic cleavage of water. J. Mater. Chem. 2010, 20, 627–641. [Google Scholar] [CrossRef]
- Kouser, S.; Thannikoth, A.; Gupta, U.; Waghmare, U.V.; Rao, C.N.R. 2D-gas as a photocatalyst for water splitting to produce h2. Small 2015, 11, 4723–4730. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef]
- Zhuiykov, S. Nanostructured two-dimensional materials. In Modeling, Characterization and Production of Nanomaterials: Electronics, Photonics and Energy Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 477–524. [Google Scholar]
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2d materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef]
- Lu, L.; Xu, S.; Luo, Z.; Wang, S.; Li, G.; Feng, C. Synthesis of znco2o4 microspheres with zn0.33co0.67co3 precursor and their electrochemical performance. J. Nanopart. Res. 2016, 18, 183. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on g-c3n4 for photocatalytic water splitting and co2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2d photocatalysts: Electronic-structure tailoring, hybridization, and applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Qin, Z.; Ji, H.; Wu, Z. An overview of photocatalysis facilitated by 2d heterojunctions. Nanotechnology 2019, 30, 502002. [Google Scholar] [CrossRef]
- Zhan, W.; Sun, L.; Han, X. Recent progress on engineering highly efficient porous semiconductor photocatalysts derived from metal–organic frameworks. Nano-Micro Lett. 2019, 11, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, L.; Xiao, C.; Hua, X.; Li, Z.; Pan, B.; Xie, Y. Promoting photogenerated holes utilization in pore-rich wo3 ultrathin nanosheets for efficient oxygen-evolving photoanode. Adv. Energy Mater. 2016, 6, 1600437. [Google Scholar] [CrossRef]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Z.; Gao, S.; Cheng, H.; Liu, Q.; Lei, F.; Wei, S.; Xie, Y. All-surface-atomic-metal chalcogenide sheets for high-efficiency visible-light photoelectrochemical water splitting. Adv. Energy Mater. 2014, 4, 1300611. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Liu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; et al. Freestanding tin disulfide single-layers realizing efficient visible-light water splitting. Angew. Chem. 2012, 51, 8727–8731. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Z.; Gao, S.; Cheng, H.; Liu, Q.; Piao, J.; Yao, T.; Wu, C.; Hu, S.; Wei, S.; et al. Fabrication of flexible and freestanding zinc chalcogenide single layers. Nat. Commun. 2012, 3, 1057. [Google Scholar] [CrossRef]
- Xiong, J.; Wen, L.; Jiang, F.; Liu, Y.; Liang, S.; Wu, L. Ultrathin hnb3o8 nanosheet: An efficient photocatalyst for the hydrogen production. J. Mater. Chem. A 2015, 3, 20627–20632. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, J.; Luo, S.; Liang, R.; Qin, N.; Liang, S.; Wu, L. Ultrathin hnbwo6 nanosheets: Facile synthesis and enhanced hydrogen evolution performance from photocatalytic water splitting. Chem. Commun. 2015, 51, 15125–15128. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, Y.; Song, Y.; Li, Y.; Wu, G.; Tang, H.; Zhao, J. Study on the oxidation process of cobalt hydroxide to cobalt oxides at low temperatures. RSC Adv. 2016, 6, 80059–80064. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of ultrathin cds nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, L.; Xie, J.; Zhang, X.; Liu, Q.; Yao, T.; Wei, S.; Zhang, Q.; Xie, Y. Enhanced photoexcited carrier separation in oxygen-doped znin2s4 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 6716–6720. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yu, Y.; Zhang, L. Superior visible light hydrogen evolution of janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 2016, 7, 11480. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ida, S.; Hyodo, J.; Hagiwara, H.; Ishihara, T. Synthesis and photocatalytic activity of rhodium-doped calcium niobate nanosheets for hydrogen production from a water/methanol system without cocatalyst loading. J. Am. Chem. Soc. 2011, 133, 18034–18037. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.; Zhang, M.; Lin, L.; Ye, X.; Lin, S.; Antonietti, M.; Wang, X. Carbon-doped bn nanosheets for metal-free photoredox catalysis. Nat. Commun. 2015, 6, 7698. [Google Scholar] [CrossRef]
- Ida, S.; Kim, N.; Ertekin, E.; Takenaka, S.; Ishihara, T. Photocatalytic reaction centers in two-dimensional titanium oxide crystals. J. Am. Chem. Soc. 2015, 137, 239–244. [Google Scholar] [CrossRef]
- Yang, M.Q.; Xu, Y.J.; Lu, W.; Zeng, K.; Zhu, H.; Xu, Q.H.; Ho, G.W. Self-surface charge exfoliation and electrostatically coordinated 2d hetero-layered hybrids. Nat. Commun. 2017, 8, 14224. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. 2d/2d heterojunction of ti3c2/g-c3n4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Constructing 2d/2d fe2o3/g-c3n4 direct z-scheme photocatalysts with enhanced h2 generation performance. Solar RRL 2018, 2, 1800006. [Google Scholar] [CrossRef]
- Tan, P.; Zhu, A.; Qiao, L.; Zeng, W.; Ma, Y.; Dong, H.; Xie, J.; Pan, J. Constructing a direct z-scheme photocatalytic system based on 2d/2d wo3/znin2s4 nanocomposite for efficient hydrogen evolution under visible light. Inorg. Chem. Front. 2019, 6, 929–939. [Google Scholar] [CrossRef]
- Shi, J.; Li, S.; Wang, F.; Gao, L.; Li, Y.; Zhang, X.; Lu, J. In situ topotactic formation of 2d/2d direct z-scheme cu2s/zn0.67cd0.33s in-plane intergrowth nanosheet heterojunctions for enhanced photocatalytic hydrogen production. Dalton Trans. 2019, 48, 3327–3337. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2d/2d wo3/g-c3n4 step-scheme h2-production photocatalyst. Appl. Catal. B 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Cao, A.; Zhang, L.; Wang, Y.; Zhao, H.; Deng, H.; Liu, X.; Lin, Z.; Su, X.; Yue, F. 2d–2d heterostructured unimof/g-c3n4 for enhanced photocatalytic h2 production under visible-light irradiation. ACS Sustain. Chem. Eng. 2019, 7, 2492–2499. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.-T.; et al. Liquid exfoliation of g-c3n4 nanosheets to construct 2d-2d mos2/g-C3N4 photocatalyst for enhanced photocatalytic h2 production activity. Appl. Catal. B 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Jiang, D.; Wen, B.; Zhang, Y.; Jin, Y.; Li, D.; Chen, M. Mos2/snnb2o6 2d/2d nanosheet heterojunctions with enhanced interfacial charge separation for boosting photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 536, 1–8. [Google Scholar] [CrossRef]
- Gu, W.; Li, X.; Zhang, W.; Wang, J.; Yin, X.; Zhu, L.; Chen, Z.; Zou, W.; Fu, Z.; Lu, Y. Self-limited ion-exchange grown bi6fe2ti3o18-biobr ferroelectric heterostructure and the enhanced photocatalytic oxygen evolution. Appl. Surf. Sci. 2019, 479, 137–147. [Google Scholar] [CrossRef]
- Ning, S.; Shi, X.; Zhang, H.; Lin, H.; Zhang, Z.; Long, J.; Li, Y.; Wang, X. Reconstructing dual-induced {0 0 1} facets bismuth oxychloride nanosheets heterostructures: An effective strategy to promote photocatalytic oxygen evolution. Solar RRL 2019, 3, 1900059. [Google Scholar] [CrossRef]
- Sun, B.; Qian, Y.; Liang, Z.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H. Oxygen vacancy-rich bio2-x ultra-thin nanosheet for efficient full-spectrum responsive photocatalytic oxygen evolution from water splitting. Sol. Energy Mater. Sol. Cells 2019, 195, 309–317. [Google Scholar] [CrossRef]
- Li, L.; She, X.; Yi, J.; Pan, L.; Xia, K.; Wei, W.; Zhu, X.; Chen, Z.; Xu, H.; Li, H. Integrating coox cocatalyst on hexagonal α-fe2o3 for effective photocatalytic oxygen evolution. Appl. Surf. Sci. 2019, 469, 933–940. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, J.-H.; Li, Y.-H.; Zhou, Z.-J.; Li, K.; Liu, F.-T.; Lan, Y.-Q. Co-doped zn1−xcdxs nanocrystals from metal–organic framework precursors: Porous microstructure and efficient photocatalytic hydrogen evolution. Dalton Trans. 2017, 46, 10553–10557. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.-L.; Wang, J.-W.; Lu, T.-B. A facile method for the synthesis of a porous cobalt oxide–carbon hybrid as a highly efficient water oxidation catalyst. J. Mater. Chem. A 2016, 4, 1819–1827. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic c3n4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Xu, J.; Tang, H.; Chen, K.; Jiang, Y. Tuning the morphology of g-c3n4 for improvement of z-scheme photocatalytic water oxidation. ACS Appl. Mater. Interfaces 2015, 7, 15285–15293. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. G-c3n4-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. A review on photocatalytic application of g-c3n4/semiconductor (cns) nanocomposites towards the erasure of dyeing wastewater. Mater. Sci. Semicond. Process. 2016, 47, 62–84. [Google Scholar] [CrossRef]
- Hu, W.; Yuan, X.; Liu, X.; Guan, Y.; Wu, X. Hierarchical sno2 nanostructures as high efficient photocatalysts for the degradation of organic dyes. J. Sol-Gel Sci. Technol. 2017, 84, 316–322. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.; Zhao, H.; Lou, Z.; Liao, Y.; Xue, C. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. RSC Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Zhang, G.; Wang, X. A facile synthesis of br-modified g-c3n4 semiconductors for photoredox water splitting. Appl. Catal. B 2016, 192, 116–125. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Chen, H.; Zhang, Y.; Zhang, F.; Liu, S.F. Fabrication of tio2/c3n4 heterostructure for enhanced photocatalytic z-scheme overall water splitting. Appl. Catal. B 2016, 191, 130–137. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, L.; Wan, J.; Yang, R.; Yu, X.; Li, H.; Chen, J.; Wang, L.; Lu, X. Microwave-enhanced catalytic degradation of p-nitrophenol in soil using mgfe2o4. Chem. Eng. J. 2016, 284, 54–60. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Lu, C.; Huang, W. G-c3n4 nanosheets coupled with tio2 nanosheets as 2d/2d heterojunction photocatalysts toward high photocatalytic activity for hydrogen production. Catal. Lett. 2019, 149, 2930–2939. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Chen, J.; Jiang, Y.; Kiani, M.; Li, B.; Wang, R. Fabrication of high-activity hybrid nitio3/g-c3n4 heterostructured photocatalysts for water splitting to enhanced hydrogen production. Ceram. Int. 2016, 42, 12297–12305. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-free synthesis of 2d porous ultrathin nonmetal-doped g-c3n4 nanosheets with highly efficient photocatalytic h2 evolution from water under visible light. Appl. Catal. B 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M. Increasing visible light water splitting efficiency through synthesis route and charge separation in measoporous g-c3n4 decorated with wo3 nanoparticles. Ceram. Int. 2019, 45, 3886–3893. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; Xia, T.; Fan, W.; Wang, F.; Bai, H.; Shi, W. Heterojunction composites of g-c3n4/knbo3 enhanced photocatalytic properties for water splitting. Int. J. Hydrogen Energy 2018, 43, 16566–16572. [Google Scholar] [CrossRef]
- Seza, A.; Soleimani, F.; Naseri, N.; Soltaninejad, M.; Montazeri, S.; Sadrnezhaad, S.; Mohammadi, M.; Moghadam, H.A.; Forouzandeh, M.; Amin, M. Novel microwave-assisted synthesis of porous g-c3n4/sno2 nanocomposite for solar water-splitting. Appl. Surf. Sci. 2018, 440, 153–161. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Li, Y. A ternary g-c3n4/pt/zno photoanode for efficient photoelectrochemical water splitting. Int. J. Hydrogen Energy 2015, 40, 9080–9087. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Zhu, C.; Li, H.; Kang, Z. Coo and g-c3n4 complement each other for highly efficient overall water splitting under visible light. Appl. Catal. B 2018, 226, 412–420. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Liu, Z.-Q.; Li, N.; Chen, X.; Hu, J.; Li, X. Multi-functional ni3c cocatalyst/g-c3n4 nanoheterojunctions for robust photocatalytic h2 evolution under visible light. J. Mater. Chem. A 2018, 6, 13110–13122. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Zheng, Y.; Sun, W.; Zhao, Y.; Francis, P.S.; Wang, X. Noble-metal-free ni3n/g-c3n4 photocatalysts with enhanced hydrogen production under visible light irradiation. Dalton Trans. 2018, 47, 12188–12196. [Google Scholar] [CrossRef]
- Chen, S.-H.; Wang, J.-J.; Huang, J.; Li, Q.-X. G-c3n4/sns2 heterostructure: A promising water splitting photocatalyst. Chin. J. Chem. Phys. 2017, 30, 36–42. [Google Scholar] [CrossRef]
- Shen, R.; Xie, J.; Zhang, H.; Zhang, A.; Chen, X.; Li, X. Enhanced solar fuel h2 generation over g-c3n4 nanosheet photocatalysts by the synergetic effect of noble metal-free co2p cocatalyst and the environmental phosphorylation strategy. ACS Sustain. Chem. Eng. 2018, 6, 816–826. [Google Scholar] [CrossRef]
- Sun, X.-J.; Yang, D.-D.; Dong, H.; Meng, X.-B.; Sheng, J.-L.; Zhang, X.; Wei, J.-Z.; Zhang, F.-M. Zif-derived cop as a cocatalyst for enhanced photocatalytic h2 production activity of g-c3n4. Sustain. Energy Fuels 2018, 2, 1356–1361. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. 2018, 57, 5765–5771. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Wu, L.; Li, X.; Shu, J. Mno2 and carbon nanotube co-modified c3n4 composite catalyst for enhanced water splitting activity under visible light irradiation. Int. J. Hydrogen Energy 2016, 41, 22743–22750. [Google Scholar] [CrossRef]
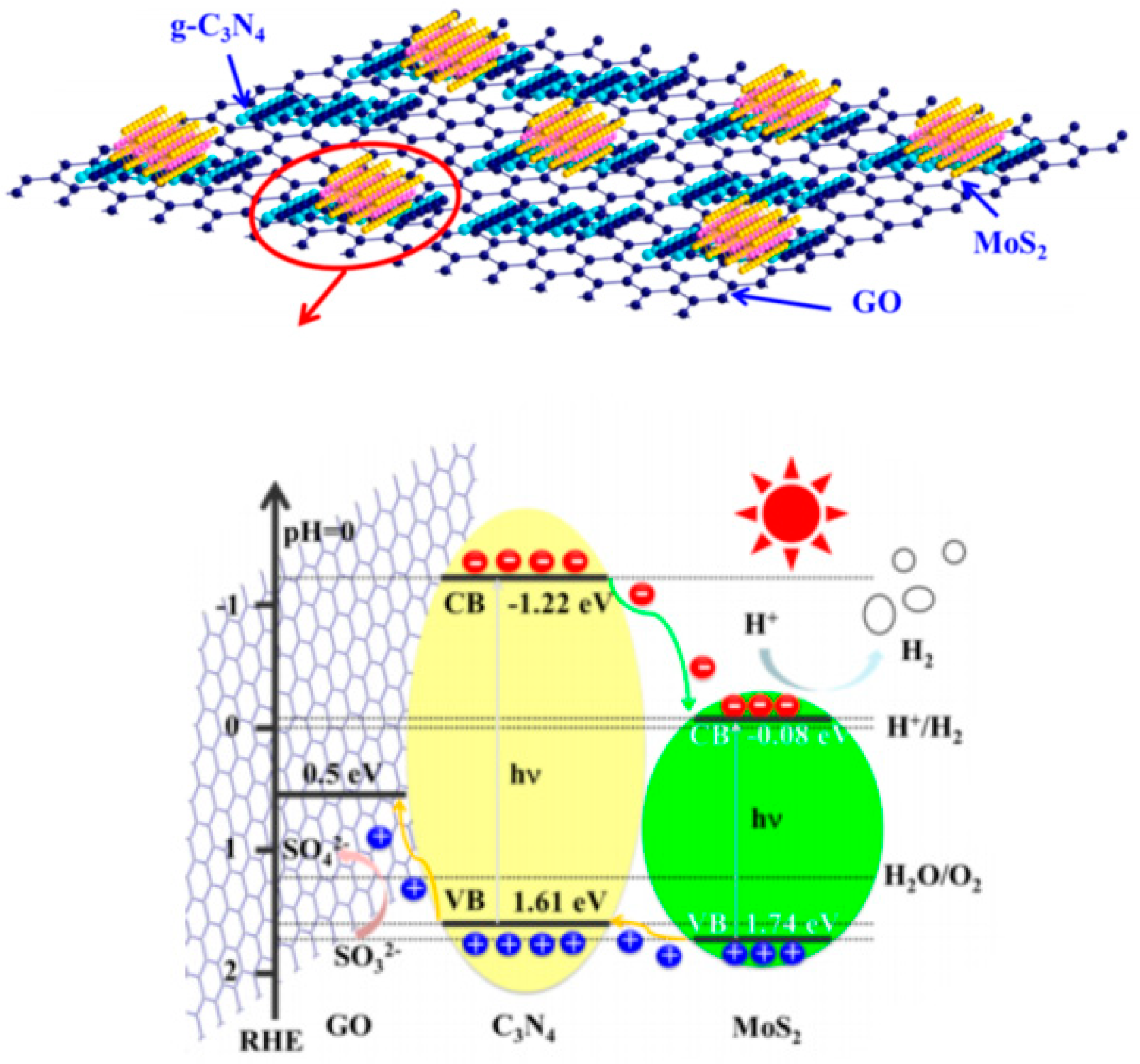
- Wang, M.; Ju, P.; Li, J.; Zhao, Y.; Han, X.; Hao, Z. Facile synthesis of mos2/g-c3n4/go ternary heterojunction with enhanced photocatalytic activity for water splitting. ACS Sustain. Chem. Eng. 2017, 5, 7878–7886. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.; Morozov, S.; Hill, E.; Blake, P.; Katsnelson, M.; Novoselov, K. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Schäffel, F.; Bachmatiuk, A.; Rümmeli, M.H. (Eds.) Chapter 3—Properties of graphene. In Graphene, Warner; Woodhead Publishing: Cambridge, UK, 2013; pp. 61–127. [Google Scholar]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaria, R.; Menendez, R.; Corma, A.; Garcia, H. Surface area measurement of graphene oxide in aqueous solutions. Langmuir ACS J. Surf. Colloids 2013, 29, 13443–13448. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhou, S.-X.; Zhang, C.; Chang, H.-X.; Chen, Y.; Fu, W.-F. Synergetic effect of cu and graphene as cocatalyst on tio 2 for enhanced photocatalytic hydrogen evolution from solar water splitting. J. Mater. Chem. 2012, 22, 18542–18549. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent advances in graphene based tio2 nanocomposites (gtio2ns) for photocatalytic degradation of synthetic dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and co2 reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef]
- Del Pino, A.P.; González-Campo, A.; Giraldo, S.; Peral, J.; György, E.; Logofatu, C.; Puigmartí-Luis, J. Synthesis of graphene-based photocatalysts for water splitting by laser-induced doping with ionic liquids. Carbon 2018, 130, 48–58. [Google Scholar] [CrossRef]
- Min, S.; Wang, F.; Lu, G. Graphene-induced spatial charge separation for selective water splitting over tio2 photocatalyst. Catal. Commun. 2016, 80, 28–32. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Thangavel, N.; Hafeez, H.Y.; Neppolian, B.; Rao, G.R. Highly active and stable multi-walled carbon nanotubes-graphene-tio2 nanohybrid: An efficient non-noble metal photocatalyst for water splitting. Catal. Today 2019, 321, 120–127. [Google Scholar] [CrossRef]
- Mateo, D.; Esteve-Adell, I.; Albero, J.; Royo, J.F.S.; Primo, A.; Garcia, H. 111 oriented gold nanoplatelets on multilayer graphene as visible light photocatalyst for overall water splitting. Nat. Commun. 2016, 7, 11819. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Ibrahim, I.; Salama, T.M. Rational design of manganese ferrite-graphene hybrid photocatalysts: Efficient water splitting and effective elimination of organic pollutants. Appl. Catal. A 2016, 524, 182–191. [Google Scholar] [CrossRef]
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-schematic water splitting into h2 and o2 using metal sulfide as a hydrogen-evolving photocatalyst and reduced graphene oxide as a solid-state electron mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Hisatomi, T.; Wang, Q.; Chen, S.; Iwase, A.; Nakabayashi, M.; Shibata, N.; Takata, T.; Katayama, M.; Minegishi, T. Photoreduced graphene oxide as a conductive binder to improve the water splitting activity of photocatalyst sheets. Adv. Funct. Mater. 2016, 26, 7011–7019. [Google Scholar] [CrossRef]
- Shudo, Y.; Karim, M.R.; Wakata, K.; Ohmagari, H.; Kameda, N.; Hayami, S. Reduced graphene oxide-transition metal hybrids for hydrogen generation by photocatalytic water splitting. J. Incl. Phenom. Macrocycl. Chem. 2018, 1–4. [Google Scholar] [CrossRef]
- Fan, X.; Peng, Z.; Ye, R.; Zhou, H.; Guo, X. M3c (m: Fe, co, ni) nanocrystals encased in graphene nanoribbons: An active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Nano 2015, 9, 7407–7418. [Google Scholar] [CrossRef]
- Jing, Y.; Tang, Q.; He, P.; Zhou, Z.; Shen, P. Small molecules make big differences: Molecular doping effects on electronic and optical properties of phosphorene. Nanotechnology 2015, 26, 095201. [Google Scholar] [CrossRef]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phosphorene–the two-dimensional black phosphorous: Properties, synthesis and applications. Mater. Sci. Eng. B 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Churchill, H.O.; Jarillo-Herrero, P. Two-dimensional crystals: Phosphorus joins the family. Nat. Nanotechnol. 2014, 9, 330. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, S.Y.; Jang, H.W. Black phosphorus: Critical review and potential for water splitting photocatalyst. Nanomaterials 2016, 6, 194. [Google Scholar] [CrossRef]
- Hu, J.; Chen, D.; Mo, Z.; Li, N.; Xu, Q.; Li, H.; He, J.; Xu, H.; Lu, J. Z-scheme 2d/2d heterojunction of black phosphorus/monolayer bi2wo6 nanosheets with enhanced photocatalytic activities. Angew. Chem. Int. Ed. 2019, 58, 2073–2077. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Chen, C.; Smith, S.C. Phosphorene: Fabrication, properties, and applications. J. Phys. Chem. Lett. 2015, 6, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Lee, S.C.; Won, J.; Son, B.-C.; Choi, S.; Kim, Y.; Park, S.Y.; Kim, H.-S.; Lee, Y.-C.; Lee, J. Stable semiconductor black phosphorus (bp)@ titanium dioxide (tio 2) hybrid photocatalysts. Sci. Rep. 2015, 5, 8691. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Electronic properties of phosphorene/graphene and phosphorene/hexagonal boron nitride heterostructures. J. Phys. Chem. C 2015, 119, 13929–13936. [Google Scholar] [CrossRef]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Hua, R.; Lei, Q.; Tian, Y. Supported black phosphorus nanosheets as hydrogen-evolving photocatalyst achieving 5.4% energy conversion efficiency at 353 k. Nat. Commun. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Guo, H.; Lu, N.; Dai, J.; Wu, X.; Zeng, X.C. Phosphorene nanoribbons, phosphorus nanotubes, and van der waals multilayers. J. Phys. Chem. C 2014, 118, 14051–14059. [Google Scholar] [CrossRef]
- Huang, L.; Huo, N.; Li, Y.; Chen, H.; Yang, J.; Wei, Z.; Li, J.; Li, S.-S. Electric-field tunable band offsets in black phosphorus and mos2 van der waals pn heterostructure. J. Phys. Chem. Lett. 2015, 6, 2483–2488. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Ruan, L.-W.; Barber, J.; Loo, S.C.J.; Xue, C. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ. Sci. 2014, 7, 3934–3951. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zhao, J.; Yang, K.; Liang, J.; Liu, D.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Monodispersed nickel phosphide nanocrystals with different phases: Synthesis, characterization and electrocatalytic properties for hydrogen evolution. J. Mater. Chem. A 2015, 3, 1656–1665. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of fe, co, and ni: A review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Ledendecker, M.; Krick Calderón, S.; Papp, C.; Steinrück, H.-P.; Antonietti, M.; Shalom, M. The synthesis of nanostructured ni5p4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.-A.; Feng, L.; Song, F.; Hu, X. Ni2p as a janus catalyst for water splitting: The oxygen evolution activity of ni2p nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Tour, J.M. Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv. Mater. 2015, 27, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Sumboja, A.; An, T.; Goh, H.Y.; Lubke, M.; Howard, D.P.; Xu, Y.; Handoko, A.D.; Zong, Y.; Liu, Z. One-step facile synthesis of cobalt phosphides for hydrogen evolution reaction catalysts in acidic and alkaline medium. ACS Appl. Mater. Interfaces 2018, 10, 15673–15680. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cui, X.; He, T.; Zeng, L.; Tian, H.; Song, Y.; Qi, K.; Xia, B.Y. Surface reconstruction of cobalt phosphide nanosheets by electrochemical activation for enhanced hydrogen evolution in alkaline solution. Chem. Sci. 2019, 10, 2019–2024. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Liu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Jiang, M.; Kuang, Y.; Sun, X.; Duan, X. Ternary nicop nanosheet arrays: An excellent bifunctional catalyst for alkaline overall water splitting. Nano Res. 2016, 9, 2251–2259. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlogl, U.; Alshareef, H.N. Plasma-assisted synthesis of nicop for efficient overall water splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, B.; Gao, Q.; Zheng, Y.-R.; Zheng, X.-S.; Zhu, J.-F.; Gao, M.-R.; Yu, S.-H. A janus nickel cobalt phosphide catalyst for high-efficiency neutral-ph water splitting. Angew. Chem. Int. Ed. 2018, 57, 15445–15449. [Google Scholar] [CrossRef]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like nicop for highly efficient overall water splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org. Process Res. Dev. 2016, 22, 430–445. [Google Scholar] [CrossRef]
- Ma, X.; Chang, Y.; Zhang, Z.; Tang, J. Forest-like nicop@cu3p supported on copper foam as a bifunctional catalyst for efficient water splitting. J. Mater. Chem. A 2018, 6, 2100–2106. [Google Scholar] [CrossRef]
- Tong, M.; Wang, L.; Yu, P.; Liu, X.; Fu, H. 3d network nanostructured nicop nanosheets supported on n-doped carbon coated ni foam as a highly active bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. Front. Chem. Sci. Eng. 2018, 12, 417–424. [Google Scholar] [CrossRef]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, S.; Lamberti, C.; Ricchiardi, G.; Regli, L.; Bonino, F.; Damin, A.; Lillerud, K.P.; Bjorgen, M.; Zecchina, A. Electronic and vibrational properties of a mof-5 metal-organic framework: Zno quantum dot behaviour. Chem. Commun. (Camb. Engl.) 2004, 2300–2301. [Google Scholar] [CrossRef]
- Tachikawa, T.; Choi, J.R.; Fujitsuka, M.; Majima, T. Photoinduced charge-transfer processes on mof-5 nanoparticles: Elucidating differences between metal-organic frameworks and semiconductor metal oxides. J. Phys. Chem. C 2008, 112, 14090–14101. [Google Scholar] [CrossRef]
- Bauer, C.A.; Timofeeva, T.V.; Settersten, T.B.; Patterson, B.D.; Liu, V.H.; Simmons, B.A.; Allendorf, M.D. Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Zang, Y.; Liu, R.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Co/co 9 s 8 @s,n-doped porous graphene sheets derived from s, n dual organic ligands assembled co-mofs as superior electrocatalysts for full water splitting in alkaline media. Nano Energy 2016, 30, 93–102. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Shang, Q.; Xiong, Y.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Jiang, H.-L. Boosting photocatalytic hydrogen production of a metal–organic framework decorated with platinum nanoparticles: The platinum location matters. Angew. Chem. 2016, 128, 9535–9539. [Google Scholar] [CrossRef]
- Qin, J.S.; Zhang, S.R.; Du, D.Y.; Shen, P.; Bao, S.J.; Lan, Y.Q.; Su, Z.M. A microporous anionic metal-organic framework for sensing luminescence of lanthanide(iii) ions and selective absorption of dyes by ionic exchange. Chemistry 2014, 20, 5625–5630. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Tian, H.R.; Ai, J.; Li, L.J.; Dang, S.; Lan, Y.Q.; Sun, Z.M. A microporous cu-mof with optimized open metal sites and pore spaces for high gas storage and active chemical fixation of co2. Chem. Commun. (Camb. Engl.) 2016, 52, 11147–11150. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X. Metal organic framework catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Lin, W. Metal-organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Song, F.; Li, W.; Sun, Y. Metal–organic frameworks and their derivatives for photocatalytic water splitting. Inorganics 2017, 5, 40. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef]
- Long, J.; Wang, S.; Ding, Z.; Wang, S.; Zhou, Y.; Huang, L.; Wang, X. Amine-functionalized zirconium metal-organic framework as efficient visible-light photocatalyst for aerobic organic transformations. Chem. Commun. (Camb. Engl.) 2012, 48, 11656–11658. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized ti(iv) metal–organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A water-stable porphyrin-based metal-organic framework active for visible-light photocatalysis. Angew. Chem. 2012, 51, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.J. Nanoparticle/metal-organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Mondal, I.; Goswami, A.; Pal, U.; Mondal, R. Co-mof as a sacrificial template: Manifesting a new co3o4/tio2 system with a p–n heterojunction for photocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 20288–20296. [Google Scholar] [CrossRef]
- Lan, M.; Guo, R.-M.; Dou, Y.; Zhou, J.; Zhou, A.; Li, J.-R. Fabrication of porous pt-doping heterojunctions by using bimetallic mof template for photocatalytic hydrogen generation. Nano Energy 2017, 33, 238–246. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, W.; Li, Z.; Ho, K.H.; Li, X.; Jiao, X.; Chen, D. Synthesis of amorphous cobalt sulfide polyhedral nanocages for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 8603–8606. [Google Scholar] [CrossRef]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Structural evolution of zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, D.; Du, S.; Wang, Y.; Chen, M.; Hou, J.; Xu, S.; Wu, S.; Gong, J. Phase transfer directed synthesis of hollow zeolitic imidazolate frameworks-67 nanocages. Cryst. Growth Des. 2016, 17, 3–6. [Google Scholar] [CrossRef]
- Xu, W.; Li, T.-T.; Zheng, Y.-Q. Porous co3o4 nanoparticles derived from a co(ii)-cyclohexanehexacarboxylate metal–organic framework and used in a supercapacitor with good cycling stability. RSC Adv. 2016, 6, 86447–86454. [Google Scholar] [CrossRef]
- Wang, C.; deKrafft, K.E.; Lin, W. Pt nanoparticles@photoactive metal–organic frameworks: Efficient hydrogen evolution via synergistic photoexcitation and electron injection. J. Am. Chem. Soc. 2012, 134, 7211–7214. [Google Scholar] [CrossRef]
- Wang, D.; Song, Y.; Cai, J.; Wu, L.; Li, Z. Effective photo-reduction to deposit pt nanoparticles on mil-100(fe) for visible-light-induced hydrogen evolution. New J. Chem. 2016, 40, 9170–9175. [Google Scholar] [CrossRef]
- Laurier, K.G.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B. Iron(iii)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Shi, X.; Du, G.; Asiri, A.M.; Luo, Y.; Sun, X. High-performance water oxidation electrocatalysis enabled by a ni-mof nanosheet array. Inorg. Chem. Front. 2018, 5, 1570–1574. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef]
- Lin, R.; Shen, L.; Ren, Z.; Wu, W.; Tan, Y.; Fu, H.; Zhang, J.; Wu, L. Enhanced photocatalytic hydrogen production activity via dual modification of mof and reduced graphene oxide on cds. Chem. Commun. 2014, 50, 8533–8535. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Gomes Silva, C.; Luz, I.; Llabres i Xamena, F.X.; Corma, A.; Garcia, H. Water stable zr-benzenedicarboxylate metal-organic frameworks as photocatalysts for hydrogen generation. Chemistry 2010, 16, 11133–11138. [Google Scholar] [CrossRef]
- Shen, L.; Luo, M.; Liu, Y.; Liang, R.; Jing, F.; Wu, L. Noble-metal-free mos2 co-catalyst decorated uio-66/cds hybrids for efficient photocatalytic h2 production. Appl. Catal. B 2015, 166–167, 445–453. [Google Scholar] [CrossRef]
- He, J.; Yan, Z.; Wang, J.; Xie, J.; Jiang, L.; Shi, Y.; Yuan, F.; Yu, F.; Sun, Y. Significantly enhanced photocatalytic hydrogen evolution under visible light over cds embedded on metal–organic frameworks. Chem. Commun. 2013, 49, 6761–6763. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kamegawa, T.; Yamashita, H. Amine-functionalized mil-101(cr) with imbedded platinum nanoparticles as a durable photocatalyst for hydrogen production from water. Chem. Commun. 2014, 50, 11645–11648. [Google Scholar] [CrossRef]
- Han, J.; Wang, D.; Du, Y.; Xi, S.; Hong, J.; Yin, S.; Chen, Z.; Zhou, T.; Xu, R. Metal–organic framework immobilized cobalt oxide nanoparticles for efficient photocatalytic water oxidation. J. Mater. Chem. A 2015, 3, 20607–20613. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Q.; Zhang, F.; Wei, J.; Yang, P. A dye-like ligand-based metal–organic framework for efficient photocatalytic hydrogen production from aqueous solution. Catal. Sci. Technol. 2016, 6, 3840–3844. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; Tong, Z.; Tian, Y.; Li, Y.; Jiang, Z. Synthesis of ag/tio2 nanotube heterojunction with improved visible-light photocatalytic performance inspired by bioadhesion. J. Phys. Chem. C 2015, 119, 5827–5835. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized pt@uio-66(zr) metal-organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. (Camb. Engl.) 2014, 50, 7063–7066. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Kampouri, S.; Valizadeh, B.; Luo, W.; Ongari, D.; Planes, O.M.; Zuttel, A.; Smit, B.; Stylianou, K.C. Photocatalytic hydrogen generation from a visible-light-responsive metal-organic framework system: Stability versus activity of molybdenum sulfide cocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 30035–30039. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional mos2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical h2 evolution from mos2 nanocatalysts. Science (N. Y.) 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Ataca, C.; Ciraci, S. Dissociation of h2o at the vacancies of single-layer mos2. Biophys. Rev. B 2012, 85, 195410. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides—efficient and viable materials for electro - and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577. [Google Scholar] [CrossRef]
- Van Gastel, M.; Shaw, J.L.; Blake, A.J.; Flores, M.; Schröder, M.; McMaster, J.; Lubitz, W. Electronic structure of a binuclear nickel complex of relevance to [nife] hydrogenase. Inorg. Chem. 2008, 47, 11688–11697. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, C.; Liu, Z.; Fei, B.; Lin, P.; Li, Q.; Sun, S.; Du, S. Application of a ni mercaptopyrimidine mof as highly efficient catalyst for sunlight-driven hydrogen generation. J. Mater. Chem. A 2015, 3, 7163–7169. [Google Scholar] [CrossRef]
- An, Y.; Liu, Y.; An, P.; Dong, J.; Xu, B.; Dai, Y.; Qin, X.; Zhang, X.; Whangbo, M.H.; Huang, B. Ni(ii) coordination to an al-based metal-organic framework made from 2-aminoterephthalate for photocatalytic overall water splitting. Angew. Chem. 2017, 56, 3036–3040. [Google Scholar] [CrossRef]
- Zhou, T.; Du, Y.; Borgna, A.; Hong, J.; Wang, Y.; Han, J.; Zhang, W.; Xu, R. Post-synthesis modification of a metal–organic framework to construct a bifunctional photocatalyst for hydrogen production. Energy Environ. Sci. 2013, 6, 3229. [Google Scholar] [CrossRef]
- Bloch, E.D.; Britt, D.; Lee, C.; Doonan, C.J.; Uribe-Romo, F.J.; Furukawa, H.; Long, J.R.; Yaghi, O.M. Metal insertion in a microporous metal-organic framework lined with 2,2’-bipyridine. J. Am. Chem. Soc. 2010, 132, 14382–14384. [Google Scholar] [CrossRef]
- Toyao, T.; Saito, M.; Dohshi, S.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Horiuchi, Y.; Matsuoka, M. Development of a ru complex-incorporated mof photocatalyst for hydrogen production under visible-light irradiation. Chem. Commun. (Camb. Engl.) 2014, 50, 6779–6781. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. 2d metal–organic frameworks as multifunctional materials in heterogeneous catalysis and electro/photocatalysis. Adv. Mater. 2019, 31, 1900617. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]


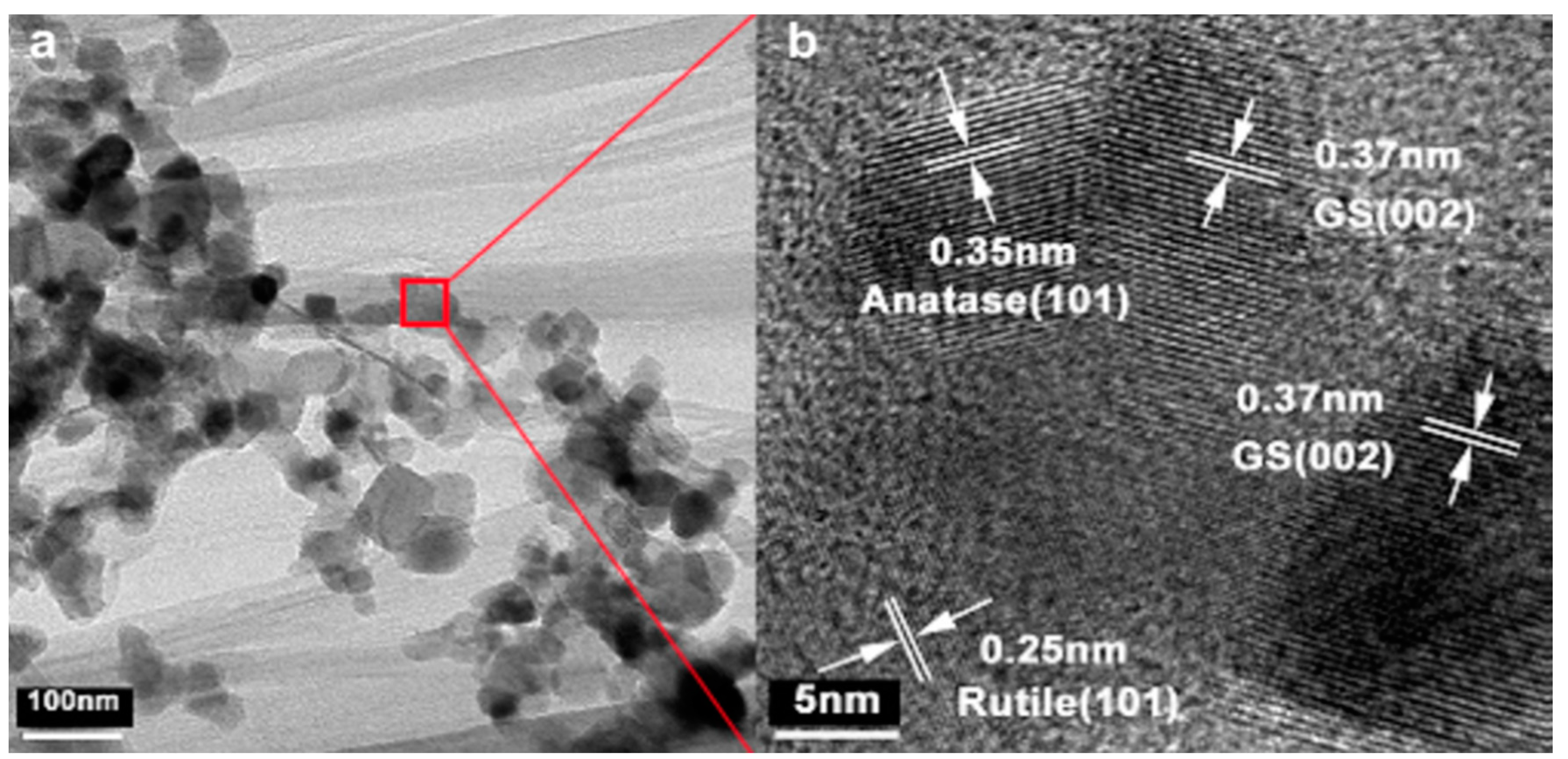


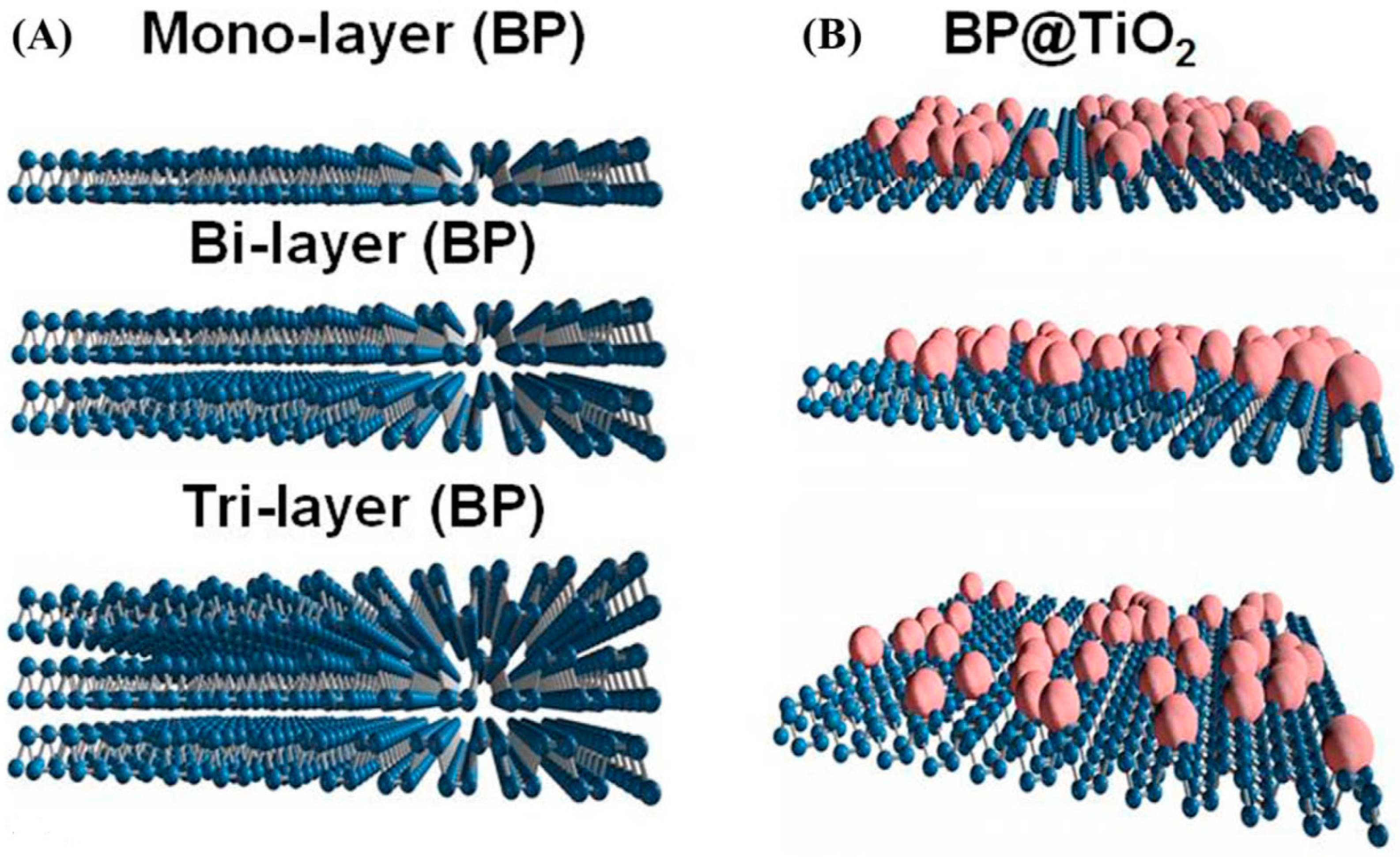
| Photocatalyst | Thickness (nm) | Bandgap (eV) | Sacrificial Agent | Co-Catalyst | Light Source | Type of Reaction | Activity (µmol/g·h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pore rich WO3 ultra-thin nanosheets | 3.8 | 2.89, CB −0.40, VB 2.48 | 0.5 mol/L Na2SO4 | - | 300 W Xe lamp | OER | Photocurrent density 2.14 mA/cm2 at 1.0 V | [49] |
| O-vacancy-rich In2O3 nanosheets | 0.9 | 2.18 | - | - | 300 W Xe lamp with 420 nm cutoff filter | OER | Photocurrent density 1.73 mA/cm2 at 0.576 V | [50] |
| Surface atomic SnS sheets | 0.57 | 1.47 | 0.5 mol/L Na2SO4 | - | 300 W Xe lamp with 420 nm cutoff filter | Overall | Photocurrent density of 5.27 mA/cm2 at 0.8 V | [51] |
| Single layer SnS2 | 0.61 | 2.23 | - | - | 300 W Xe lamp with 420 nm cutoff filter | Overall | Photocurrent density of 2.75 mA/cm2 at 1.0 V | [52] |
| ZnSe | 0.85 | 3.5 | 0.5 mol/L Na2SO4 | - | 300 W Xe lamp | Overall | Photocurrent density of 2.14 mA/cm2 at 0.72 V | [53] |
| Cu2O | 0.62 | 1.92 | 0.5 mol/L Na2SO4 | - | 300 W Xe lamp with 420 nm cutoff filter | HER | Photocurrent density of 3.98 mA/cm2 at −1.0 V | |
| HNb3O8 | 1.3 | 3.68 | 10 vol% TEOA | 1 wt% Pt | 125 W Hg lamp | HER | ≈610 | [54] |
| HNbWO6 | 1.8–2.0 | 3.13, CB −0.68, VB 2.45 | TEOA | 1 wt% Pt | 300 W Xe lamp | HER | 1986.25 | [55] |
| SnNb2O6 | ≈3 | 2.43 | 20 vol% lactic acid | 0.3 wt% Pt | 300 W Xe lamp with 400 nm cutoff filter | HER | 264 | [9] |
| CoOOH | 1.5 | 2.4 | 0.5 M Na2SO3 | - | 300 W Xe lamp | HER | 1200 | [56] |
| CdS | ≈4 | ≈2.86 | 0.25 M Na2S, 0.25 M Na2SO3 | - | 300 W Xe lamp with 420 nm cutoff filter | HER | 41,100 | [57] |
| O-doped ZnIn2S4 | 6 | 2.07, CB −1.34, VB 0.73 | 0.25 M Na2SO3, 0.35 M Na2S | - | 300 W Xe lamp with 420 nm cutoff filter | HER | 2120 | [58] |
| g-C3N4 | ≈2 | 2.97 | 10 vol% TEOA | 6 wt% Pt | 300 W Xe lamp | HER | 170.5 | [39] |
| g-C3N4 | ≈2 | 2.65 | 10 vol% TEOA | 3 wt% Pt | 300 W Xe lamp with 420 nm cutoff filter | HER | 1860 | [59] |
| MoS2/Bi12O17Cl2 | 0.71 of Bi12O17Cl2 | ≈2.5 | 0.3 M Ascorbic acid | - | 300 W Xe lamp with 420 nm cutoff filter | HER | 33,000 | [60] |
| Rh-doped calcium niobate | ≈2.8–3.0 | ≈1.9 | 10 vol% CH3OH | Rh | 500 W Xe lamp | HER | 76,960 | [61] |
| C-BN | 3–4 | 2.72, CB −1.24, VB 1.48 | 10 vol% TEOA | 1 wt% Pt | 300 W Xe lamp | HER | ≈920 | [62] |
| Rh single atoms/TiO2 | 0.7 | - | 20 vol% CH3OH | Single atom Rh | 500 W Xe lamp | HER | 2550 | [63] |
| MoSe2/ZnIn2S4 | 2.5 of ZnIn2S4 | ≈2.75 | 10 vol% Lactic acid | 1 wt% MoSe2 | 300 W Xe lamp with 400 nm cutoff filter | HER | 6454 | [64] |
| Ti3C2/g-C3N4 | 1.7–2.6 of Ti3C2 | - | 10 vol% TEOA | 3 wt% Pt, 3 wt% Ti3C2 | 200 W Hg lamp λ > 400 nm | HER | 72.3, 0.81% quantum efficiency at 400 nm | [65] |
| 10% Fe2O3/g-C3N4 (1.0 wt% Pt) | 21 Fe2O3 | 2.8 of g-C3N4, 2.1 of Fe2O3 | 15 vol% TEOA | 1 wt% Pt | 350 W xenon lamp λ > 420 nm | HER | 398.0 | [66] |
| WO3/ZnIn2S4 | - | 2.4 of ZnIn2S4 | 0.25 M Na2SO3/0.35 M Na2S | - | 300 W xenon lamp λ > 420 nm | HER | 2202.9 | [67] |
| Cu2S/Zn0.67Cd0.33S | 5 | 2.28–2.5 | 0.1 M Na2S/Na2SO3 | Cu2S | 300 W xenon lamp λ > 420 nm | HER | 15270, quantum efficiency 18.15% at 420 nm | [68] |
| 15% WO3/g-C3N4 | 2.5–3.5 | 2.77 of WO3 and 2.68 of g-C3N4 | 20 vol% Lactic acid | 2 wt% Pt | 350 W xenon lamp | HER | 982 | [69] |
| 25.0% UNiMOF/g-C3N4 | 3.04 UNiMOF | - | 10 vol% TEOA | - | 300 W xenon lamp λ > 420 nm | HER | 400.6, quantum efficiency 0.979% at 420 nm | [70] |
| 0.75% MoS2/g-C3N4 | 8–10 of g-C3N4 | - | 0.1 M TEOA | 0.75 wt% MoS2 | 300 W xenon lamp λ > 420 nm | HER | 1155, quantum efficiency 6.8% at 420 nm nm | [71] |
| MoS2/SnNb2O6 (1.0 wt% Pt) | - | 2.59 of SNO, 1.8 of MoS2 | 20 vol% CH3OH | 1 wt% Pt | 300 W xenon lamp λ > 420 nm | HER | 258 | [72] |
| Bi6Fe2Ti3O18/BiOBr ferroelectric heterostructure | 20–50 | 2.5 of BFTO | 0.2 g AgNO3 | - | 300 W Xe arc lamp λ > 420 nm | OER | 13.8 | [73] |
| Ultrathin Bi3O4Cl/BiOCl | 0.799–0.961 | 2.6 | AgNO3 and FeCl3 | - | 300 W Xe lamp with the 400 nm cut-off filter | OER | 58.6 | [74] |
| MoSe2/Ag3PO4 Heterojunction | - | - | 0.5 mol/L Na2SO4 | - | white LED | OER | 182 | [65] |
| BiO2-x Ultra-thin nanosheet | 5–10 | 1.3 | Methyl viologen (2:20 ratio) | - | 300 W Xenon arc lamp | OER | 2715.443 | [75] |
| CoOx/hexagonal α-Fe2O3 | - | 2.09 of α-Fe2O3 | 0.1 g AgNO3 | 5 wt% CoOx | 300 W Xenon lamp with λ 400 nm cut-off filter | OER | 195.19 | [76] |
| Co-Zn0.5Cd0.5S | - | 2.45 | Na2S–Na2SO3 | 0.5 wt% Co | visible light (>420 nm) | HER | 17.36 | [77] |
| 700-CoOx-C | - | - | [Ru(bpy)3]2+–Na2S2O8 | - | visible light (>420 nm) | OER | 0.039 | [78] |
| Photocatalyst | Bandgap (eV) | Thickness (nm) | Co-Catalyst | Sacrificial Agent | Light Source | Type of Reaction | Activity (µmol/g·h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| g-C3N4 nanosheet | 2.85 | - | 3 wt% Pt | 15 vol% TEOA, AgNO3 (0.1 M) | 300 W Xenon lamp, λ > 420 nm cutoff filter | HER OER | 297 120 | [85] |
| Br modified g-C3N4 | 2.82 | - | 3 wt% Pt, 0.3 wt% CoOx | 10 vol% TEOA, AgNO3 (0.01 M) | 300 W Xenon lamp, λ > 420 nm cutoff filter | HER OER | 600 80 | [86] |
| Ti3C2/g-C3N4 Heterojunction | 2.77 of g-C3N4 nanosheets | 1.7–2.6 | 3 wt% Pt | 10 vol% TEOA | 200 W Hg lamp, λ > 400 nm cutoff filter | HER | 72.3 | [89] |
| NiTiO3/g-C3N4 | 2.18 NiTiO3, 2.7 g-C3N4 | - | 1 wt% Pt | 10 vol% TEOA | 300 W Xenon lamp, λ > 420 nm cutoff filter | HER | 835 | [90] |
| O-doped g-C3N4 nanosheets | 2.95 | - | 3 wt% Pt | - | 300 W Xenon lamp, λ > 400 nm cutoff filter | HER | 3786 | [91] |
| WO3 decorated g-C3N4 | 2.39 | - | - | 10 vol% glycerol | 500 W Xenon lamp | HER | 1111 | [92] |
| KNbO3/g-C3N4 Heterojunctioon | - | - | 2 wt% Pt | 5 vol% TEOA | 300 W Xenon lamp | HER | 1019.38 | [93] |
| CoO/g-C3N4 Heterojunctioon | 2.52 CoO, 2.75 g-C3N4 | - | - | - | White LED, λ > 400 nm | HER OER | 50.2 27.8 | [96] |
| Ni3C/g-C3N4 Heterojunctioon | 2.65 | - | 15 wt% Ni3C | 15 vol% TEOA | 350 W Xenon lamp, λ > 420 nm cutoff filter | HER | 1518 | [97] |
| Ni3N/g-C3N4 Heterojunctioon | - | - | 3 wt% Ni3N | 10 vol% TEOA | 300 W Xenon lamp, λ > 400 nm cutoff filter | HER | 169 | [98] |
| Co2P/g-C3N4 | 2.61 | - | 2 wt% Co2P | 15 vol% TEOA, 0.1mM K2HPO4 proton carrier | 300 W Xenon lamp | HER | 556 | [100] |
| ZIF-67 derived CoP/g-C3N4 | 2.85 | - | 1.42 wt% CoP | - | Light irradiation λ > 320 nm | HER | 201.5 | [101] |
| MnO2/CNT/g-C3N4 | 2.64 | - | MnO2/CNT | - | 70 W metal halide lamp (380 nm < λ < 780 nm) | HER | 4067 | [103] |
| GO thin film/MoS2/g-C3N4 Quantum dots | 1.82 MoS2, 2.83 g-C3N4 | - | MoS2/g-C3N4 Quantum dots | 0.25 M Na2SO3 | 450 W Xenon lamp | HER | 1650 | [104] |
| TiO2/CoOx/rGO nanosheet | - | - | TiO2 nanoparticles/CoOx | 20 vol% CH3OH, 0.05 M AgNO3 | 250 W Hg lamp | HER OER | 3800 1616 | [113] |
| TiO2/Graphene nanosheet | 2.96 | 1 | 0.5 wt% Pt | 25 vol% CH3OH | 300 W Xenon lamp | HER | 6680 | [39] |
| CNT/Graphene/TiO2 nanohybrid | 2.79 | 7 | Graphene | 10 vol% CH3OH | 350 W Xenon lamp | HER | 29,000 | [114] |
| 111-oriented Au nanoplatelets/Graphene | - | 20 | Au nanoplatelets | TEOA | 150 W Xenon lamp | HER OER | 12,000 9000 | [115] |
| rGO-transition metal hybrids | - | - | Al, Co, Fe, Ni, Mn | - | 500 W Xenon lamp | HER | 24.74 | [119] |
| Black Phosphorus/Monolayer Bi2WO6 Nanosheets | 0.3–2 eV BP, 2.67 MBWO | - | 3 wt% Pt | - | Visible light | HER | 4208 | [125] |
| BP/CoP nanosheets | 1.14 | 1.4 | CoP | None | 300 W Xenon lamp, λ > 420 nm cutoff filter | HER | 735 | [129] |
| Pt@UiO-66-NH2 MOF | 2.76 | - | 2.87 wt% Pt | TEOA, CH3CN | Visible light | HER | 257.38 | [155] |
| Pt/Amine functionalized Ti-MOF | - | - | Pt | 0.01 M TEOA | Xenon lamp, λ > 420 nm | HER | 366.7 | [164] |
| Co3O4/TiO2 heterojunction using Co-MOF sacrificial template | - | - | 2 wt% Co | 15 vol% CH3OH | 400 W Xenon lamp | HER | 7000 | [168] |
| Pt heterojunction using bimetallic MOF template | - | - | Pt | 10 vol% CH3OH | 300 W Xenon lamp | HER | 9150 | [169] |
| Pt/MIL—100(Fe) | - | - | 0.8 wt% Pt | CH3OH (1:3,CH3OH:H2O) | 300 W Xenon lamp, λ > 420 nm cutoff filter | HER | 109 | [175] |
| UiO-66/CdS/1%rGO | - | - | Pt | 0.1 M Na2S, 0.1 M Na2SO3 | 300 W Xenon lamp, λ > 400 nm cutoff filter | HER | 13,800 | [180] |
| MoS2/UiO-66/CdS hybrid | - | - | 1.5 wt% MoS2 | 10 vol% LA | 300 W Xenon lamp, λ > 420 nm cutoff filter | HER | 32,500 | [183] |
| CdS(10wt%)/MIL-101 | - | - | 0.5 wt% Pt | LA | Visible light λ > 420 nm | HER | 75,500 | [184] |
| Pt/NH2-MIL-101(Cr) | - | - | 1.5 wt% Pt | 25 vol% TEOA | Visible light | HER | 50,000 | [185] |
| MoS/MIL125-NH2 | 1.29 MoS2 | - | 0.8 wt% Mo3S132−, 1T-MoS2 | 16.1 vol% TEA | Xenon lamp λ > 420 nm | HER | 2094, 1454 | [190] |
| 2D Ni mercaptopyrimidine MOF | - | - | - | 15 vol% TEA | White LED | HER | 6017 | [196] |
| Al-based MOF derived from 2-aminoterephthalic acid | 2.75 | - | Ni2+ | CH3OH, AgNO3 | Xenon lamp | HER OER | 1166.7 5000 | [197] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M.B.K. Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts 2020, 10, 464. https://doi.org/10.3390/catal10040464
Saleem Z, Pervaiz E, Yousaf MU, Niazi MBK. Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts. 2020; 10(4):464. https://doi.org/10.3390/catal10040464
Chicago/Turabian StyleSaleem, Zubia, Erum Pervaiz, M. Usman Yousaf, and M. Bilal Khan Niazi. 2020. "Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review" Catalysts 10, no. 4: 464. https://doi.org/10.3390/catal10040464
APA StyleSaleem, Z., Pervaiz, E., Yousaf, M. U., & Niazi, M. B. K. (2020). Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts, 10(4), 464. https://doi.org/10.3390/catal10040464






