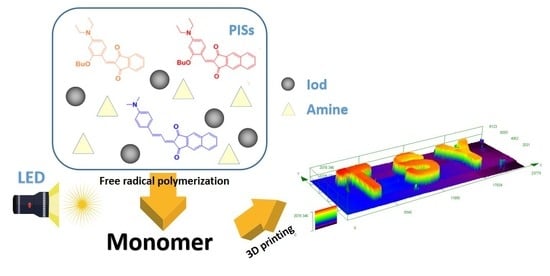
Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems
Abstract
1. Introduction
2. Results
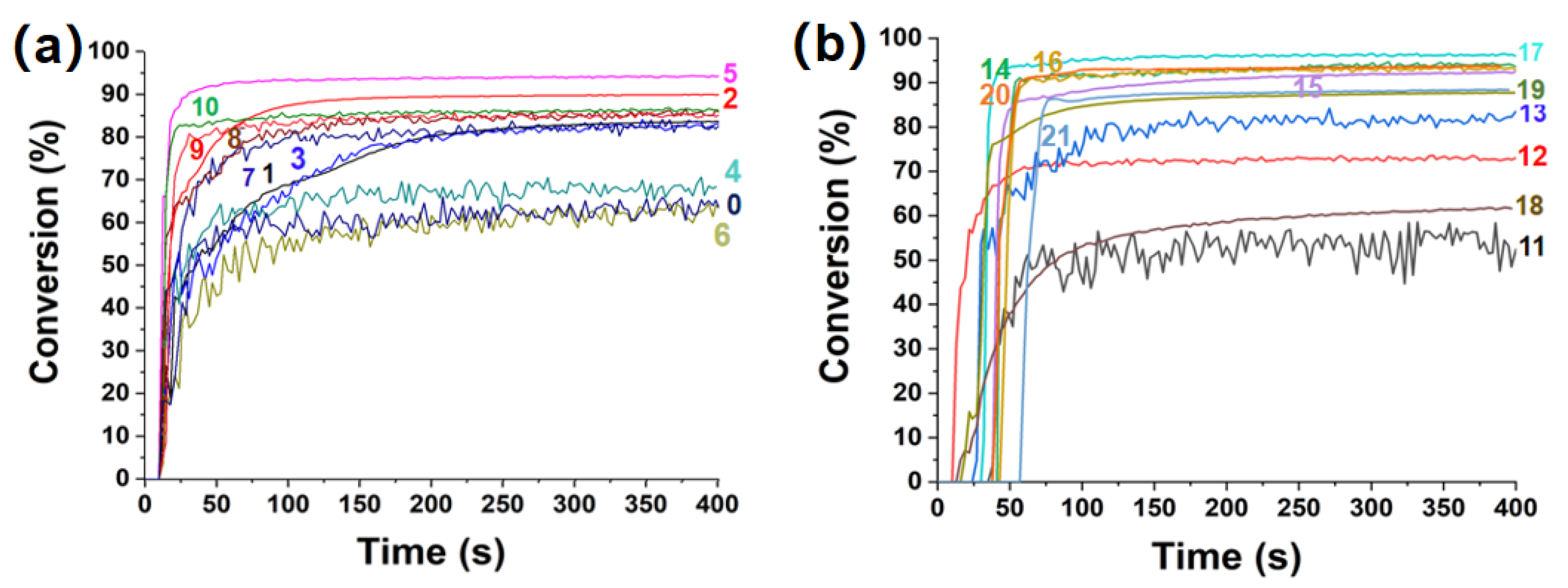
Photopolymerization Kinetics for the Proposed Dyes in Three-Component Photoinitiating Systems
3. Discussion
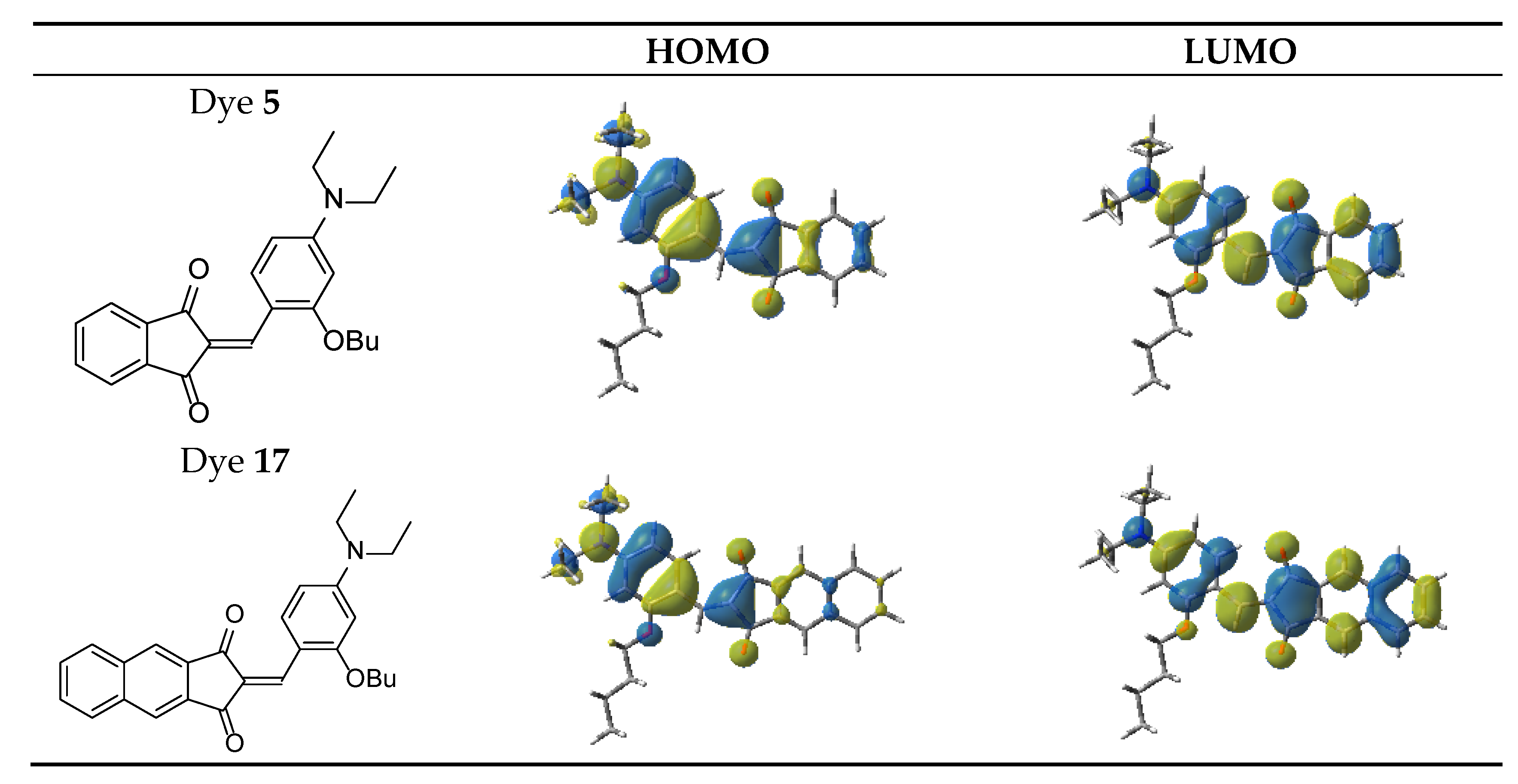
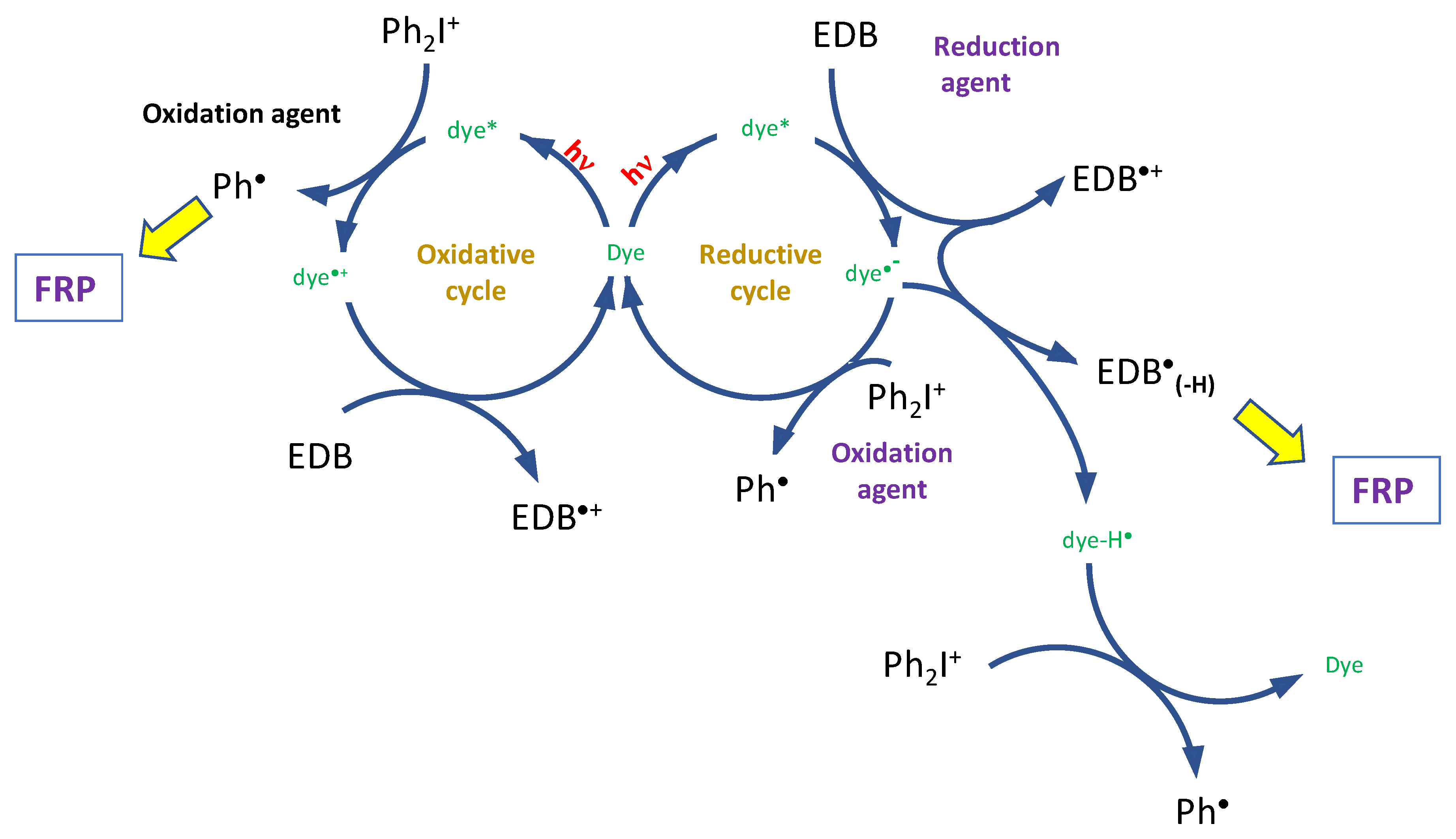
3.1. Proposed Chemical Mechanisms
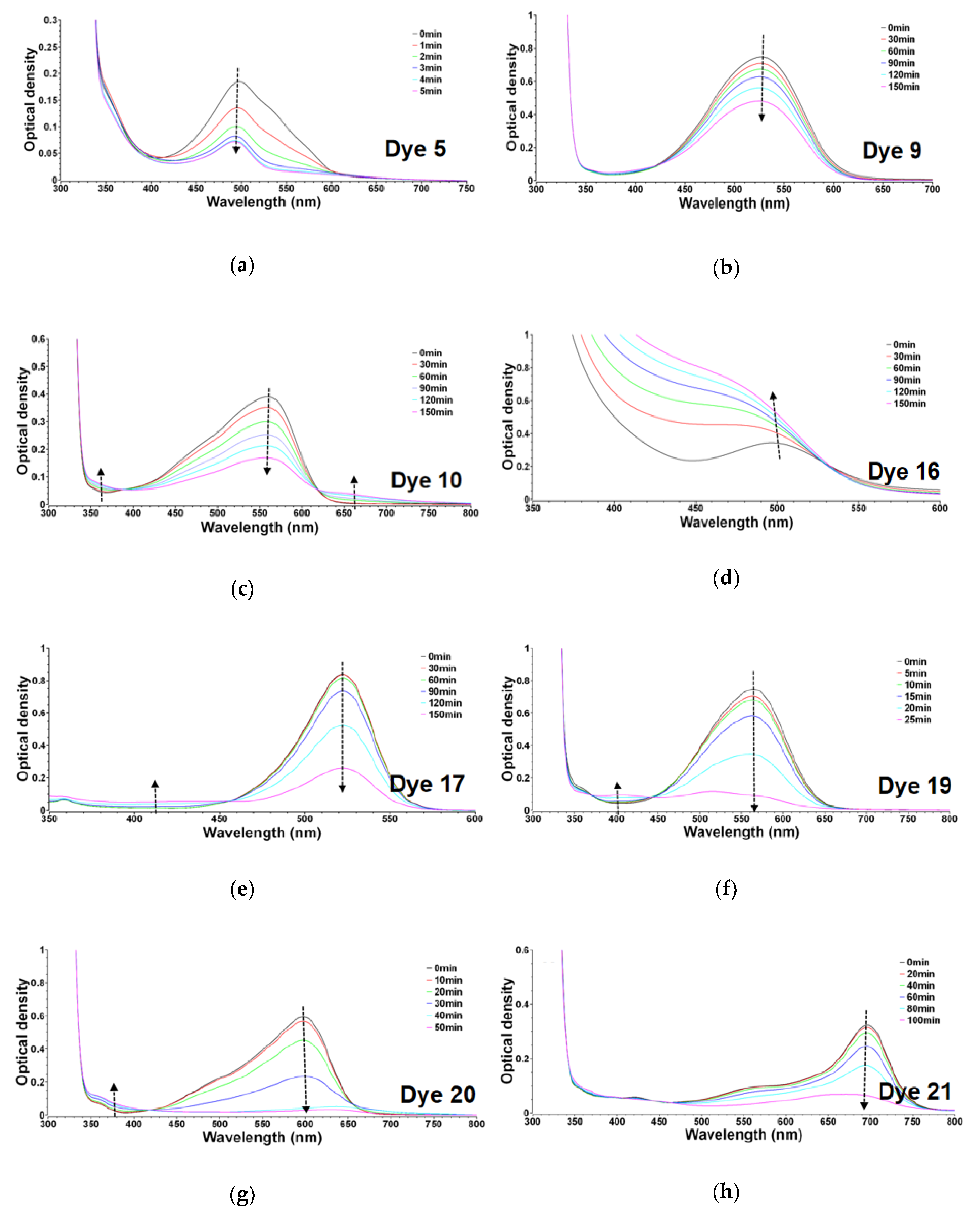
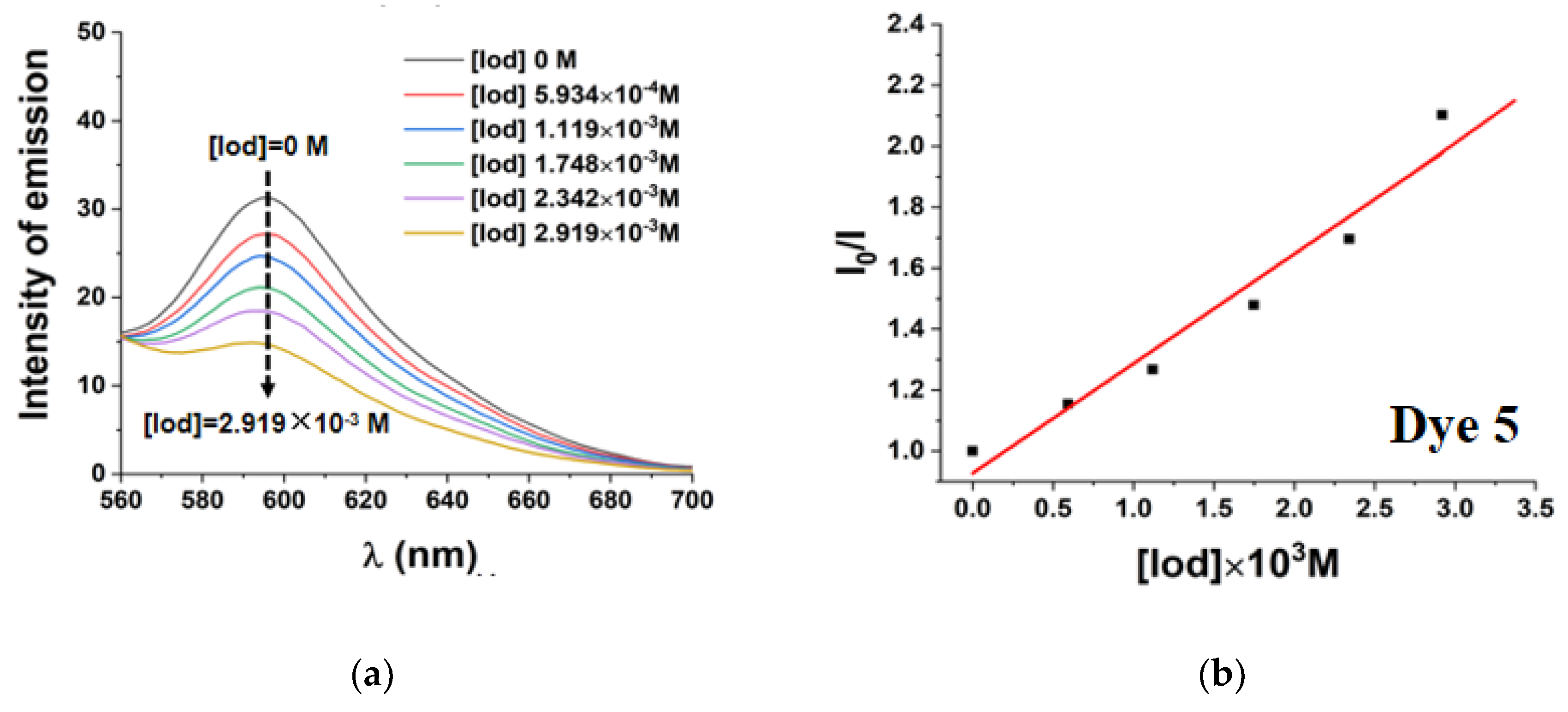
3.1.1. UV-Visible Absorption and Steady State Photolysis of the Selected Ten Dyes
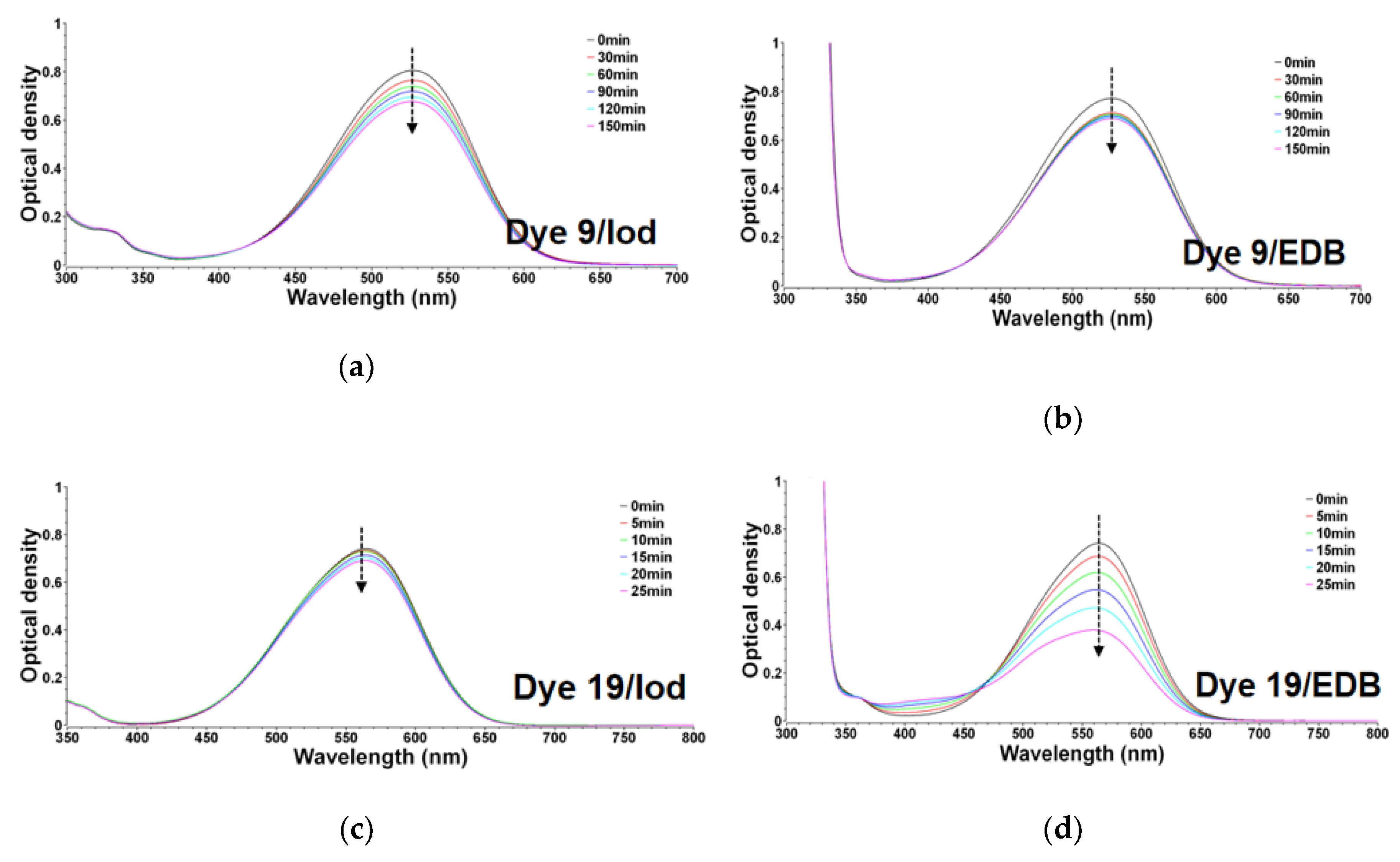
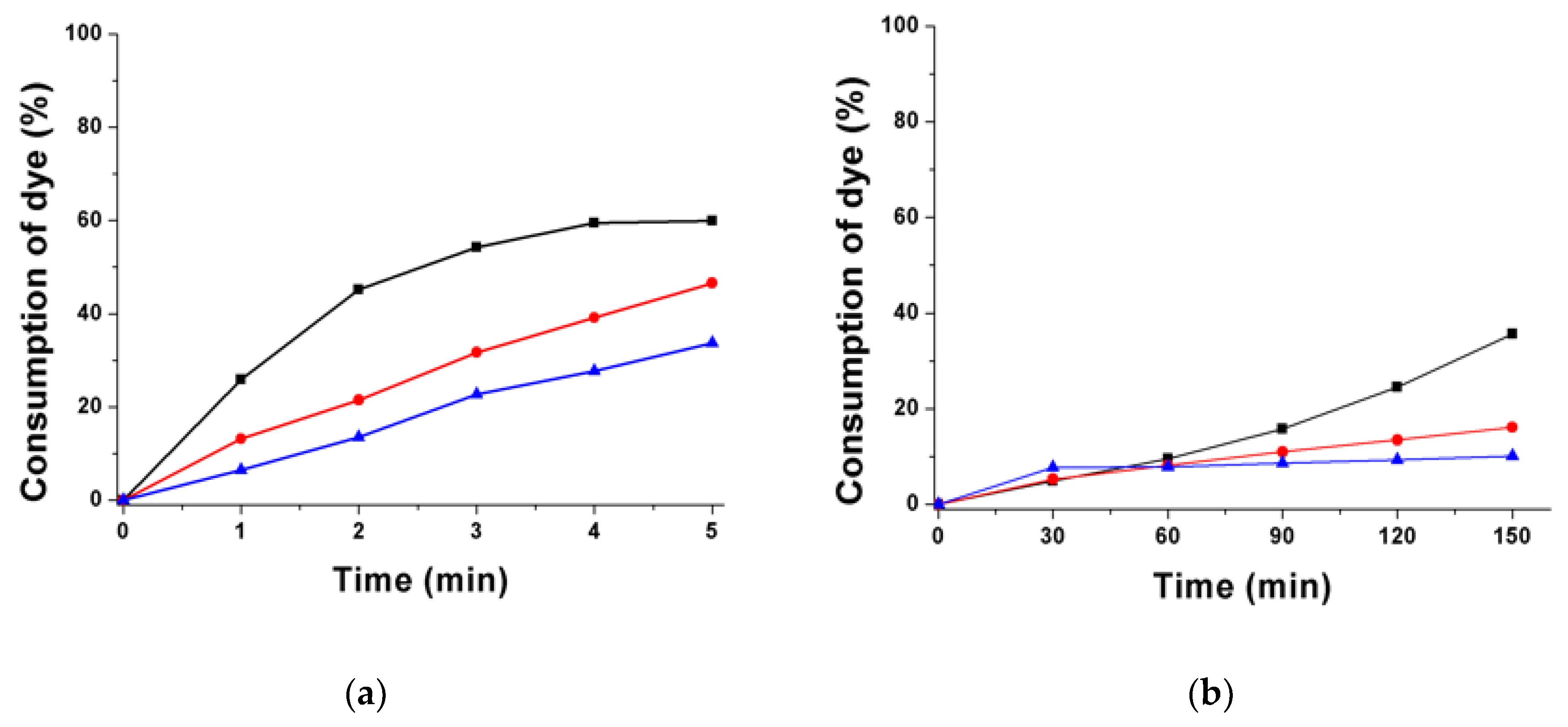
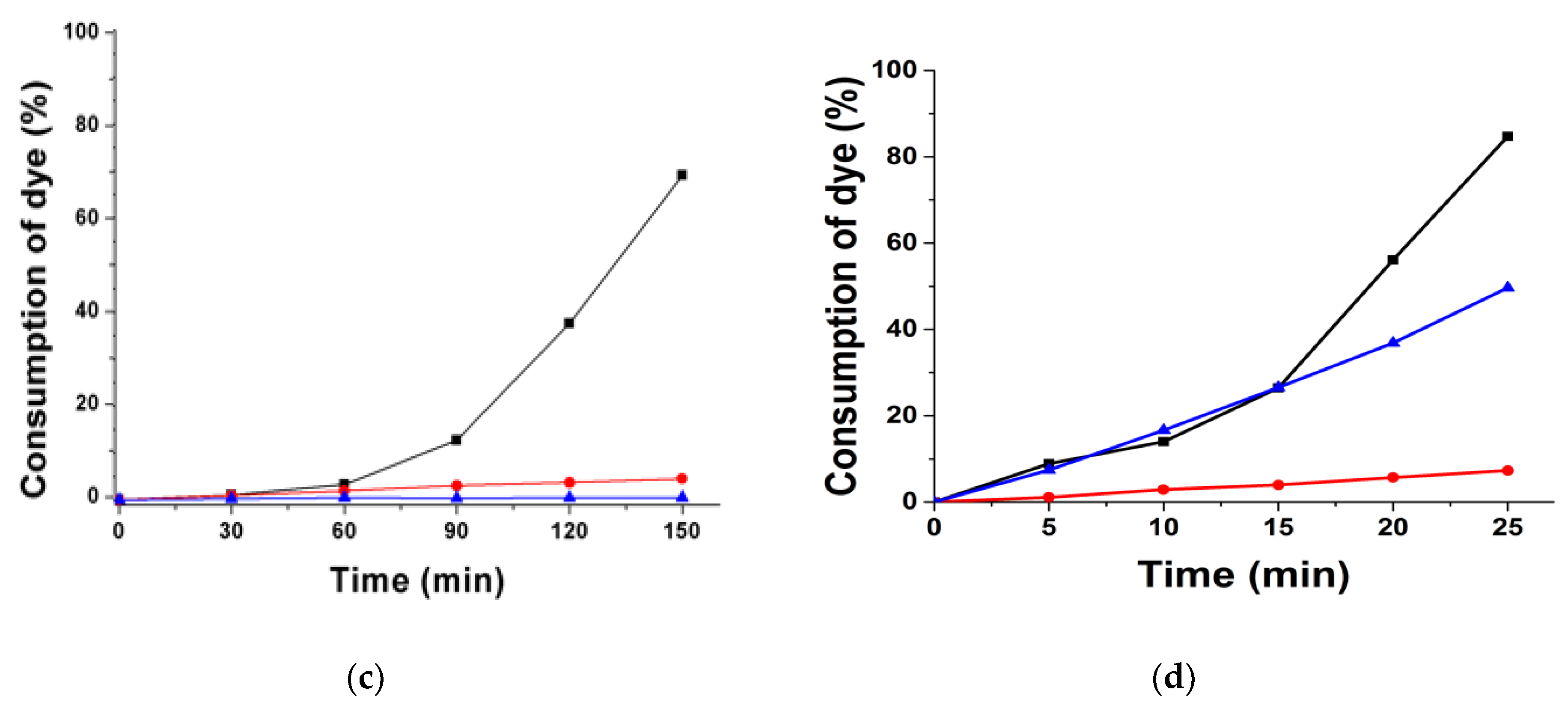
3.1.2. Consumption of Dyes in Photolysis Reactions
3.1.3. Chemical Mechanisms in Electron Transfer Reactions for Dyes
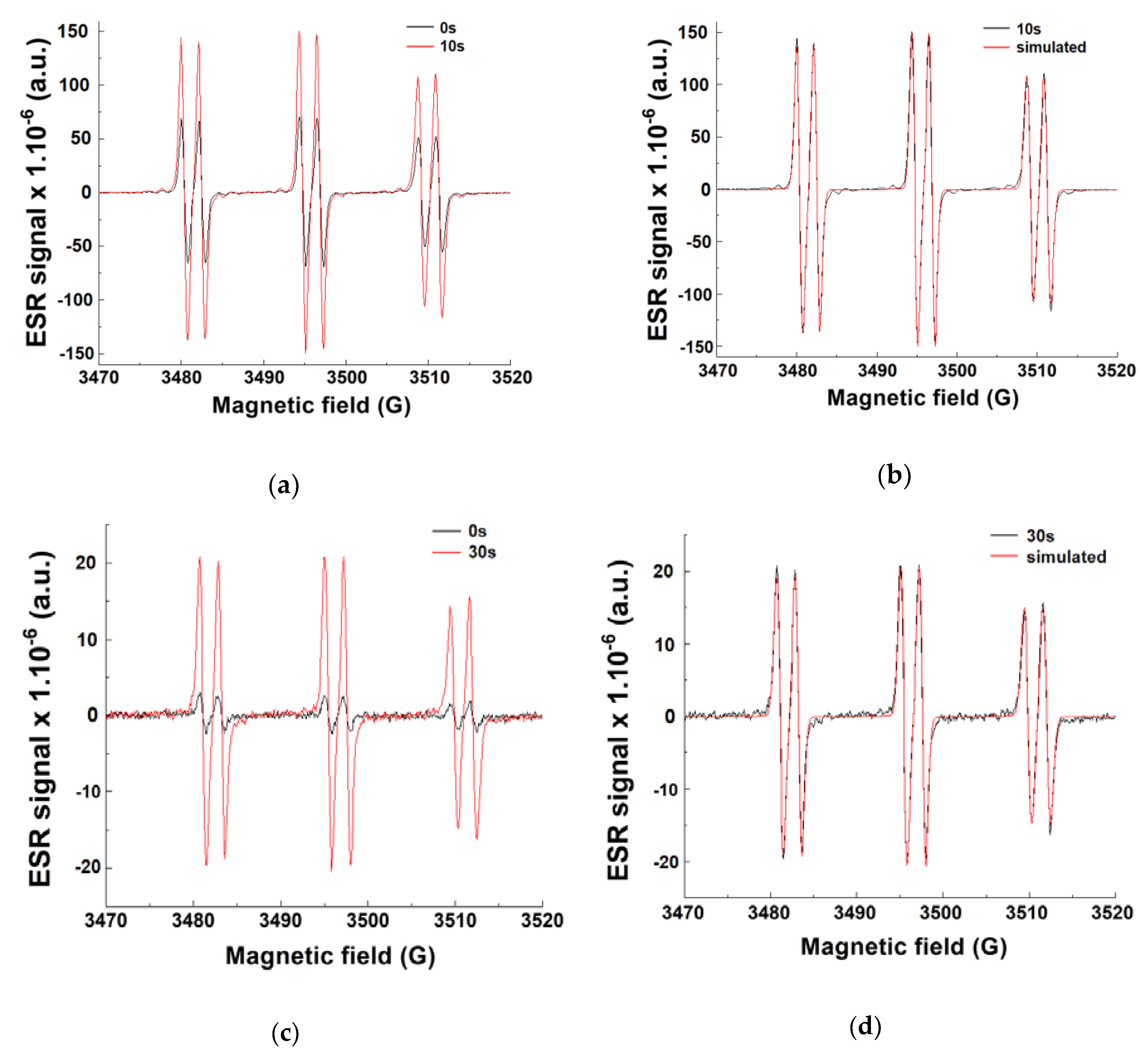
3.1.4. ESR Spin-Trapping Experiments
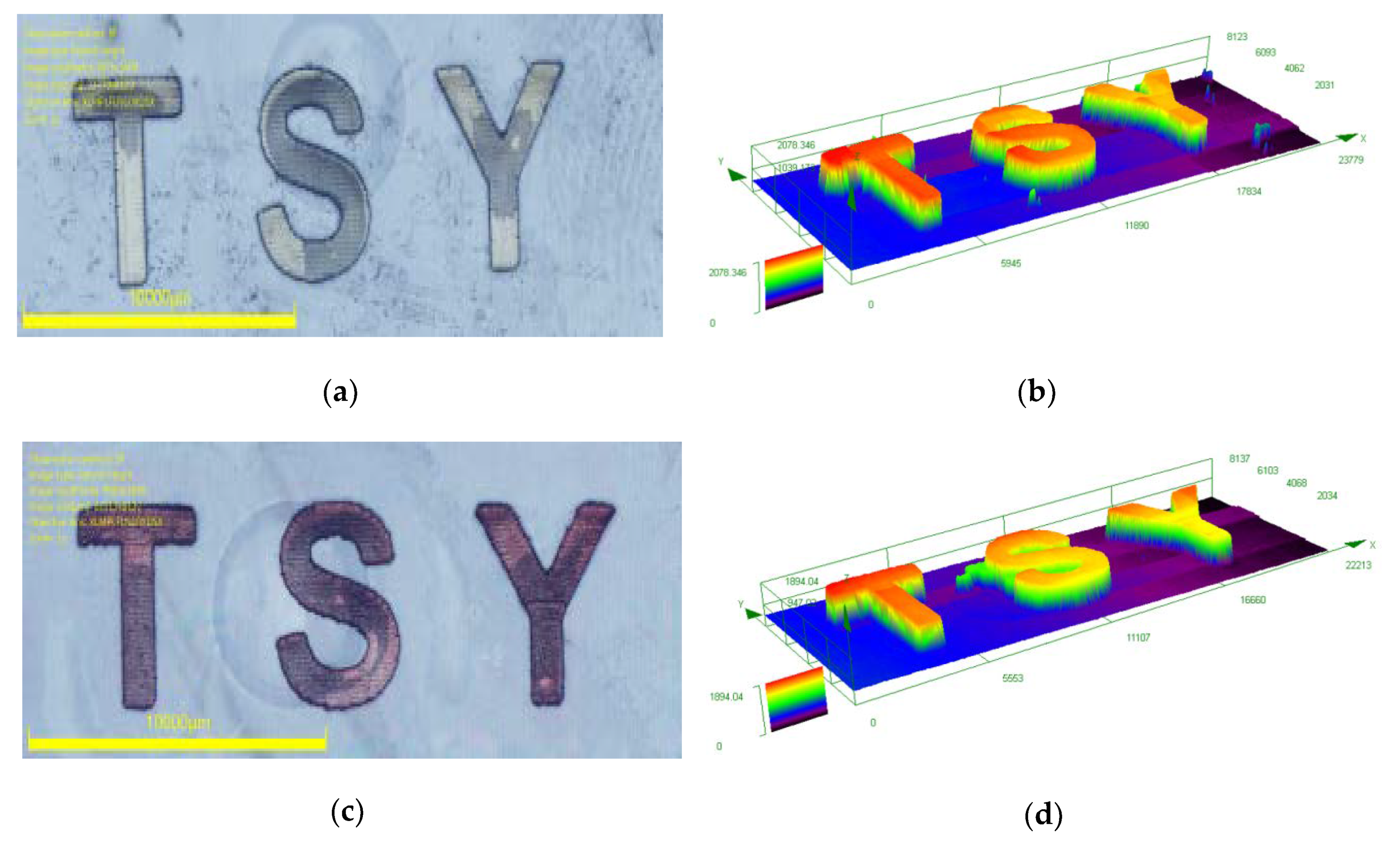
3.2. Laser Write Experiments Based on Dyes 5 and 19
4. Materials and Methods
4.1. Dyes
Synthesis of 2-(2-(3,3-bis(4-(dimethylamino)phenyl)allylidene)-3-oxo-2,3-dihydro-1H-cyclopenta[b] naphthalen-1-ylidene)malononitrile (dye 21)
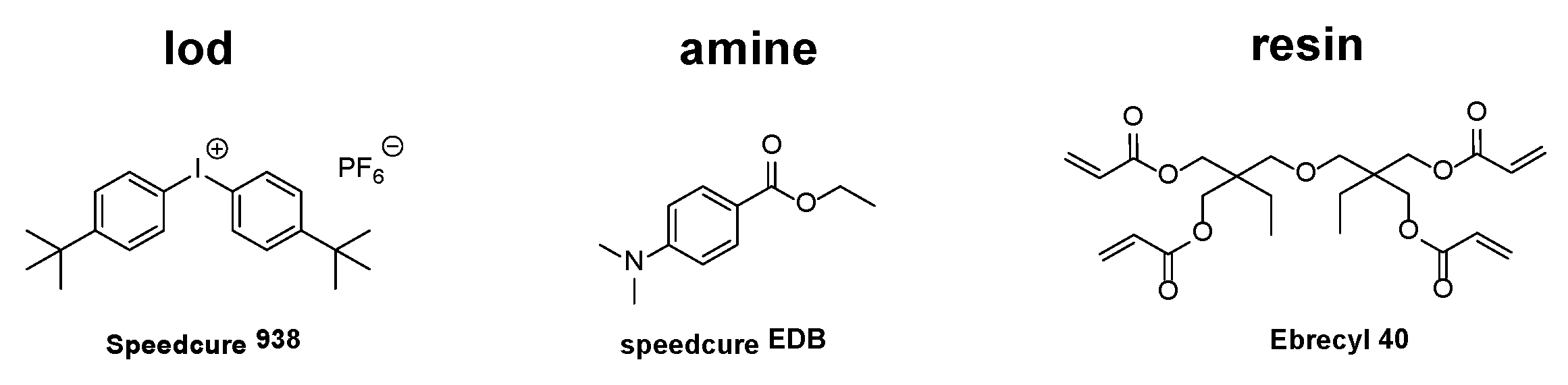
4.2. Other Materials
4.3. Free Radical Polymerization (FRP) Process Monitored by Real Time Fourier Transformed Infrared Spectroscopy (RT-FTIR)
4.4. UV-Visible Absorption, Photolysis and Fluorescent Properties
4.5. Redox Potentials
4.6. 3D Printing Experiments
4.7. Electron Spin Resonance (ESR) Spin Trapping (ESR-ST)
4.8. Computational Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures. Diacylgermane-based photoinitiators for visible light curing. Macromolecules 2008, 41, 2394–2400. [Google Scholar] [CrossRef]
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New Photocleavable Structures, 4: Acylgermane-Based Photoinitiator for Visible Light Curing. Macromol. Rapid Commun. 2008, 29, 57–62. [Google Scholar] [CrossRef]
- Cook, W.D.; Chen, F. Enhanced visible radiation photopolymerization of dimethacrylates with the three component thioxanthone (CPTXO)–amine–iodonium salt system. Polym. Chem. 2015, 6, 1325–1338. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, Y.; Dai, M.; Wu, G.; Shi, S.; Nie, J. Synthesis and photopolymerization kinetics of benzophenone piperazine one-component initiator. Polym. Adv. Technol. 2008, 19, 409–413. [Google Scholar] [CrossRef]
- Karaca, N.; Balta, D.K.; Ocal, N.; Arsu, N.J. Mechanistic studies of thioxanthone–carbazole as a one-component type II photoinitiator. J. Lumin. 2014, 146, 424–429. [Google Scholar] [CrossRef]
- Esen, D.S.; Temel, G.; Balta, D.K.; Allonas, X.; Arsu, N. One-component thioxanthone acetic acid derivative photoinitiator for free radical polymerization. Photochem. Photobiol. 2014, 90, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Sangermano, M.; Bongiovanni, R.; Burtscher, P.; Moszner, N. Visible light curable restorative composites for dental applications based on epoxy monomer. Materials 2014, 7, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Fors, B.P.; Hawker, C. Control of a living radical polymerization of methacrylates by light. J. Angew. Chem. Int. Ed. 2012, 51, 8850–8853. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Schroöder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible light and sunlight photoinduced ATRP with ppm of Cu catalyst. ACS Macro Lett. 2012, 1, 1219–1223. [Google Scholar] [CrossRef]
- Haddleton, D.M. Polymer chemistry: Rooftop reactions. Nat. Chem. 2013, 5, 366–368. [Google Scholar] [CrossRef]
- Treat, N.J.; Fors, B.P.; Kramer, J.W.; Christianson, M.; Chiu, C.-Y.; Alaniz, J.R.D.; Hawker, C.J. Controlled radical polymerization of acrylates regulated by visible light. ACS Macro Lett. 2014, 3, 580–584. [Google Scholar] [CrossRef]
- Xu, J.; Boyer, C. Visible light photocatalytic thiol–ene reaction: An elegant approach for fast polymer post-functionalization and step-growth polymerization. Macromolecules 2015, 48, 520–529. [Google Scholar] [CrossRef]
- Kenning, N.S.; Ficek, B.A.; Hoppe, C.C.; Scranton, A.B. Spatial and temporal evolution of the photoinitiation rate for thick polymer systems illuminated by polychromatic light: Selection of efficient photoinitiators for LED or mercury lamps. Polym. Int. 2008, 57, 1134–1140. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A novel photopolymerization initiating system based on an iridium complex photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Metal and metal-free photocatalysts: Mechanistic approach and application as photoinitiators of photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Allonas, X.; Fouassier, J.P. Addition of carbon-centered radicals to double bonds: Influence of the alkene structure. J. Org. Chem. 2005, 70, 814–819. [Google Scholar] [CrossRef]
- Geérard, V.; Ay, E.; Morlet-Savary, F.; Graff, B.; Galopin, C.; Ogren, T.; Mutilangi, W.; Lalevée, J. Thermal and photochemical stability of anthocyanins from black carrot, grape juice, and purple sweet potato in model beverages in the presence of ascorbic acid. J. Agric. Food Chem. 2019, 67, 5647–5660. [Google Scholar] [CrossRef]
- Aparicio, J.L.; Elizalde, M. Migration of photoinitiators in food packaging: A Review. Packag. Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- Lago, M.A.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Bustos, J.; Nieto, M.T.; Paseiro, P. Photoinitiators: A food safety review. Food Addit. Contam. Part A 2015, 32, 779–798. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Roth, M.; Hennen, D.; Mostegel, F.H.; Edler, M.; Kappaun, S.; Griesser, T. Low migration type I photoinitiators for biocompatible thiol-ene formulations. Eur. Polym. J. 2017, 88, 393–402. [Google Scholar] [CrossRef]
- Taschner, R.; Gauss, P.; Knaack, P.; Liska, R. Biocompatible photoinitiators based on poly-α-ketoesters. J. Polym. Sci. 2020, 58, 242–253. [Google Scholar] [CrossRef]
- Leonhardt, S.; Klare, M.; Scheer, M.; Fischer, T.; Cordes, B.; Eblenkamp, M. Biocompatibility of photopolymers for additive manufacturing. Curr. Dir. Biomed. Eng. 2016, 2, 113–116. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Ruhlmann, D.; Graff, B.; Morlet-Savary, F.; Wieder, F. Excited state processes in polymerization photoinitiators. Progr. Org. Coat. 1995, 25, 235–271. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Guillaume, N.; Goubard, F.; Bui, T.-T.; Villotte, S.; Dietlin, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Dumur, F.; et al. A novel class of photoinitiators with a thermally activated delayed fluorescence (TADF) property. New J. Chem. 2018, 42, 8261–8270. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Photocatalysts in polymerization reactions. ChemCatChem 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogana, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Mitterbauer, M.; Knaack, P.; Naumov, S.; Markovic, M.; Ovsianikov, A.; Moszner, N.; Liska, R. Acylstannanes: Cleavable and highly reactive photoinitiators for radical photopolymerization at wavelengths above 500 nm with excellent photobleaching behavior. Angew. Chem. Int. Ed. 2018, 57, 12146–12150. [Google Scholar] [CrossRef]
- Radebner, J.; Eibel, A.; Leypold, M.; Jungwirth, N.; Pickl, T.; Torvisco, A.; Fischer, R.; Fischer, U.K.; Moszner, N.; Gescheidt, G.; et al. Tetraacylstannanes as long-wavelength visible-light photoinitiators with intriguing low toxicity. Chem. Eur. J. 2018, 24, 8281–8285. [Google Scholar] [CrossRef]
- Eibel, A.; Fast, D.E.; Gescheidt, G. Choosing the ideal photoinitiator for free radical photopolymerizations: Predictions based on simulations using established data. Polym. Chem. 2018, 9, 5107–5115. [Google Scholar] [CrossRef]
- Eibel, A.; Radebner, J.; Haas, M.; Fast, D.E.; Freißmuth, H.; Stadler, E.; Faschauner, P.; Torvisco, A.; Lamparth, I.; Moszner, N.; et al. From mono-to tetraacylgermanes: Extending the scope of visible light photoinitiators. Polym. Chem. 2018, 9, 38–47. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Zivic, N.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.-P.; Lalevée, J. New violet to yellow light sensitive diketopyrrolo-pyrrole photoinitiators: High performance systems with unusual bleaching properties and solubility in water. Polym. Chem. 2017, 8, 2028–2040. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of furan derivative as LED light photoinitiator: One-pot, low usage, photobleaching for light color 3D printing. Dyes Pigm. 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. A benzophenone-naphthalimide derivative as versatile photoinitiator for near UV and visible lights. J. Polym. Sci. A Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High performance near infrared (NIR) photoinitiating systems operating under low light intensity and in presence of oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Guda, R.; Bhaskar, A.; Goodson, T. Ultrafast excited state relaxation dynamics of branched donor-π-acceptor chromophore: Evidence of a charge-delocalized state. J. Phys. Chem. B 2006, 110, 20872–20878. [Google Scholar]
- Bures, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Nechab, M.; Gigmes, D.; Dumur, F. Unprecedented Nucleophilic Attack of Piperidine on the Electron Acceptor during the Synthesis of Push-Pull Dyes by a Knoevenagel Reaction. Helv. Chim. Acta 2019, 102, e1900229. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis—Scope, Reactivity, and Efficiency; John Wiley & Sons: Weinheim, Germany, 2012. [Google Scholar]
- Tehfe, M.A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Blanchard, N.; Fouassier, J.P. Organic photocatalyst for polymerization reactions: 9,10-bis[(triisopropylsilyl)ethynyl]anthracene. ACS Macro Lett. 2012, 1, 198–203. [Google Scholar] [CrossRef]
- Oster, G. Dye-Sensitized Photopolymerization. Nature 1954, 173, 300–301. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-red light sensitive push-pull structured photoinitiators: Indanedione derivatives for radical and cationic photopolymerization reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push-pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm Laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Julolidine or fluorenone based push-pull dyes for polymerization upon soft polychromatic visible light or green light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New push-pull dyes derived from Michler’s ketone for polymerization reactions upon visible lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Thirion, D.; Fagour, D.; Vacher, A.; Sallenave, X.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Multicolor photoinitiators for radical and cationic polymerization: Mono vs. poly functional thiophene derivatives. Macromolecules 2013, 46, 6786–6793. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Green light sensitive diketopyrrolopyrrole derivatives used in versatile photoinitiating systems for photo-polymerizations. Polym. Chem. 2014, 5, 2293–2300. [Google Scholar] [CrossRef]
- Sanguinet, L.; Williams, J.C.; Yang, Z.; Twieg, R.J.; Mao, G.; Singer, K.D.; Wiggers, G.; Petschek, R.G. Synthesis and characterization of new truxenones for nonlinear optical applications. Chem. Mater. 2006, 18, 4259–4269. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.P. Intra- and intermolecular hydrogen bonds in pyrrolylindandione derivatives and their interaction with fluoride and acetate: Possible anion sensing properties. J. Phys. Chem. A 2013, 117, 11346–11356. [Google Scholar] [CrossRef]
- Feng, H.; Qiu, N.; Wang, X.; Wang, Y.; Kan, B.; Wan, X.; Zhang, M.; Xia, A.; Li, C.; Liu, F.; et al. An A-D-A type small-molecule electron acceptor with end-extended conjugation for high performance organic solar cells. Chem. Mater. 2017, 29, 7908–7917. [Google Scholar] [CrossRef]
- Li, R.; Liu, G.; Xiao, M.; Yang, X.; Liu, X.; Wang, Z.; Ying, L.; Huang, F.; Cao, Y. Non-fullerene acceptors based on fused-ring oligomers for efficient polymer solar cells via complementary light-absorption. J. Mater. Chem. A 2017, 5, 23926–23936. [Google Scholar] [CrossRef]
- Knoevenagel, E. Ueber eine Darstellungsweise des Benzylidenacetessigesters. Ber. Dtsch. Chem. Ges. 1896, 29, 172–174. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Naphthalic anhydride derivatives: Structural effects on their initiating abilities in radical and/or cationic photopolymerizations under visible light. J. Polym. Sci. A Polym. Chem. 2015, 53, 2860–2866. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Amino and nitro substituted 2-amino-1H-benzo-[de]isoquinoline-1,3(2H)-diones: As versatile photoinitiators of polymerization from violet-blue LED absorption to a panchromatic behavior. Polym. Chem. 2015, 6, 1171–1179. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Naphthalimide-phthalimide derivative based photoinitiating systems for polymerization reactions under blue lights. J. Polym. Sci. A Polym. Chem. 2015, 53, 665–674. [Google Scholar] [CrossRef]
- Toba, Y.; Saito, M.; Usui, Y. Cationic photopolymerization of epoxides by direct and sensitized photolysis of onium tetrakis(pentafluorophenyl)borate initiators. Macromolecules 1999, 32, 3209–3215. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Dye-sensitized photoinitiated cationic polymerization. The system: Perylene-triarylsulfonium salts. J. Polym. Sci. Part A Polym. Chem. 1979, 17, 1059–1065. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Design of high performance photoinitiators at 385−405 nm: Search around the naphthalene scaffold. Macromolecules 2014, 47, 973–978. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on pyrene-based photoinitiators of polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Cationic and thiol-ene photopoly-merization upon red lights using anthraquinone derivatives as photoinitiators. Macromolecules 2013, 46, 6744–6750. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Vidal, L.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural effects in the indanedione skeleton for the design of low intensity 300–500 nm light sensitive initiators. Macromolecules 2014, 47, 26–34. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Red-light-induced cationic photopolymerization: Perylene derivatives as efficient photoinitiators. Macromol. Rapid. Commun. 2013, 34, 1452–1458. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Arar, A.; Ibrahim-Ouali, M.; Duval, S.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; et al. Carbazole-based compounds as photoinitiators for free radical and cationic polymerization upon near visible light illumination. Photochem. Photobiol. Sci. 2018, 17, 578–585. [Google Scholar] [CrossRef]
- Zhang, J.; Sallenave, X.; Bui, T.-T.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. LED-Induced Polymerization (385, 405, and 455 nm) Using Star-Shaped Tris(4-(thiophen-2-yl)phenyl) amine Derivatives as Light-Harvesting Photoinitiators. Macromol. Chem. Phys. 2015, 216, 218–227. [Google Scholar] [CrossRef]
- Romanczyk, P.P.; Kurek, S.S. The Reduction Potential of Diphenyliodonium Polymerisation Photoinitiator Is Not −0.2 V vs. SCE. A Computational Study. Electrochim. Acta 2017, 225, 482–485. [Google Scholar] [CrossRef]
- Haire, L.D.; Krygsman, P.H.; Janzen, E.G.; Oehler, U.M. Correlation of radical structure with EPR spin adduct parameters: Utility of the proton, carbon-13, and nitrogen-14 hyperfine splitting constants of aminoxyl adducts of PBN-nitronyl-13C for three-parameter scatter plots. J. Org. Chem. 1988, 53, 4535–4542. [Google Scholar] [CrossRef]
- Ohto, N.; Niki, E.; Kamiya, Y. Study of autoxidation by spin trapping. Spin trapping of peroxyl radicals by phenyl N-t-butyl nitrone. J. Chem. Soc. Perkin Trans. 2 1977, 13, 1770–1774. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.-P. Green bulb light source induced epoxy cationic polymerization under air using tris(2, 2′-bipyridine) ruthenium (II) and silyl radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P. Efficient dual radical/cationic photoinitiator under visible light: A new concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structure design of naphthalimide derivatives: Toward versatile photoinitiators for near-UV/visible LEDs, 3D printing, and water-soluble photoinitiating systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Copper complexes in radical photoinitiating systems: Applications to free radical and cationic polymerization upon visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- James, B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods; Gaussian Inc.: Wallingford, CT, USA, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 03, Revision B-2; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
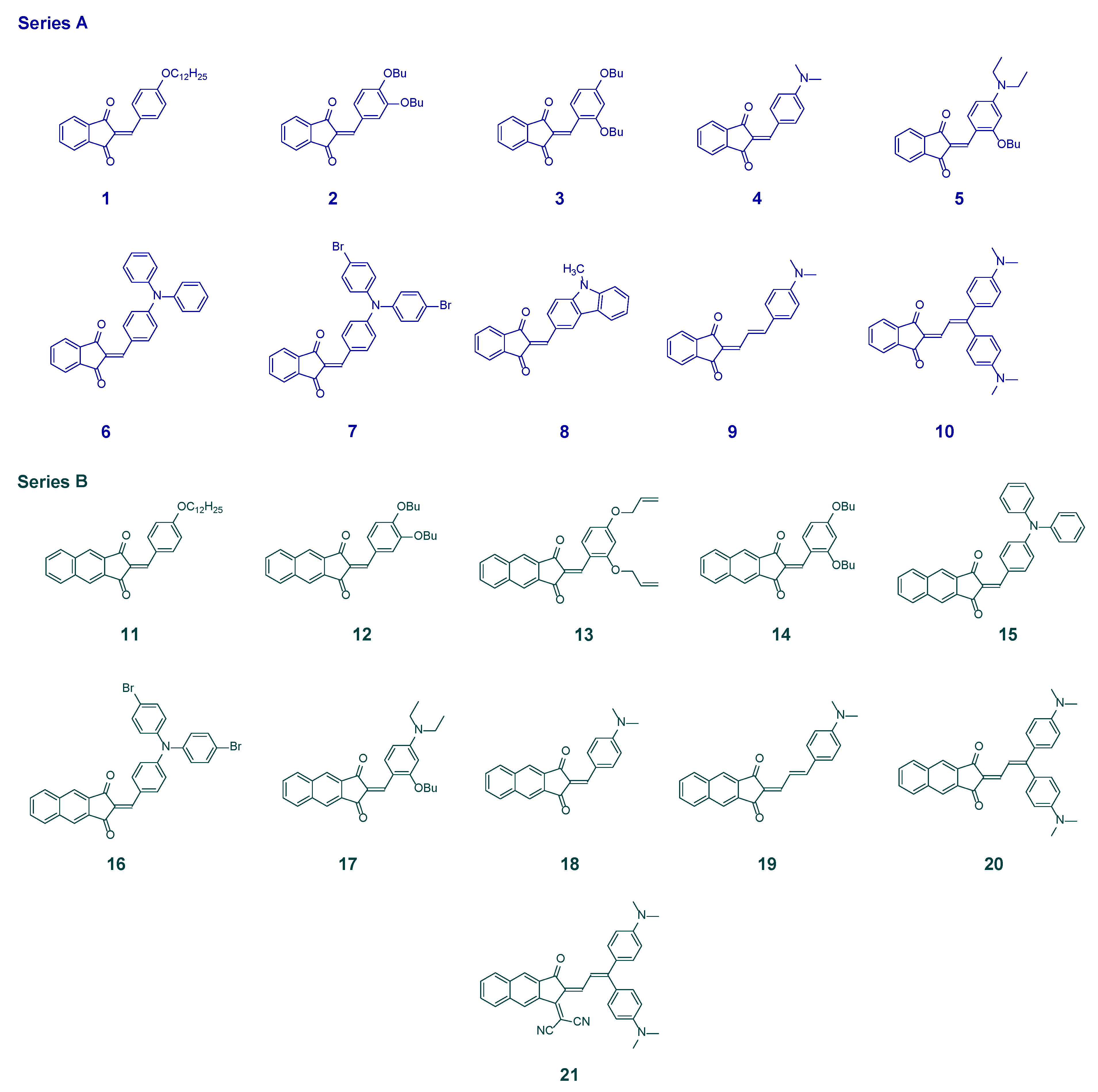



| Dye | 5 | 9 | 10 | 14 | 15 |
| FCs | 93% | 82% | 84% | 92% | 90% |
| Dye | 16 | 17 | 19 | 20 | 21 |
| FCs | 92% | 95% | 85% | 91% | 86% |
| Dyes | λmax (nm) | εmax (M−1 cm−1) | ε@405nm (M−1 cm−1) |
|---|---|---|---|
| Dye 5 | 498 | 19,900 | 6510 |
| Dye 9 | 526 | 51,110 | 350 |
| Dye 10 | 559 | 41,820 | 5520 |
| Dye 14 | 449 | 23,180 | 10,930 |
| Dye 15 | 504 | 56,620 | 3130 |
| Dye 16 | 498 | 33,740 | 6790 |
| Dye 17 | 522 | 106,650 | 2180 |
| Dye 19 | 564 | 55,720 | 3790 |
| Dye 20 | 598 | 56,280 | 1810 |
| Dye 21 | 696 | 56,330 | 7210 |
| Dye 5 | Dye 9 | Dye 16 | Dye 17 | Dye 19 | |
|---|---|---|---|---|---|
| Eox (eV) | 0.54 | 0.49 | 0.79 | 0.79 | 0.49 |
| Ered (eV) | −1.30 | −1.30 | −1.31 | −1.31 | −1.22 |
| ES1 (eV) b | 2.32 | 2.02 | 2.29 | 2.29 | 2.12 |
| ET1 (eV) c | 2.1 | 1.6 | 1.9 | 2.0 | 1.6 |
| ΔGS1Iod (eV) | −1.08 | −0.83 | −0.80 | −0.80 | −0.93 |
| ΔGS1EDB (eV) | −0.02 | 0.29 | 0.02 | 0.02 | 0.10 |
| ΔGetT1Iod (eV) | −0.86 | −0.41 | −0.41 | −0.51 | −0.41 |
| ΔGetT1EDB (eV) | 0.21 | 0.7 | 0.41 | 0.31 | 0.62 |
| Ksv Iod(M−1) | 359 | 2.62 | 64 | - | 1.86 |
| ϕet(S1) Iod d | 0.939 | 0.101 | 0.732 | - | 0.074 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems. Catalysts 2020, 10, 463. https://doi.org/10.3390/catal10040463
Sun K, Pigot C, Chen H, Nechab M, Gigmes D, Morlet-Savary F, Graff B, Liu S, Xiao P, Dumur F, et al. Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems. Catalysts. 2020; 10(4):463. https://doi.org/10.3390/catal10040463
Chicago/Turabian StyleSun, Ke, Corentin Pigot, Hong Chen, Malek Nechab, Didier Gigmes, Fabrice Morlet-Savary, Bernadette Graff, Shaohui Liu, Pu Xiao, Frédéric Dumur, and et al. 2020. "Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems" Catalysts 10, no. 4: 463. https://doi.org/10.3390/catal10040463
APA StyleSun, K., Pigot, C., Chen, H., Nechab, M., Gigmes, D., Morlet-Savary, F., Graff, B., Liu, S., Xiao, P., Dumur, F., & Lalevée, J. (2020). Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems. Catalysts, 10(4), 463. https://doi.org/10.3390/catal10040463