A 3D Hierarchical Pancake-Like Porous Carbon Nitride for Highly Enhanced Visible-Light Photocatalytic H2 Evolution
Abstract
1. Introduction
2. Results and Discussion
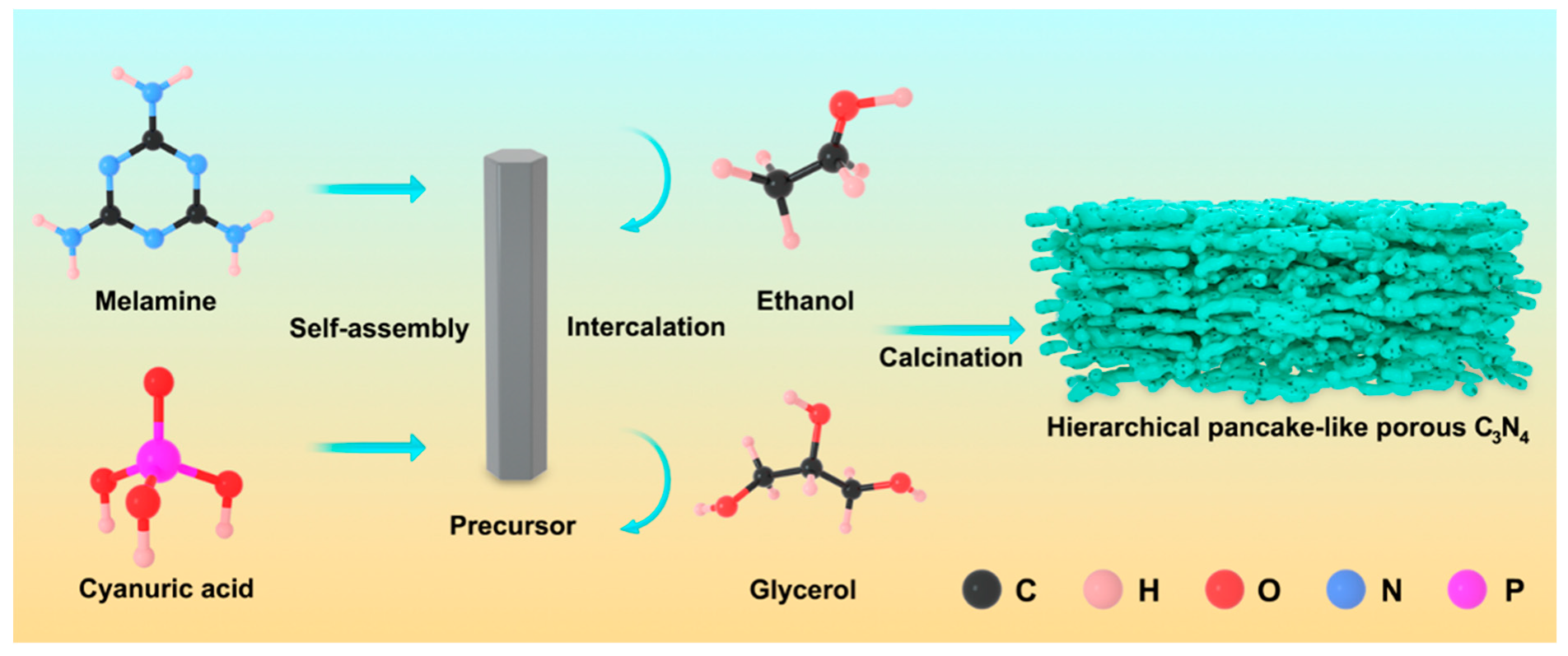
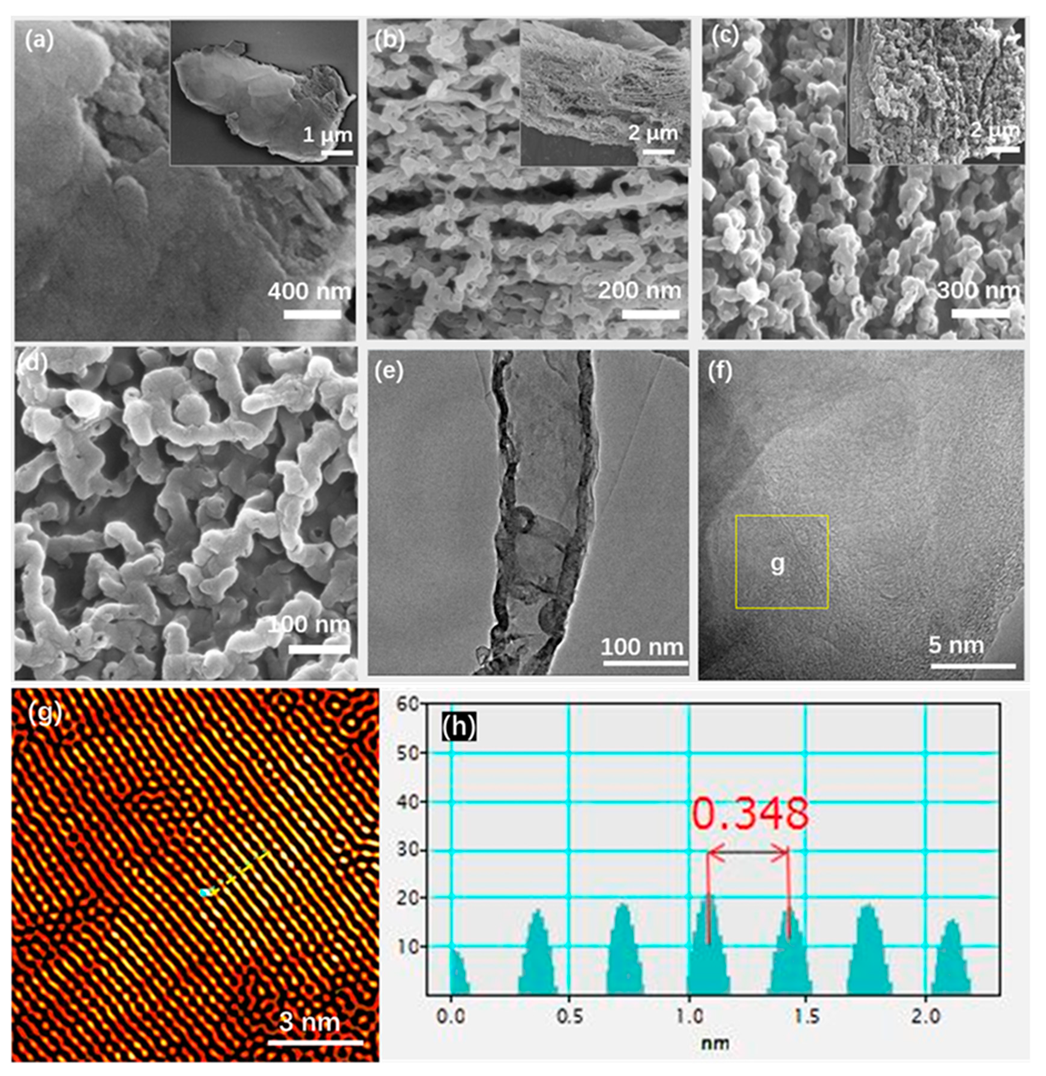
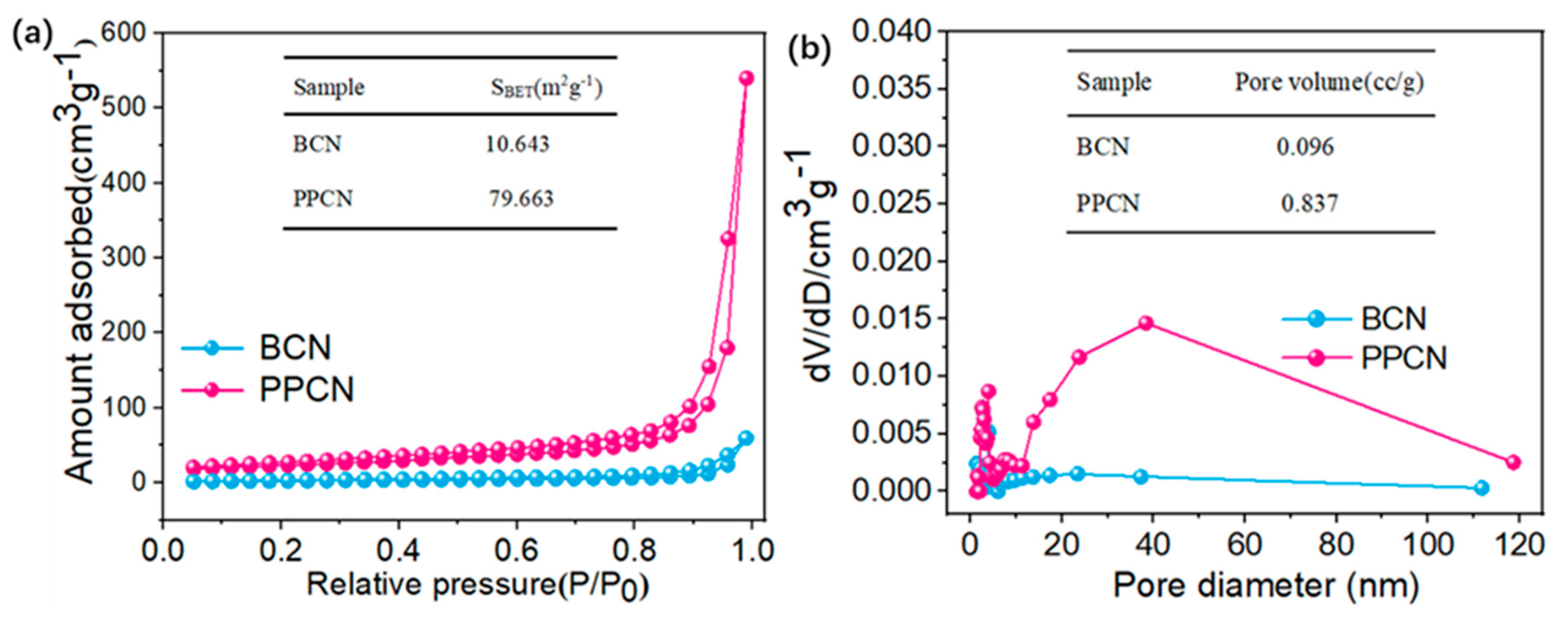
2.1. Morphology and Structure Characterization
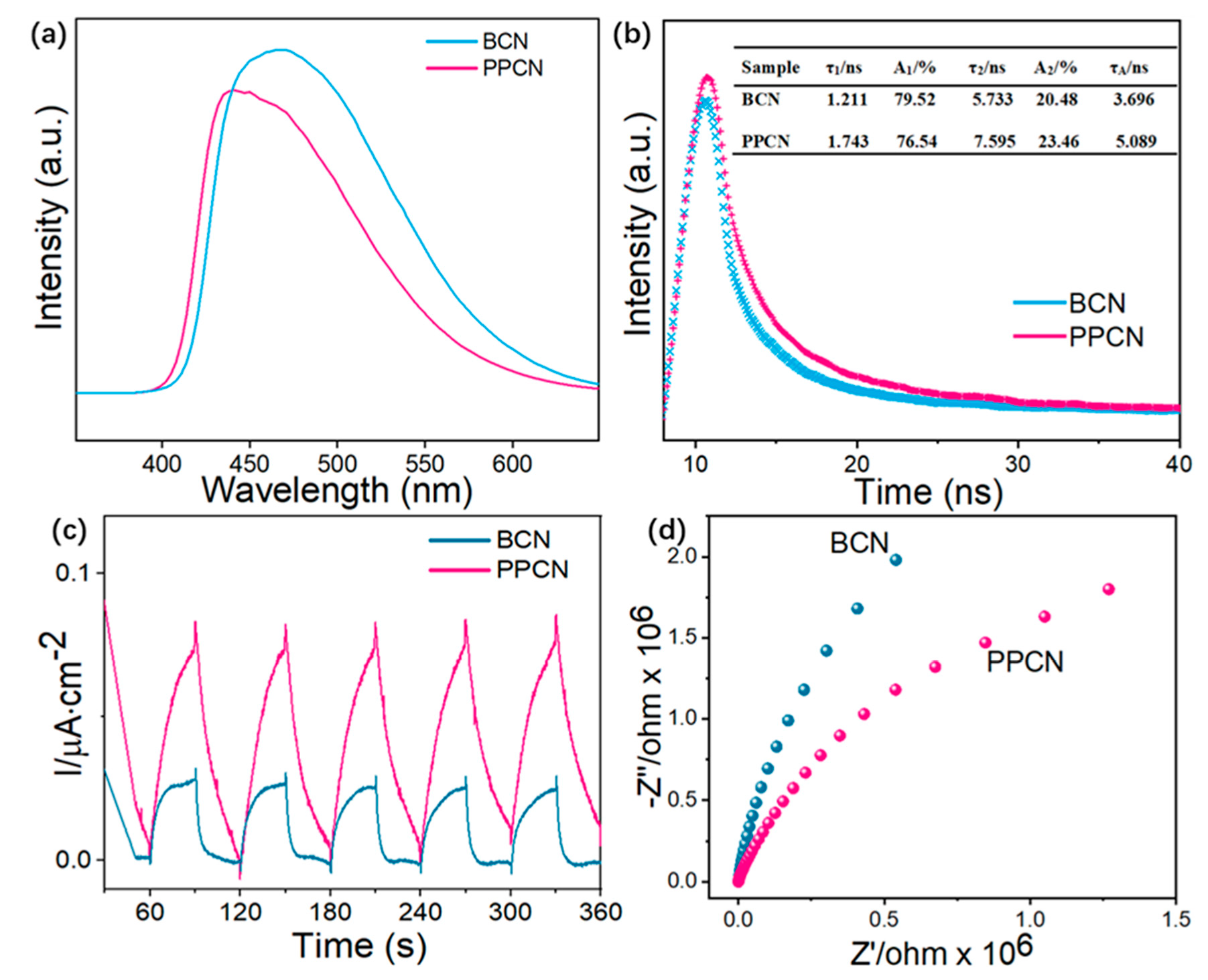
2.2. Photoelectric Property
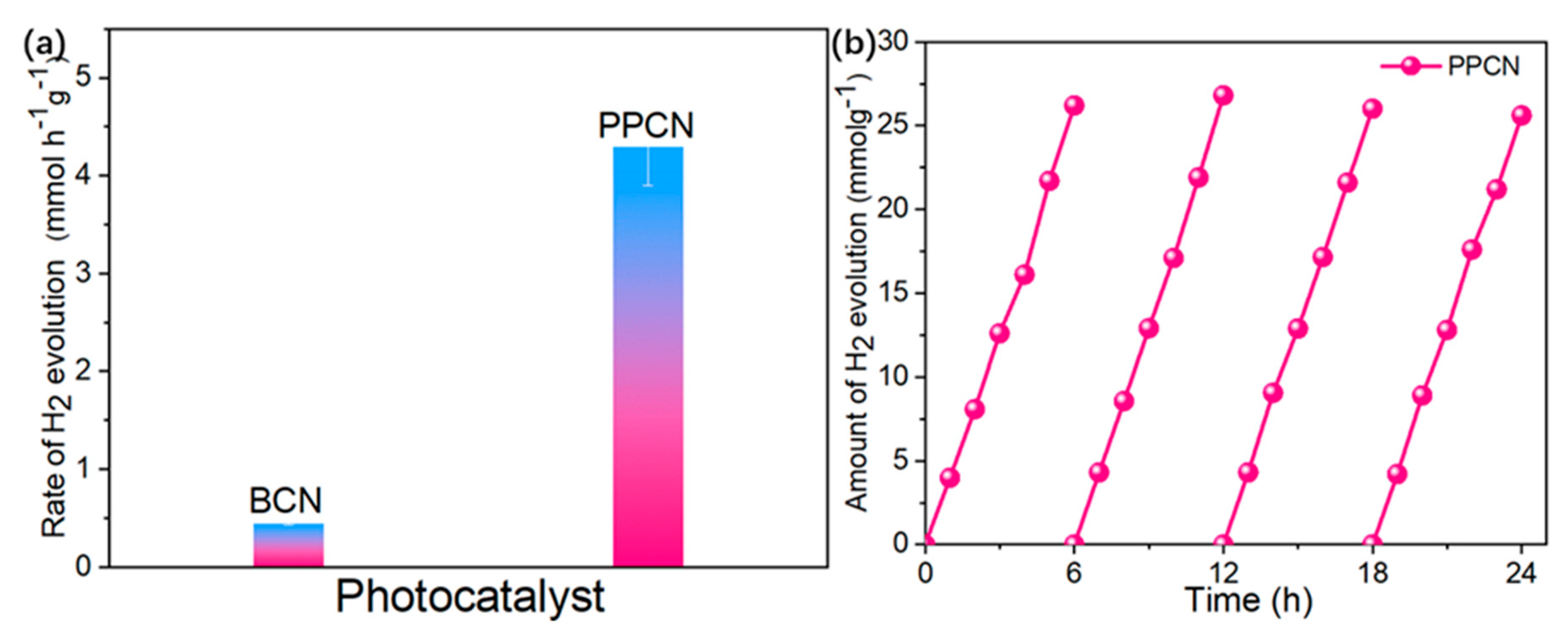
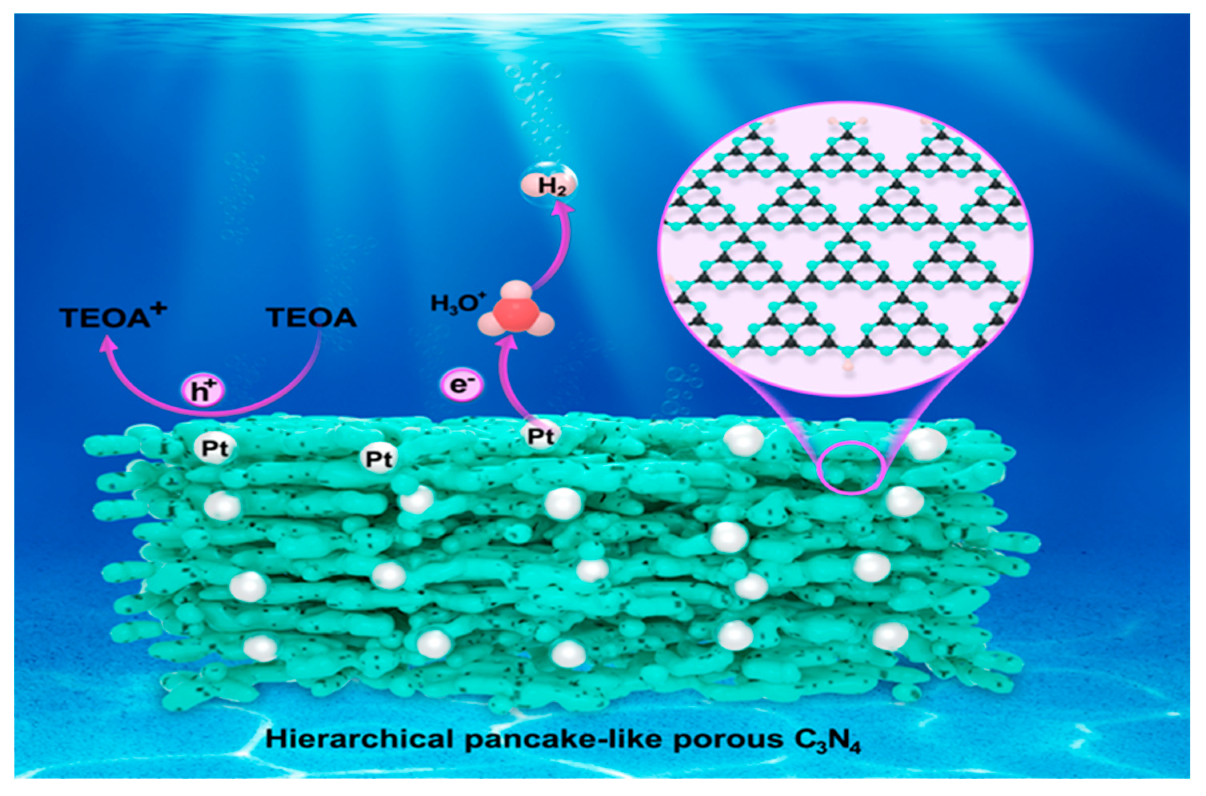
2.3. Photocatalytic Performance
3. Experimental
3.1. Reagents
3.2. Synthesis of Bulk C3N4
3.3. Synthesis of Precursor
3.4. Preparation of Hierarchical Pancake-Like Porous Carbon Nitride
3.5. Characterization
3.6. Electrochemical Analysis
3.7. Photocatalytic Hydrogen Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.M.; Okyay, M.S.; Ahmad, I.; Kim, S.J.; Park, N.; Jeong, H.Y.; Baek, J.B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, L.; Shi, J. Anion-Containing Noble-Metal-Free Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2018, 8, 3688–3707. [Google Scholar] [CrossRef]
- Wu, J.; Li, N.; Fang, H.-B.; Li, X.; Zheng, Y.-Z.; Tao, X. Nitrogen vacancies modified graphitic carbon nitride: Scalable and one-step fabrication with efficient visible-light-driven hydrogen evolution. Chem. Eng. J. 2019, 358, 20–29. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Davey, K.; Qiao, S.Z. Carbon, nitrogen and phosphorus containing metal-free photocatalysts for hydrogen production: Progress and challenges. J. Mater. Chem. A 2018, 6, 1305–1322. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Davey, K.; Mullins, C.B. Tuning the intrinsic properties of carbon nitride for high Quantum yield photocatalytic hydrogen production. Adv. Sci. 2018, 5, 1800820. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Mullins, C.B. Understanding charge transport in carbon nitride for enhanced photocatalytic solar fuel production. Acc. Chem. Res. 2019, 52, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Versatile graphene-promoting photocatalytic performance of semiconductors: Basic principles, synthesis, solar energy conversion, and environmental applications. Adv. Funct. Mater. 2013, 23, 4996–5008. [Google Scholar] [CrossRef]
- Kapilashrami, M.; Zhang, Y.; Liu, Y.S.; Hagfeldt, A.; Guo, J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Duan, P.; Baldini, M.; Huang, H.T.; Chen, B.; Juhl, S.J.; Koeplinger, D.; Crespi, V.H.; Schmidt-Rohr, K.; et al. Carbon nitride nanothread crystals derived from pyridine. J. Am. Chem. Soc. 2018, 140, 4969–4972. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Cui, W.; Wang, H.; Cen, W.; Johnson, G.; Jiang, G.; Zhang, S.; Dong, F. The spatially oriented charge flow and photocatalysis mechanism on internal van der waals heterostructures enhanced g-C3N4. ACS Catal. 2018, 8, 8376–8385. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Han, C.; Meng, P.; Waclawik, E.R.; Zhang, C.; Li, X.H.; Yang, H.; Antonietti, M.; Xu, J. Palladium/graphitic carbon nitride (g-C3N4) stabilized emulsion microreactor as a store for hydrogen from ammonia borane for use in alkene hydrogenation. Angew. Chem. Int. Ed. 2018, 57, 14857–14861. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Li, H.; Tong, T.; Chen, H.-C.; Yu, A.; Yu, J.; Chen, H.M. Single-atom engineering of directional charge transfer channels and active sites for photocatalytic hydrogen evolution. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Sun, N.; Tan, Y.; Wang, W.; Yan, C.; Wang, H. One-step synthesis of petals-like graphitic carbon nitride nanosheets with triazole defects for highly improved photocatalytic hydrogen production. Int. J. Hydrogen Energy 2019, 44, 2675–2684. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, Y.; Wang, W.; Zhou, J.; Li, T.; Liu, X.; Tan, Y.; Zhao, Z. A green one-pot approach for mesoporous g-C3N4 nanosheets with in situ sodium doping for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 748–756. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yu, X.; Yang, X.; Liu, W.; Yang, J.; Tang, H.; Xu, H.; Li, H.; Li, Y.; et al. Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-codoped and exfoliated ultrathin g-C3N4 nanosheets. Appl. Catal. B Environ. 2019, 248, 84–94. [Google Scholar] [CrossRef]
- Kessler, F.K.; Zheng, Y.; Schwarz, D.; Merschjann, C.; Schnick, W.; Wang, X.; Bojdys, M.J. Functional carbon nitride materials-design strategies for electrochemical devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, X.; Antonietti, M.; Li, H. Boron- and fluorine-containing mesoporous carbon nitride polymers: Metal-free catalysts for cyclohexane oxidation. Angew. Chem. Int. Ed. 2010, 49, 3356–3359. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lu, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-shell nanostructured black rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping. J. Am. Chem. Soc. 2013, 135, 17831–17838. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Shan, Y.; Hu, Y.; Xu, X.; Long, L.; Zhang, J.; Dai, J.; Guo, J.; Shen, J.; Li, S.; et al. Half-metallic carbon nitride nanosheets with micro grid mode resonance structure for efficient photocatalytic hydrogen evolution. Nat. Commun. 2018, 9, 3366. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, D.; Li, Z.; Nan, Y.; Ding, F.; Shen, Y.; Jiang, Z. Thylakoid-inspired multishell g-C3N4 nanocapsules with enhanced visible-Light harvesting and electron transfer properties for high-Efficiency photocatalysis. ACS Nano 2017, 11, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, W.J.; Lee, S.-H.A.; Kobayashi, Y.; Hernandez-Pagan, E.A.; Hoertz, P.G.; Moore, T.A.; Moore, A.L.; Gust, D.; Mallouk, T.E. Photoassisted overall water splitting in a visible light-absorbing dye-sensitized photoelectrochemical Cell. J. Am. Chem. Soc. 2009, 131, 926–927. [Google Scholar] [CrossRef]
- Xue, Y.; Lei, Y.; Liu, X.; Li, Y.; Deng, W.; Wang, F.; Min, S. Highly active dye-sensitized photocatalytic H2 evolution catalyzed by a single-atom Pt cocatalyst anchored onto g-C3N4 nanosheets under long-wavelength visible light irradiation. New J. Chem. 2018, 42, 14083–14086. [Google Scholar] [CrossRef]
- Wang, P.; Guan, Z.; Li, Q.; Yang, J. Efficient visible-light-driven photocatalytic hydrogen production from water by using Eosin Y-sensitized novel g-C3N4/Pt/GO composites. J. Mater. Sci. 2017, 53, 774–786. [Google Scholar] [CrossRef]
- Zhu, M.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.; Wang, X.; Majima, T. Metal-Free Photocatalyst for H2 evolution in visible to near-infrared region: Black phosphorus/graphitic carbon nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Wang, Y.; Cao, D.; Tian, S.; Xiao, K.; Mao, R.; Zhao, X. H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at g-C3N4/BiVO4 photoanode under visible light irradiation. Chem. Eng. J. 2018, 332, 312–320. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Yin, S.-N.; Feng, L.; Zang, Y.; Xue, H. In situ construction of fibrous AgNPs/g-C3N4 aerogel toward light-driven COx-free methanol dehydrogenation at room temperature. Chem. Eng. J. 2018, 334, 2401–2407. [Google Scholar] [CrossRef]
- Bafaqeer, A.; Tahir, M.; Amin, N.A.S. Well-designed ZnV2O6/g-C3N4 2D/2D nanosheets heterojunction with faster charges separation via pCN as mediator towards enhanced photocatalytic reduction of CO2 to fuels. Appl. Catal. B Environ. 2019, 242, 312–326. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S.Z. 2D phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yang, H.; Chen, Z.; Lin, J.; Shen, Z.X. Active sites-enriched hierarchical MoS2 nanotubes: Highly active and stable architecture for boosting hydrogen evolution and lithium storage. J. Mater. Chem. A 2016, 4, 7565–7572. [Google Scholar] [CrossRef][Green Version]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Tang, C.; Zhou, Y.; Zeng, L.; Huang, L. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B Environ. 2019, 240, 30–38. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.; Xue, C. Self-sensitized carbon nitride microspheres for long-lasting visible-light-driven hydrogen generation. Small 2016, 12, 3543–3549. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Liu, G.; Cheng, H.M. An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Tapping, P.C.; Kee, T.W.; Smernik, R.; Spooner, N.; Moffatt, J.; Tang, Y.; Davey, K.; Qiao, S.-Z. A benchmark quantum yield for water photoreduction on amorphous carbon nitride. Adv. Funct. Mater. 2017, 27, 1702384. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.H.; Yu, J.Y.; Kim, D.H.; Lee, S.U.; Lee, J.H. Hierarchically designed 3D holey C2N aerogels as bifunctional oxygen electrodes for flexible and rechargeable Zn-Air batteries. ACS Nano 2018, 12, 596–608. [Google Scholar] [CrossRef]
- Wulan, B.-R.; Yi, S.-S.; Li, S.-J.; Duan, Y.-X.; Yan, J.-M.; Jiang, Q. Amorphous nickel pyrophosphate modified graphitic carbon nitride: An efficient photocatalyst for hydrogen generation from water splitting. Appl. Catal. B Environ. 2018, 231, 43–50. [Google Scholar] [CrossRef]
- Shinde, S.S.; Sami, A.; Lee, J.-H. Sulfur mediated graphitic carbon nitride/S-Se-graphene as a metal-free hybrid photocatalyst for pollutant degradation and water splitting. Carbon 2016, 96, 929–936. [Google Scholar] [CrossRef]
- Shen, R.; Xie, J.; Zhang, H.; Zhang, A.; Chen, X.; Li, X. Enhanced solar fuel H2 generation over g-C3N4 nanosheet photocatalysts by the synergetic effect of noble metal-free Co2P cocatalyst and the environmental phosphorylation strategy. ACS Sustain. Chem. Eng. 2017, 6, 816–826. [Google Scholar] [CrossRef]
- Shinde, S.S.; Sami, A.; Lee, J.-H. Nitrogen- and phosphorus-doped nanoporous graphene/graphitic carbon nitride hybrids as efficient electrocatalysts for hydrogen evolution. ChemCatChem 2015, 7, 3873–3880. [Google Scholar] [CrossRef]
- Tang, H.; Wang, R.; Zhao, C.; Chen, Z.; Yang, X.; Bukhvalov, D.; Lin, Z.; Liu, Q. Oxamide-modified g-C3N4 nanostructures: Tailoring surface topography for high-performance visible light photocatalysis. Chem. Eng. J. 2019, 374, 1064–1075. [Google Scholar] [CrossRef]
- Marci, G.; Garcia-Lopez, E.I.; Pomilla, F.R.; Palmisano, L.; Zaffora, A.; Santamaria, M.; Krivtsov, I.; Ilkaeva, M.; Barbierikova, Z.; Brezova, V. Photoelectrochemical and EPR features of polymeric C3N4 and O-modified C3N4 employed for selective photocatalytic oxidation of alcohols to aldehydes. Catal. Today 2019, 328, 21–28. [Google Scholar] [CrossRef]
- Rahman, M.; Davey, K.; Qiao, S.Z. Counteracting blueshift optical absorption and maximizing photon harvest in carbon nitride nanosheet photocatalyst. Small 2017, 13, 1700376. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Shi, L.; Cao, L.; Yu, Q.; Jie, Y.; Fei, J.; Ouyang, H.; Ye, J. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 204, 335–345. [Google Scholar] [CrossRef]
- Rahman, M.; Davey, K. Enabling Pt-free photocatalytic hydrogen evolution on polymeric melon: Role of amorphization for overcoming the limiting factors. Phys. Rev. Mater. 2018, 2, 125402. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Tang, Y.; Kwong, P. Reduced recombination and low-resistive transport of electrons for photo-redox reactions in metal-free hybrid photocatalyst. Appl. Phys. Lett. 2018, 112, 253902. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Moffatt, J.; Spooner, N. Topological carbon nitride: Localized photon absorption and delocalized charge carrier separation at intertwined photocatalyst interfaces. Mater. Horiz. 2018, 5, 553–559. [Google Scholar] [CrossRef]
- Zhu, M.; Osakada, Y.; Kim, S.; Fujitsuka, M.; Majima, T. Black phosphorus: A promising two dimensional visible and near-infrared-activated photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2017, 217, 285–292. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, Y.; Liao, F.; Shen, W.; Lin, H.; Wang, H.; Shao, M. Rh-Ag-Si ternary composites: Highly active hydrogen evolution electrocatalysts over Pt–Ag–Si. J. Mater. Chem. A 2017, 5, 1623–1628. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Qiu, L.; Ma, M.; Hou, Y.; Duo, S. A 3D Hierarchical Pancake-Like Porous Carbon Nitride for Highly Enhanced Visible-Light Photocatalytic H2 Evolution. Catalysts 2020, 10, 77. https://doi.org/10.3390/catal10010077
Qiu X, Qiu L, Ma M, Hou Y, Duo S. A 3D Hierarchical Pancake-Like Porous Carbon Nitride for Highly Enhanced Visible-Light Photocatalytic H2 Evolution. Catalysts. 2020; 10(1):77. https://doi.org/10.3390/catal10010077
Chicago/Turabian StyleQiu, Xiaobin, Lingfang Qiu, Mengfan Ma, Yingying Hou, and Shuwang Duo. 2020. "A 3D Hierarchical Pancake-Like Porous Carbon Nitride for Highly Enhanced Visible-Light Photocatalytic H2 Evolution" Catalysts 10, no. 1: 77. https://doi.org/10.3390/catal10010077
APA StyleQiu, X., Qiu, L., Ma, M., Hou, Y., & Duo, S. (2020). A 3D Hierarchical Pancake-Like Porous Carbon Nitride for Highly Enhanced Visible-Light Photocatalytic H2 Evolution. Catalysts, 10(1), 77. https://doi.org/10.3390/catal10010077





