Abstract
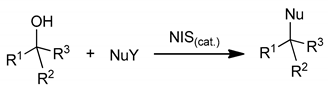
N-halosuccinimides (chloro, bromo, and iodo, respectively) were introduced, tested, and applied as efficient and non-metal precatalysts for C-, N-, O-, and X-nucleophilic substitution reactions of alcohols under solvent-free reaction conditions (SFRC) or under high substrate concentration reaction conditions (HCRC) efficiently and selectively, into the corresponding products.
1. Introduction
The development of protocols for the transformation of organic compounds following the principles of green chemistry [1] is currently one of the main trends in organic synthesis. In accordance with the principle of atom economy, effective catalytic approaches, replacement of volatile or toxic organic compounds used as solvents with safer reaction media is one of the main challenges in organic synthesis.
Since alcohols are readily available and inexpensive alkylating agents, their nucleophilic substitution is an important and attractive process used in the synthesis of organic compounds, which offers a potential impact on the environment, resulting in water as the only side product of the reaction.
In order to apply the direct transformation of a hydroxyl group often an additional activation is inescapable [2].
Many related transformations, including the use of Brønsted and Lewis acids, metal ions, or other supporters in a substoichiometric amount, have been reported by several reviews [3,4,5,6,7,8,9,10,11,12,13] and recent related advanced reports [14,15,16,17,18]. However, the requirement of toxic or expensive reagents, environmentally undesirable solvents, a high concentration of the mediator, prolonged reaction time, or high temperature make such a method less attractive from the green chemical aspect. Accordingly, it was essential to design environmentally friendly synthetic protocols for the C–C and C–heteroatom bond formation. A group of organic compounds bearing an active N-halogen bond, N-halosuccinimides (NXSs) (chloro, bromo, and iodo), are an inexpensive, commercially available, easy-to-handle, and metal-free compounds, employed for oxidation, hydroxyhalogenation or halogenation reactions [19,20]. NXSs have attracted significant interest as mediators for comprehensive organic transformations [21,22,23,24,25,26,27,28]. However, the use of NXSs as the mediator for the direct transformation of alcohols forming C–C or C–heteroatom bonds has not been discovered so far.
In our continuous research on developing greener synthetic routes [29,30,31], we wish to report herein the introduction of NXSs as a non-metal substoichiometric mediator for the comprehensive transformations of alcohols bearing newly C–C or C–heteroatom bonds, under SFRC or under HCRC.
2. Results and Discussion
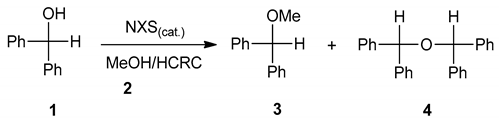
Initially, diphenylmethanol 1 has been chosen as the model substrate to investigate the efficiency of N-halosuccinimides as a mediator of the process and to find the best reaction conditions for alcohol transformation (Table 1). As can be seen from Table 1 the transformation of diphenylmethanol 1 in the presence of methyl alcohol 2 in the absence of any of the NXSs, no reaction has occurred (entry 1, Table 1). The transformation of diphenylmethanol 1 mediated by N-halosuccinimides in the presence of MeOH 2 under HCRC gave ether 3 (entries 2–4), while in the absence of MeOH under SFRC, dimerization has been observed, affording the dimeric ether 4, (entry 5, Table 1). Under the typical reaction conditions in the dark or the presence of radical scavenger, the quantitative conversion of starting material 1 into the corresponding product 3 was established (entries 6–7, Table 1).

Table 1.
Optimal reaction conditions for the highest conversion of diphenylmethanol 1 into the (methoxymethylene)dibenzene 3 in the presence of NXSs as mediators under HCRC a.
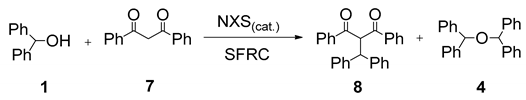
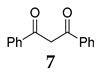
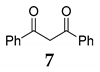
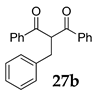
Furthermore, we investigated the role of N-halosuccinimides as the mediators for the direct C–C bond formation by direct coupling reactions of secondary benzyl alcohol with various types of electron-rich compounds, including 1,3-dicarbonyl compounds or electron-rich alkene under SFRC. We have examined the reaction of diphenylmethanol 1 with dibenzoylmethane 7 under SFRC, and results are summarized in Table 2. The transformation of diphenylmethanol 1 in the presence of dibenzoylmethane 7 under SFRC in the absence of any of the NXSs, no reaction has occurred (entry 1, Table 2). In the presence of N-chlorosuccinimide (NCS) as the mediator, we did not observe any conversion of the diphenylmethanol 1 (entry 2), while in the presence of N-bromosuccinimide (NBS) a low conversion of the diphenylmethanol 1 into the dimeric ether 4 was observed (entry 3). Moreover, in the presence of N-iodosuccinimide (NIS) as the mediator, the coupling of benzyl moiety with 7 took place, providing efficiently and selectively the corresponding product 8 with the new C–C bond formed between the benzyl carbon atom and C-2 carbon of dicarbonyl target 7 (entries 4 and 5). Under the typical reaction conditions in the dark or the presence of radical scavenger, the efficient and selective transformation of diphenylmethanol 1 into the corresponding product 8 was observed (entries 6–7, Table 2).

Table 2.
The effect of substoichiometric amounts of NXS on conversion of diphenylmethanol 1 with dibenzoylmethane 7 under SFRC a.
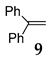
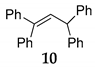
In order to support the assumption that the dimeric ether 4 might be the intermediate of this dehydrative coupling [32], few control reactions were performed (Scheme 1). Under the typical conditions when diphenylmethanol 1 was efficiently and selectively converted into dimeric ether 4, it was applied as a starting material in the reaction with 1,1-diphenylethene 9 providing the corresponding substituted alkene 10 in quantitative yield. With 9 providing the corresponding substituted alkene 10 in quantitative yield.
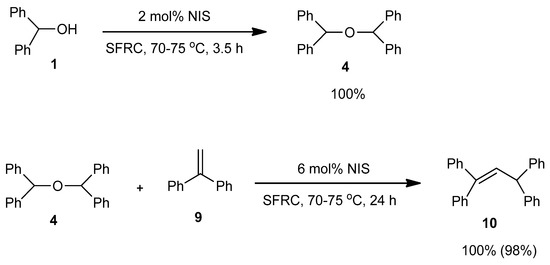
Scheme 1.
Control reactions.
Based on the results of the control experiment presented in (Scheme 1), the proposed reaction pathway is presented in (Scheme 2). It was reported that the R-X bond of halosuccinimides as the precatalysts was activated by its reaction with the addition of Lewis base. Therefore, it seems plausible that transient halogen bonding could be responsible for the catalytic effect of NXS [33,34].
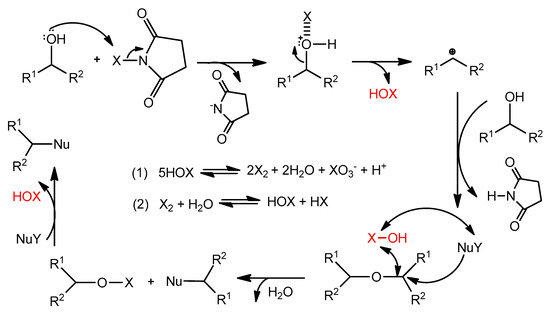
Scheme 2.
Plausible reaction course.
The term halogen bonding which is defined as non-covalent interaction of a halogen atom X in one molecule with a negative site in another, such as the lone pair electrons of a Lewis base [35,36]. The halogen bonding adducts are not the activated species. Rather, halonium (X+) transfer will generate the intermediate forming succinimide anion and HOX, which further promotes the course of the reaction. HOX decomposes by disproportionation to X2 and HXO3. It is well known that X2 forms HOX and HX in reactions with water, where HOX regenerate for following catalysis. Thus, the water resulting as an only side product of the reaction might be acting as a supporter in acceleration of the reaction.
The assumption that NXSs are actually precatalysts producing HOX, X2, and protons during the process while these species could catalyze nucleophilic substitutions seems to be reasonable [37]. The discrimination between the oxidation process observed in the case of the use of molar excess of NXSs (1.3 to 2 equivalent excess) [26,27,28] was achieved using substoichiometric amounts of NIS and by the selection of alcohols, which could after realizing hydroxyl group form, through resonance or strong inductive effect, stabilize carbocation intermediates which readily collapse with present nucleophile sources. Alkyl alcohols or some primary benzyl alcohols were inactive under our reaction conditions. In contrast, in some cases of secondary alcohols, the formations of trace amounts of oxidation products were observed besides target products (see Tables).
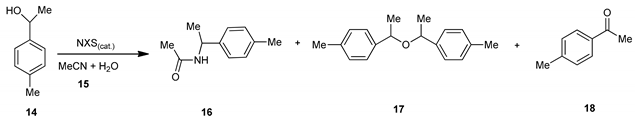
Furthermore, we studied the role of N-halosuccinimides as mediators for direct C-N bond formation in the reaction of the alcohol with acetonitrile and water solution, and results are summarized in Table 3. The transformation of 1-(p-tolyl)ethan-1-ol 14, with acetonitrile 15 in the absence of any of the NXSs, no reaction occurred (entry 1, Table 3), while, in the presence of N-halosuccinimides as the mediator the corresponding N-acyl benzyl product 16 (entries 2–4), in moderate to high yield was observed, accompanied with the formation of a small amount of dimeric ether 17 (entry 4) and oxidized alcohol 18 (entries 2–4, Table 3). We found that NCS and NIS were slightly more convenient mediators for this transformation than NBS. Under the typical reaction conditions in the dark or the presence of radical scavenger, the high conversion of starting material 14 into the corresponding product 16 was established, accompanied by the formation of a small amount of dimeric ether 17 and oxidized alcohol 18 [38] (entries 5–6, Table 3).

Table 3.
The effect of NXS as the mediator on conversion of 1-(p-tolyl)ethan-1-ol 14 in acetonitrile solution a.
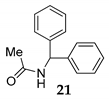
In order to support the assumption that the dimeric ether 4 might be the intermediate of this dehydrative coupling [32], few control reactions were performed (Scheme 3). Under the typical conditions when diphenylmethanol 1 was efficiently and selectively converted into dimeric ether 4 In order to support the assumption that the dimeric ether 4 might be the intermediate of the reaction course, few control reactions were performed (Scheme 3). Under the typical conditions when diphenylmethanol 1 was efficiently and selectively converted into dimeric ether 4 (Scheme 1), which was used as a starting material instead of 1 in the reaction with MeCN/H2O 15 providing the corresponding N-acyl benzyl product 21 in nearly quantitative yield (Scheme 3).
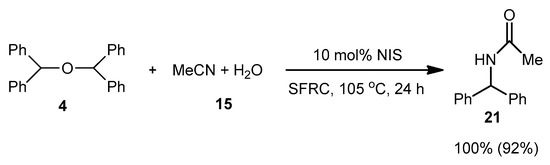
Scheme 3.
Control experiment.
According to these results, the dimeric ether 4 can generate the benzylic carbocation which can be trapped by MeCN to generate a nitrilium ion followed by the presence of water providing the corresponding product 21. However, another probable pathway could be a direct reaction between carbocation and MeCN [39,40] (Scheme 4).
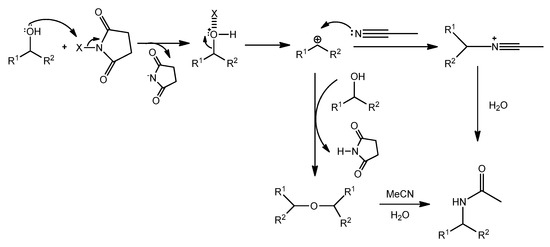
Scheme 4.
Plausible reaction pathway.
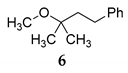
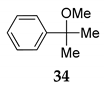
The catalytic effect of NIS was applied for tertiary alkyl alcohol, in the reaction of etherification of α,α-dimethylbenzenepropanol 5 with MeOH under HCRC (entry 1, Table 4). The efficient and selective transformation was observed in the reaction with tertiary benzyl alcohol. The 2-phenylpropan-2-ol 33 was readily catalyzed by NIS providing the corresponding product 34 in nearly quantitative yield (entry 2, Table 4).

Table 4.
Nucleophilic substitution of alcohols using NIS mediator a.
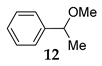
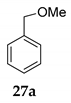
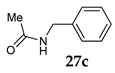
The stereochemical course of the etherification was monitored by the reaction between (S)-(-)-1-phenylethanol 11 and MeOH 2 in the presence of NIS providing the corresponding product 12 in moderate yield (entry 3, Table 4) accompanied with a small amount of acetophenone 13. The specific rotation of pure product 12 gave the value [α] = +15°, revealing that we are not dealing with completely SN1 or SN2 processes but with the combination of both. It seems that the dimerization is the SN1, and final etherification is the SN2 process. The details of this analysis are given in Supplementary Materials. In the case of the reaction of primary benzyl alcohol 27, only a trace amount of benzaldehyde was observed (entry 4, Table 1).
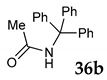
Furthermore, under the typical reaction conditions, we checked the reaction of primary and tertiary alcohol with dibenzoylmethane 7 in the presence of NIS as the mediator under SFRC. In contrast, no reaction was observed with benzyl alcohol 27 as the type of primary alcohol due to lower reactivity and triphenylmethanol 36 as the type of tertiary alcohol, which may be attributed to the steric hindrance (entries 6 and 7, Table 4).
Additionally, the catalytic effect of NIS for direct C–C bond formation by direct coupling of phenyl substituted alkene with secondary benzyl alcohol under SFRC was studied. NIS as the mediator was found to most efficiently and selectively promote the direct coupling of diphenylmethanol 1 with 1,1-diphenylethene 9 under SFRC, furnishing the corresponding substituted alkene 10 in nearly quantitative yield (entry 8, Table 4).
On the other hand, we checked the reactions of primary and tertiary alcohol with aqueous acetonitrile 15 in the presence of NIS as the mediator under SFRC, no reaction was observed with benzyl alcohol 27 (only a trace amount of benzaldehyde was observed) and triphenylmethanol 36, which may be attributed to the steric hindrance (entries 9 and 10, Table 4).
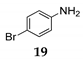
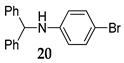
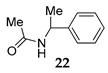
Additionally, the direct coupling of aniline [41], bearing deactivated group 19, with diphenylmethanol 1 in the presence of NIS as the mediator under SFRC, afforded the corresponding N-alkylated compound in high yield 20 accompanied with a small amount of oxidized alcohol (entry 10, Table 4). By increasing the temperature to 105 °C, the direct coupling of diphenylmethanol 1, 1- phenylethanol 11 with acetonitrile and water solution 15 in the presence of NIS as the mediator afforded the corresponding N-acyl benzyl products 21 and 22 (entries 12 and 13, Table 4) in nearly quantitative yield.
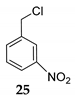
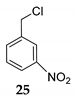
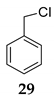
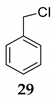
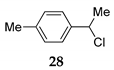
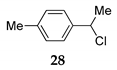
We further investigated the impact of N-halosuccinimide as a mediator in reactions of primary benzyl alcohol with trimethylchlorosilane (TMSCl) 23a under SFRC. Primary benzyl alcohol bearing a strong deactivated group was not converted into the corresponding product using TMSCl [42]. Thus, we studied the impact of N-halosuccinimides as a mediator on the course of reaction of 3-nitrobenzyl alcohol 24 with TMSCl under SFRC. Gratifyingly, the addition of a substoichiometric amount of NIS was found to effectively and selectively promote the reaction of 3-nitrobenzyl alcohol 24 with TMSCl under SFRC (entries 15 and 16, Table 4). To increase the yield of 1-(chloromethyl)-3-nitrobenzene 25, different concentrations of the mediator were employed, and the results are given in the Supplementary Materials (Table S1). The role of NIS as the mediator was further examined in the improvement in yields of the chlorinated products. In the case of benzyl alcohol 27, 1-(p-tolyl)ethanol 14 using TMSCl under mediator-free and SFRC the good- to high-yielding formation of the corresponding chlorides 28 and 29 were accompanied by 10% of the symmetric ethers in both cases [42]. In the case of the reaction of benzyl alcohol 27 by adding NIS as the mediator, quantitative conversion of the starting material into the corresponding chloride 29 was observed, while only a small amount of dimer was detected as the side product (entries 17 and 18, Table 4). In the case of 1-(p-tolyl)ethanol 14, the improvement of 98% yield 28 without the formation of the dimeric ether was attained by adding the NIS as the mediator (entries 19 and 20, Table 4).
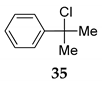
The efficient and selective transformation was observed in the case with 2-phenylpropan-2-ol 33 into the corresponding product 35 [42] (entry 21, Table 4).
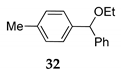
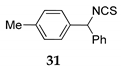
Inspired by this results with TMSCl 23a, we performed the reactions of phenyl(p-tolyl)methanol 30 as the model compound under the mentioned reaction conditions, using (trimethylsilyl)isothiocyanate (TMSNCS) 23b or ethoxytrimethylsilane (TMSOEt) 23c as the sources of nucleophiles where isothiocyanate and ethoxy were introduced successfully into organic molecules 31 and 32 (entry 14 and 5, Table 4).
The collected results in Table 4 are organized primarily stressing the type of bond formation mediated by NIS as the most efficient and selective catalyst among the NXSs, enhancing the green chemical profiles of these transformations.
In order to check the thermal stability of N-halosuccinimides under reaction conditions, the thermal gravimetric analysis (TGA) on the NCS, NBS, and NIS catalysts were performed. It was detected that the degradation had occurred at none of the catalysts at the temperature range 25–200 °C.
3. Materials and Methods
All chemicals used for synthetic procedures were purchased from commercial sources ((Merck, Darmstadt, Germany; Sigma Aldrich, St. Louis, MO, USA) Reactions were monitored by thin-layer chromatography (TLC) (with silica gel/TLC cards, DC-Alufolien-Kieselgel, Sigma-Aldrich, St. Louis, MO, USA). For the detection of compounds on chromatographic plates, a UV (Camag, Muttenz, Switzerland) lamp (254 nm) was used. Column chromatography (CC) was performed with silica gel Kieselgel 60 (particle size: 0.063–0.200 mm, Fluka, Sigma Aldrich). Nuclear magnetic resonance (Varian INOVA 300 NMR instrument, 1H: at 303.0 MHz, 13C: at 76.2 MHz) using CDCl3 as the solvent with SiMe4 (TMS) as an internal reference and melting points (open capillary tube methodology; uncorrected, by Buchi 535 equipment) were used for identification and structure elucidation. General procedure for etherification of alcohols mediated by N-halosuccinimide on half mmol scale:
A mixture of benzyl alcohol (0.5–1 mmol) and N-halosuccinimide as a precatalyst (3–10 mol %), which had been powdered in a mortar in the case of solid-state reactants, was transferred to a 4 mL screw-capped vial, finally added eventual liquid component alkyl alcohol (1 mmol–1 mL) and heated at 70–75 °C for 6–24 h.
The reaction was observed by TLC. After reaction completion, the reaction mixture was cooled to room temperature; dissolved in EtOAc (3 × 5 mL); washed with saturated Na2S2O3 (2 × 3 mL), saturated NaHCO3 (2 × 3 mL), and water (2 × 5 mL); and the collected organic layers were dried over anhydrous Na2SO4 and the solvent was removed by evaporation under reduced pressure providing the corresponding product.
General procedure for new carbon-carbon bond formation through ß-dicarbonyl compound in organic molecule mediated by NIS on half mmol scale:
A mixture of benzyl alcohol (0.5 mmol), ß-dicarbonyl compound (0.5 mmol), and NIS (1 mol %) which had been powdered in a mortar, was transferred to a 4 mL screw-capped vial and heated at 70–75 °C for 24 h. The reaction was observed by TLC. After reaction completion, the reaction mixture was cooled to room temperature; dissolved in EtOAc (3 × 5 mL); washed with saturated Na2S2O3 (2 × 3 mL), saturated NaHCO3 (2 × 3 mL) and water (2 × 5 mL); and the collected organic layers were dried over anhydrous Na2SO4 and the solvent was removed by evaporation under reduced pressure providing the corresponding product.
General procedure for new carbon-carbon bond formation through electron rich C=C bond in organic molecules mediated by NIS on half mmol scale:
A mixture of benzyl alcohol or dimeric ether (0.5 mmol), alkene (0.5 mmol), and NIS (6 mol %), which had been powdered in a mortar in the case of solid-state reactants was transferred to a 4 mL screw-capped vial, finally, eventual liquid component was added, and it was heated at 70–75 °C for 24 h.
The reaction was observed by TLC. After reaction completion, the reaction mixture was cooled to room temperature, dissolved in EtOAc (3 × 5 mL), washed with saturated Na2S2O3 (2 × 3 mL), saturated NaHCO3 (2 × 3 mL) and water (2 × 5 mL). Through the anhydrous Na2SO4 the collected organic layers were dried, and the solvent was voided by evaporation under reduced pressure.
General procedure for new carbon-nitrogen bond formation in organic molecules mediated by NIS on half mmol scale:
In a 4 mL screw-capped vial, a mixture of benzyl alcohol (0.5 mmol), acetonitrile (0.5 mL), water (2 mmol) and NIS (10 mol %), or a mixture of solid reaction components previously powdered in a mortar was transferred: benzyl alcohol (0.5 mmol), aniline (0.55 mmol) and NIS (10 mol %). The vial was then heated at 70–105 °C for 24–48 h. The reaction was observed by TLC. When the reaction was completed, the reaction mixture was cooled to room temperature, dissolved in EtOAc (3 × 5 mL), washed with saturated Na2S2O3 (2 × 3 mL), saturated NaHCO3 (2 × 3 mL) and water (2 × 5 mL), and the collected organic layers were dried over anhydrous Na2SO4 and the solvent was removed by evaporation under reduced pressure providing the corresponding product.
General procedure for halo functionalization of organic compounds using trimethylsilyl derivatives mediated by NIS on half mmol scale:
A mixture of benzyl alcohol (0.5 mmol) and NIS (2–10 mol %) which had been powdered in a mortar in the case of solid-state reactants, was transferred to a 4 mL screw-capped vial, then trimethylsilyl derivatives (0.55 mmol) added and stirred at rt (room temperature) or heated at 70–75 °C for 6.5–25 h. The progress of the reaction mixture was monitored by TLC. When the reaction was completed, the reaction mixture was cooled to room temperature, dissolved in ethyl acetate (15 mL), washed with saturated Na2S2O3 (6 mL), saturated NaHCO3 (6 mL) and water (10 mL), and the collected organic layers were dried over anhydrous Na2SO4 and the solvent was removed by evaporation under reduced pressure providing the corresponding product.
4. Conclusions
In conclusion, N-halosuccinimides (chloro, bromo, and iodo, respectively), were introduced, tested, and applied as efficient and metal-free substoichiometric mediators for reactions of a comprehensive range of alcohols with various type of electron-rich organic molecules or reactive anionic species thus forming new carbon-carbon or carbon-heteroatom bonds in target alcohol molecules. Leading reactions enhanced green chemical profiles of these valuable transformations under solvent-free reaction conditions or high concentration reaction conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/4/460/s1, detailed experimental data, 1H-NMR and 13C-NMR spectra of isolated final products and experimental data related to thermal analysis.
Author Contributions
Conceptualization, S.S.; formal analysis, N.A. and S.S.; investigation, N.A. and S.S.; methodology, N.A. and S.S.; writing—original draft, N.A. and S.S.; writing—review and editing, N.A. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovene Human Resources Development and Scholarship Fund (contract: 11011-9/2011), Slovenian Research Agency (contract: Programme P1-0134).
Acknowledgments
The authors are grateful to the Slovenian NMR Centre at the National Institute of Chemistry, Ljubljana, Slovenia, Prof. U. Grošelj for the specific rotation measurements, Prof. P. Bukovec for the TGA analysis of N-halosuccinimides, and K. Čebular for sharing her knowledge.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Yasuda, M.; Saito, T.; Ueba, M.; Baba, A. Direct Substitution of the Hydroxy Group in Alcohols with Silyl Nucleophiles Catalyzed by Indium Trichloride. Angew. Chem. Int. Ed. 2004, 43, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Emer, E.; Sinisi, R.; Capdevila, M.G.; Petruzziello, D.; De Vincentiis, F.; Cozzi, P.G. Direct Nucleophilic SN1-Type Reactions of Alcohols. Eur. J. Org. Chem. 2011, 2011, 647–666. [Google Scholar] [CrossRef]
- Kumar, R.; Van der Eycken, E.V. Recent approaches for C-C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 2013, 42, 1121–1146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; He, T.; Ma, L.; Wang, Z. Recent developments in Ritter reaction. RSC Advances 2014, 4, 64936–64946. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.-P.; Wang, C.-H.; Zhou, J. Catalytic functionalization of tertiary alcohols to fully substituted carbon centres. Org. Biomol. Chem. 2014, 12, 6033–6048. [Google Scholar] [CrossRef]
- Uchuskin, M.G.; Makarov, A.S.; Butin, A.V. Catalytic Alkylation of Furans by π-Activated Alcohols (Review). Chem. Heterocycl. Compd. 2014, 50, 791–806. [Google Scholar] [CrossRef]
- Chaskar, A.; Murugan, K. Direct allylation of alcohols using allyltrimethylsilane: A move towards an economical and ecological protocol for C-C bond formation. Catal. Sci. Tech. 2014, 4, 1852–1868. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Z.-Q. Iron-Catalyzed Alkylation of Alkenes and Alkynes Using Alcohols as the Alkylating Reagent. Synthesis 2015, 47, 1706–1708. [Google Scholar] [CrossRef]
- Dryzhakov, M.; Richmond, E.; Moran, J. Recent Advances in Direct Catalytic Dehydrative Substitution of Alcohols. Synthesis 2016, 48, 935–959. [Google Scholar]
- Ajvazi, N.; Stavber, S. Alcohols in direct carbon-carbon and carbon-heteroatom bond-forming reactions: Recent advances. Arkivoc 2018, part ii, 288–329. [Google Scholar] [CrossRef]
- Guillena, G.; Ramón, D.J.; Yus, M. Alcohols as Electrophiles in C–C Bond-Forming Reactions: The Hydrogen Autotransfer Process. Angew. Chem. Int. Ed. 2007, 46, 2358–2364. [Google Scholar] [CrossRef]
- Dobereiner, G.E.; Crabtree, R.H. Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev. 2010, 110, 681–703. [Google Scholar] [CrossRef]
- Yang, G.-P.; Jiang, N.; Huang, X.-Q.; Yu, B.; Hu, C.-W. Non-corrosive heteropolyacid-based recyclable ionic liquid catalyzed direct dehydrative coupling of alcohols with alcohols or alkenes. Mol. Catal. 2019, 468, 80–85. [Google Scholar] [CrossRef]
- Chevella, D.; Macharla, A.K.; Kodumuri, S.; Banothu, R.; Gajula, K.S.; Amrutham, V.; Gennadievna, G.E.N.; Nama, N. Synthesis of internal olefins by direct coupling of alcohols and olefins over Moβ zeolite. Catal. Commun. 2019, 123, 114–118. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bora, U. Molecular Iodine-Catalyzed Selective C-3 Benzylation of Indoles with Benzylic Alcohols: A Greener Approach toward Benzylated Indoles. ACS Omega 2019, 4, 11770–11776. [Google Scholar] [CrossRef] [PubMed]
- Veenboer, R.M.P.; Nolan, S.P. Gold(i)-catalysed dehydrative formation of ethers from benzylic alcohols and phenols. Green Chem. 2015, 17, 3819–3825. [Google Scholar] [CrossRef]
- Böldl, M.; Fleischer, I. Dehydrative Coupling of Benzylic Alcohols Catalyzed by Brønsted Acid/Lewis Base. Eur. J. Org. Chem. 2019, 2019, 5856–5861. [Google Scholar] [CrossRef]
- Kolvari, E.; Ghorbani-Choghamarani, A.; Salehi, P.; Shirini, F.; Zolfigol, M.A. Application of N-halo reagents in organic synthesis. J. Iran Chem. Soc. 2007, 4, 126–174. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides. Molecules 2016, 21, 1325. [Google Scholar] [CrossRef]
- Karimi, B.; Ebrahimian, G.R.; Seradj, H. Efficient and Chemoselective Conversion of Carbonyl Compounds to 1,3-Dioxanes Catalyzed with N-Bromosuccinimide under Almost Neutral Reaction Conditions. Org. Lett. 1999, 1, 1737–1739. [Google Scholar] [CrossRef]
- Karimi, B.; Seradj, H. N-Bromosuccinimide (NBS), a Novel and Highly Effective Catalyst for Acetylation of Alcohols under Mild Reaction Conditions. Synlett 2001, 2001, 0519–0520. [Google Scholar] [CrossRef]
- Čebular, K.; Božić, B.Đ.; Stavber, S. Esterification of Aryl/Alkyl Acids Catalysed by N-bromosuccinimide under Mild Reaction Conditions. Molecules 2018, 23, 2235. [Google Scholar] [CrossRef] [PubMed]
- Wagh, Y.S.; Sawant, D.N.; Bhanage, B.M. Metal-free N-iodosuccinimide-catalyzed mild oxidative C–H bond amination of benzoxazoles. Tetrahedron Lett. 2012, 53, 3482–3485. [Google Scholar] [CrossRef]
- Kadam, S.T.; Kim, S.S. N-Iodosuccinimide (NIS) a novel and effective catalyst for the cyanosilylation of aldehydes under mild reaction conditions. Catal. Commun. 2008, 9, 1342–1345. [Google Scholar] [CrossRef]
- Guha, S.; Rajeshkumar, V.; Kotha, S.S.; Sekar, G. A Versatile and One-Pot Strategy to Synthesize α-Amino Ketones from Benzylic Secondary Alcohols Using N-Bromosuccinimide. Org. Lett. 2015, 17, 406–409. [Google Scholar] [CrossRef]
- Muneeswara, M.; Muthukumar, A.; Sekar, G. Dual Role of N-Bromosuccinimide as Oxidant and Succinimide Surrogate in Domino One-Pot Oxidative Amination of Benzyl Alcohols for the Synthesis of α–Imido Ketones. ChemistrySelect 2018, 3, 12524–12529. [Google Scholar] [CrossRef]
- Muneeswara, M.; Sundaravelu, N.; Sekar, G. NBS-mediated synthesis of β-keto sulfones from benzyl alcohols and sodium arenesulfinates. Tetrahedron 2019, 75, 3479–3484. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic Oxidative Iodination of Organic Compounds with Iodide Catalyzed by Sodium Nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite “on water” or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262–1267. [Google Scholar] [CrossRef]
- Stavber, G.; Stavber, S. Towards Greener Fluorine Organic Chemistry: Direct Electrophilic Fluorination of Carbonyl Compounds in Water and Under Solvent-Free Reaction Conditions. Adv. Synth. Catal. 2010, 352, 2838–2846. [Google Scholar] [CrossRef]
- Chun, S.; Chung, Y.K. Silver/NBS-Catalyzed Synthesis of α-Alkylated Aryl Ketones from Internal Alkynes and Benzyl Alcohols via Ether Intermediates. Org. Lett. 2018, 20, 5583–5586. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Breugst, M.; von der Heiden, D. Mechanisms in Iodine Catalysis. Chem. Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2006, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Čebular, K.; Božić, B.Đ.; Stavber, S. 1,3-Dibromo-5,5-dimethylhydantoin as a Precatalyst for Activation of Carbonyl Functionality. Molecules 2019, 24, 2608. [Google Scholar] [CrossRef]
- Beebe, T.R.; Adkins, R.L.; Bogardus, C.C.; Champney, B.; Hii, P.S.; Reinking, P.; Shadday, J.; Weatherford, W.D.; Webb, M.W.; Yates, S.W. Primary alcohol oxidation with N-iodosuccinimide. J. Org. Chem. 1983, 48, 3126–3128. [Google Scholar] [CrossRef]
- Anxionnat, B.; Guérinot, A.; Reymond, S.; Cossy, J. FeCl3-catalyzed Ritter reaction. Synthesis of amides. Tetrahedron Lett. 2009, 50, 3470–3473. [Google Scholar] [CrossRef]
- Theerthagiri, P.; Lalitha, A.; Arunachalam, P.N. Iodine-catalyzed one-pot synthesis of amides from nitriles via Ritter reaction. Tetrahedron Lett. 2010, 51, 2813–2819. [Google Scholar] [CrossRef]
- Ogata, O.; Nara, H.; Fujiwhara, M.; Matsumura, K.; Kayaki, Y. N-Monomethylation of Aromatic Amines with Methanol via PNHP-Pincer Ru Catalysts. Org. Lett. 2018, 20, 3866–3870. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Direct halogenation of alcohols with halosilanes under catalyst- and organic solvent-free reaction conditions. Tetrahedron Lett. 2016, 57, 2430–2433. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).