Ru Nanoparticles Embedded in Cubic Mesoporous Silica SBA-1 as Highly Efficient Catalysts for Hydrogen Generation from Ammonia Borane
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
3.2. Synthesis of S1B-C0 and S1B-C10
3.3. Synthesis of Ru(x)S1B-C0 and Ru(x)@S1B-C10
3.4. Characterization Methods
3.5. Hydrolysis of Ammonia Borane by Ru(x)@S1B-C0 and Ru(x)@S1B-C10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B-N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Nunes, H.; Ferreira, M.; Rangel, C.; Pinto, A. Hydrogen generation and storage by aqueous sodium borohydride (NaBH4) hydrolysis for small portable fuel cells (H2–PEMFC). Int. J. Hydrogen Energy 2016, 41, 15426–15432. [Google Scholar] [CrossRef]
- Li, X.; Fan, G.; Zeng, C. Synthesis of ruthenium nanoparticles deposited on graphene-like transition metal carbide as an effective catalyst for the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2014, 39, 14927–14934. [Google Scholar] [CrossRef]
- Wu, Z.; Duan, Y.; Ge, S.; Yip, A.C.K.; Yang, F.; Li, Y.; Dou, T. Promoting hydrolysis of ammonia borane over multiwalled carbon nanotube-supported Ru catalysts via hydrogen spillover. Catal. Commun. 2017, 91, 10–15. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Wu, D.; Wen, M.; Gu, C.; Wu, Q. 2D NiFe/CeO2 basic-site-enhanced catalyst via in-situ topotactic reduction for selectively catalyzing the H2 generation from N2H4·H2O. ACS Appl. Mater. Interfaces 2017, 9, 16103–16108. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, C.L.; Zhang, Y.; Li, H.; Min, S.; Guo, X.; Zheng, B.; Chen, H.; Anders, A.; Lai, Z.; et al. Selective hydrogen generation from formic acid with well-defined complexes of ruthenium and phosphorus-nitrogen PN3-pincer ligand. Chem. Asian J. 2016, 11, 1357–1360. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Jiang, H.L.; Yadav, M.; Aranishi, K.; Xu, Q. Synergistic catalysis of Au-Co@SiO2 nanospehere in hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. J. Mater. Chem. 2012, 22, 5065–5071. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- Ramzan, M.; Silvearv, F.; Blomqvist, A.; Scheicher, R.H.; Lebegue, S.; Ahuja, R. Structural and energetic analysis of the hydrogen storage materials LiNH2BH3 and NaNH2BH3 from ab initio calculations. Phys. Rev. B 2009, 79, 132102. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydrogen generation from the hydrolysis of ammonia borane using intrazeolite cobalt(0) nanoclusters catalyst. Int. J. Hydrogen Energy 2010, 35, 3341–3346. [Google Scholar] [CrossRef]
- Jiang, H.L.; Xu, Q. Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 2011, 170, 56–63. [Google Scholar] [CrossRef]
- Mohajeri, N.; Raissi, A.T.; Adebiyi, O. Hydrolytic cleavage of ammonia-borane complex for hydrogen production. J. Power Sources 2007, 167, 482–485. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Gagare, P.D. Preparation of ammonia borane in high yield and purity, methanolysis, and regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef]
- Erdogan, H.; Metin, Ö.; Özkar, S. In situ-generated PVP-stabilized palladium (0) nanocluster catalyst in hydrogen generation from the methanolysis of ammonia–borane. Phys. Chem. Chem. Phys. 2009, 11, 10519–10525. [Google Scholar] [CrossRef]
- Sun, D.H.; Mazumder, V.; Metin, Ö.; Sun, S.H. Methanolysis of ammonia borane by CoPd nanoparticles. ACS Catal. 2012, 2, 1290–1295. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Lu, G.; Qiu, S.; Yao, X. Ammonia borane confined by a metal-organic framework for chemical hydrogen storage: Enhancing kinetics and eliminating ammonia. J. Am. Chem. Soc. 2010, 132, 1490–1491. [Google Scholar] [CrossRef]
- Moury, R.; Moussa, G.; Demirci, U.B.; Hannauer, J.; Bernard, S.; Petit, E.; van der Lee, A.; Miele, P. Hydrazine borane: Synthesis, characterization, and application prospects in chemical hydrogen storage. Phys. Chem. Chem. Phys. 2012, 14, 1768–1777. [Google Scholar] [CrossRef]
- Marder, T.B. Will we soon be fueling our automobiles with ammonia–borane? Angew. Chem. Int. Ed. 2007, 46, 8116–8118. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Özkar, S. Transition metal nanoparticles in catalysis for the hydrogen generation from the hydrolysis of ammonia-borane. Top. Catal. 2013, 56, 1171–1183. [Google Scholar] [CrossRef]
- Aijaz, A.; Karkamkar, A.; Choi, Y.J.; Tsumori, N.; Rönnebro, E.; Autrey, T.; Shioyama, H.; Xu, Q. Immobilizing highly catalytically active pt nanoparticles inside the pores of metal–organic framework: A double solvents approach. J. Am. Chem. Soc. 2012, 134, 13926–13929. [Google Scholar] [CrossRef]
- Xi, P.; Chen, F.; Xie, G.; Ma, C.; Liu, H.; Shao, C.; Wang, J.; Xu, Z.; Xu, X.; Zhang, Z. Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. Nanoscale 2014, 4, 5597–5601. [Google Scholar] [CrossRef]
- Fuku, R.; Hayashi, K.; Takakura, S.; Kamegawa, T.; Mori, K.; Yamashita, H. The synthesis of size- and color-controlled silver nanoparticles by using microwave heating and their enhanced catalytic activity by localized surface plasmon resonance. Angew. Chem. Int. Ed. 2013, 52, 7446–7450. [Google Scholar] [CrossRef]
- Fan, G.; Liu, Q.; Tang, D.; Li, X.; Bi, J.; Gao, D. Nanodiamond supported Ru nanoparticles as an effective catalyst for hydrogen evolution from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2016, 41, 1542–1549. [Google Scholar] [CrossRef]
- Zhong, F.; Wang, Q.; Xu, C.; Yang, Y.; Wang, Y.; Zhang, Y.; Gao, D.; Jian, B.; Fan, G. Ultrafine and highly dispersed Ru nanoparticles supported on nitrogen-doped carbon nanosheets: Efficient catalysts for ammonia borane hydrolysis. Appl. Surf. Sci. 2018, 455, 326–332. [Google Scholar] [CrossRef]
- Xu, C.; Ming, M.; Wang, Q.; Yang, C.; Fan, G.; Wang, Y.; Gao, D.; Bi, J.; Zhang, Y. Facile synthesis of effective Ru nanoparticles on carbon by adsorption-low temperature pyrolysis strategy for hydrogen evolution. J. Mater. Chem. A 2018, 6, 14380–14386. [Google Scholar] [CrossRef]
- Yao, Q.L.; Lu, Z.H.; Huang, W.; Chen, X.; Zhu, J. High Pt-like activity of the Ni–Mo/graphene catalyst for hydrogen evolution from hydrolysis of ammonia borane. J. Mater. Chem. A 2016, 4, 8579–8583. [Google Scholar] [CrossRef]
- Xu, D.Y.; Dai, P.; Liu, X.M.; Cao, C.Q.; Guo, Q.J. Carbon-supported cobalt catalyst for hydrogen generation from alkaline sodium borohydride solution. J. Power Sources 2008, 182, 616–620. [Google Scholar] [CrossRef]
- Zou, X.X.; Huang, X.X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmekova, E.; Asefa, T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef]
- Yu, J.; Hsieh, C.-C.; Chen, P.-Y.; Weng, B.-J.; Chen-Yang, Y.W. Highly active and reusable silica-aerogel-supported platinum–cobalt bimetallic catalysts for the dehydrogenation of ammonia borane. RSC Adv. 2016, 6, 112109–112116. [Google Scholar] [CrossRef]
- Luo, Y.C.; Liu, Y.S.; Huang, Y.; Liu, X.Y.; Mou, C.Y. Mesoporous silica supported cobalt catalysts for hydrogen generation in hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2013, 37, 7280–7290. [Google Scholar] [CrossRef]
- Arbiol, J.; Cabot, A.; Morante, J.R.; Chen, F.; Liu, M. Distributions of noble metal Pd and Pt in mesoporous silica. Appl. Phys. Lett. 2002, 81, 3449–3451. [Google Scholar] [CrossRef]
- Yang, C.; Sheu, H.; Chao, K. Templated synthesis and structural study of densely packed metal nanostructures in MCM-41 and MCM-48. Adv. Funct. Mater. 2002, 12, 143–148. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Yang, Y.; Zhao, J.; Li, C.; Yang, Q. Entrapment of metal nanoparticles within nanocages of mesoporous silicas aided by co-surfactants. J. Mater. Chem. 2012, 22, 21045–21050. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhang, Y.H.; Chen, J.; Li, J.L.; Liew, K.; Nordin, M.R.B. SBA-16-Supported Cobalt catalyst with high activity and stability for Fischer–Tropsch synthesis. ChemCatChem 2012, 4, 265–272. [Google Scholar] [CrossRef]
- Minieri, L.; Esposito, S.; Russo, V.; Bonelli, B.; Di Serio, M.; Silvestri, B.; Vergara, A.; Aronne, A. A sol–gel ruthenium–niobium–silicon mixed-oxide bifunctional catalyst for the hydrogenation of levulinic acid in the aqueous phase. ChemCatChem 2017, 9, 1476–1486. [Google Scholar] [CrossRef]
- Esposito, S.; Silvestri, B.; Russo, V.; Bonelli, B.; Manzoli, M.; Deorsola, F.A.; Vergara, A.; Aronne, A.; Di Serio, M. Self-activating catalyst for glucose hydrogenation in the aqueous phase under mild conditions. ACS Catal. 2019, 9, 3426–3436. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, L.; Zhong, L.; Yang, Q.; Li, C. Enhanced cooperative activation effect in the hydrolytic kinetic resolution of epoxides on [co(salen)] catalysts confined in nanocages. Angew. Chem. Int. Ed. 2007, 46, 6861–6865. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, Z.C.; Wang, Y.K.; Wang, Y.W.; Fang, L. Hoveyda–Grubbs catalyst confined in the nanocages of SBA-1: Enhanced recyclability for olefinmetathesis. Chem. Commun. 2010, 46, 8659–8661. [Google Scholar] [CrossRef] [PubMed]
- Deka, J.R.; Kao, H.M.; Huang, S.Y.; Chang, W.; Ting, C.C.; Rath, P.C.; Chen, C.S. Ethane-bridged periodic mesoporous organosilicas functionalized with high loadings of carboxylic acid groups: Synthesis, bifunctionalization, and fabrication of metal nanoparticles. Chem. Eur. J. 2014, 20, 894–903. [Google Scholar] [CrossRef]
- Zhu, H.; Lee, B.; Dai, S.; Overbury, S.H. Coassembly synthesis of ordered mesoporous silica materials containing Au nanoparticles. Langmuir 2003, 19, 3974–3980. [Google Scholar] [CrossRef]
- Wu, P.; Bai, P.; Yan, Z.; Zhao, G.X.S. Gold nanoparticles supported on mesoporous silica: Origin of high activity and role of Au NPs in selective oxidation of cyclohexane. Sci. Rep. 2016, 6, 18817. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Ao, Q.; Cao, N.; Yang, L.; Luo, W.; Cheng, G. Facile synthesis of monodisperse ruthenium nanoparticles supported on graphene for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2015, 40, 6180–6187. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Li, G.; Zhang, M. Controlled synthesis of highly dispersed and nanosized Ru catalysts supported on carbonaceous materials via supercritical fluid deposition. RSC Adv. 2016, 6, 16851–16858. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia–borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Yao, Q.L.; Shi, W.M.; Feng, G.; Lu, Z.H.; Zhang, X.L.; Tao, D.J.; Kong, D.J.; Chen, X.S. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2014, 257, 293–299. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.-H.; Yang, K.; Chen, X.; Zhu, M. Ruthenium nanoparticles confined in SBA-15 as highly efficient catalyst for hydrolytic dehydrogenation of ammonia borane and hydrazine borane. Sci. Rep. 2015, 5, 15186–15196. [Google Scholar] [CrossRef]
- Cui, Z.; Guo, Y.; Feng, Z.; Xu, D.; Ma, J. Ruthenium nanoparticles supported on nitrogen-doped porous carbon as a highly efficient catalyst for hydrogen evolution from ammonia borane. New J. Chem. 2019, 43, 4377–4384. [Google Scholar] [CrossRef]
- Tonbul, Y.; Akbayrak, S.; Özkar, S. Nanozirconia supported ruthenium (0) nanoparticles: Highly active and reusable catalyst in hydrolytic dehydrogenation of ammonia borane. J. Coll. Int. Sci. 2018, 513, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Taşçı, E.; Akbayrak, S.; Özkar, S. Ruthenium (0) nanoparticles supported on silica coated Fe3O4 as magnetically separable catalysts for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2018, 43, 15124–15134. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts. J. Power Sources 2007, 168, 135–142. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Yu, Y.; Du, J.; Li, N.; Xu, L. Synthesis of mono-dispersed mesoporous SBA-1 nanoparticles with tunable pore size and their application in lysozyme immobilization. RSC Adv. 2014, 4, 37470–37478. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yan, W.; Duan, F.; Zhang, B.; Gao, Z.; Qin, Y. Ni nanoparticles supported on CNTs with excellent activity produced by atomic layer deposition for hydrogen generation from the hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 2112–2119. [Google Scholar] [CrossRef]


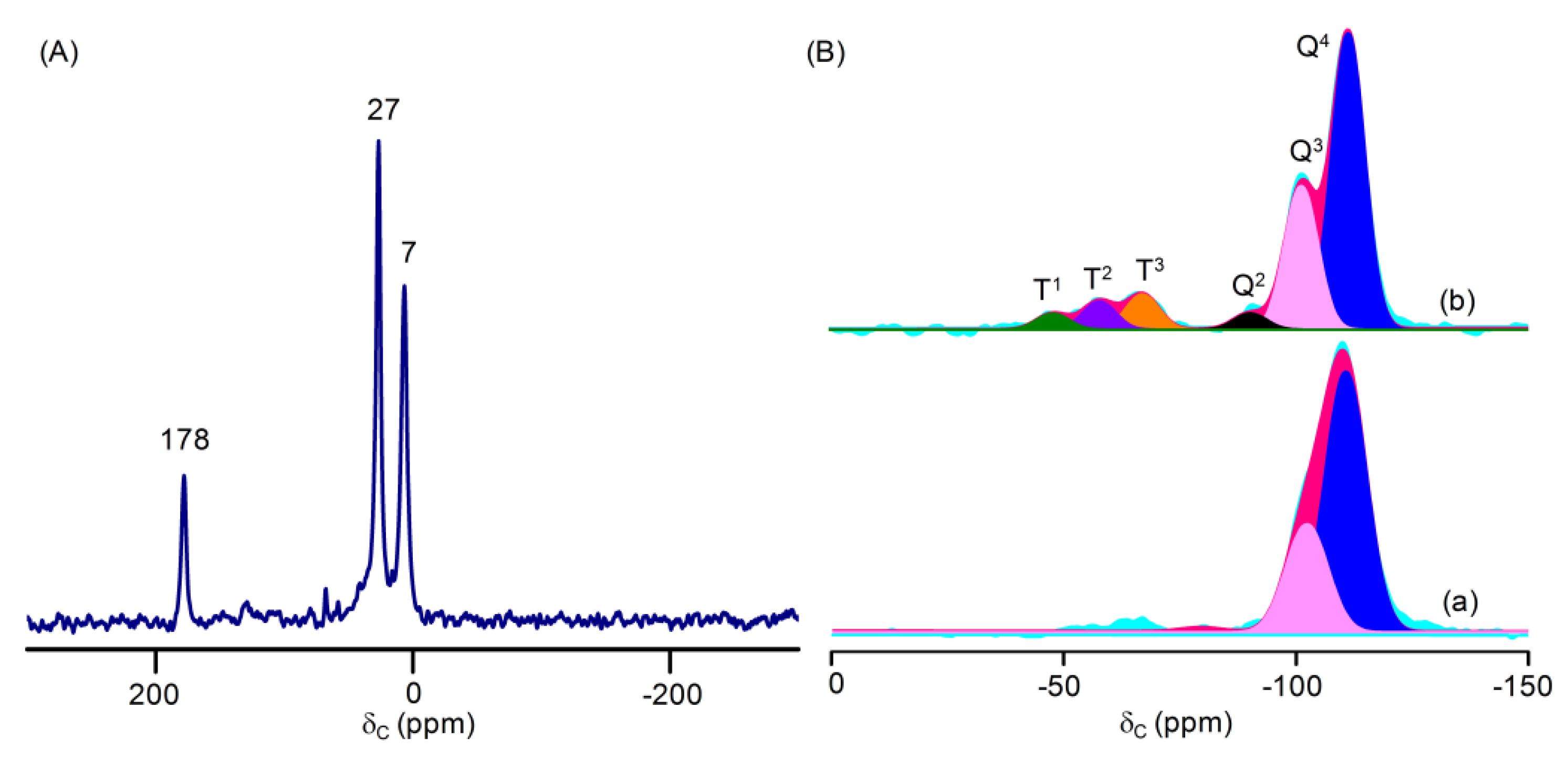

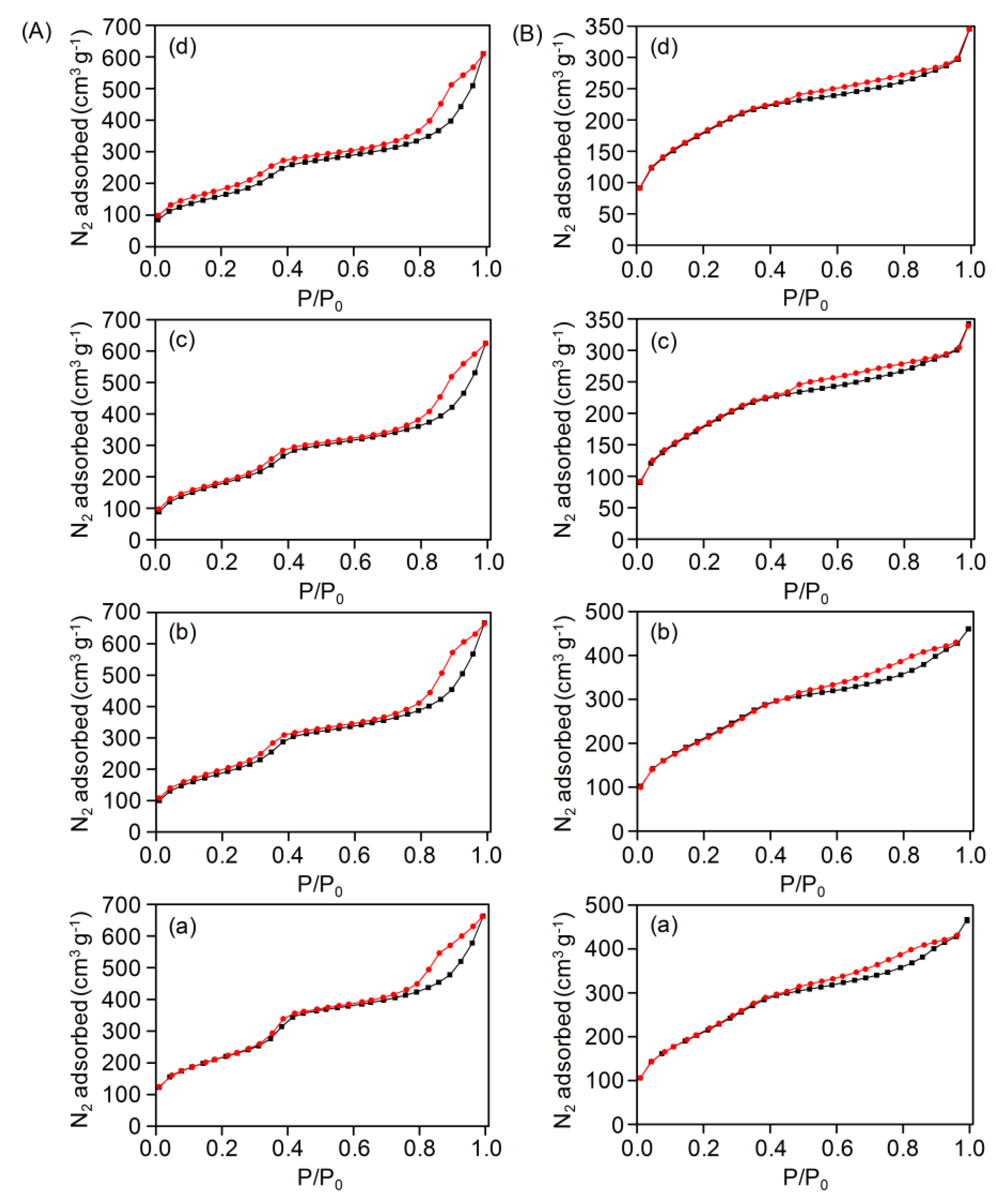
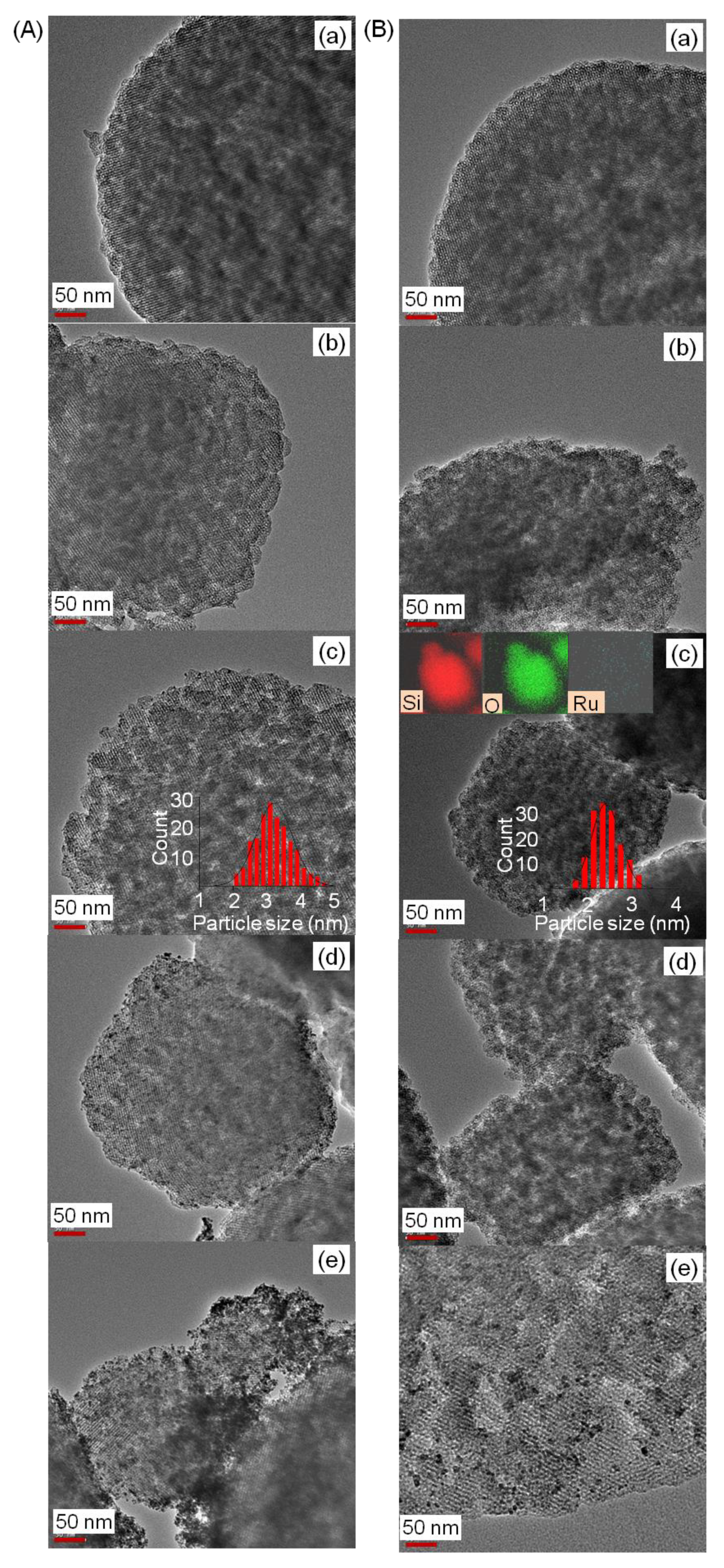

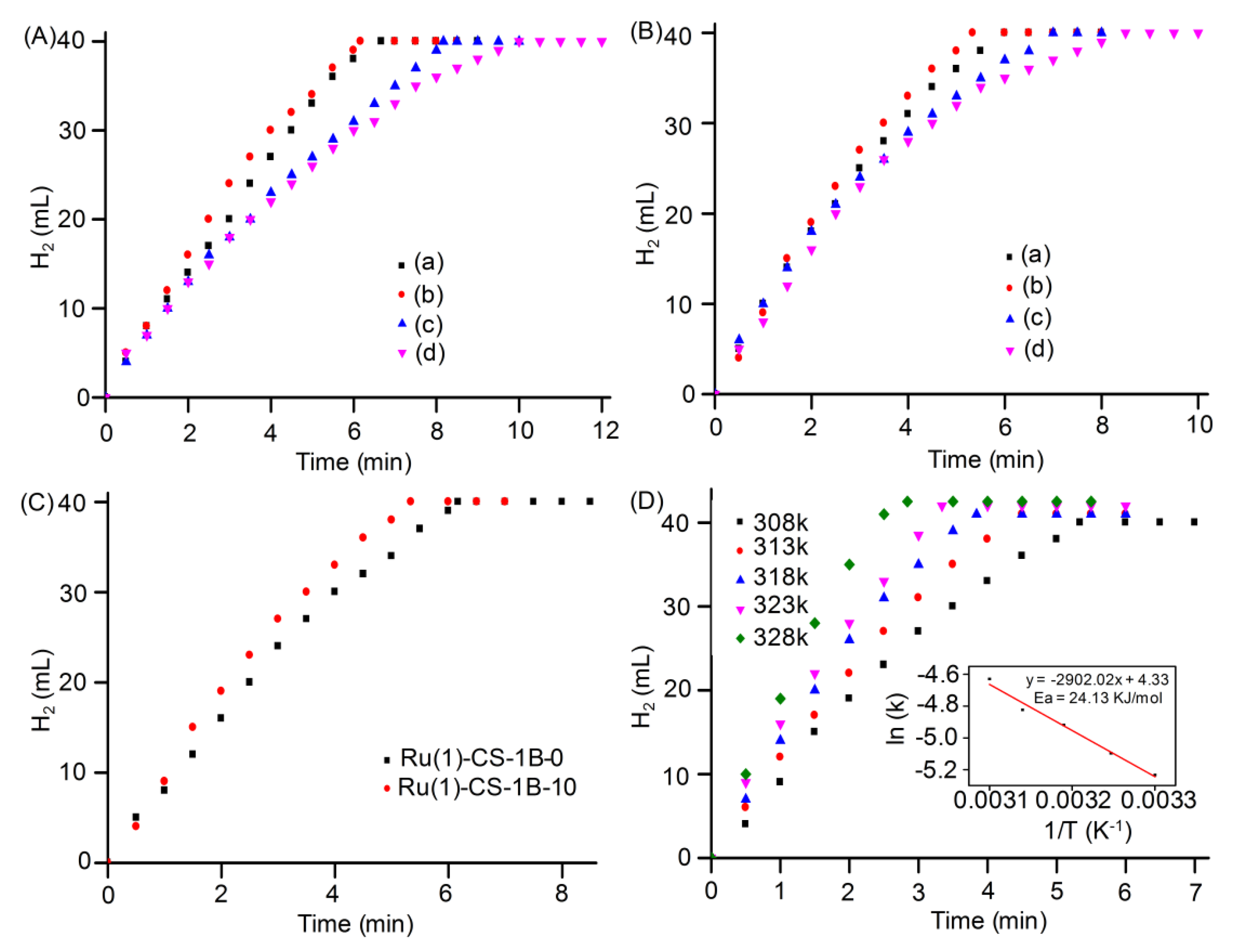

| Sample | Surface Area (m2g−1) | Pore Volume (cm3g−1) | Pore Size (nm) | Ru Loading (wt %) |
|---|---|---|---|---|
| S1B-C0 | 834 | 1.23 | 3.2 | - |
| Ru(0.5)@S1B-C0 | 775 | 1.03 | 3.0 | 0.223 |
| Ru(1)@S1B-C0 | 698 | 1.03 | 3.0 | 0.779 |
| Ru(2)@S1B-C0 | 664 | 0.96 | 3.0 | 1.020 |
| Ru(5)@ S1B-C0 | 602 | 0.94 | 3.0 | 2.760 |
| S1B-C10 | 850 | 1.84 | 3.0 | - |
| Ru(0.5)@S1B-C10 | 808 | 0.72 | 3.0 | 0.224 |
| Ru(1)@S1B-C10 | 792 | 0.71 | 3.0 | 0.639 |
| Ru(2)@S1B-C10 | 658 | 0.53 | 3.0 | 1.190 |
| Ru(5)@S1B-C10 | 624 | 0.48 | 3.0 | 2.880 |
| Catalysts | TOF (mol H2 min−1 molRu−1) | Ea (kJ mol−1) | Ref. |
|---|---|---|---|
| Ru(1)@S1B-C10 | 202.4 | 24.13 | This work |
| Ru(1)@S1B-C0 | 175.0 | 30.06 | This work |
| Ru/HPCM | 440 | 43.0 | [28] |
| Ru@SiO2 | 200 | 38.2 | [49] |
| Ru@SBA-15 | 316 | 34.8 | [50] |
| Ru/NC-Fe | 102.9 | 47.42 | [51] |
| Ru0/ZrO2 | 173 | 58 | [52] |
| Ru(0)/SiO2-Fe2O3 | 127 | 54 | [53] |
| Ru@ϒ-Al2O3 | 77 | 23 | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, J.R.; Saikia, D.; Hsia, K.-S.; Kao, H.-M.; Yang, Y.-C.; Chen, C.-S. Ru Nanoparticles Embedded in Cubic Mesoporous Silica SBA-1 as Highly Efficient Catalysts for Hydrogen Generation from Ammonia Borane. Catalysts 2020, 10, 267. https://doi.org/10.3390/catal10030267
Deka JR, Saikia D, Hsia K-S, Kao H-M, Yang Y-C, Chen C-S. Ru Nanoparticles Embedded in Cubic Mesoporous Silica SBA-1 as Highly Efficient Catalysts for Hydrogen Generation from Ammonia Borane. Catalysts. 2020; 10(3):267. https://doi.org/10.3390/catal10030267
Chicago/Turabian StyleDeka, Juti Rani, Diganta Saikia, Kuo-Shu Hsia, Hsien-Ming Kao, Yung-Chin Yang, and Ching-Shiun Chen. 2020. "Ru Nanoparticles Embedded in Cubic Mesoporous Silica SBA-1 as Highly Efficient Catalysts for Hydrogen Generation from Ammonia Borane" Catalysts 10, no. 3: 267. https://doi.org/10.3390/catal10030267
APA StyleDeka, J. R., Saikia, D., Hsia, K.-S., Kao, H.-M., Yang, Y.-C., & Chen, C.-S. (2020). Ru Nanoparticles Embedded in Cubic Mesoporous Silica SBA-1 as Highly Efficient Catalysts for Hydrogen Generation from Ammonia Borane. Catalysts, 10(3), 267. https://doi.org/10.3390/catal10030267







