Selective Oxidation of Benzyl Alcohol by Ag/Pd/m-BiVO4 Microspheres under Visible Light Irradiation
Abstract
1. Introduction
2. Results and Discussion
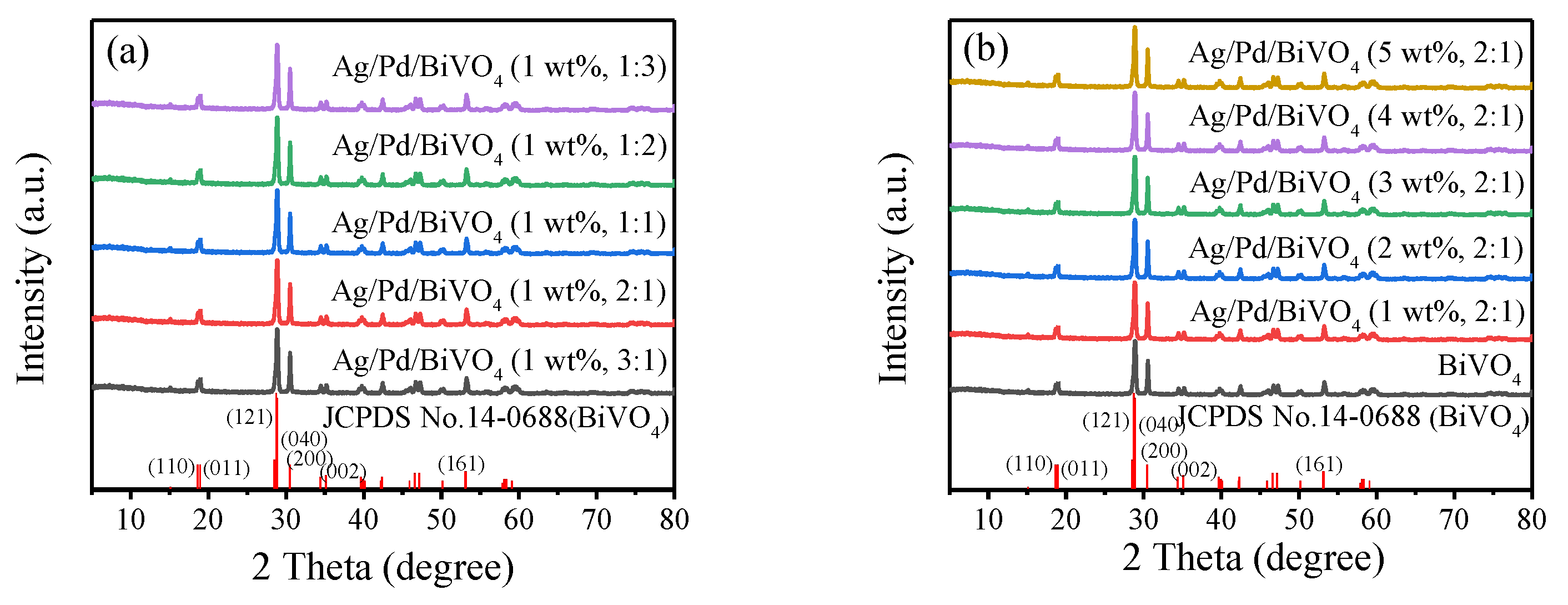
2.1. XRD Analysis
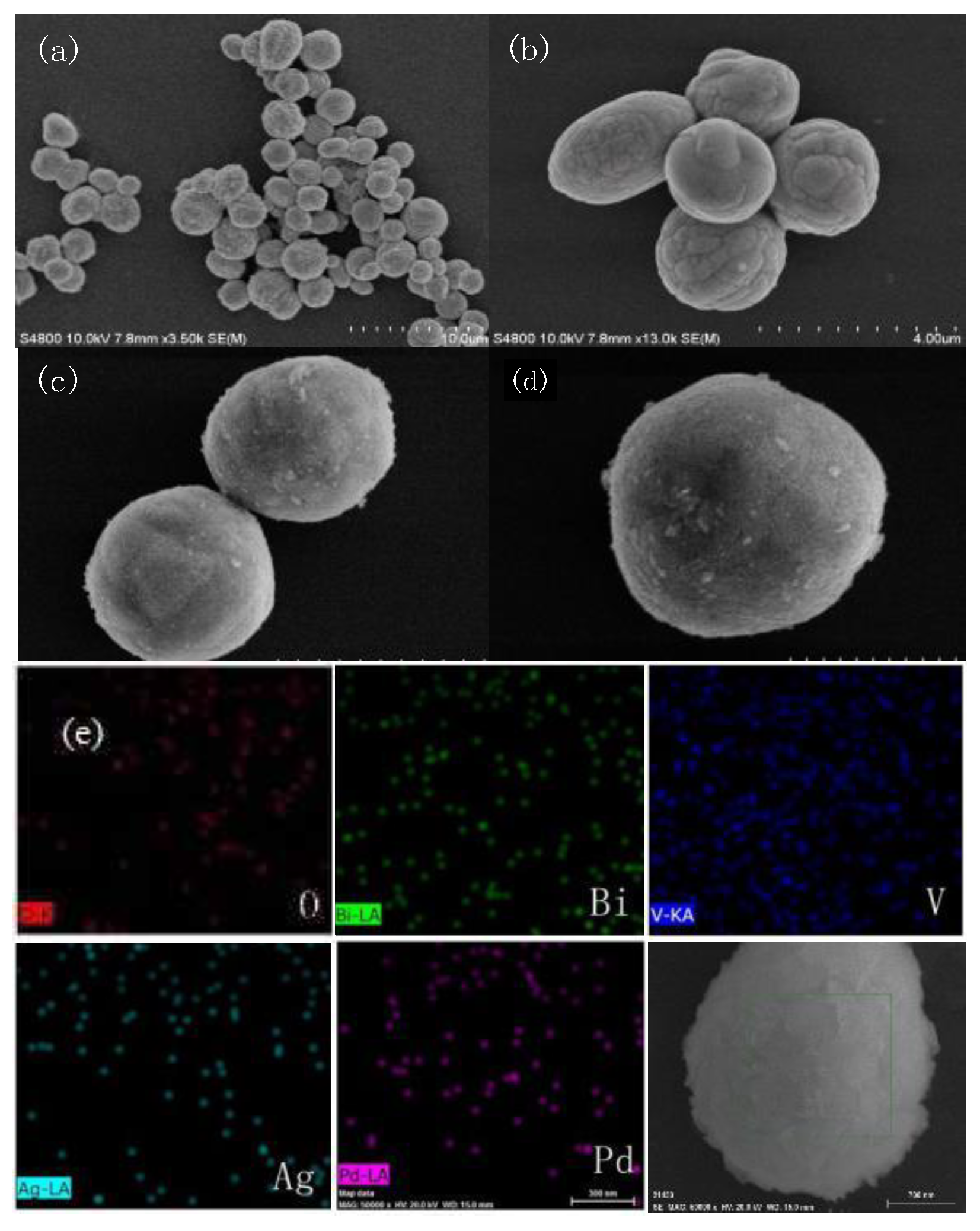
2.2. SEM Analysis
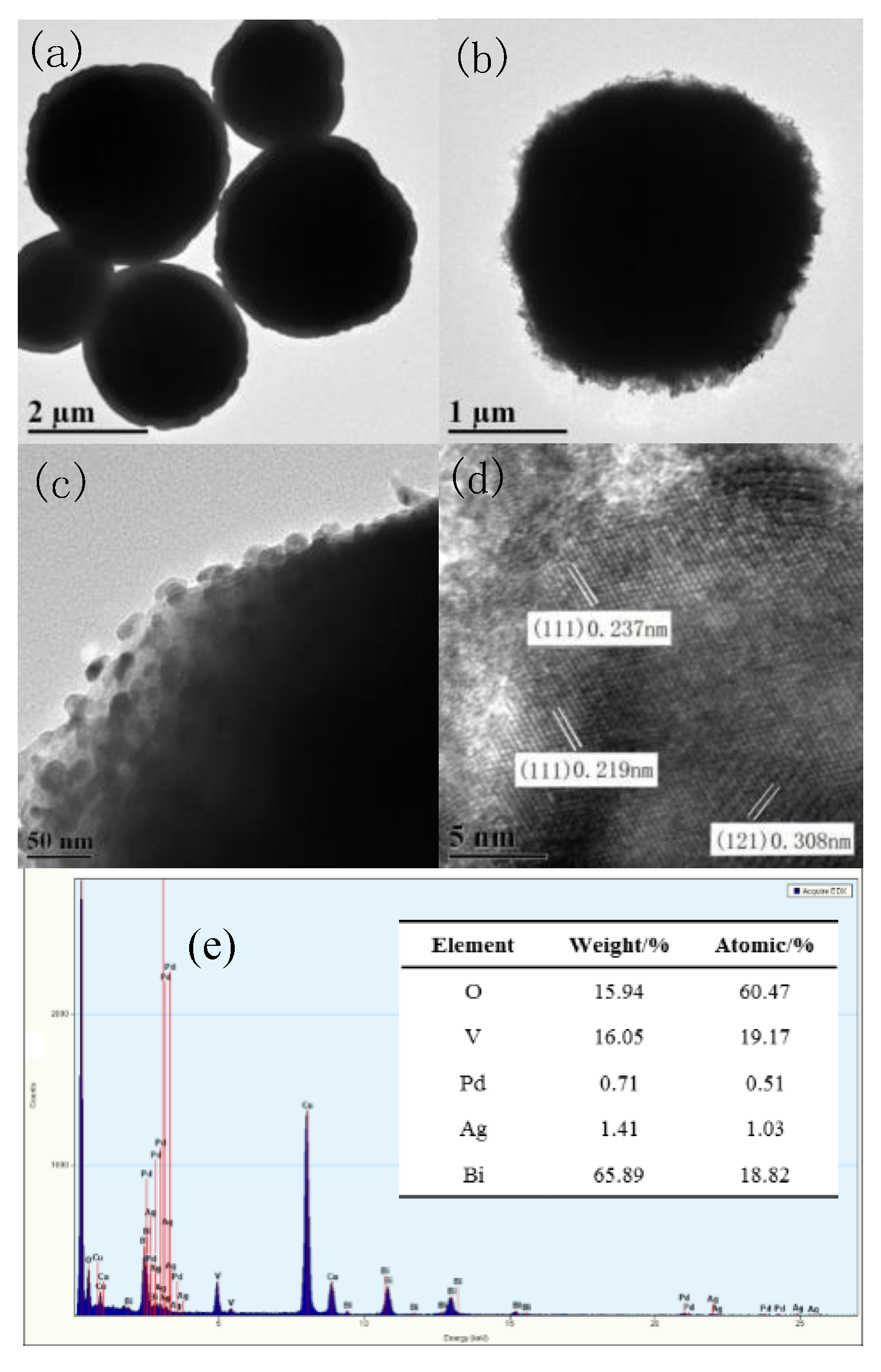
2.3. TEM Analysis
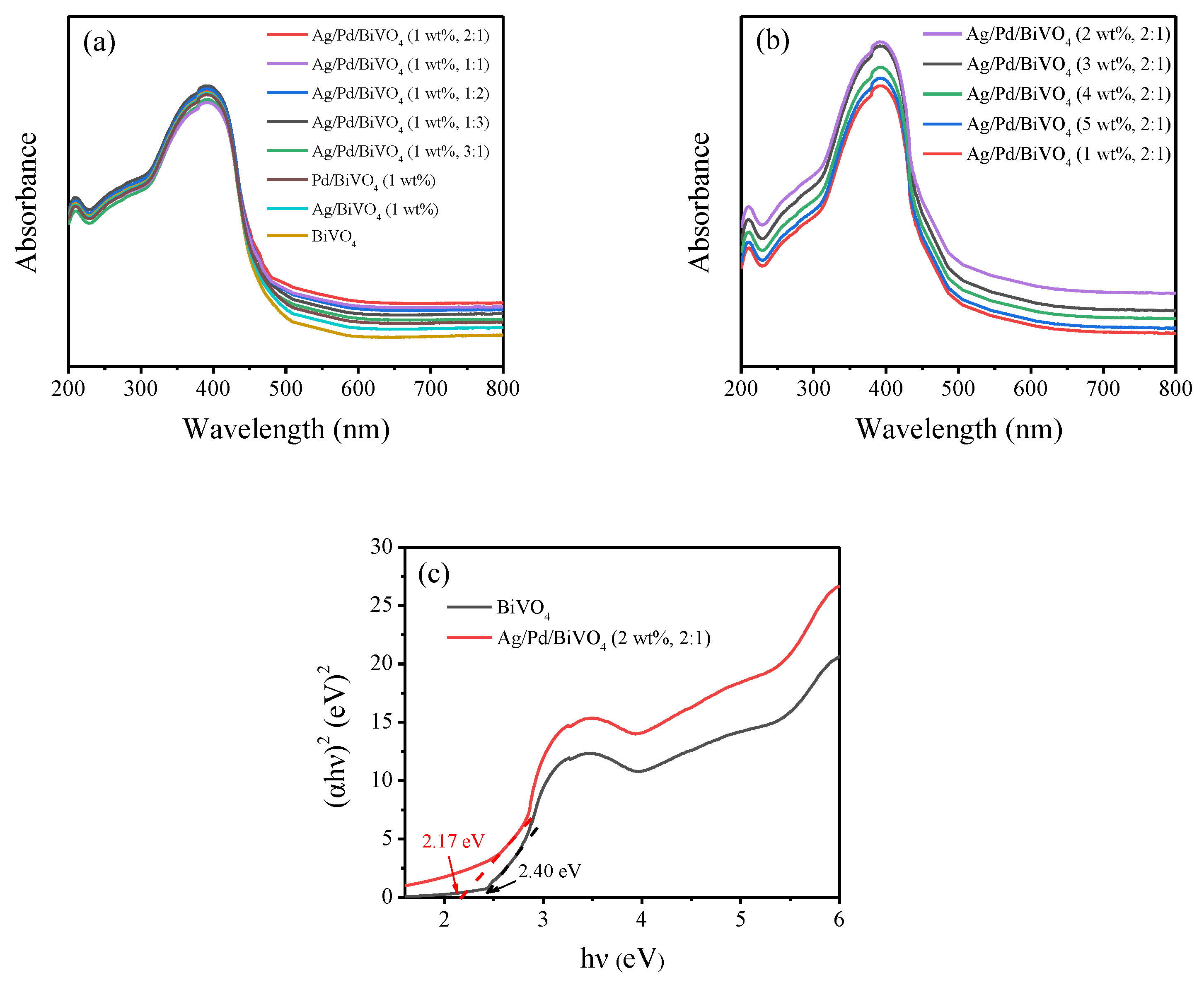
2.4. UV-vis DRS Analysis
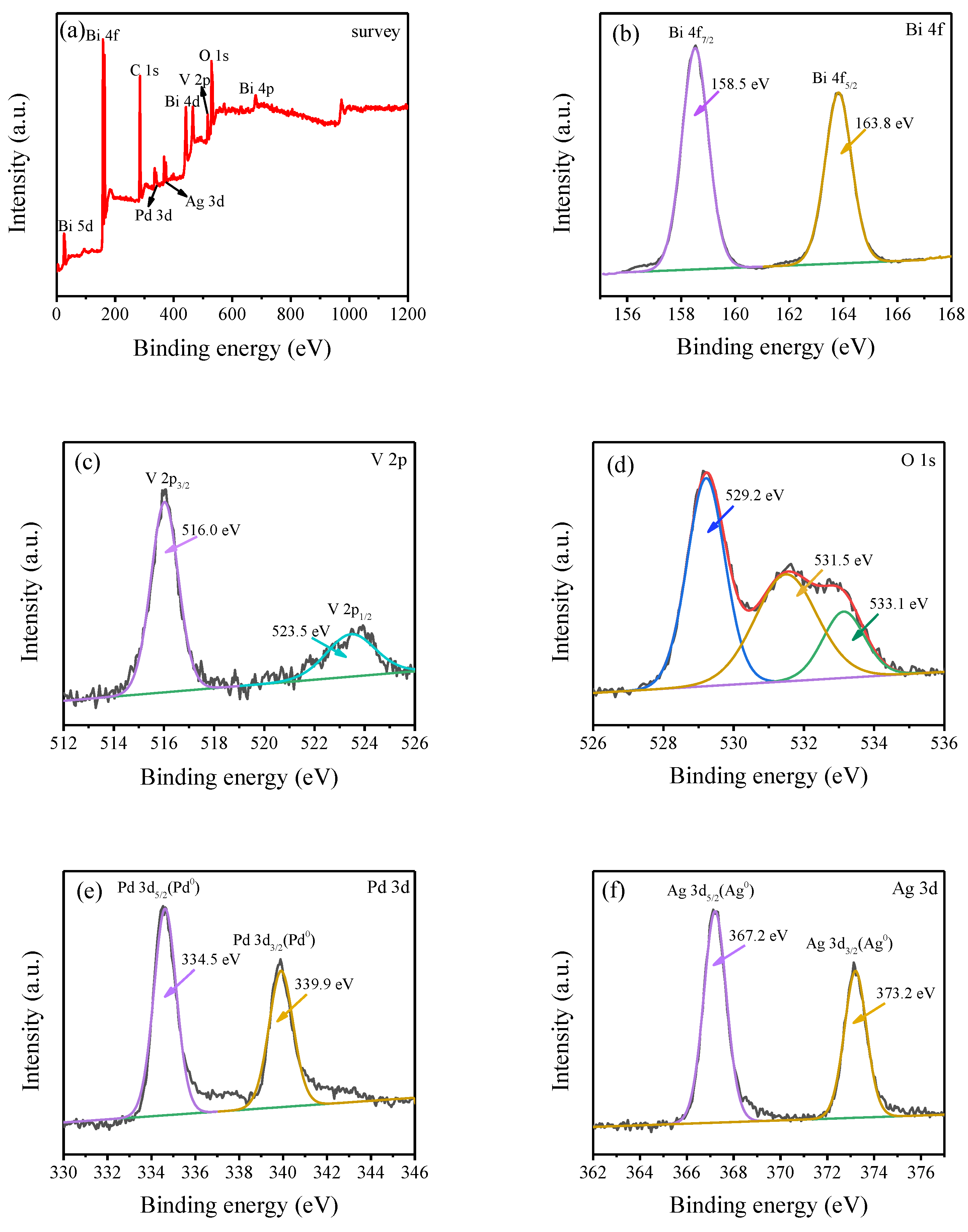
2.5. XPS Analysis
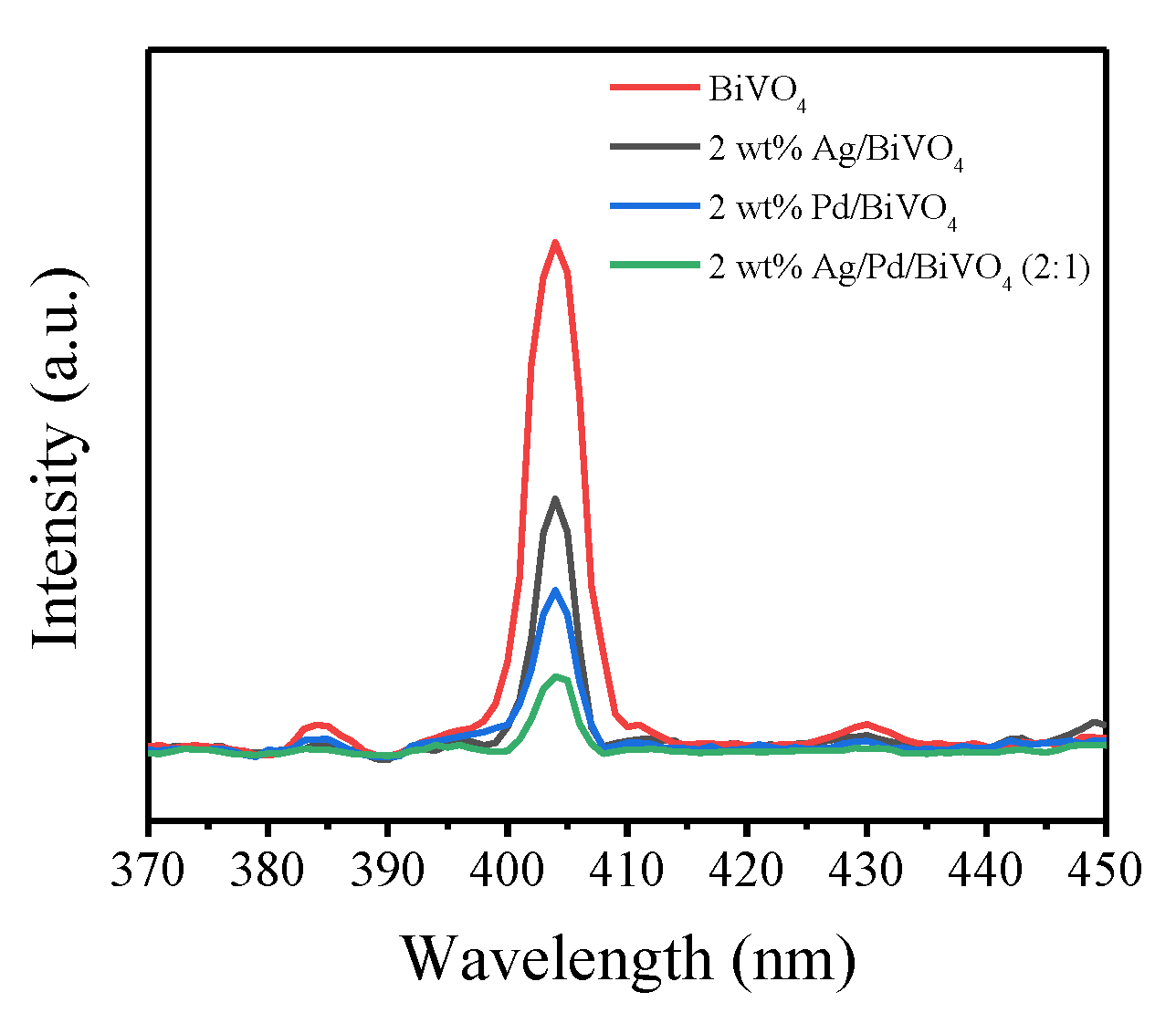
2.6. PL Analysis
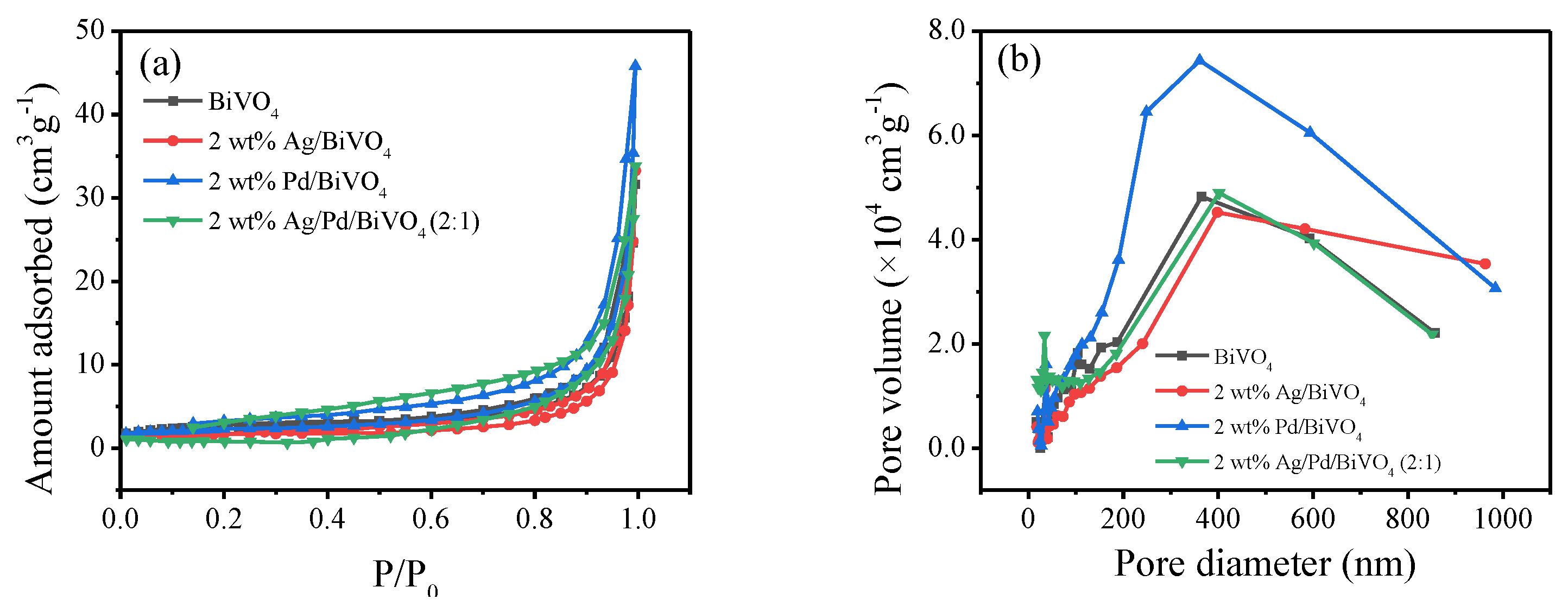
2.7. BET Analysis
2.8. Catalytic Activity
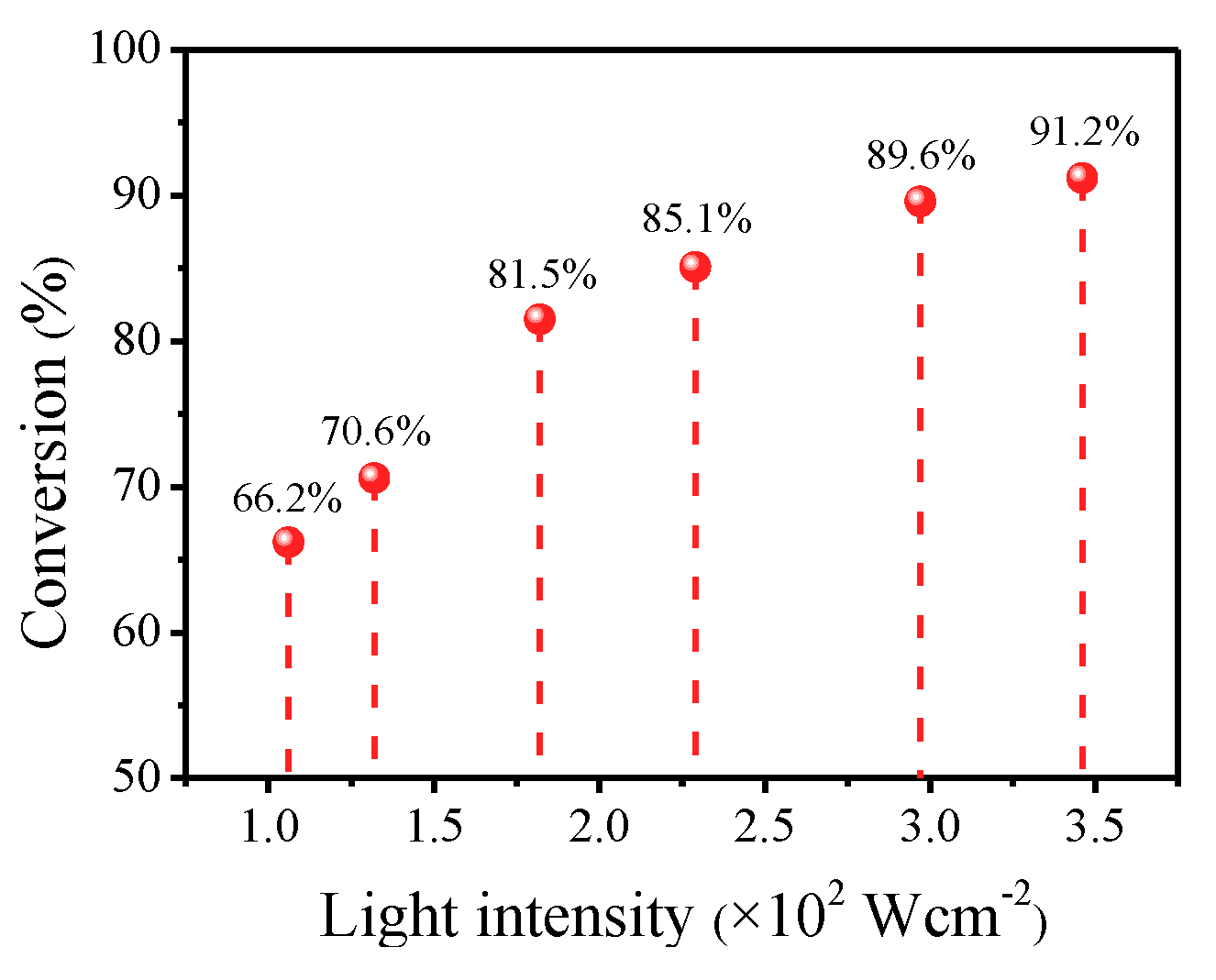
2.9. Effect of Light Intensity on The Selective Oxidation of Benzyl Alcohol
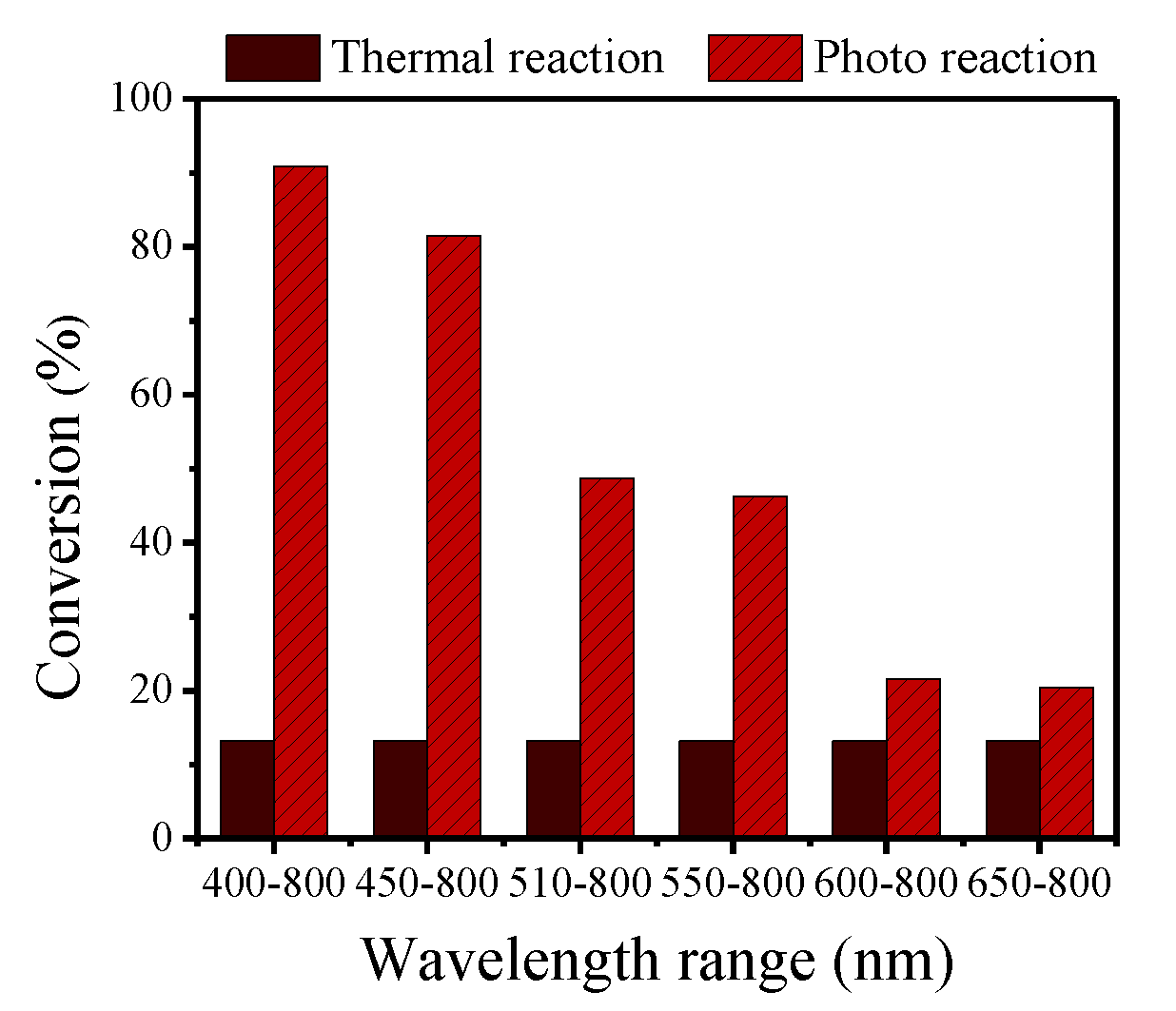
2.10. Effect of Light Wavelength on The Selective Oxidation of Benzyl Alcohol
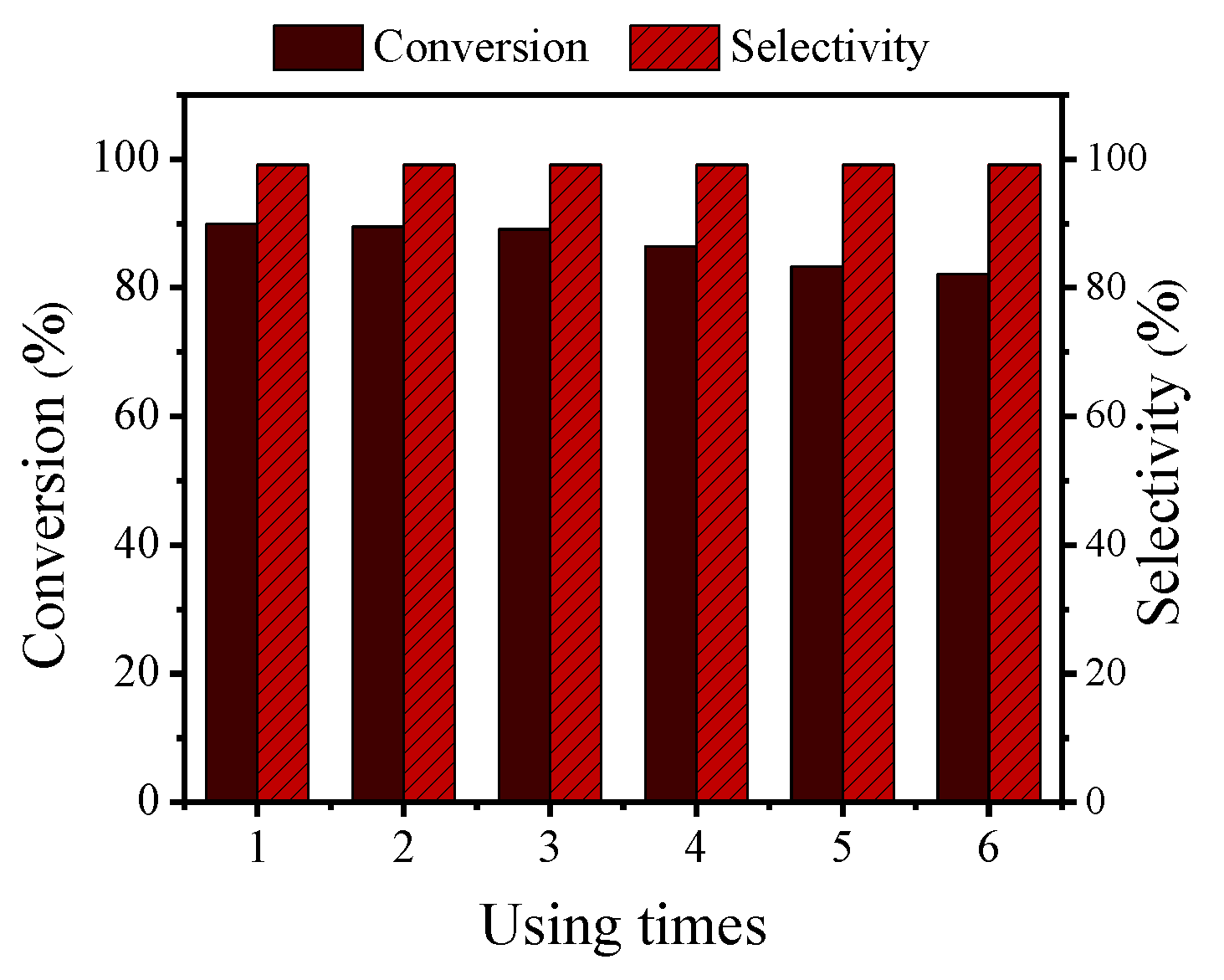
2.11. Cycle Capability Test of the Catalyst
2.12. Active Species Test
3. Materials and Methods
3.1. Preparation of Photocatalysts
3.1.1. Preparation of M-BiVO4
3.1.2. Preparation of Ag/Pd/m-BiVO4
3.2. Characterization Methods
3.3. Photocatalytic Activity Analysis
3.4. Recycling Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R.S.; Li, B.X.; Xiao, Y.; Tao, X.Q.; Su, X.T.; Dong, X.P. Optimizing Pd and Au-Pd decorated Bi2WO6 ultrathin nanosheets for photocatalytic selective oxidation of aromatic alcohols. J. Catal. 2018, 364, 154–165. [Google Scholar] [CrossRef]
- Wang, J.Z.; Jin, J.; Wang, X.G.; Yang, S.N.; Zhao, Y.L.; Wu, Y.W.; Dong, S.Y.; Sun, J.Y.; Sun, J.H. Facile fabrication of novel BiVO4/Bi2S3/MoS2 n-p heterojunction with enhanced photocatalytic activities towards pollutant degradation under natural sunlight. J. Colloid Interf. Sci. 2017, 505, 805–815. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, S.B.; Xiao, K.; Ma, T.Y.; Zhang, Y.H.; Huang, H.W. Z-scheme g-C3N4/Bi4NbO8Cl heterojunction for enhanced photocatalytic hydrogen production. ACS Sustain. Chem. Eng. 2018, 6, 16219–16227. [Google Scholar] [CrossRef]
- Jin, B.B.; Yao, G.D.; Wang, X.G.; Ding, K.F.; Jin, F.M. Photocatalytic oxidation of glucose into formate on nano TiO2 catalyst. ACS Sustain. Chem. Eng. 2017, 5, 6377–6381. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Serendipitous assembly of mixed phase BiVO4 on B-Doped g-C3N4: An appropriate p-n heterojunction for photocatalytic O2 evolution and Cr (VI) reduction. Inorg. Chem. 2019, 58, 12480–12491. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Khilari, S.; Pradhan, D.; Srivastava, R. An efficient, visible light driven, selective oxidation of aromatic alcohols and amines with O2 using BiVO4/g-C3N4 nanocomposite: A systematic and comprehensive study toward the development of a photocatalytic process. ACS Sustain. Chem. Eng. 2017, 5, 2562–2577. [Google Scholar] [CrossRef]
- Chong, B.; Chen, L.; Wang, W.T.; Han, D.Z.; Wang, L.; Feng, L.J.; Li, Q.; Li, C.H. Visible-light-driven Ag-decorated g-C3N4/Bi2WO6 Z-scheme composite for high photocatalytic activity. Mater. Lett. 2017, 204, 149–153. [Google Scholar] [CrossRef]
- Wang, J.J.; Tang, L.; Zeng, G.G.; Liu, Y.N.; Zhou, Y.Y.; Deng, Y.C.; Wang, J.J.; Peng, B. Plasmonic Bi metal deposition and g-C3N4 coating on Bi2WO6 microspheres for efficient visible-light photocatalysis. ACS Sustain. Chem. Eng. 2016, 5, 1062–1072. [Google Scholar] [CrossRef]
- He, W.J.; Sun, Y.J.; Jiang, G.M.; Huang, H.W.; Zhang, X.M.; Dong, F. Activation of amorphous Bi2WO6 with synchronous Bi metal and Bi2O3 coupling: Photocatalysis mechanism and reaction pathway. Appl. Catal. B Environ. 2018, 232, 340–347. [Google Scholar] [CrossRef]
- Song, J.; Seo, M.J.; Lee, T.H.; Jo, Y.R.; Lee, J.; Kim, T.L.; Kim, S.Y.; Kim, S.M.; Jeong, S.Y.; An, H.; et al. Tailoring crystallographic orientations to substantially enhance charge separation efficiency in anisotropic BiVO4 photoanodes. ACS Catal. 2018, 8, 5952–5962. [Google Scholar] [CrossRef]
- Jing, Q.F.; Feng, X.Y.; Zhao, X.J.; Duan, Z.Y.; Pan, J.L.; Chen, L.M.; Liu, Y.N. Bi/BiVO4 chain-like hollow microstructures: Synthesis, characterization and application as visible-light-active photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2653–2661. [Google Scholar] [CrossRef]
- He, B.; Li, Z.P.; Zhao, D.; Liu, H.H.; Zhong, Y.J.; Ning, J.Q.; Zhang, Z.Y.; Wang, Y.J.; Hu, Y. Fabrication of porous Cu-doped BiVO4 nanotubes as efficient oxygen-evolving photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2589–2599. [Google Scholar] [CrossRef]
- Dong, C.W.; Lu, S.Y.; Yao, S.Y.; Ge, R.; Wang, Z.D.; Wang, Z.; An, P.F.; Liu, Y.; Yang, B.; Zhang, H. Colloidal synthesis of ultrathin monoclinic BiVO4 nanosheets for Z-scheme overall water splitting under visible light. ACS Catal. 2018, 8, 8649–8658. [Google Scholar] [CrossRef]
- Sajid, M.M.; Khan, S.B.; Shad, N.A.; Amin, N.; Zhang, Z.J. Visible light assisted photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid using a BiVO4/FeVO4 heterojunction composite. RSC Adv. 2018, 8, 23489–23498. [Google Scholar] [CrossRef]
- Grigioni, I.; Stamplecoskie, K.G.; Selli, E.; Kamat, P.V. Dynamics of photogenerated charge carriers in WO3/BiVO4 heterojunction photoanodes. J. Phys. Chem. C. 2015, 119, 20792–20800. [Google Scholar] [CrossRef]
- Pan, Q.G.; Zhang, C.; Xiong, Y.J.; Mi, Q.X.; Li, D.D.; Zou, L.L.; Huang, Q.H.; Zou, Z.Q.; Yang, H. Boosting charge separation and transfer by plasmon-enhanced MoS2/BiVO4 p–n heterojunction composite for efficient photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 2018, 6, 6378–6387. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Dai, Z.X.; Zheng, G.H.; Yao, Z.F.; Mu, J.J. Superior visible light photocatalytic performance of reticular BiVO4 synthesized via a modified sol-gel method. RSC Adv. 2018, 8, 10654–10664. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.Z.; Xiao, C.X.; Chen, L.; Guo, J.K.; Shen, S.; Au, C.T.; Yin, S.F. Preparation of helical BiVO4/Ag/C3N4 for selective oxidation of C-H bond under visible light irradiation. ACS Sustain. Chem. Eng. 2019, 7, 17500–17506. [Google Scholar] [CrossRef]
- Ni, S.N.; Zhou, T.T.; Zhang, H.N.; Cao, Y.Q.; Yang, P. BiOI/BiVO4 two-dimensional hetero-nanostructures for visible-light photocatalytic degradation of rhodamine B. ACS Appl. Nano Mater. 2018, 1, 5128–5141. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.G.; Mu, L.C.; Li, C. The Significance of crystal morphology controlling in semiconductor-based photocatalysis: A case study on BiVO4 photocatalyst. Cryst. Growth Des. 2017, 17, 2923–2928. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Y.; Zhang, J.; Zhu, F.X.; Sheng, Z.H.; Dai, B.L.; Dennis, Y.C.L.; Zhang, L.L.; Xu, J.M. A novel Ag/p-AgBr/n-BiVO4 plasmonic heterojunction photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. ACS Appl. Energy Mater. 2018, 2, 694–704. [Google Scholar]
- Tao, X.Q.; Shao, L.Z.; Wang, R.S.; Xiang, H.P.; Li, B.X. Synthesis of BiVO4 nanoflakes decorated with AuPd nanoparticles as selective oxidation photocatalysts. J. Colloid Interf. sci. 2019, 541, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Chen, X.X.; Yi, Z.G. Defective, porous TiO2 nanosheets with Pt decoration as an efficient photocatalyst for ethylene oxidation synthesized by a C3N4 templating method. ACS Appl. Mater. Interf. 2016, 8, 10104–10108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Zheng, X.Y.; Feng, X.H.; Li, Y. CO2 reduction by plasmonic Au nanoparticles decorated TiO2 photocatalyst with an ultrathin Al2O3 interlayer. J. Phys. Chem. C. 2018, 122, 18949–18956. [Google Scholar] [CrossRef]
- Sharma, M.; Das, B.; Sharma, M.; Deka, B.K.; Park, Y.B.; Bhargava, S.K.; Bania, K.K. Pd/Cu-oxide nanoconjugate at zeolite-Y crystallite crafting the mesoporous channels for selective oxidation of benzyl-alcohols. ACS appl. Mater. Interf. 2017, 9, 35453–35462. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.F.; Xu, D.B.; Li, D.; Wu, G.L.; Wu, M.M.; Shi, W.D.; Chen, M. Fabrication of a Ag/Bi3TaO7 plasmonic photocatalyst with enhanced photocatalytic activity for degradation tetracycline. ACS appl. Mater. Interf. 2015, 7, 17061–17069. [Google Scholar] [CrossRef] [PubMed]
- She, H.D.; Zhou, H.; Li, L.S.; Wang, L.; Huang, J.W.; Wang, Q.Z. Nickel-doped excess oxygen defect titanium dioxide for efficient selective photocatalytic oxidation of benzyl alcohol. ACS Sustain. Chem. Eng. 2018, 6, 11939–11948. [Google Scholar] [CrossRef]
- Hayashido, Y.; Naya, S.I.; Tada, H. Local electric field-enhanced plasmonic photocatalyst: Formation of Ag cluster-incorporated AgBr nanoparticles on TiO2. J. Phys. Chem. C. 2016, 120, 19663–19669. [Google Scholar] [CrossRef]
- Su, R.; Dimitratos, N.; Liu, J.J.; Carter, E.; Althahban, S.; Wang, X.Q.; Shen, Y.B.; Wendt, S.; Wen, X.D.; Niemantsverdriet, J.W.; et al. Mechanistic insight into the interaction between a titanium dioxide photocatalyst and Pd cocatalyst for improved photocatalytic performance. ACS Catal. 2016, 6, 4239–4247. [Google Scholar] [CrossRef]
- Liu, H.X.; Wang, M.; Zhang, X.Q.; Ma, J.T.; Lu, G.X. High efficient photocatalytic hydrogen evolution from formaldehyde over sensitized Ag@Ag-Pd alloy catalyst under visible light irradiation. Appl. Catal. B Environ. 2018, 237, 563–573. [Google Scholar] [CrossRef]
- Wang, W.Z.; Huang, X.W.; Wu, S.; Zhou, Y.X.; Wang, L.J.; Shi, H.L.; Liang, Y.J.; Zou, B. Preparation of p-n junction Cu2O/BiVO4 heterogeneous nanostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2013, 134, 293–301. [Google Scholar] [CrossRef]
- Deng, Y.C.; Tang, L.; Zeng, G.G.; Feng, C.Y.; Dong, H.R.; Wang, J.J.; Feng, H.P.; Liu, Y.N.; Zhou, Y.Y.; Pang, Y. Plasmonic resonance excited dual Z-scheme BiVO4/Ag/Cu2O nanocomposite: Synthesis and mechanism for enhanced photocatalytic performance in recalcitrant antibiotic degradation. Environ. Sci. Nano 2017, 4, 1494–1511. [Google Scholar] [CrossRef]
- Gao, J.; Si, Z.C.; Xu, Y.F.; Liu, L.P.; Zhang, Y.Y.; Wu, X.D.; Ran, R.; Weng, D. Pd-Ag@CeO2 catalyst of core-shell structure for low temperature oxidation of toluene under visible light irradiation. J. Phys. Chem. C. 2018, 123, 1761–1769. [Google Scholar] [CrossRef]

| No. | Solvents | Polar Index | Light Irradiation | In Dark | ||
|---|---|---|---|---|---|---|
| Conv. (%) | Sel. (%) | Conv. (%) | Sel. (%) | |||
| 1 | DMF | 6.4 | 30.1 | >99 | 5.2 | >99 |
| 2 | 1,4-Dioxane | 4.8 | 61.4 | >99 | 10.4 | >99 |
| 3 | Isopropanol | 4.3 | 63.8 | >99 | 10.5 | >99 |
| 4 | Toluene | 2.4 | 70.7 | >99 | 12.2 | >99 |
| 5 | n-heptane | 0.2 | 51.2 | 92.1 | 8.6 | 90.4 |
| 6 | Cyclohexane | 0.1 | 40.7 | 88.0 | 8.2 | 87.7 |
| 7 | n-Hexane | 0.06 | 36.9 | 86.2 | 7.0 | 87.0 |
| 8 | Petroleum ether | 0.01 | 31.2 | 85.3 | 5.9 | 86.1 |
| No. | Bases | Light Irradiation | In Dark | ||
|---|---|---|---|---|---|
| Conv. (%) | Sel. (%) | Conv. (%) | Sel. (%) | ||
| 1 | 1.0 mmol CH3ONa | 23.5 | >99 | 3.4 | >99 |
| 2 | 1.0 mmol Cs2CO3 | 30.4 | >99 | 3.6 | >99 |
| 3 | 1.0 mmol K2CO3 | 15.6 | >99 | 2.4 | >99 |
| 4 | 1.0 mmol Na2CO3 | 26.1 | >99 | 5.3 | >99 |
| 5 | 1.0 mmol KOH | 70.7 | >99 | 12.2 | >99 |
| 6 | 1.0 mmol LiOH | 40.9 | >99 | 10.4 | >99 |
| 7 | 1.2 mmol NaOH | 75.1 | >99 | 13.3 | >99 |
| 8 | 1.0 mmol NaOH | 75.9 | >99 | 13.1 | >99 |
| 9 | 0.8 mmol NaOH | 71.6 | >99 | 13.0 | >99 |
| 10 | 0.6 mmol NaOH | 50.1 | >99 | 12.1 | >99 |
| 11 | 0.4 mmol NaOH | 29.2 | >99 | 7.6 | >99 |
| 12 | None | 8.4 | >99 | 0 | >0 |
| No. | Catalysts | Light Irradiation | In Dark | ||
|---|---|---|---|---|---|
| Conv. (%) | Sel. (%) | Conv. (%) | Sel. (%) | ||
| 1 | BiVO4 | 30.7 | >99 | 4.2 | >99 |
| 2 | Ag/BiVO4(1wt%) | 56.4 | >99 | 6.9 | >99 |
| 3 | Pd/BiVO4(1wt%) | 65.8 | >99 | 7.2 | >99 |
| 4 | Ag/Pd/BiVO4(1wt%,3:1) | 76.3 | >99 | 9.8 | >99 |
| 5 | Ag/Pd/BiVO4(1wt%,2:1) | 79.8 | >99 | 10.9 | >99 |
| 6 | Ag/Pd/BiVO4(1wt%,1:1) | 75.9 | >99 | 13.1 | >99 |
| 7 | Ag/Pd/BiVO4(1wt%,1:2) | 72.7 | >99 | 10.0 | >99 |
| 8 | Ag/Pd/BiVO4(1wt%,1:3) | 71.9 | >99 | 10.4 | >99 |
| 9 | Ag/Pd/BiVO4(2wt%,2:1) | 89.9 | >99 | 11.2 | >99 |
| 10 | Ag/Pd/BiVO4(3wt%,2:1) | 86.8 | >99 | 10.7 | >99 |
| 11 | Ag/Pd/BiVO4(4wt%,2:1) | 83.1 | >99 | 10.4 | >99 |
| 12 | Ag/Pd/BiVO4(5wt%,2:1) | 81.6 | >99 | 10.2 | >99 |
| No. | Catalyst (mg) | Light Irradiation | In Dark | ||
|---|---|---|---|---|---|
| Conv. (%) | Sel. (%) | Conv. (%) | Sel. (%) | ||
| 1 | 25 | 57.3 | >99 | 8.4 | >99 |
| 2 | 50 | 89.9 | >99 | 11.2 | >99 |
| 3 | 75 | 84.1 | >99 | 11.9 | >99 |
| 4 | 100 | 70.2 | >99 | 12.1 | >99 |
| No. | Reaction Times (h) | Light Irradiation | In Dark | ||
|---|---|---|---|---|---|
| Conv. (%) | Sel. (%) | Conv. (%) | Sel. (%) | ||
| 1 | 8 | 78.1 | >99 | 10.7 | >99 |
| 2 | 10 | 83.5 | >99 | 12.2 | >99 |
| 3 | 12 | 89.9 | >99 | 11.2 | >99 |
| 4 | 15 | 90.2 | >99 | 13.3 | >99 |
| 5 | 24 | 90.7 | >99 | 13.9 | >99 |
| No. | 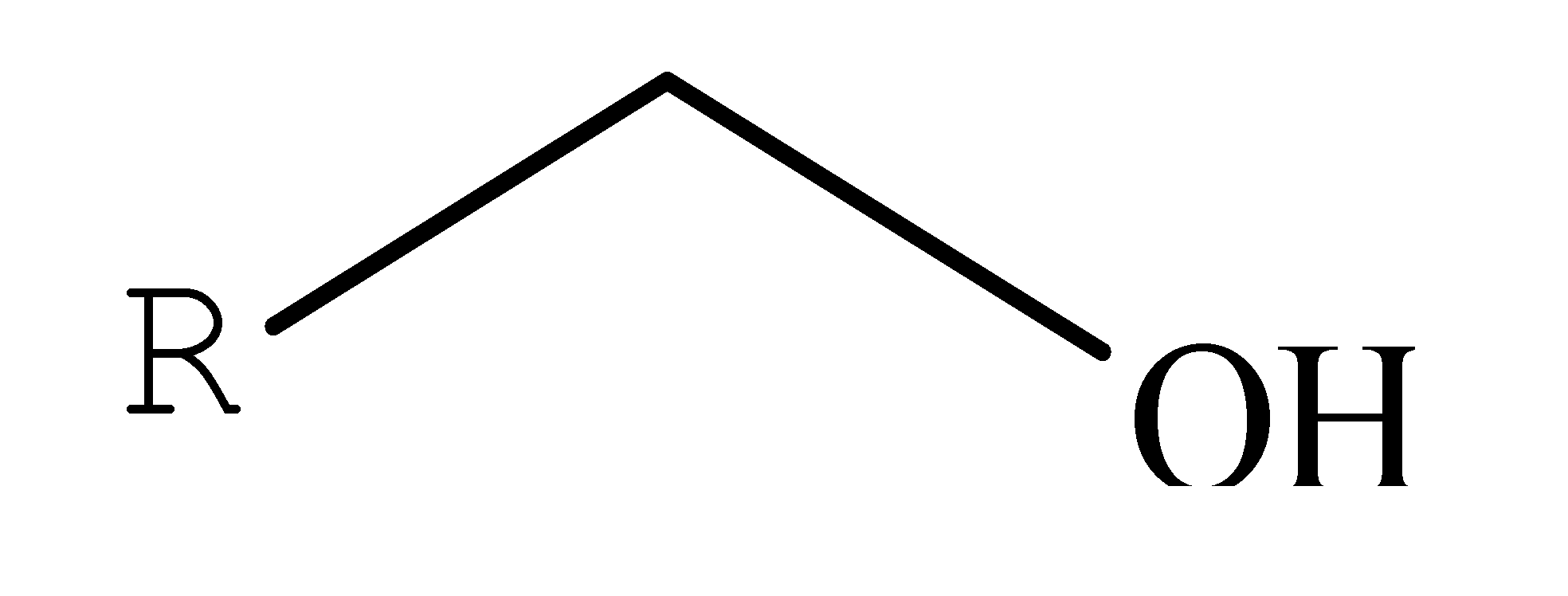 | Light Irradiation | In Dark | ||
|---|---|---|---|---|---|
| Conv. (%) | Sel. (%) | Conv. (%) | Sel. (%) | ||
| 1 | 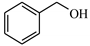 | 89.9 | >99 | 11.2 | >99 |
| 2 | 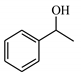 | 35.1 | >99 | 10.7 | >99 |
| 3 |  | 9.8 | >99 | 3.1 | >99 |
| 4 | 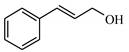 | 90.0 | >99 | 13.5 | >99 |
| 5 |  | 92.1 | >99 | 13.8 | >99 |
| 6 | 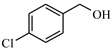 | 90.4 | >99 | 13.6 | >99 |
| 7 |  | 82.3 | >99 | 20.6 | >99 |
| 8 | 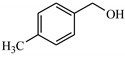 | 65.1 | >99 | 21.9 | >99 |
| 9 | 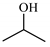 | 6.9 | >99 | 0 | 0 |
| 10 | 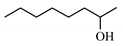 | 3.7 | >99 | 0 | 0 |
| 11 | 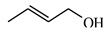 | 64.9 | >99 | 23.9 | >99 |
| 12 |  | 4.2 | >99 | 1.0 | >99 |
| 13 | 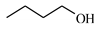 | 20.7 | >99 | 7.3 | >99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Li, H.; Hao, X.; Zhang, Z.; Wang, Y.; Li, J.; Wang, K. Selective Oxidation of Benzyl Alcohol by Ag/Pd/m-BiVO4 Microspheres under Visible Light Irradiation. Catalysts 2020, 10, 266. https://doi.org/10.3390/catal10020266
Yu X, Li H, Hao X, Zhang Z, Wang Y, Li J, Wang K. Selective Oxidation of Benzyl Alcohol by Ag/Pd/m-BiVO4 Microspheres under Visible Light Irradiation. Catalysts. 2020; 10(2):266. https://doi.org/10.3390/catal10020266
Chicago/Turabian StyleYu, Xiujuan, Haiying Li, Xueli Hao, Zhiying Zhang, Yan Wang, Jingyi Li, and Kai Wang. 2020. "Selective Oxidation of Benzyl Alcohol by Ag/Pd/m-BiVO4 Microspheres under Visible Light Irradiation" Catalysts 10, no. 2: 266. https://doi.org/10.3390/catal10020266
APA StyleYu, X., Li, H., Hao, X., Zhang, Z., Wang, Y., Li, J., & Wang, K. (2020). Selective Oxidation of Benzyl Alcohol by Ag/Pd/m-BiVO4 Microspheres under Visible Light Irradiation. Catalysts, 10(2), 266. https://doi.org/10.3390/catal10020266