Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides
Abstract
1. Introduction
2. Technologies for Biodiesel Production
3. Mixed Metal Oxides in Biodiesel Production
3.1. ZrO2-Based Catalysts
3.2. ZnO-Based Catalysts
3.3. TiO2-Based Catalysts
3.4. MgO-Based Catalysts
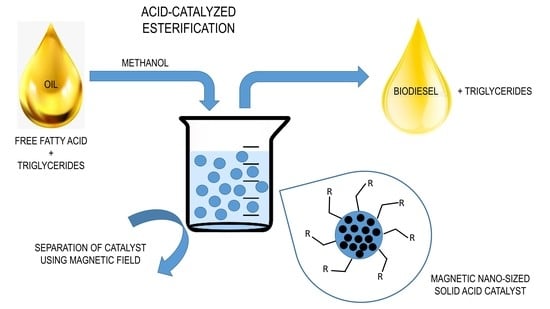
3.5. Magnetic Catalysts
4. Use of Solid Acid Catalysts in Recent Studies
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Quah, R.V.; Tan, Y.H.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Nolasco-Hipolito, C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.M. Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: A review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef]
- Wong, K.Y.; Ng, J.-H.; Chong, C.T.; Lam, S.S.; Chong, W.T. Biodiesel process intensification through catalytic enhancement and emerging reactor designs: A critical review. Renew. Sustain. Energy Rev. 2019, 116, 1–32. [Google Scholar] [CrossRef]
- Shote, A.S. Biofuel: An Environmental Friendly Fuel. In Anaerobic Digestion; IntechOpen: London, UK, 2019; Volume 1, pp. 1–14. [Google Scholar]
- Oh, K.K.; Kim, Y.S.; Yoon, H.H.; Tae, B.S. Pretreatment of Lignocellulosic Biomass using Combination of Ammonia Recycled Percolation and Dilute-Acid Process. J. Ind. Eng. Chem. 2002, 8, 64–70. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: a review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Bhatti, H.; Hanif, M.; Qasim, M.; Rehman, A. Biodiesel production from waste tallow. Fuel 2019, 87, 2961–2966. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Gerpen, J. The Biodiesel Handbook, 2nd ed.; Academic Press and AOCS Press: Cambridge, MA, USA, 2010; ISBN 978-1-893997-62-2. [Google Scholar]
- Pruszko, R. Chapter 20—Biodiesel Production. In Bioenergy; Dahiya, A., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 339–359. ISBN 978-0-12-407909-0. [Google Scholar]
- Luque, R.; Melero, J.A. Introduction to advanced biodiesel production. In Advances in Biodiesel Production; Luque, R., Melero, J.A., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, UK, 2012; pp. 1–9. ISBN 978-0-85709-117-8. [Google Scholar]
- Ahmad, I.; Dhar, R. Sulfonic Acid-Functionalized Solid Acid Catalyst in Esterification and Transesterification Reactions. Catal. Surv. Asia 2017, 21, 53–69. [Google Scholar]
- Diamantopoulos, N.; Panagiotaras, D.; Nikolopoulos, D. Comprehensive Review on the Biodiesel Production using Solid Acid Heterogeneous Catalysts. J. Thermodyn. Catal. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.V.; Nachiyar, V.; Farizah, A.B.; Nityasree, J. Production and characterization of biodiesel obtained from transesterification of lipid from goat tallow. J. Environ. Biol. 2019, 40, 601–606. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Y.-C.; Song, B.-A.; Shi, X.; Wang, J.; Yin, S.-T.; Hu, D.-Y.; Jin, L.-H.; Yang, S. Synthesis of biodiesel from Jatropha curcas L. seed oil using artificial zeolites loaded with CH3COOK as a heterogeneous catalyst. Nat. Sci. 2009, 1, 55–62. [Google Scholar] [CrossRef]
- Ferdous, K.; Uddin, M.R.; Uddin, M.R.; Khan, M.R.; Islam, M.A. Preparation and Optimization of Biodiesel Production from Mixed Feedstock Oil. Chem. Eng. Sci. 2013, 1, 62–66. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.H.; Mounts, T.L. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Descorme, C.; Gallezot, P.; Geantet, C.; George, C. Heterogeneous Catalysis: A Key Tool toward Sustainability. ChemCatChem 2012, 4, 1897–1906. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic applications in the production of biodiesel from vegetable oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Kaewchada, A.; Jaree, A. Enhancement of continuous supercritical biodiesel production: influence of co-solvent types. Energy Procedia 2019, 156, 48–52. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, S.Y.; Fu, Y.; Chang, J. A quick method for producing biodiesel from soy sauce residue under supercritical carbon dioxide. Renew. Energy 2019, 134, 739–744. [Google Scholar] [CrossRef]
- Meira, M.; Quintella, C.M.; Ribeiro, E.M.O.; Silva, H.R.G.; Guimarães, A.K. Overview of the challenges in the production of biodiesel. Biomass Convers. Biorefinery 2015, 5, 321–329. [Google Scholar] [CrossRef]
- Priyadarshi, D.; Paul, K.K. Single phase blend: An advanced microwave process for improved quality low-cost biodiesel production from kitchen food waste. Biochem. Eng. J. 2018, 137, 273–283. [Google Scholar] [CrossRef]
- Shuit, S.H.; Ong, Y.T.; Lee, K.T.; Subhash, B.; Tan, S.H. Membrane technology as a promising alternative in biodiesel production: A review. Biotechnol. Adv. 2012, 30, 1364–1380. [Google Scholar] [CrossRef]
- Anantapinitwatna, A.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Assabumrungrat, S. Effect of Water Content in Waste Cooking Oil on Biodiesel Production via Ester-transesterification in a Single Reactive Distillation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 559, 1–10. [Google Scholar] [CrossRef]
- Kojima, Y.; Takai, S. Transesterification of vegetable oil with methanol using solid base catalyst of calcium oxide under ultrasonication. Chem. Eng. Process. Process Intensif. 2019, 136, 101–106. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T. Biodiesel Production in Supercritical Fluids (Chapter 22); Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Biomass, Biofuels, Biochemicals; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-12-816856-1. [Google Scholar]
- Wei, C.-Y.; Huang, T.-C.; Chen, H.-H. Biodiesel Production Using Supercritical Methanol with Carbon Dioxide and Acetic Acid. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Salis, A.; Pinna, M.; Monduzzi, M.; Solinas, V. Biodiesel production from triolein and short chain alcohols through biocatalysis. J. Biotechnol. 2005, 119, 291–299. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Mujeeb, A.; Ankalabasappa, V. Current strategies and prospects of biodiesel production: A review. Appl. Sci. Res. 2016, 7, 120–133. [Google Scholar]
- Abbasi, S.; Diwekar, U.M. Characterization and stochastic modeling of uncertainties in the biodiesel production. Clean Technol. Environ. Policy 2014, 16, 79–94. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Camacho, L.M.; Deng, S. Microwave-Assisted Catalytic Transesterification of Camelina Sativa Oil. Energy Fuels 2010, 24, 1298–1304. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Cao, P.; Dubé, M.A.; Tremblay, A.Y. Methanol recycling in the production of biodiesel in a membrane reactor. Fuel 2008, 87, 825–833. [Google Scholar] [CrossRef]
- Guerreiro, L.; Pereira, P.M.; Fonseca, I.M.; Martin-Aranda, R.M.; Ramos, A.M.; Dias, J.M.L.; Oliveira, R.; Vital, J. PVA embedded hydrotalcite membranes as basic catalysts for biodiesel synthesis by soybean oil methanolysis. Catal. Today 2010, 156, 191–197. [Google Scholar] [CrossRef]
- Sarmento, L.A.V.; Spricigo, C.B.; Petrus, J.C.C.; Carlson, L.H.C.; Machado, R.A.F. Performance of reverse osmosis membranes in the separation of supercritical CO2 and essential oils. J. Membr. Sci. 2004, 237, 71–76. [Google Scholar] [CrossRef]
- Saleh, J.; Tremblay, A.Y.; Dubé, M.A. Glycerol removal from biodiesel using membrane separation technology. Fuel 2010, 89, 2260–2266. [Google Scholar] [CrossRef]
- Noshadi, I.; Amin, N.A.S.; Parnas, R.S. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM). Fuel 2012, 94, 156–164. [Google Scholar] [CrossRef]
- Limniyakul, W.; Srinophakun, T.; Wanganusorn, S. Application of Reactive Distillation for Biodiesel Production Enhancement: An alkyl process. Asian J. Appl. Sci. 2019, 7, 303–312. [Google Scholar] [CrossRef][Green Version]
- Joda, F.; Ahmadi, F. Exergoeconomic analysis of conventional and using reactive distillation biodiesel production scenarios thermally integrated with a combined power plant. Renew. Energy 2019, 132, 898–910. [Google Scholar] [CrossRef]
- Ali, S.S.; Asif, M.; Basu, A. Design and simulation of high purity biodiesel reactive distillation process. Pol. J. Chem. Technol. 2019, 21, 1–7. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Intensification Approaches for Biodiesel Synthesis from Waste Cooking Oil: A Review. Ind. Eng. Chem. Res. 2012, 51, 14610–14628. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Shinde, K.; Kaliaguine, S. A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering 2019, 3, 18. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. Appl. Catal. B Environ. 2018, 234, 268–278. [Google Scholar] [CrossRef]
- Kiss, A.A.; Omota, F.; Dimian, A.C.; Rothenberg, G. The heterogeneous advantage: biodiesel by catalytic reactive distillation. Top. Catal. 2006, 40, 141–150. [Google Scholar] [CrossRef]
- Nage, S.D.; Kulkarni, K.S.; Kulkarni, A.D.; Topare, N.S. Biodiesel production by a continuous process using a heterogeneous catalyst. J. Curr. Chem. Pharm. Sci. 2012, 2, 12–16. [Google Scholar]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 33. [Google Scholar] [CrossRef]
- Sulaiman, N.F.; Wan Abu Bakar, W.A.; Toemen, S.; Kamal, N.M.; Nadarajan, R. In depth investigation of bi-functional, Cu/Zn/γ-Al2O3 catalyst in biodiesel production from low-grade cooking oil: Optimization using response surface methodology. Renew. Energy 2019, 135, 408–416. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of mixed metal oxides in catalysis science—versatile applications in organic synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Abdul Aziz, A.R. Activity of solid acid catalysts for biodiesel production: A critical review. Appl. Catal. Gen. 2014, 470, 140–161. [Google Scholar] [CrossRef]
- Pappu, V.K.S.; Kanyi, V.; Santhanakrishnan, A.; Lira, C.T.; Miller, D.J. Butyric acid esterification kinetics over Amberlyst solid acid catalysts: The effect of alcohol carbon chain length. Bioresour. Technol. 2013, 130, 793–797. [Google Scholar] [CrossRef]
- Li, K.-T.; Wang, C.-K.; Wang, I.; Wang, C.-M. Esterification of lactic acid over TiO2–ZrO2 catalysts. Appl. Catal. Gen. 2011, 392, 180–183. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics, 3rd ed.; Wiley-VCH Verlag: Weinheim, Germany, 2017; ISBN 978-3-527-33268-7. [Google Scholar]
- Clark, J.H.; Monks, G.L.; Nightingale, D.J.; Price, P.M.; White, J.F. A New Solid Acid-Based Route to Linear Alkylbenzenes. J. Catal. 2000, 193, 348–350. [Google Scholar] [CrossRef]
- Ni, J.; Meunier, F.C. Esterification of free fatty acids in sunflower oil over solid acid catalysts using batch and fixed bed-reactors. Appl. Catal. Gen. 2007, 333, 122–130. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.V.M.; Fierro, J.L.G. Study of the surface and redox properties of ceria–zirconia oxides. Appl. Catal. Gen. 2008, 337, 86–96. [Google Scholar] [CrossRef]
- Sinha Majumdar, S.; Celik, G.; Alexander, A.-M.; Gawade, P.; Ozkan, U.S. In-situ incorporation of binder during sol-gel preparation of Pd-based sulfated zirconia for reduction of nitrogen oxides under lean-burn conditions: Effect on activity and wash-coating characteristics. Appl. Catal. B Environ. 2017, 202, 134–146. [Google Scholar] [CrossRef]
- Ma, L.; Lv, E.; Du, L.; Han, Y.; Lu, J.; Ding, J. A flow-through tubular catalytic membrane reactor using zirconium sulfate tetrahydrate-impregnated carbon membranes for acidified oil esterification. J. Energy Inst. 2017, 90, 875–883. [Google Scholar] [CrossRef]
- Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.S.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification. Catalysts 2018, 8, 17. [Google Scholar] [CrossRef]
- Gopinath, S.; Kumar, P.S.M.; Arafath, K.A.Y.; Thiruvengadaravi, K.V.; Sivanesan, S.; Baskaralingam, P. Efficient mesoporous SO42−/Zr-KIT-6 solid acid catalyst for green diesel production from esterification of oleic acid. Fuel 2017, 203, 488–500. [Google Scholar] [CrossRef]
- Enascuta, C.E.; Stepan, E.; Bolocan, I.; Bombos, D.; Calin, C.; Oprescu, E.-E.; Lavric, V. Simultaneous production of oil enriched in ω-3 polyunsaturated fatty acids and biodiesel from fish wastes. Waste Manag. 2018, 75, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Yu, F.; Yan, X.; Li, R. Synthesis of tetragonal sulfated zirconia via a novel route for biodiesel production. J. Fuel Chem. Technol. 2017, 45, 311–316. [Google Scholar] [CrossRef]
- Dehghani, S.; Haghighi, M. Sono-sulfated zirconia nanocatalyst supported on MCM-41 for biodiesel production from sunflower oil: Influence of ultrasound irradiation power on catalytic properties and performance. Ultrason. Sonochem. 2017, 35, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Rubiyanto, D.; Taushiyah, A.; Najah, F.B.; Azmi, U.; Sim, Y.-L. Use of ZrO2 supported on bamboo leaf ash as a heterogeneous catalyst in microwave-assisted biodiesel conversion. Sustain. Chem. Pharm. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Zhang, F. Esterification of oleic acid to biodiesel catalyzed by a highly acidic carbonaceous catalyst. Catal. Today 2019, 319, 172–181. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Efficient waste Gallus domesticus shell derived calcium-based catalyst for biodiesel production. Fuel 2018, 211, 67–75. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Muhtaseb, A.; Myint, M.T.Z.; Al-Hinai, M.; Al-Haj, L.; Baawain, M.; Al-Abri, M.; Kumar, G.; Atabani, A.E. Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@MgO-ZrO2). Energy Convers. Manag. 2018, 155, 128–137. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Bakkali, B.E.; Trautwein, G.; Reinoso, S. Zirconia-supported tungstophosphoric heteropolyacid as heterogeneous acid catalyst for biodiesel production. Appl. Catal. B Environ. 2018, 224, 194–203. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ansari, F.A.; Singh, B.; Bux, F. Biodiesel synthesis from microalgal lipids using tungstated zirconia as a heterogeneous acid catalyst and its comparison with homogeneous acid and enzyme catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Kalavathy, G.; Baskar, G. Synergism of clay with zinc oxide as nanocatalyst for production of biodiesel from marine Ulva lactuca. Bioresour. Technol. 2019, 281, 234–238. [Google Scholar] [CrossRef]
- Malhotra, R.; Ali, A. 5-Na/ZnO doped mesoporous silica as reusable solid catalyst for biodiesel production via transesterification of virgin cottonseed oil. Renew. Energy 2019, 133, 606–619. [Google Scholar] [CrossRef]
- Baskar, G.; Gurugulladevi, A.; Nishanthini, T.; Aiswarya, R.; Tamilarasan, K. Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst. Renew. Energy 2017, 103, 641–646. [Google Scholar] [CrossRef]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and application of Co doped ZnO as heterogeneous nanocatalyst for biodiesel production from non-edible oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Nehdi, I.A.; Al-Resayes, S.I.; Al-Muhtaseb, A.H. Sulfonated mesoporous zinc aluminate catalyst for biodiesel production from high free fatty acid feedstock using microwave heating system. J. Taiwan Inst. Chem. Eng. 2017, 70, 219–228. [Google Scholar] [CrossRef]
- Vinoth Arul Raj, J.; Bharathiraja, B.; Vijayakumar, B.; Arokiyaraj, S.; Iyyappan, J.; Praveen Kumar, R. Biodiesel production from microalgae Nannochloropsis oculata using heterogeneous Poly Ethylene Glycol (PEG) encapsulated ZnOMn2+ nanocatalyst. Bioresour. Technol. 2019, 282, 348–352. [Google Scholar] [CrossRef]
- AlSharifi, M.; Znad, H. Transesterification of waste canola oil by lithium/zinc composite supported on waste chicken bone as an effective catalyst. Renew. Energy 2019, in press. [Google Scholar]
- Escorsim, A.M.; Hamerski, F.; Ramos, L.P.; Corazza, M.L.; Cordeiro, C.S. Multifunctionality of zinc carboxylate to produce acylglycerols, free fatty acids and fatty acids methyl esters. Fuel 2019, 244, 569–579. [Google Scholar] [CrossRef]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel production from used cooking oil using a novel surface functionalised TiO2 nano-catalyst. Appl. Catal. B Environ. 2017, 207, 297–310. [Google Scholar] [CrossRef]
- Patil, A.; Baral, S.S.; Dhanke, P.; Kore, V. Biodiesel production using prepared novel surface functionalised TiO2 nano-catalyst in hydrodynamic cavitation reactor. Mater. Today Proc. 2019, in press. [Google Scholar] [CrossRef]
- Gurusamy, S.; Kulanthaisamy, M.R.; Hari, D.G.; Veleeswaran, A.; Thulasinathan, B.; Muthuramalingam, J.B.; Balasubramani, R.; Chang, S.W.; Arasu, M.V.; Al-Dhabi, N.A.; et al. Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol. B 2019, 193, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Malhotra, R.; Ali, A. Tungsten supported Ti/SiO2 nanoflowers as reusable heterogeneous catalyst for biodiesel production. Renew. Energy 2018, 116, 109–119. [Google Scholar] [CrossRef]
- Fan, M.; Si, Z.; Sun, W.; Zhang, P. Sulfonated ZrO2-TiO2 nanorods as efficient solid acid catalysts for heterogeneous esterification of palmitic acid. Fuel 2019, 252, 254–261. [Google Scholar] [CrossRef]
- Hossain, M.N.; Siddik Bhuyan, M.S.U.; Md Ashraful Alam, A.H.; Seo, Y.C. Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst. Catalysts 2019, 9, 67. [Google Scholar] [CrossRef]
- Dai, Y.-M.; Kao, I.-H.; Chen, C.-C. Evaluating the optimum operating parameters of biodiesel production process from soybean oil using the Li2TiO3 catalyst. J. Taiwan Inst. Chem. Eng. 2017, 70, 260–266. [Google Scholar] [CrossRef]
- Alsharifi, M.; Znad, H.; Hena, S.; Ang, M. Biodiesel production from canola oil using novel Li/TiO2 as a heterogeneous catalyst prepared via impregnation method. Renew. Energy 2017, 114, 1077–1089. [Google Scholar] [CrossRef]
- Shaheen, A.; Sultana, S.; Lu, H.; Ahmad, M.; Asma, M.; Mahmood, T. Assessing the potential of different nano-composite (MgO, Al2O3-CaO and TiO2) for efficient conversion of Silybum eburneum seed oil to liquid biodiesel. J. Mol. Liq. 2018, 249, 511–521. [Google Scholar] [CrossRef]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51–59. [Google Scholar] [CrossRef]
- Moghanian, H.; Mobinikhaledi, A.; Blackman, A.G.; Sarough-Farahani, E. Sulfanilic acid-functionalized silica-coated magnetite nanoparticles as an efficient, reusable and magnetically separable catalyst for the solvent-free synthesis of 1-amido- and 1-aminoalkyl-2-naphthols. RSC Adv. 2014, 4, 28176–28185. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic Conversion of Biomass into Chemicals and Fuels over Magnetic Catalysts. ACS Catal. 2016, 6, 326–338. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; Jiang, H.; Yu, H.-Q. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salimi, Z.; Hosseini, S.A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 2019, 239, 1204–1212. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Silva, M.R.; Kiminami, R.H.G.A.; Costa, A.C.F.M.; Daramola, M.O. Catalytic performance of NiFe2O4 and Ni0.3Zn0.7Fe2O4 magnetic nanoparticles during biodiesel production. Arab. J. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Gardy, J.; Nourafkan, E.; Osatiashtiani, A.; Lee, A.F.; Wilson, K.; Hassanpour, A.; Lai, X. A core-shell SO4/Mg-Al-Fe3O4 catalyst for biodiesel production. Appl. Catal. B Environ. 2019, 259, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Liang, X. Magnetic solid acid catalyst for biodiesel synthesis from waste oil. Energy Convers. Manag. 2017, 141, 126–132. [Google Scholar] [CrossRef]
- Chellappan, S.; Aparna, K.; Chingakham, C.; Sajith, V.; Nair, V. Microwave assisted biodiesel production using a novel Brønsted acid catalyst based on nanomagnetic biocomposite. Fuel 2019, 246, 268–276. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Pan, H.; Wang, A.; Souzanchi, S.; Xu, C.; Yang, S. Magnetically recyclable acidic polymeric ionic liquids decorated with hydrophobic regulators as highly efficient and stable catalysts for biodiesel production. Appl. Energy 2018, 223, 416–429. [Google Scholar] [CrossRef]
- Han, Y.; Ye, L.; Gu, X.; Zhu, P.; Lu, X. Lignin-based solid acid catalyst for the conversion of cellulose to levulinic acid using γ-valerolactone as solvent. Ind. Crops Prod. 2019, 127, 88–93. [Google Scholar] [CrossRef]
- Gaurav, A.; Dumas, S.; Mai, C.T.Q.; Ng, F.T.T. A kinetic model for a single step biodiesel production from a high free fatty acid (FFA) biodiesel feedstock over a solid heteropolyacid catalyst. Green Energy Environ. 2019, 4, 328–341. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Yang, X.-X.; Xu, J.; Wang, H.-L.; Wang, Z.-B.; Zhang, L.; Wang, S.-L.; Liang, J.-L. Biodiesel production from esterification of oleic acid by a sulfonated magnetic solid acid catalyst. Renew. Energy 2019, 139, 688–695. [Google Scholar] [CrossRef]
- Sandouqa, A.; Al-Hamamre, Z.; Asfar, J. Preparation and performance investigation of a lignin-based solid acid catalyst manufactured from olive cake for biodiesel production. Renew. Energy 2019, 132, 667–682. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mahmoud, H.R.; El-Molla, S.A. Influence of support on physicochemical properties of ZrO2 based solid acid heterogeneous catalysts for biodiesel production. Catal. Commun. 2019, 122, 10–15. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Hosseini, M.-S.; Forouzeshfar, N. Propyl-SO3H functionalized graphene oxide as multipurpose solid acid catalyst for biodiesel synthesis and acid-catalyzed esterification and acetalization reactions. Renew. Energy 2019, 1–10. [Google Scholar] [CrossRef]
- Yang, X.-X.; Wang, Y.-T.; Yang, Y.-T.; Feng, E.-Z.; Luo, J.; Zhang, F.; Yang, W.-J.; Bao, G.-R. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Convers. Manag. 2018, 164, 112–121. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, F.; Dong, Y.; Wu, X.; Sun, Y.; Zhang, D.; Zhang, T.; Han, M. Function promotion of SO42−/Al2O3–SnO2 catalyst for biodiesel production from sewage sludge. Renew. Energy 2020, 147, 275–283. [Google Scholar] [CrossRef]
- Mahmoud, H.R. Bismuth silicate (Bi4Si3O12 and Bi2SiO5) prepared by ultrasonic-assisted hydrothermal method as novel catalysts for biodiesel production via oleic acid esterification with methanol. Fuel 2019, 256, 1–10. [Google Scholar] [CrossRef]
- Saravanan Arumugamurthy, S.; Sivanandi, P.; Pandian, S.; Choksi, H.; Subramanian, D. Conversion of a low value industrial waste into biodiesel using a catalyst derived from brewery waste: An activation and deactivation kinetic study. Waste Manag. 2019, 100, 318–326. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Owolabi, J.O. Synthesis and characterization of alumina supported coconut chaff catalyst for biodiesel production from waste frying oil. South Afr. J. Chem. Eng. 2019, 30, 42–49. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Chingakham, C.; David, A.; Sajith, V. Fe3O4 nanoparticles impregnated eggshell as a novel catalyst for enhanced biodiesel production. Chin. J. Chem. Eng. 2019, 27, 2835–2843. [Google Scholar] [CrossRef]
- Lim, S.; Yap, C.Y.; Pang, Y.L.; Wong, K.H. Biodiesel synthesis from oil palm empty fruit bunch biochar derived heterogeneous solid catalyst using 4-benzenediazonium sulfonate. J. Hazard. Mater. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Santos, E.C.S.; Dias, J.P.; Barreto, J.; Stavale, F.L.; Ronconi, C.M. Reduced graphene oxide as an excellent platform to produce a stable Brønsted acid catalyst for biodiesel production. Fuel 2019, 256, 1–10. [Google Scholar] [CrossRef]
- Du, L.; Li, Z.; Ding, S.; Chen, C.; Qu, S.; Yi, W.; Lu, J.; Ding, J. Synthesis and characterization of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 2019, 258, 1–7. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Adeniyi, O.D.; Azeez, S.O.; Olutoye, M.A.; Akpan, U.G. Synthesis and characterization of anthill-eggshell-Ni-Co mixed oxides composite catalyst for biodiesel production from waste frying oil. Biofuels Bioprod. Biorefining 2019, 13, 37–47. [Google Scholar] [CrossRef]
- Sai, B.; Subramaniapillai, N.; Sheriffa Begum, K.M.M.; Narayanan, A. Optimization of Continuous Biodiesel Production from Rubber Seed Oil (RSO) using Calcined Eggshells as Heterogeneous Catalyst. J. Environ. Chem. Eng. 2019, 8, 1–14. [Google Scholar]
- Nizami, A.S.; Shahzad, K.; Rehan, M.; Ouda, O.K.M.; Khan, M.Z.; Ismail, I.M.I.; Almeelbi, T.; Basahi, J.M.; Demirbas, A. Developing waste biorefinery in Makkah: A way forward to convert urban waste into renewable energy. Appl. Energy 2017, 186, 189–196. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef]
- Sánchez Faba, E.M.; Ferrero, G.O.; Dias, J.M.; Eimer, G.A. Alternative Raw Materials to Produce Biodiesel through Alkaline Heterogeneous Catalysis. Catalysts 2019, 9, 690. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Ahmad, M.; Shaheen, A.; Zafar, M.; Ullah, R.; Asma, M.; Sultana, S.; Munir, M.; Rashid, N.; Malik, K.; et al. Comparative Study of Liquid Biodiesel From Sterculia foetida (Bottle Tree) Using CuO-CeO2 and Fe2O3 Nano Catalysts. Front. Energy Res. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Rathore, D.; Nizami, A.-S.; Singh, A.; Pant, D. Key issues in estimating energy and greenhouse gas savings of biofuels: challenges and perspectives. Biofuel Res. J. 2016, 3, 380–393. [Google Scholar] [CrossRef]
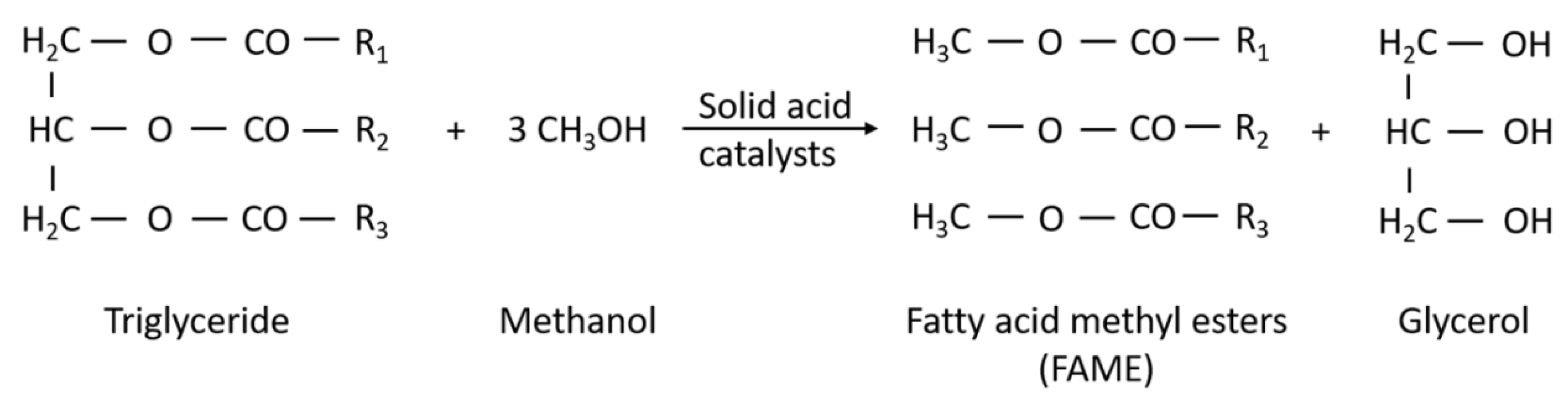

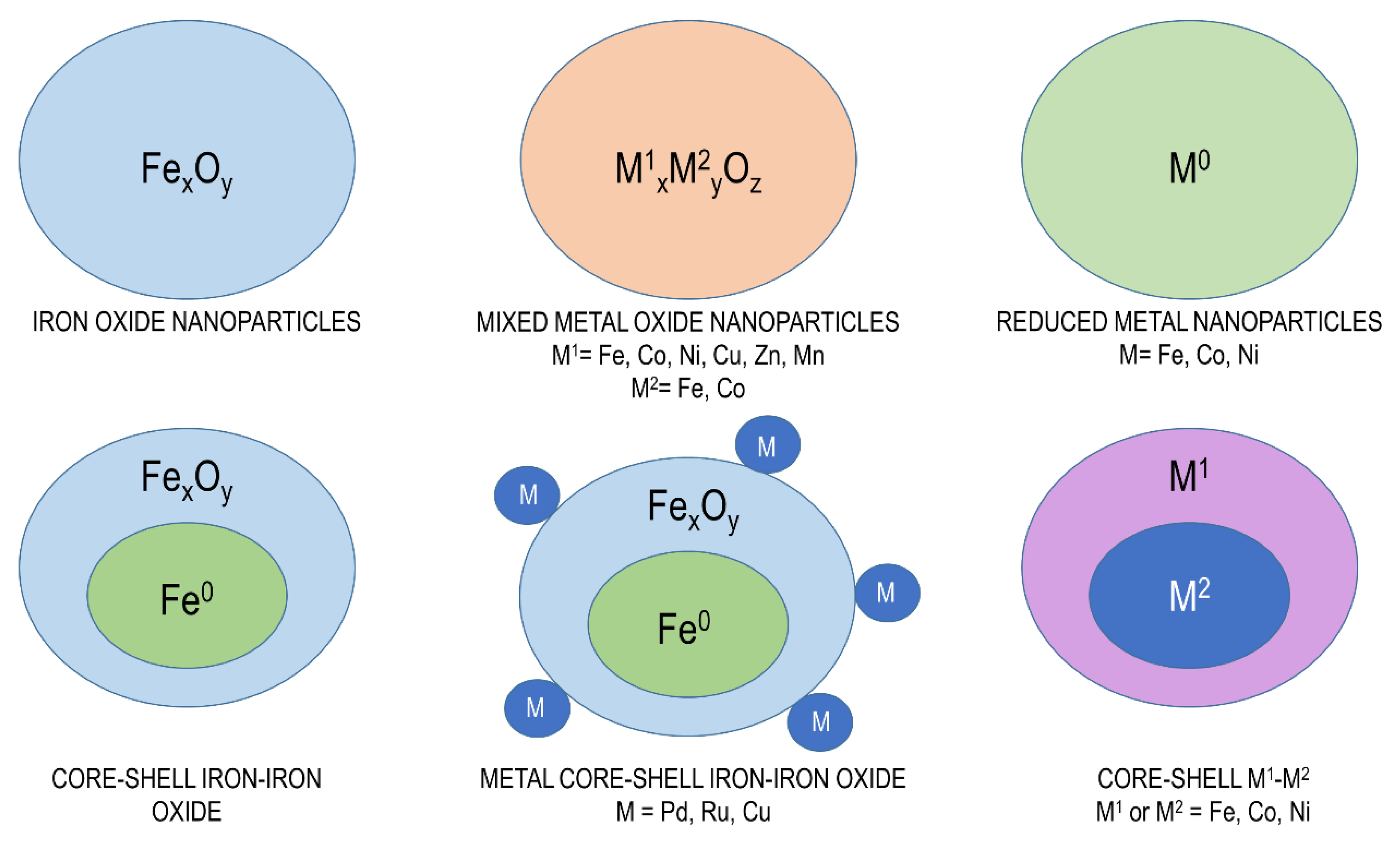
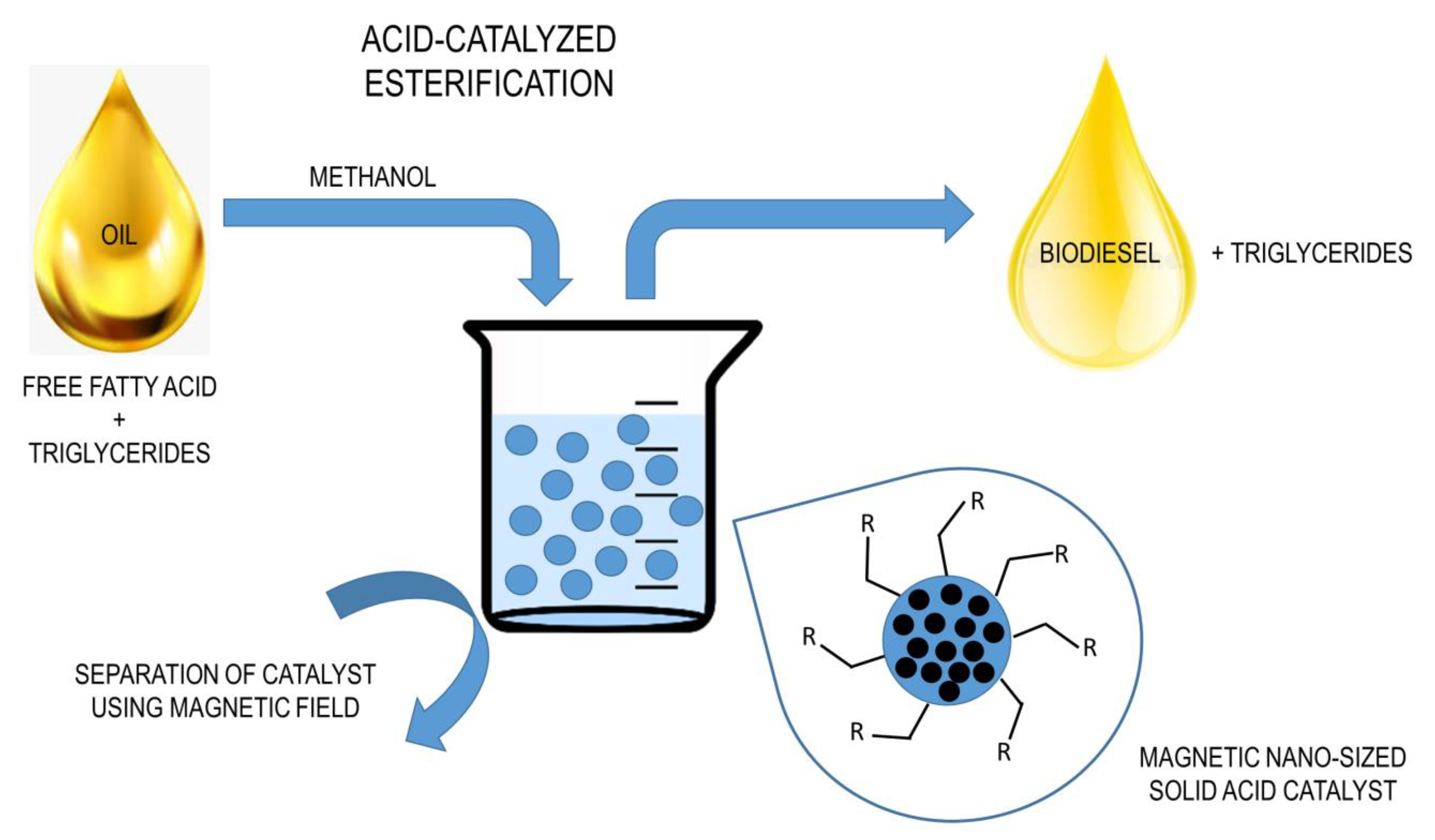
| Oil Feedstock (Molar Ratio) | Catalyst | Operating Conditions | Conversion/Yield | References |
|---|---|---|---|---|
| Sewage sludge + methanol | SO42−/Al2O3-SnO2 | 403.15 K 4 h 8 wt. % catalyst | 73.3% yield | [112] |
| Oleic acid + methanol (120:1) | Bismuth silicate (BS30) | 353.15 K 2 h 0.3 g of catalyst | 90% conversion | [113] |
| Palm fatty acid distillate (PFAD) + methanol (21:1) | BSY-SO3H (brewer’s spent yeast (BSY)) | 338.15 K 3 h 8 wt. % catalyst | 87.8% conversion | [114] |
| Waste frying oil + methanol (12:1) | Al2O3-supported coconut chaff | 338.15 K 2.5 h 1.5 wt. % catalyst | 91.05% yield | [115] |
| Soybean oil + methanol (35:1) | Fe3O4/SiO2 | 393.15 K 6 h 9 wt. % catalyst | 93.3% conversion | [116] |
| Pongamia pinnata raw oil + methanol (12:1) | Fe3O4-loaded catalytic eggshell (CES-Fe3O4) | 338.15 K 2 h 2 wt. % catalyst | 98% yield | [117] |
| Palm oil + methanol (15:1) | 4-Benzenediazonium sulfonate; SO and SO3H sulfonic groups on carbon catalyst | 473.15 K 7 h 20 wt. % catalyst | 98.1% yield | [118] |
| Soybean oil + methanol (20:1) | Reduced graphene oxide | 353.15 K 3 h 3 wt. % catalyst | 99% yield | [119] |
| Castor oil + ethanol (12:1) | MgO-Urea-800 | 348.15 K 1 h 6 wt. % catalyst | 96.5% yield | [120] |
| Waste frying oil + methanol (12:1) | Anthill-eggshell-Ni-Co (AENiCo) | 343.15 K 2 h 3 wt. % catalyst | 90.23% yield | [121] |
| Rubber seed oil + methanol (12:1) | Calcium oxide (CaO) derived from eggshells | N.D. 4 h 5 wt. % catalyst | 97.84% conversion | [122] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. https://doi.org/10.3390/catal10020237
Vasić K, Hojnik Podrepšek G, Knez Ž, Leitgeb M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts. 2020; 10(2):237. https://doi.org/10.3390/catal10020237
Chicago/Turabian StyleVasić, Katja, Gordana Hojnik Podrepšek, Željko Knez, and Maja Leitgeb. 2020. "Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides" Catalysts 10, no. 2: 237. https://doi.org/10.3390/catal10020237
APA StyleVasić, K., Hojnik Podrepšek, G., Knez, Ž., & Leitgeb, M. (2020). Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts, 10(2), 237. https://doi.org/10.3390/catal10020237