The Influence of The Light-Activated Titania P25 on Human Breast Cancer Cells
Abstract
1. Introduction
2. Results
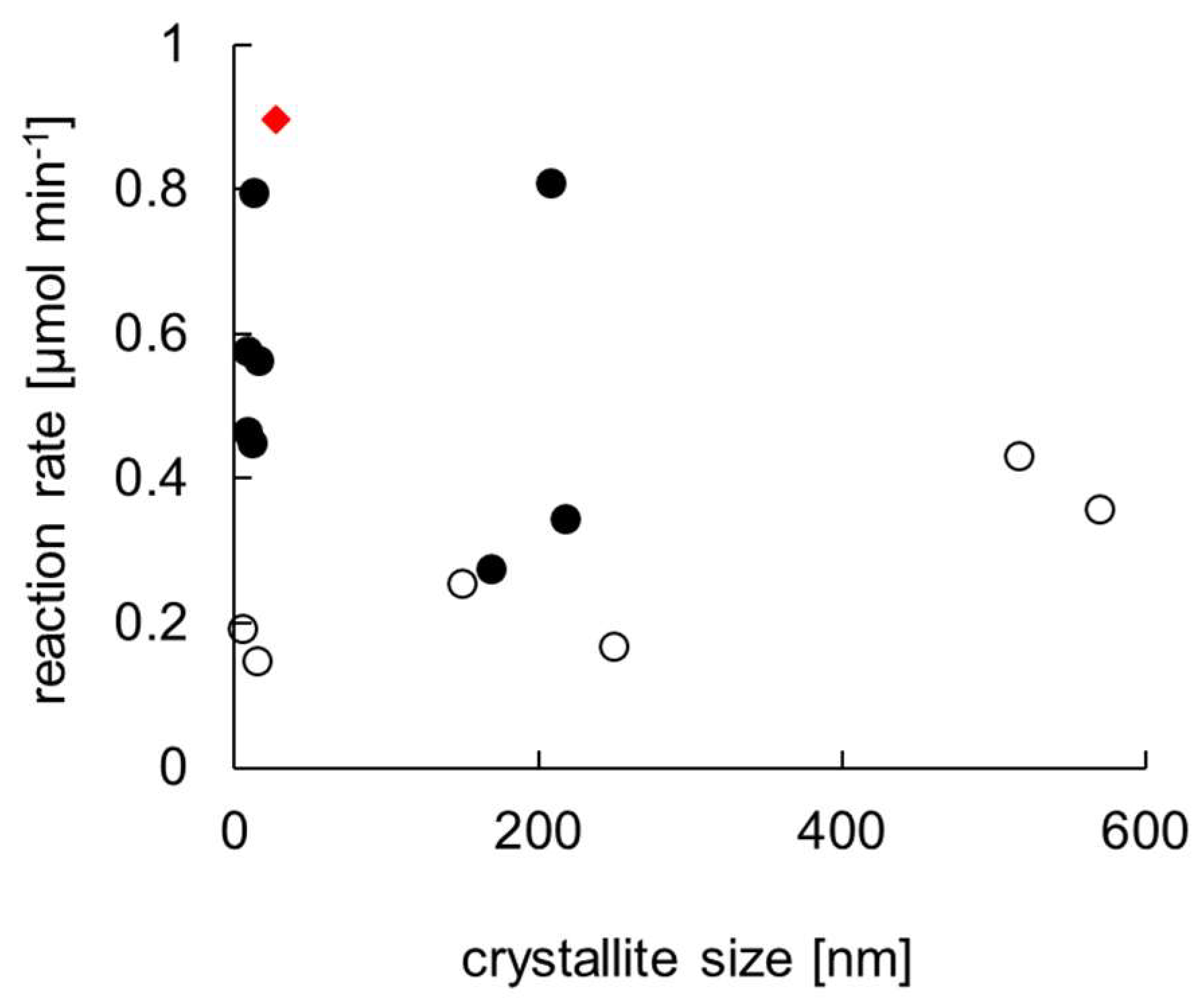
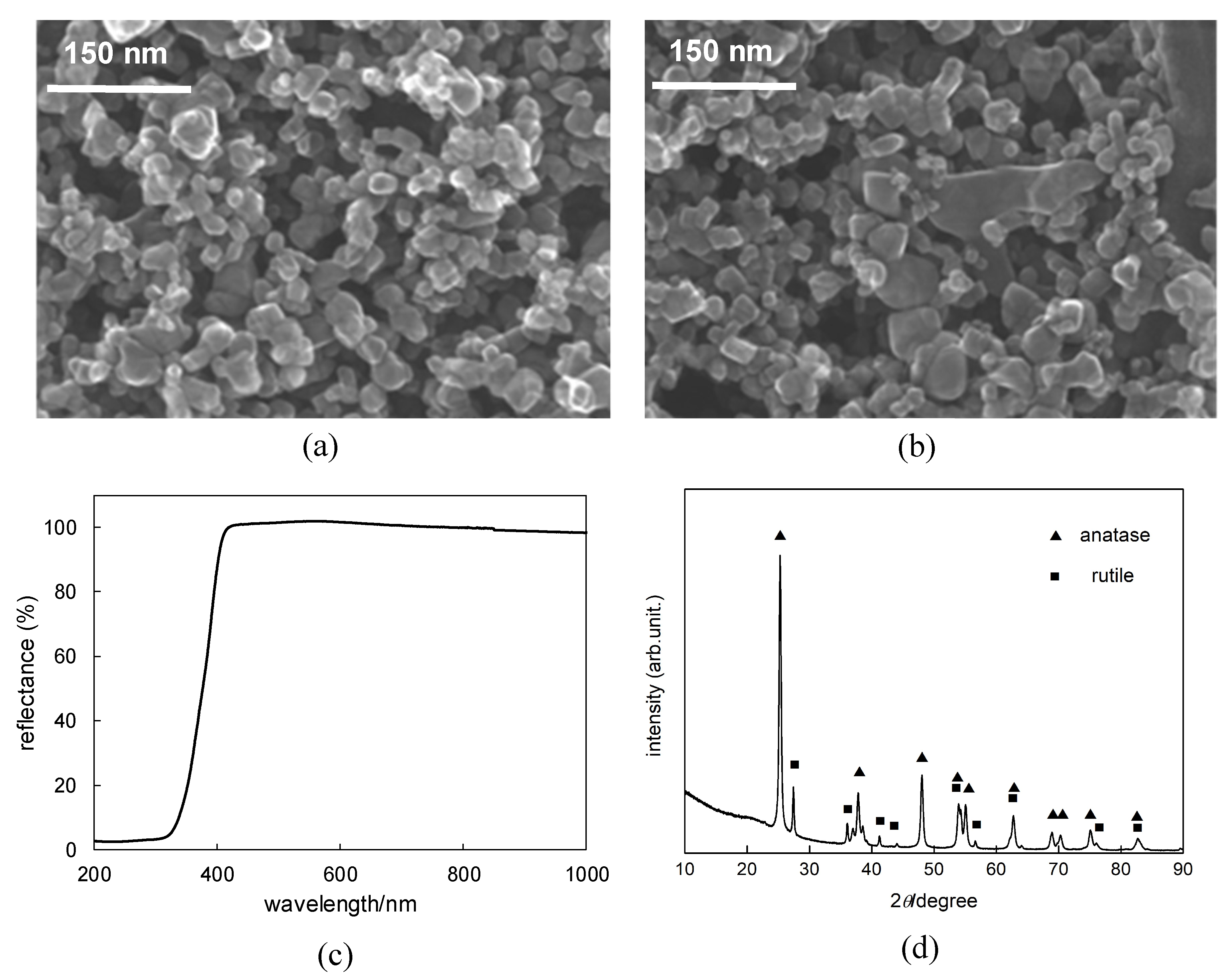
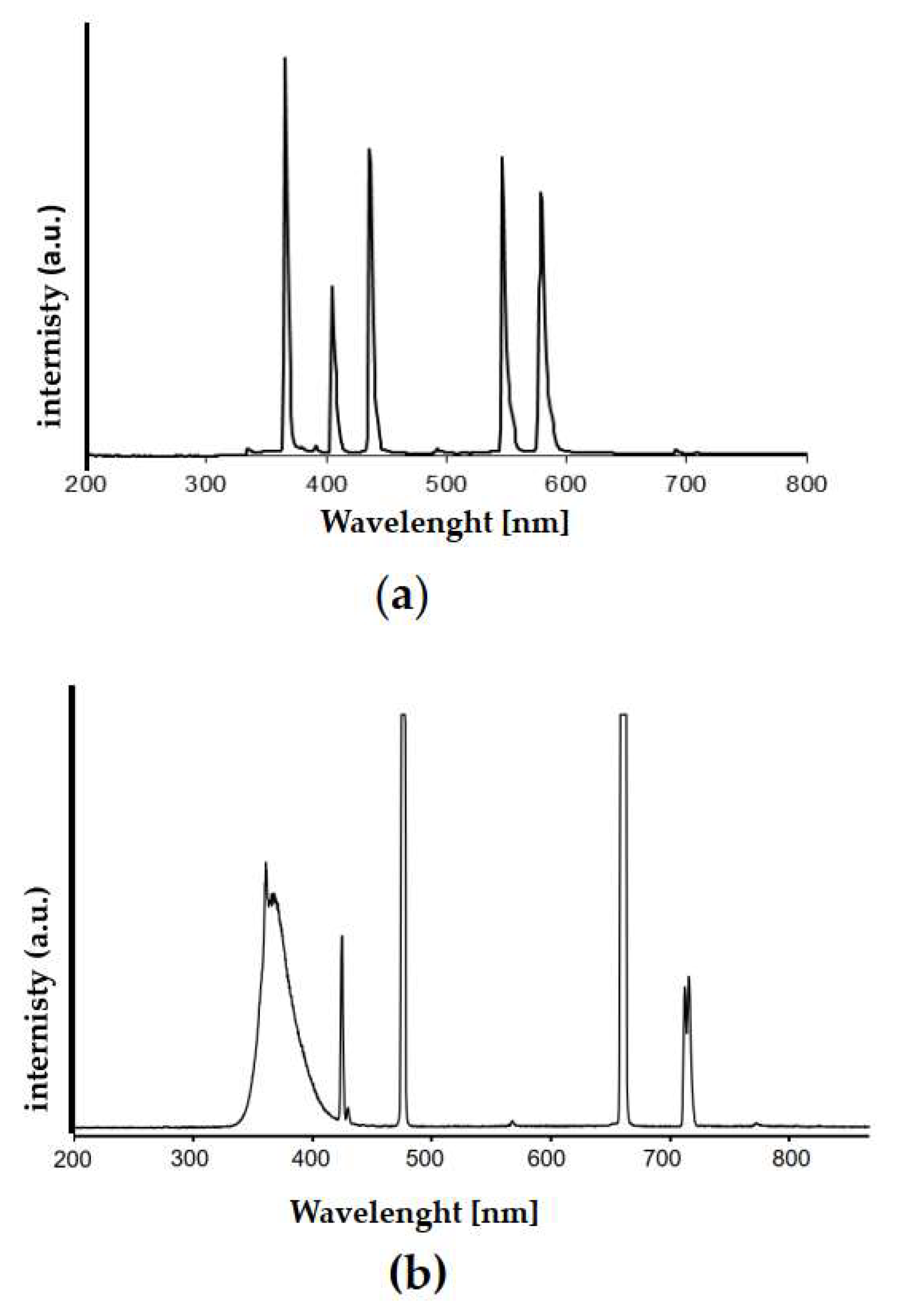
2.1. Titania P25 Photocatalyst
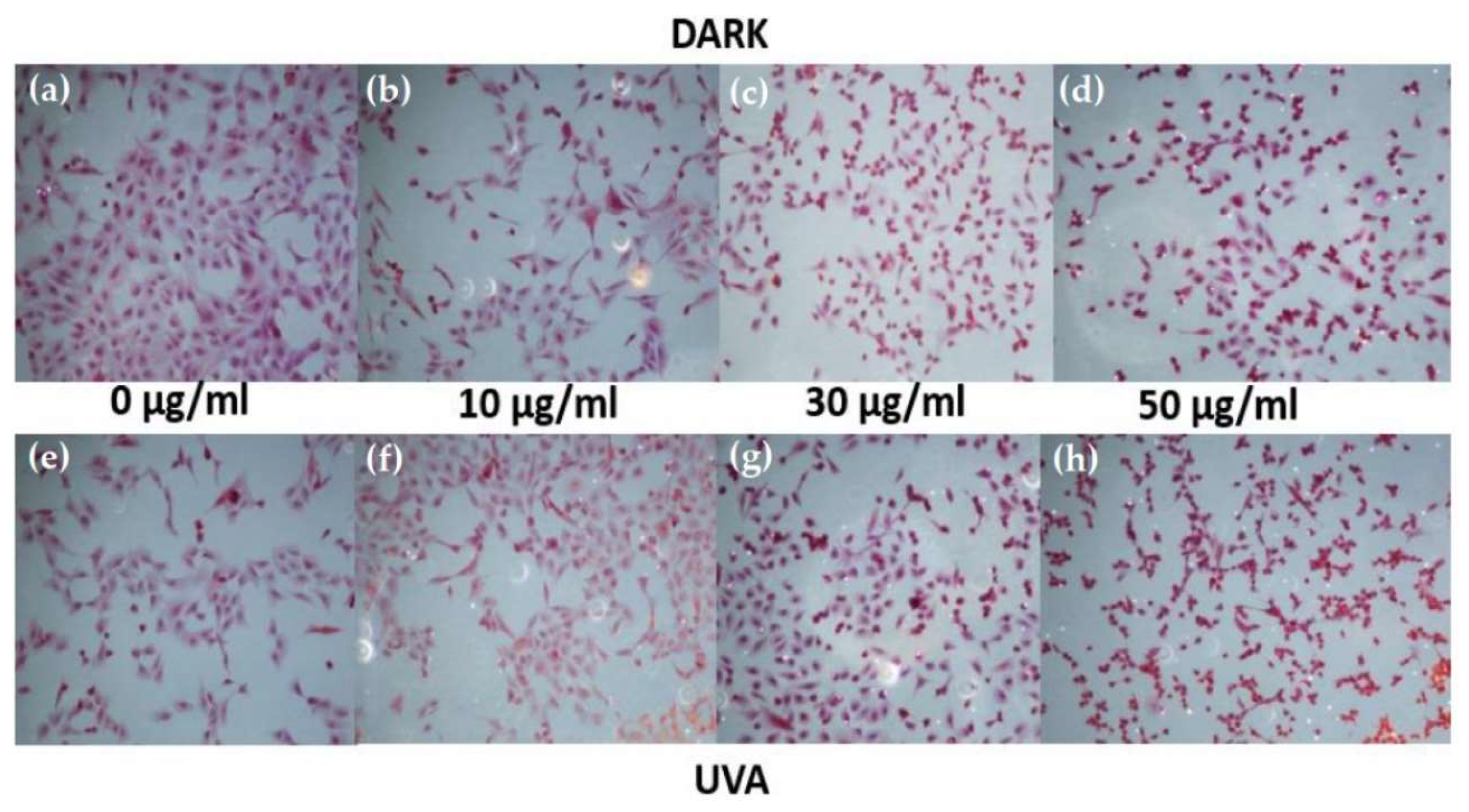
2.2. P25 Effect on Human Carcinoma Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Photocatalyst Characterization
4.4. Experimental Details
4.5. Viability Assay
4.6. Morphological Studies
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Sahu, S.C.; Hayes, A.W. Toxicity of nanomaterials found in human environment: A literature review. Toxicol. Res. Appl. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1513. [Google Scholar] [CrossRef]
- Pichat, P. A Brief survey of the potential health risks of TiO2 particles and TiO2-containing photocatalytic or non-photocatalytic materials. J. Adv. Oxid. Technol. 2010, 13, 238–246. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Ugazio, E.; Sapino, S.; Fenoglio, I.; Greco, G.; Fubini, B. Role of particle coating in controlling skin damage photoinduced by titania nanoparticles. Free Radic. Res. 2009, 43, 312–322. [Google Scholar] [CrossRef]
- Buchalska, M.; Kras, G.; Oszajca, M.; Łasocha, W.; Macyk, W. Singlet oxygen generation in the presence of titanium dioxide materials used as sunscreens in suntan lotions. J. Photochem. Photobiol. A 2010, 213, 158–163. [Google Scholar] [CrossRef]
- ANSES Website. Available online: https://www.anses.fr/fr/content/nanoparticules-de-dioxyde-de-titane-dans-l%E2%80%99alimentation-additif-e-171-des-effets-biologiques (accessed on 10 January 2020).
- Bomgardner, M.M. Europe may call TiO2 a carcinogen. Inhalation studies in rats spark push for classification. C&EN 2017, 95, 13. [Google Scholar]
- Oziel, C. EU Titanium Dioxide Classification Proposal Hits Waste Obstacle, Decision on CLP Entry Delayed Again Amid Questions over Waste Forms, Chemical Watch Global Risk & Regulation News. 2019. Available online: https://chemicalwatch.com/75209/eu-titanium-dioxide-classification-proposal-hits-waste-obstacle (accessed on 12 December 2019).
- Regulation (EC) No 1272/2008 of the European Parliament. Available online: https://ec.europa.eu/transparency/regdoc/rep/3/2019/EN/C-2019-7227-F1-EN-MAIN-PART-1.PDF (accessed on 20 January 2020).
- Varner, K.E.; Rindfusz, K.; Gaglione, A.; Viveiros, E. Nano Titanium Dioxide Environmental Matters: State of the Science Literature Review; U.S. Environmental Protection Agency: Washington, DC, USA, 2010. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=227225 (accessed on 12 December 2019).
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication–hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic methods for titanium dioxide nanoparticles: A review; Yang, D., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–181. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Hutchinson, L. Breast cancer: Challenges, controversies, breakthroughs. Nat. Rev. Clin. Oncol. 2010, 7, 669–679. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Prieto-Mahaney, O.O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between photocatalytic activities and structural and physical properties of titanium(IV) oxide powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Sclafani, A.; Hermmann, J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Thomas, A.G.; Flavell, W.R.; Mallick, A.K.; Kumarasinghe, A.R.; Tsoutsou, D.; Khan, N.; Chatwin, C.; Rayner, S.; Smith, G.C.; Stockbauer, R.L.; et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and x-ray absorption spectroscopy. Phys. Rev. B 2007, 75, 035105. [Google Scholar]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A Gen. 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Mi, Y.; Weng, X. Band alignment and controllable electron migration between rutile and anatase TiO2. Sci. Rep. 2015, 5, 11482. [Google Scholar] [CrossRef]
- Wang, K.; Janczarek, M.; Wei, Z.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.; Ohtani, B.; Kowalska, E. Morphology- and crystalline composition-governed activity of titania-based photocatalysts: Overview and perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ismail, A.A.; Kowalska, E.; Bahnemann, D.W. A comparative study of microcystin-LR degradation by UV-A, solar and visible light irradiation using bare and C/N/S-modified titania. Catalysts 2019, 9, 877. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Kisch, H. On the problem of comparing rates or apparent quantum yields in heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Wang, K.; Rokicka, P.; Endo, M.; Wei, Z.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photochem. Photobio. B Biol. 2015, 151, 54–62. [Google Scholar] [CrossRef]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyła, S.; Macyk, W. On Oxygen activation at rutile- and anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Janda, K.; Wang, K.; Morawski, A.W.; Kowalska, E. Effect of water activity and titania P25 photocatalyst on inactivation of pathogenic fungi—Contribution to the protection of public health. Cent. Eur. J. Public Health 2015, 23, 67–71. [Google Scholar]
- Guillard, C.; Bui, T.H.; Felix, C.; Moules, V.; Lina, B.; Lejeune, P. Microbiological disinfection of water and air by photocatalysis. Comptes Rendus Chim. 2008, 11, 107–113. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Ulfig, K.; Morawski, A.W. The application of titanium dioxide for deactivation of bioparticulates: An overview. Catal. Today 2011, 169, 249–257. [Google Scholar] [CrossRef]
- Endo, M.; Wei, Z.; Wang, K.; Karabiyik, B.; Yoshiiri, K.; Rokicka, P.; Ohtani, B.; Markowska-Szczupak, A. Noble-metal-modified titania with visible-light activity for the decomposition of microorganisms. Beilstein J. Nanotechnol. 2018, 9, 829–841. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2015, 22, 5519–5530. [Google Scholar] [CrossRef]
- Mohammadalipour, Z.; Rahmati, M.; Khataee, A.; Moosavi, M.A. Different concentrations of titanium dioxide nanoparticles induce autophagy followed by growth inhibition or cell death in A375 melanoma cells. J. Skin Stem Cell. 2017, 4, e63994. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kobayashi, H.; Narita, T.; Konehira, K.; Sonezaki, S.; Kubota, Y.; Terasaka, S.; Iwasaki, Y. Novel Photodynamic therapy using water-dispersed TiO2–polyethylene glycol compound: Evaluation of antitumor effect on glioma cells and spheroids in vitro. Photochem. Photobiol. 2010, 86, 964–971. [Google Scholar] [CrossRef]
- Barough, M.S.; Hasanzadeh, H.; Barati, M.; Pak, F.; Kokhaei, P.; Rezaei-Tavirani, M. Apoptosis/necrosis induction by ultraviolet, in ER Positive and ER negative breast cancer cell lines. Ira J. Cancer Prev. 2015, 8, e4193. [Google Scholar]
- Rowe, P.R.; Glazer, P.M. Emergence of rationally designed therapeutic strategies for breast cancer targeting DNA repair mechanisms. Cancer Res. 2010, 12, 203. [Google Scholar] [CrossRef]
- Cai, R.; Kubota, Y.; Shuin, T.; Sakai, H.; Hashimoto, K.; Fujishima, A. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992, 52, 2346–2348. [Google Scholar]
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Zhang, A.P.; Sun, Y.P. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World J. Gastroenterol. 2004, 10, 3191–3193. [Google Scholar] [CrossRef]
- Lotfian, H.; Nemati, F. Cytoxic effect of TiO2 nanoparticels on breast cancer cell line MCF-7. IOAB J. 2016, 7, 219–224. [Google Scholar]
- Ellingsen, J.E. A study on mechanism of protein adsorption to TiO2. Biomaterials 1991, 12, 593–596. [Google Scholar] [CrossRef]
- Topoglidis, E.; Cass, A.E.G.; Gilardi, G.; Sadeghi, S.; Beaumont, N.; Durrant, J.R. Protein adsorption on nanocrystalline TiO2 films: An immobilization strategy for bioanalytical devices. Anal. Chem. 1998, 70, 5111–5113. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Molecular modelling of protein adsorption on the surface of titanium dioxide polymorphs. Philos. Trans. R. Soc. A 2012, 370, 1444–1462. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 1997, 21, A.3B.1–A.3B.2. [Google Scholar]
- Humphreys, T.R.; Nemeth, A.; McCrevey, S.; Baer, S.C.; Goldberg, L.H. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol. Surg. 1996, 22, 693–697. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska-Szczupak, A.; Wei, Z.; Kowalska, E. The Influence of The Light-Activated Titania P25 on Human Breast Cancer Cells. Catalysts 2020, 10, 238. https://doi.org/10.3390/catal10020238
Markowska-Szczupak A, Wei Z, Kowalska E. The Influence of The Light-Activated Titania P25 on Human Breast Cancer Cells. Catalysts. 2020; 10(2):238. https://doi.org/10.3390/catal10020238
Chicago/Turabian StyleMarkowska-Szczupak, Agata, Zhishun Wei, and Ewa Kowalska. 2020. "The Influence of The Light-Activated Titania P25 on Human Breast Cancer Cells" Catalysts 10, no. 2: 238. https://doi.org/10.3390/catal10020238
APA StyleMarkowska-Szczupak, A., Wei, Z., & Kowalska, E. (2020). The Influence of The Light-Activated Titania P25 on Human Breast Cancer Cells. Catalysts, 10(2), 238. https://doi.org/10.3390/catal10020238







