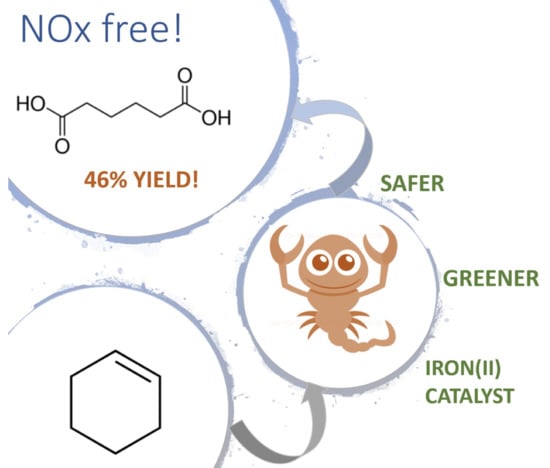
Adipic Acid Route: Oxidation of Cyclohexene vs. Cyclohexane
Abstract
1. Introduction
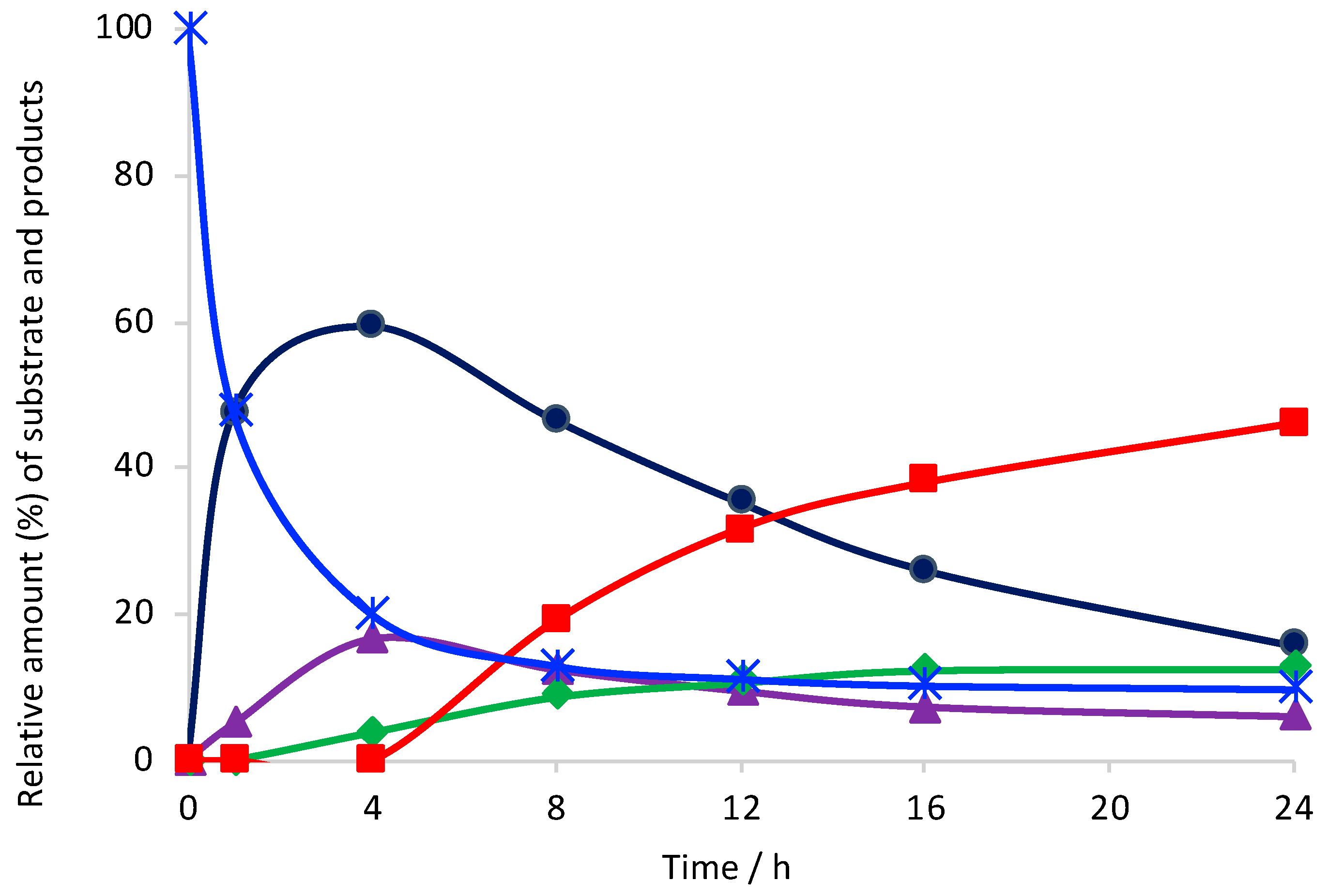
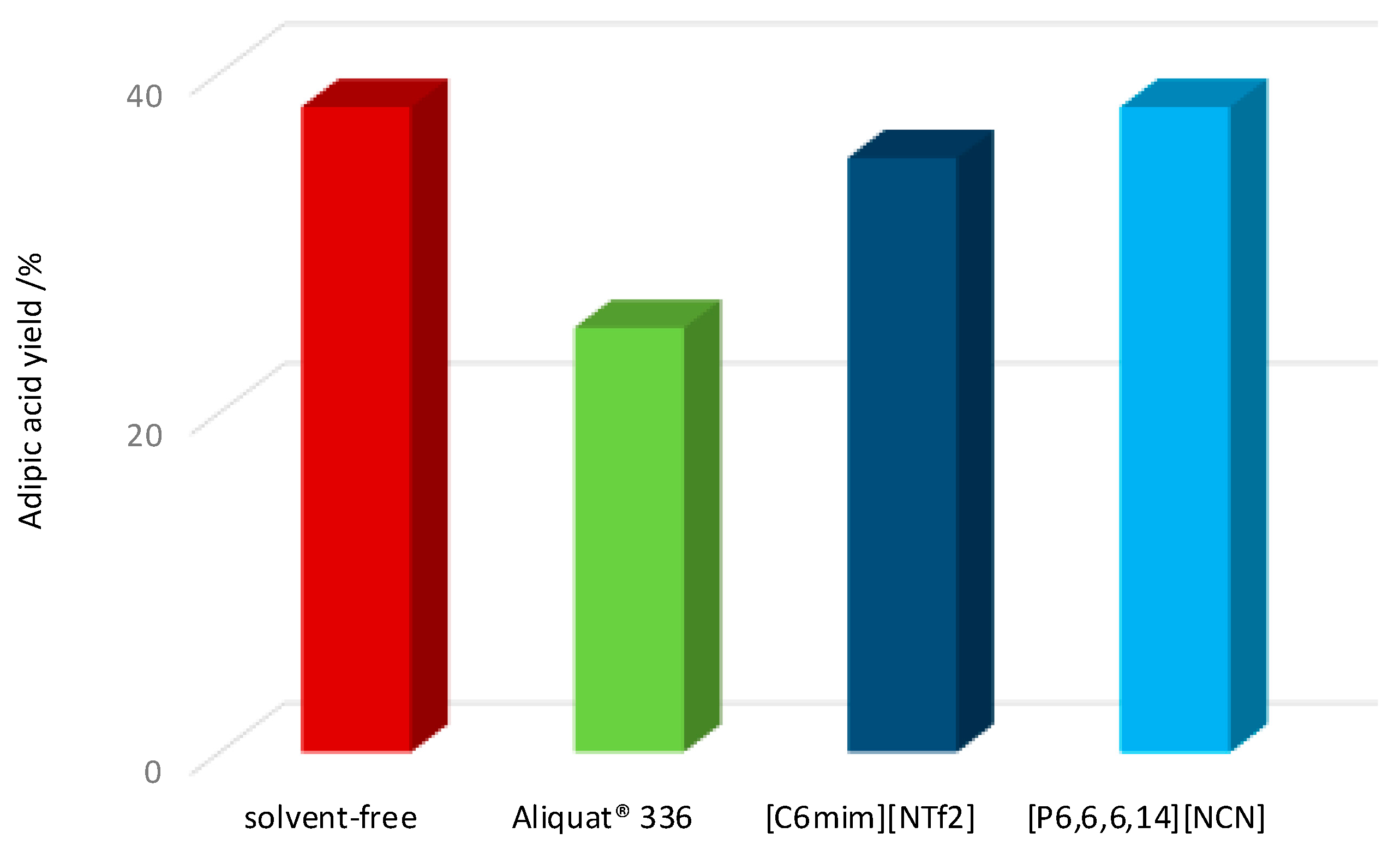
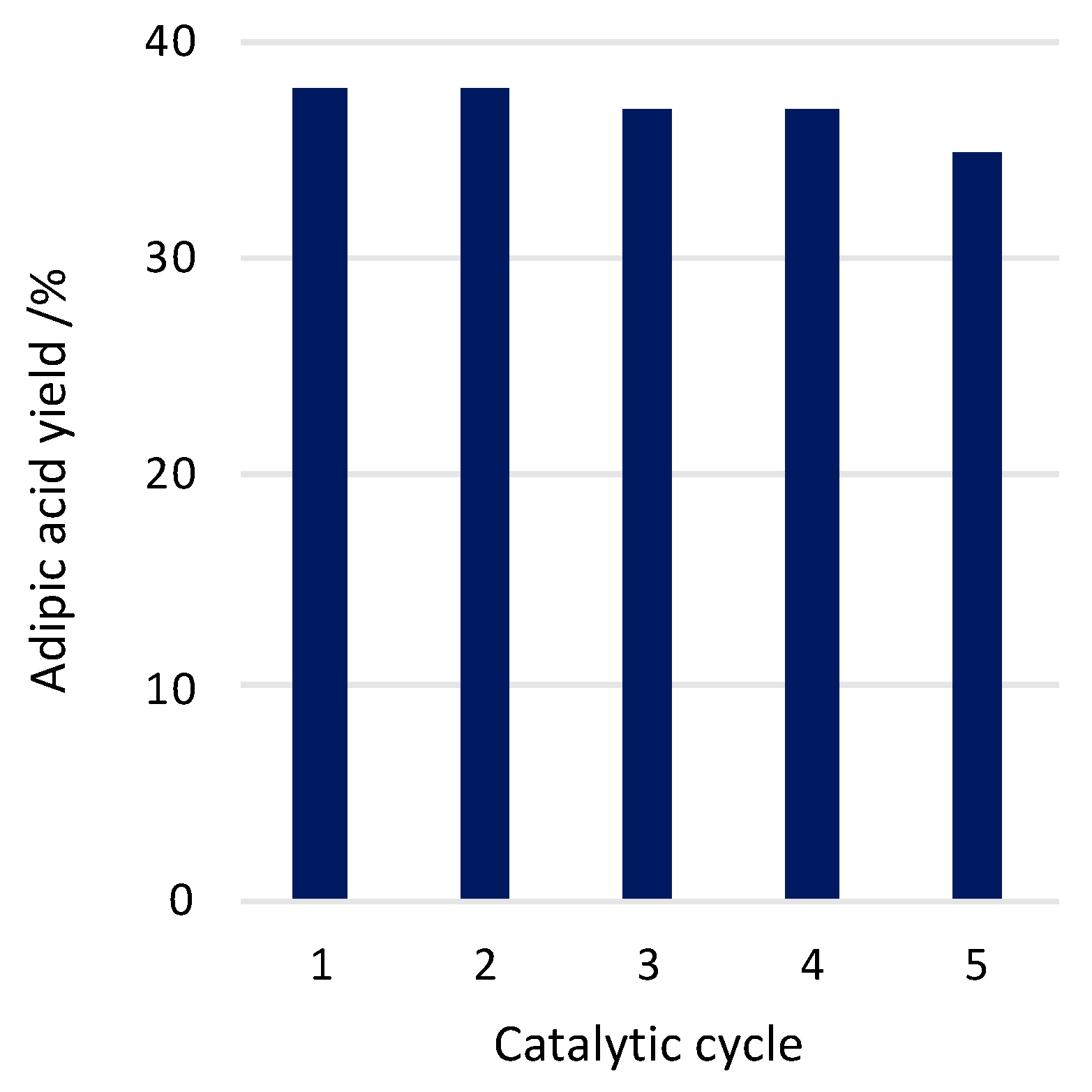
2. Results and Discussion
3. Materials and Methods
General Procedure for the Oxidation of Cyclohexene by Hydrogen Peroxide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 1999–2016.
- Adipic acid (ADPA). World Market Outlook and Forecast up to 2020; Merchant Research and Consulting: Birmingham, UK, 2016. [Google Scholar]
- Hermans, I.; Jacobs, P.A.; Peeters, J. To the core of autocatalysis in cyclohexane autoxidation. Chem. Eur. J. 2006, 12, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Van de Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl) methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Co-ord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-scorpionate complexes: Ever young catalytic tools. Co-ord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate vanadium, iron and copper complexes as selective catalysts for the peroxidative oxidation of cyclohexane under mild conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning cyclohexane oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organometallics 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Zhao, B.Z.; Han, D.K. The N-H functional group in organometallic catalysis. Angew. Chem. Int. Ed. 2013, 52, 4744–4788. [Google Scholar] [CrossRef] [PubMed]
- Dudley, G.B.; Richert, R.; Stiegman, A.E. On the existence of and mechanism for microwave-specific reaction rate enhancement. Chem. Sci. 2015, 6, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. Comptes Rendus Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Almeida, M.P.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-scorpionate Fe(II) complex in carbon materials for cyclohexane oxidation with hydrogen peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 2236–2252. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenised C-scorpionate iron(II) complex on nanostructured carbon materials as catalysts for microwave-assisted oxidation reactions. ChemCatChem 2018, 10, 1821–1828. [Google Scholar] [CrossRef]
- Van-Dúnem, V.; Carvalho, A.P.; Martins, L.M.D.R.S.; Martins, A. Improved cyclohexane oxidation catalyzed by a heterogenised iron(II) complex on hierarchical Y zeolite through surfactant mediated technology. ChemCatChem 2018, 10, 4058–4066. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, M.; Noyori, R. A “Green” Route to Adipic Acid: Direct Oxidation of Cyclohexenes with 30 Percent Hydrogen Peroxide. Science 1998, 281, 1646–1647. [Google Scholar] [CrossRef]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem.Com. 2003, 16, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Hashemi, M.M. One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nano-rods in a Brønsted acidic ionic liquid. RSC Adv. 2015, 5, 31298. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.P.C.; Spada, E.; Bertani, R.; Martins, L.M.D.R.S. Adipic Acid Route: Oxidation of Cyclohexene vs. Cyclohexane. Catalysts 2020, 10, 1443. https://doi.org/10.3390/catal10121443
Ribeiro APC, Spada E, Bertani R, Martins LMDRS. Adipic Acid Route: Oxidation of Cyclohexene vs. Cyclohexane. Catalysts. 2020; 10(12):1443. https://doi.org/10.3390/catal10121443
Chicago/Turabian StyleRibeiro, Ana P. C., Elisa Spada, Roberta Bertani, and Luísa M. D. R. S. Martins. 2020. "Adipic Acid Route: Oxidation of Cyclohexene vs. Cyclohexane" Catalysts 10, no. 12: 1443. https://doi.org/10.3390/catal10121443
APA StyleRibeiro, A. P. C., Spada, E., Bertani, R., & Martins, L. M. D. R. S. (2020). Adipic Acid Route: Oxidation of Cyclohexene vs. Cyclohexane. Catalysts, 10(12), 1443. https://doi.org/10.3390/catal10121443